Reversed-Phase (RP) and Hydrophilic Interaction (HILIC) Separation Mechanisms for the Assay of Nicotine and E-Cigarette Liquids
Abstract
1. Introduction
2. Results
2.1. RP Approach
2.2. HILIC Approach
2.3. Quantitative Studies
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Equipment
4.3. Chromatographic Conditions—Reversed Phase
4.4. Chromatographic Conditions—HILIC
4.5. Samples
4.6. Sample Preparation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RP | Reversed Phase |
| HILIC | Hydrophilic interaction liquid chromatography |
| LOQ | Limit of quantitation |
| HRMS | High resolution mass spectrometry |
| MS-MS | Tandem mass spectrometry |
| LLE | Liquid–liquid extraction |
| SPE | Solid-phase extraction |
| SLE | Supported liquid extraction |
| EP | European Pharmacopoeia |
| FID | Flame Ionization Detector |
| NPD | Nitrogen–Phosphorus Detector (Thermionic Detector) |
| DAD | Diode array detector |
| LC | Liquid chromatography |
| GC | Gas chromatography |
| AcOEt | Ethyl acetate |
| MTBE | Methyl tert-butyl ether |
| BMP | 1-methyl-1-butylpyrrolidinium hexafluorophosphate |
| S.F. | Symmetry factor |
| USP T | United States Pharmacopeia tailing factor |
| ODS, C18 | Octadecyl-functionalized silicagel |
References
- Siegmund, B.; Leitner, E.; Pfannhauser, W. Determination of the nicotine content of various edible nightshades (Solanaceae) and their products and estimation of the associated dietary nicotine intake. J. Agric. Food Chem. 1999, 47, 3113–3120. [Google Scholar] [CrossRef]
- Boyd, R.T. Nicotinic Acetylcholine Receptors in Health and Disease, 1st ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 71–187. [Google Scholar] [CrossRef]
- Hammond, C. Cellular and Molecular Neurophysiology, 4th ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 173–197. [Google Scholar] [CrossRef]
- Crestini, A.; Carbone, E.; Rivabene, R.; Ancidoni, A.; Rosa, P.; Tata, A.M.; Fabrizi, E.; Locuratolo, N.; Vanacore, N.; Lacorte, E.; et al. A Systematic Review on Drugs Acting as Nicotinic Acetylcholine Receptor Agonists in the Treatment of Dementia. Cells 2024, 13, 237. [Google Scholar] [CrossRef]
- Gipson, C.D.; Fowler, C.D. Nicotinic Receptors Underlying Nicotine Dependence: Evidence from Transgenic Mouse Models. In Behavioral Pharmacology of the Cholinergic System; Current Topics in Behavioral Neurosciences; Shoaib, M., Wallace, T., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; Volume 45, pp. 101–121. [Google Scholar] [CrossRef]
- Benowitz, N.L. Pharmacology of nicotine: Addiction, smoking-induced disease, and therapeutics. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Dongelmans, M.; Durand-de Cuttoli, R.; Nguyen, C.; Come, M.; Duranté, E.K.; Lemoine, D.; Brito, R.; Yahia, T.A.; Mondoloni, S.; Didienne, S.; et al. Chronic nicotine increases midbrain dopamine neuron activity and biases individual strategies towards reduced exploration in mice. Nat. Commun. 2021, 12, 6945. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, L.; He, Y.; Zhang, H.; Zhang, Y.; Sheng, H.; Chen, M.; Ru, J.; Gao, Y. Pharmacological effects of nicotine salts on dopamine release in the nucleus accumbens. Front. Pharmacol. 2023, 14, 1279512. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, A.H.; Levin, E.D. Cognitive effects of nicotine. Biol. Psychiat. 2021, 49, 258–267. [Google Scholar] [CrossRef]
- Mishra, A.; Chaturvedi, P.; Datta, S.; Sinukumar, S.; Joshi, P.; Garg, A. Harmful effects of nicotine. Indian. J. Med. Paediatr. Oncol. 2015, 36, 24–31. [Google Scholar] [CrossRef]
- Pinto, M.I.; Thissen, J.; Hermes, N.; Cunningham, A.; Digard, H.; Murphy, J. Chemical characterisation of the vapour emitted by an e-cigarette using a ceramic wick-based technology. Sci. Rep. 2022, 12, 16497. [Google Scholar] [CrossRef]
- Glantz, S.A.; Bareham, D.W. E-Cigarettes: Use, Effects on Smoking, Risks, and Policy Implications. Annu. Rev. Public. Health 2018, 39, 215–235. [Google Scholar] [CrossRef]
- Hahn, J.; Monakhova, Y.B.; Hengen, J.; Kohl-Himmelseher, M.; Schüssler, J.; Hahn, H.; Kuballa, T.; Lachenmeier, D.W. Electronic cigarettes: Overview of chemical composition and exposure estimation. Tob. Induc. Dis. 2014, 12, 23. [Google Scholar] [CrossRef]
- Jo, S.H.; Kim, K.H. Development of a sampling method for carbonyl compounds released due to the use of electronic cigarettes and quantitation of their conversion from liquid to aerosol. J. Chromatogr. A 2016, 1429, 369–373. [Google Scholar] [CrossRef]
- Kubica, P. Determination of Glycerol, Propylene Glycol, and Nicotine as the Main Components in Refill Liquids for Electronic Cigarettes. Molecules 2023, 28, 4425. [Google Scholar] [CrossRef]
- St Helen, G.; Dempsey, D.A.; Havel, C.M.; Jacob, P.; Benowitz, N.L. Impact of e-liquid flavors on nicotine intake and pharmacology of e-cigarettes. Drug Alcohol. Depen. 2017, 178, 391–398. [Google Scholar] [CrossRef]
- Na, C.; Jo, S.; Kim, K.; Sohn, J.; Son, Y. The transfer characteristics of heavy metals in electronic cigarette liquid. Env. Res. 2019, 174, 152–159. [Google Scholar] [CrossRef]
- Aherrera, A.; Lin, J.J.; Chen, R.; Tehrani, M.; Schultze, A.; Borole, A.; Tanda, S.; Goessler, W.; Rule, A.M. Metal Concentrations in E-Cigarette Aerosol Samples: A Comparison by Device Type and Flavor. Env. Health Persp. 2023, 131, 12. [Google Scholar] [CrossRef] [PubMed]
- Re, D.B.; Hilpert, M.; Saglimbeni, B.; Strait, M.; Ilievski, V.; Coady, M.; Talayero, M.; Wilmsen, K.; Chesnais, H.; Balac, O.; et al. Exposure to e-cigarette aerosol over two months induces accumulation of neurotoxic metals and alteration of essential metals in mouse brain. Environ. Res. 2021, 202, 111557. [Google Scholar] [CrossRef] [PubMed]
- Şpaiuc, D.; Şpac, A.; Agoroaei, L.; Butnaru, E. Nicotine Determination From Tabacco By GC/MS. Farmacia 2014, 62, 982–990. [Google Scholar]
- Goniewicz, M.L.; Gupta, R.; Lee, Y.H.; Reinhardt, S.; Kim, S.; Kim, B.; Kosmider, L.; Sobczak, A. Nicotine levels in electronic cigarette refill solutions: A comparative analysis of products from the US, Korea, and Poland. Int. J. Drug Policy 2015, 26, 583–588. [Google Scholar] [CrossRef]
- Famele, M.; Palmisani, J.; Ferranti, C.; Abenavoli, C.; Palleschi, L.; Mancinelli, R.; Fidente, R.; de Gennaro, G.; Draisci, R. Liquid chromatography with tandem mass spectrometry method for the determination of nicotine and minor tobacco alkaloids in electronic cigarette refill liquids and second-hand generated aerosol. J. Sep. Sci. 2016, 40, 1049–1056. [Google Scholar] [CrossRef]
- Gholap, V.V.; Kosmider, L.; Golshahi, L.; Halquist, M.S. Nicotine forms: Why and how do they matter in nicotine delivery from electronic cigarettes? Expert. Opin. Drug Del. 2020, 17, 1727–1736. [Google Scholar] [CrossRef]
- Yildiz, D. Nicotine, its metabolism and an overview of its biological effects. Toxicon 2024, 43, 619–632. [Google Scholar] [CrossRef]
- Bansal, M.; Sharma, M.; Bullen, C.; Svirskis, D. A Stability Indicating HPLC Method to Determine Actual Content and Stability of Nicotine within Electronic Cigarette Liquids. Int. J. Environ. Res. Public Health 2018, 15, 1737. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Kistler, K.A.; Gillman, G.; Voudris, V. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob. Res. 2015, 17, 168–174. [Google Scholar] [CrossRef]
- Barhdadi, S.; Desmedt, B.; Courselle, P.; Rogiers, V.; Vanhaecke, T.; Deconinck, E. A simple dilute-and-shoot method for screening and simultaneous quantification of nicotine and alkaloid impurities in electronic cigarette refills (e-liquids) by UHPLC-DAD. J. Pharm. Biomed. Anal. 2019, 169, 225–234. [Google Scholar] [CrossRef]
- Axente, R.E.; Stan, M.; Chitescu, C.L.; Nitescu, V.G.; Vlasceanu, A.M.; Baconi, D.L. Application of ionic liquids as mobile phase additives for simultaneous analysis of nicotine and its metabolite cotinine in human plasma by HPLC-DAD. Molecules 2023, 28, 1563. [Google Scholar] [CrossRef]
- Baj, J.; Flieger, W.; Przygodzka, D.; Buszewicz, G.; Teresiński, G.; Pizoń, M.; Maciejewski, R.; Flieger, J. Application of HPLC-QQQ-MS/MS and New RP-HPLC-DAD System Utilizing the Chaotropic Effect for Determination of Nicotine and Its Major Metabolites Cotinine, and trans-3′-Hydroxycotinine in Human Plasma Samples. Molecules 2022, 27, 682. [Google Scholar] [CrossRef]
- Taylor, C.; Woodmansey, K. Superior Separation of Nicotine and Tobacco Related Alkaloids by Utilizing The Selectivity of Hydrophilic Interaction Chromatography (HILIC). ThermoScientific Technical Note, TN21998-EN 1219S. 2019. Available online: https://assets.thermofisher.com/TFS-Assets/CMD/Technical-Notes/tn-21998-hilic_nicotine-tobacco-related-alkaloids-tn21998-en.pdf (accessed on 16 June 2025).
- Chang, M. Modified QuEChERS for HILIC LC/MS/MS Analysis of Nicotine and Its Metabolites in Fish. Agilent Technologies Application Note, 5991-2408EN. 2013. Available online: https://www.agilent.com/cs/library/applications/5991-2408EN.pdf (accessed on 16 June 2025).
- Taujenis, L.; Olšauskaitė, V.; Padarauskas, A. Determination of Nicotine and Three Minor Alkaloids in Tobacco by Hydrophilic Interaction Chromatography-Tandem Mass Spectrometry. Acta Chromatogr. 2015, 27, 373–385. [Google Scholar] [CrossRef]
- Mallock, N.; Rabenstein, A.; Laux, P.; Rüther, T.; Hutzler, C.; Parr, M.K.; Luch, A. Rapid, sensitive, and reliable quantitation of nicotine and its main metabolites cotinine and trans-3′-hydroxycotinine by LC-MS/MS: Method development and validation for human plasma. J. Chromatogr. B 2021, 1179, 122736. [Google Scholar] [CrossRef] [PubMed]
- Najafi, R.B.; Saghaie, L.; Mollabashi, N. Formulation and pharmaceutical evaluation of ferric-maltol floating table. J. Rep. Pharm. Sci. 2014, 3, 126–140. [Google Scholar] [CrossRef]
- Liteanu, C.; Rica, I. Statistical Theory and Methodology of Trace Analysis; Ellis Horwood; Halsted Press: Chichester, NY, USA, 1980; ISBN 0853121087. [Google Scholar]
- Lazăr, P.; Udrescu, Ș.; Tache, F.; Albu, F.; Grinberg, N.; Medvedovici, A. Revisiting large volume injection in non-miscible diluents: An on-line reversed phase supported liquid extraction/liquid chromatography scenario. Anal. Meth. 2015, 7, 342–352. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Validation and Verification of Analytical Testing Methods Used for Tobacco Products: Guidance for Industry. January 2025. Docket No. FDA-2021-D-0756. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/validation-and-verification-analytical-testing-methods-used-tobacco-products (accessed on 9 August 2025).
- Standard Operating Procedure for Determination of Nicotine, Glycerol and Propylene Glycol Content in the Tobacco of Heated Tobacco products. WHO TobLabNet Official Method SOP15. World Health Organization: Geneva. 2023. Available online: https://www.who.int/publications/i/item/9789240079304 (accessed on 9 August 2025).
- 01/2009:1452 corrected 6.6; Nicotine. Council of Europe European Directorate for the Quality of Medicines and Healthcare: Strasbourg, France, 2012.
- Green, B.T.; Lee, S.T.; Panter, K.E.; Brown, D.R. Piperidine alkaloids: Human and food animal teratogens. Food Chem. Toxicol. 2012, 50, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Paweł Kubica, P.; Kot-Wasik, A.; Wasik, A.; Namiesnik, J. “Dilute & Shoot” approach for rapid determination of trace amounts of nicotine in zero-level e-liquids by reversed phase liquid chromatography and hydrophilic interactions liquid chromatography coupled with tandem mass spectrometry-electrospray ionization. J. Chromatogr. A 2013, 1289, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Van der Velpen, V.; Liakoni, E.; Hirt, M.B.; Vonwyl, C.M.; Christen, S.E.; Duthaler, U.; Jacob, P.; Haschke, M. A validated single-step saliva and serum sample extraction LC-MS/MS method for the analysis of nicotine, cotinine and 3′-hydroxycotinine for clinical vaping studies. J. Pharm. Biomed. Anal. 2025, 258, 116703. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, I.A.; Hammell, D.C.; Stinchcomb, A.L.; Hassan, H.E. A fully validated LC–MS/MS method for simultaneous determination of nicotine and its metabolite cotinine in human serum and its application to a pharmacokinetic study after using nicotine transdermal delivery systems with standard heat application in adult smokers. J. Chromatogr. B 2016, 1020, 67–77. [Google Scholar] [CrossRef]
- Loukotková, L.; VonTungeln, L.S.; Vanlandingham, M.; da Costa, G.G. A simple and highly sensitive UPLC-ESI-MS/MS method for the simultaneous quantification of nicotine, cotinine, and the tobacco-specific carcinogens N’-nitrosonornicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in serum samples. J. Chromatogr. B 2018, 1072, 229–234. [Google Scholar] [CrossRef]
- Dobrinas, M.; Choong, E.; Noetzli, M.; Cornuz, J.; Ansermot, N.; Eap, C.B. Quantification of nicotine, cotinine, trans-3-hydroxycotinine and varenicline in human plasma by a sensitive and specific UPLC-tandem mass-spectrometry procedure for a clinical study on smoking cessation. J. Chromatogr. B 2011, 879, 3574–3582. [Google Scholar] [CrossRef]
- Shou, W.Z.; Naidong, W. Simple means to alleviate sensitivity loss by trifluoroacetic acid (TFA) mobile phases in the hydrophilic interaction chromatography-electrospray tandem mass spectrometric (HILIC-ESI/MS/MS) bioanalysis of basic compounds. J. Chromatogr. B 2005, 825, 186–192. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, N.; Prinyawiwatkul, W.; Xu, Z. A rapid LC-MS/MS method for simultaneous determination of nicotine and its key derivatives including hydroxylation isomers. Int. J. Mass Spectrom. 2021, 468, 116642. [Google Scholar] [CrossRef]
- Piller, M.; Gilch, G.; Scherer, G.; Scherer, M. Simple, fast and sensitive LC-MS/MS analysis for the simultaneous quantification of nicotine and 10 of its major metabolites. J. Chromatogr. B 2014, 951–952, 7–15. [Google Scholar] [CrossRef]
- Tretzel, L.; Thomas, A.; Piper, T.; Hedeland, M.; Geyer, H.; Schänzer, W.; Thevis, M. Fully automated determination of nicotine and its major metabolitesin whole blood by means of a DBS online-SPE LC-HR-MS/MS approach for sports drug testing. J. Pharm. Biomed. Anal. 2016, 123, 132–140. [Google Scholar] [CrossRef]
- Baumann, F.; Regenthal, R.; Burgos-Guerrero, I.L.; Hegerl, U.; Preiss, R. Determination of nicotine and cotinine in human serum by means of LC/MS. J. Chromatogr. B 2010, 878, 107–111. [Google Scholar] [CrossRef] [PubMed]
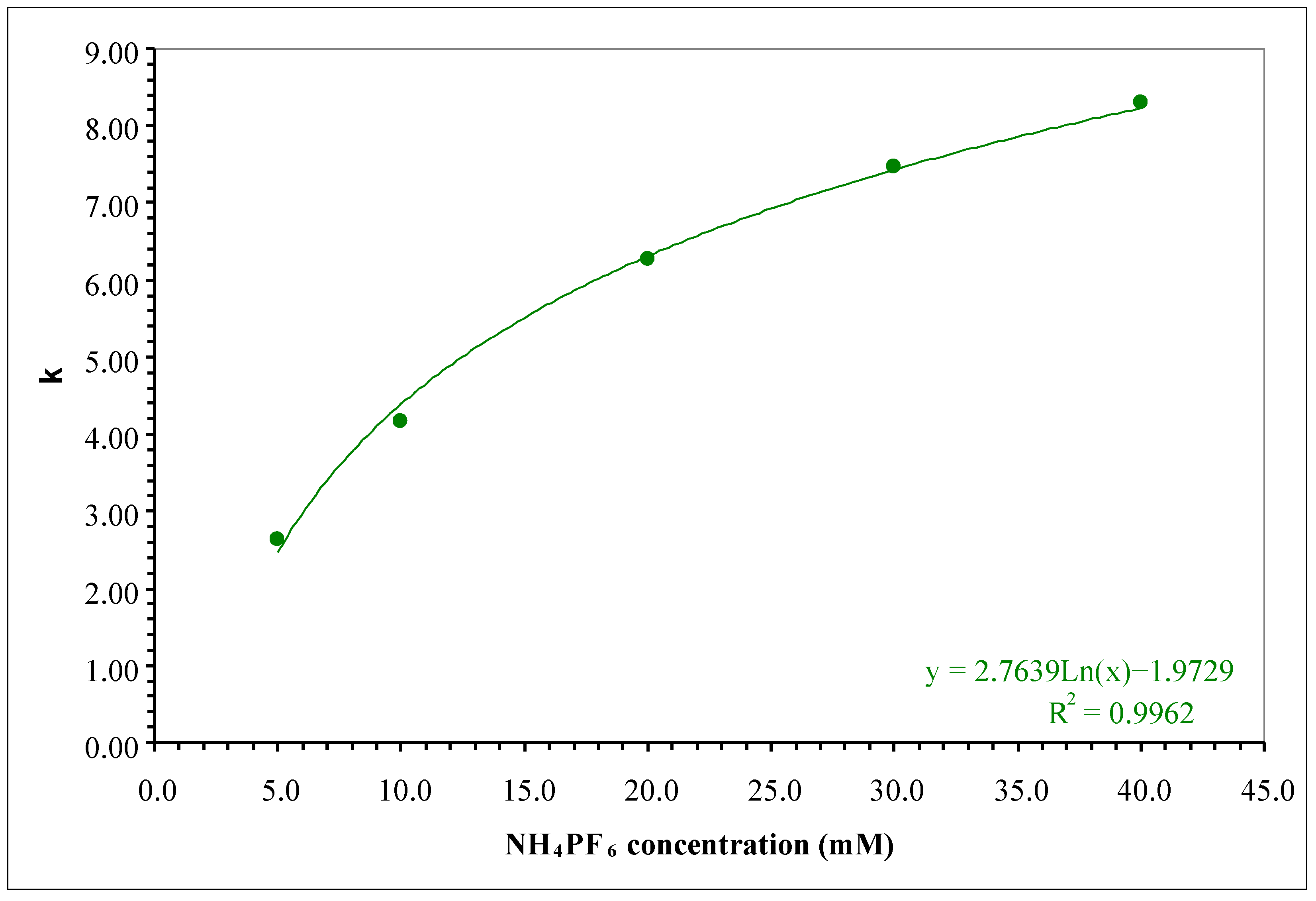

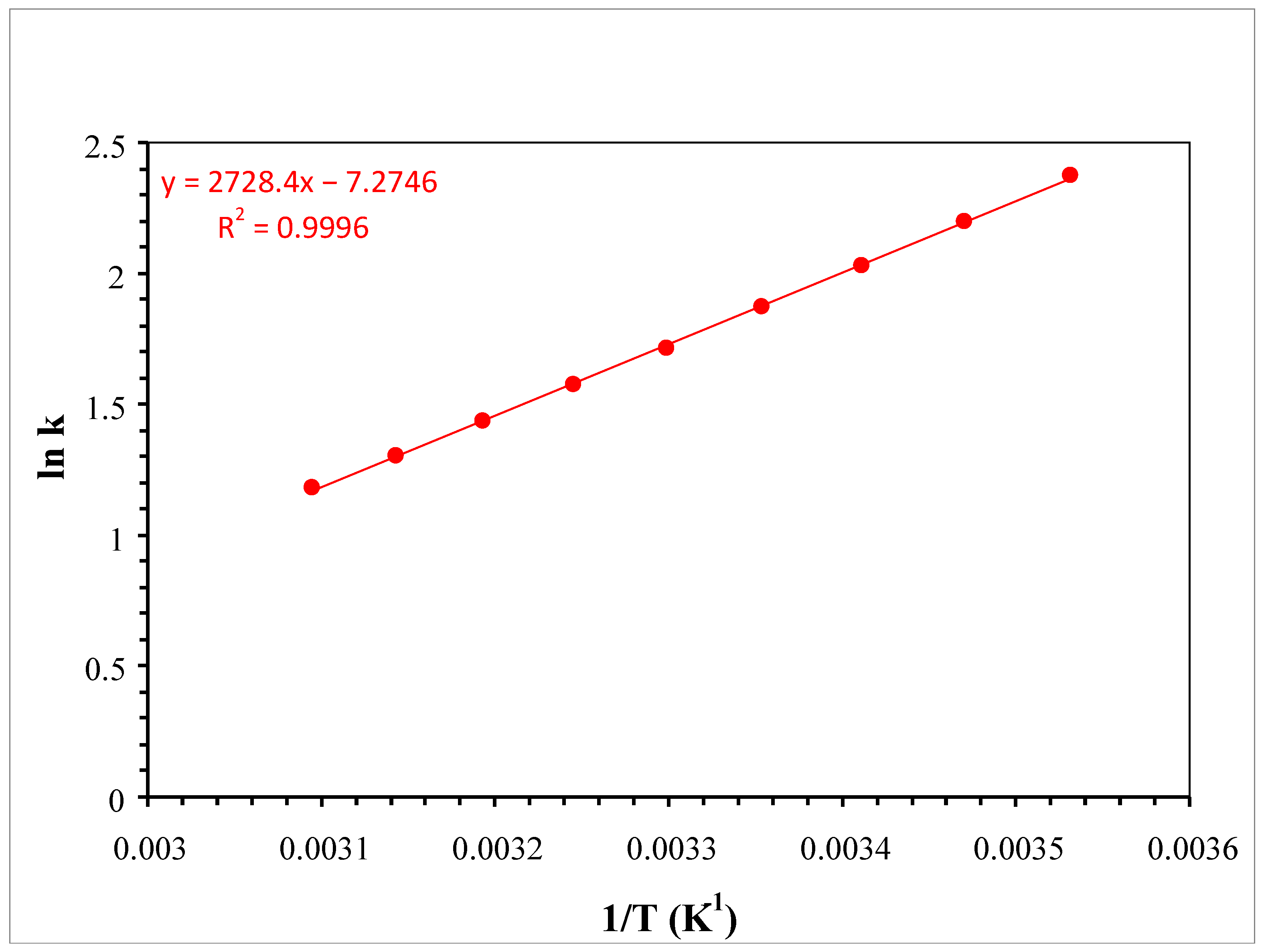
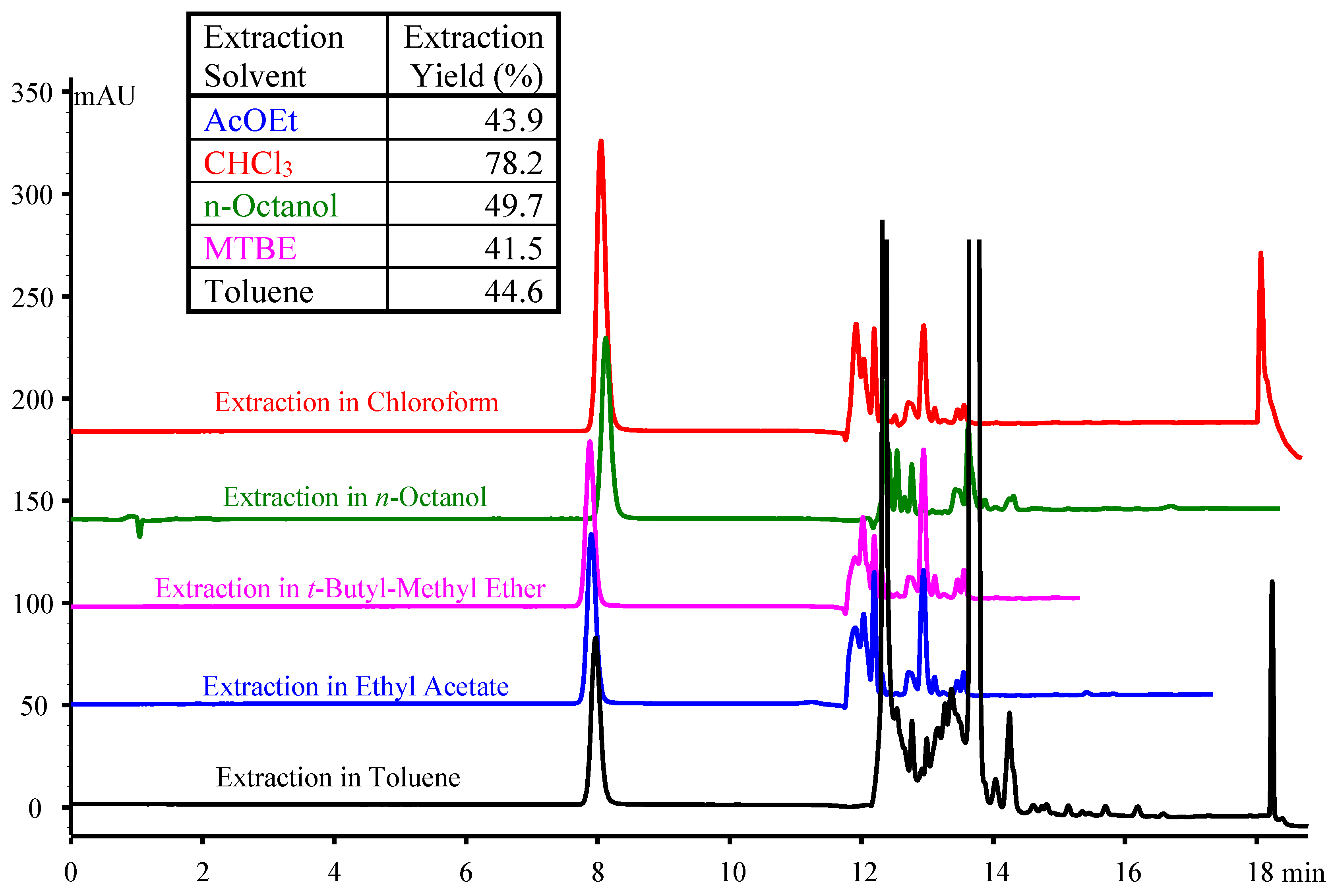

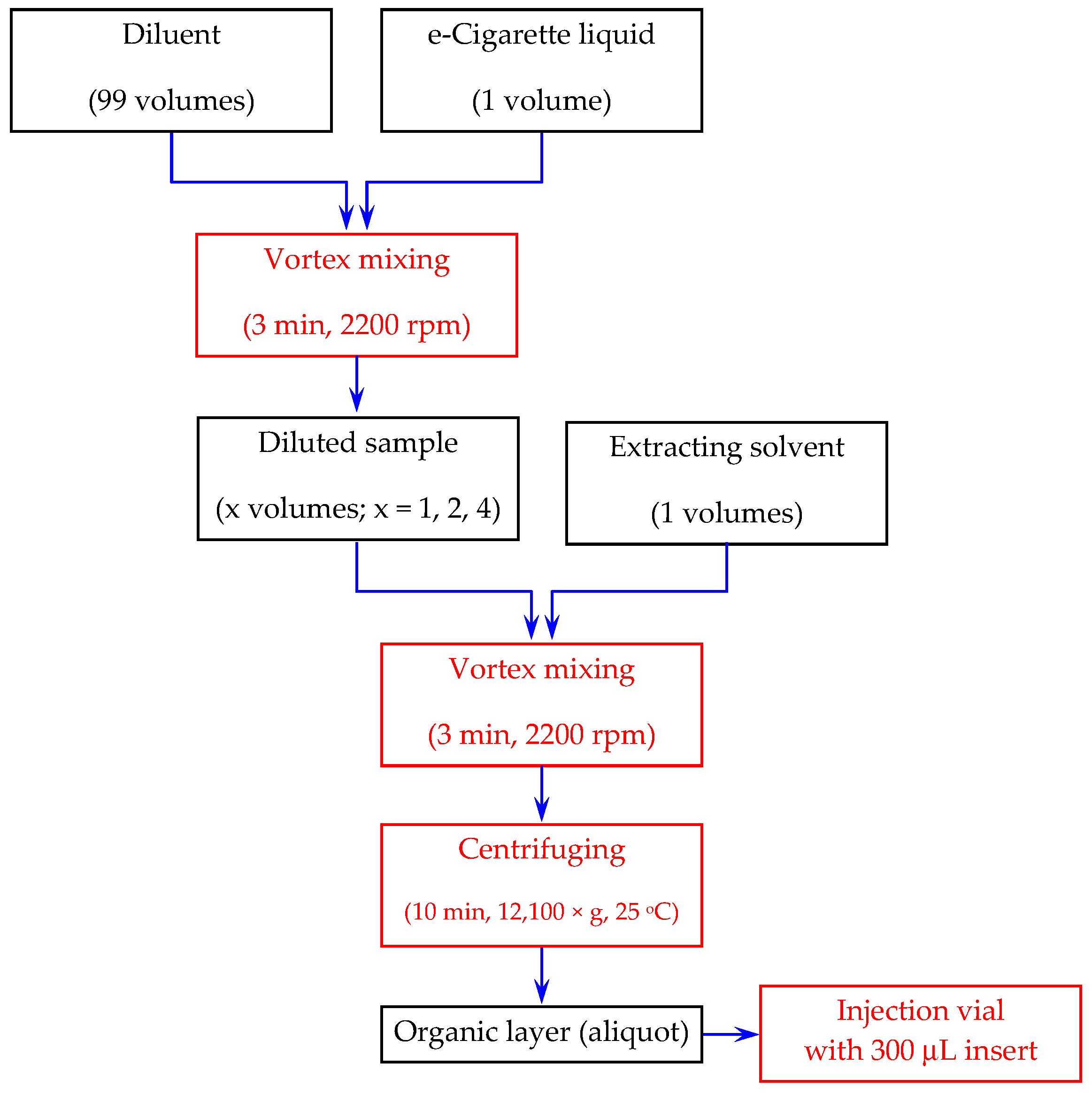
| # | Additive in the Aqueous Component of the Mobile Phase | Organic Modifier | tR (min) | k | S.F. | USP T.F. | N (Plates) |
|---|---|---|---|---|---|---|---|
| 1 | H3PO4 0.1% | ACN | 1.187 | 0.11 | 1.120 | 1.102 | 3376 |
| 2 | HClO4 0.1% | ACN | 1.774 | 0.66 | 1.253 | 1.198 | 7622 |
| 3 | HCOOH 0.1% | ACN | 1.128 | 0.05 | 0.845 | 0.929 | 2362 |
| 4 | KPF6 20 mM + HClO4 0.1% | ACN | 7.216 | 5.74 | 1.404 | 1.212 | 10,860 |
| 5 | NH4PF6 20 mM + HCOOH 0.1% | ACN | 7.792 | 6.27 | 1.398 | 1.210 | 13,960 |
| 6 | NH4PF6 20 mM + H3PO4 0.1% | ACN | 6.416 | 4.99 | 1.155 | 1.097 | 12,259 |
| 7 | NH4BF4 20 mM + HCOOH 0.1% | ACN | 2.600 | 1.43 | 0.567 | 0.787 | 8671 |
| 8 | NH4PF6 20 mM + HCOOH 0.1% | MeOH | 4.575 | 3.27 | 0.927 | 0.986 | 11,405 |
| 9 | BMPPF6 20 mM + HCOOH 0.1% | MeOH | 2.890 | 1.70 | 1.027 | 1.026 | 10,280 |
| Variable | RP | HILIC | ||||||
|---|---|---|---|---|---|---|---|---|
| Neat Solutions | Toluene Extracts | Neat Solutions | Toluene Extracts | |||||
| Linear | Linear | Linear | Linear | |||||
| Calibration levels | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| x min (μg/mL) | 1 | 1 | 1 | 1 | 1.1 | 1.1 | 1 | 1 |
| x max (μg/mL) | 1000 | 1000 | 1000 | 1000 | 550 | 550 | 1000 | 1000 |
| B (slope) | 1.5640 | 1.5206 | 2.9334 | 2.9429 | 1.3251 | 1.4527 | 2.8200 | 2.6878 |
| A (intercept) | 2.4014 | −0.5075 | 1.6295 | 1.2406 | 13.6880 | 2.1176 | −0.1448 | 7.3283 |
| rxy (correlation coeff.) | 0.9977 | 0.9934 | 0.9999 | 0.99998 | 0.9961 | 0.9948 | 0.99988 | 0.9971 |
| S0 | 40.9021 | 0.1790 | 1.8391 | 0.0210 | 26.0442 | 0.1537 | 17.2405 | 0.2087 |
| SB (st. dev. slope) | 0.0430 | 0.2032 | 0.0019 | 0.0238 | 0.0477 | 0.1717 | 0.0181 | 0.2369 |
| SA (st. dev. intercept) | 17.0775 | 0.5604 | 0.7679 | 0.0657 | 12.4704 | 0.5231 | 7.1983 | 0.6532 |
| LOQ (1) | 58.7 | 1.4 | 1.6 | 0.1 | 53.7 | 1.4 | 14.8 | 1.0 |
| LOQ (2) | 109.2 | 3.7 | 2.6 | 0.2 | 94.1 | 3.6 | 25.5 | 2.4 |
| LOQ (3) | 107.7 | 4.0 | 2.1 | -0.2 | 83.8 | 2.1 | 25.6 | −0.3 |
| Bias% min (level) | −188.9 (1) | −15.6 (3) | −13.5 (1) | −1 (4) | −785.2 (1) | −12.3 (4) | 259.8 (1) | −13.4 (3) |
| Bias% max (level) | 11.1 (7) | 14.7 (7) | 0.9 (6) | 0.8 (2) | 18.7 (6) | 14.4 (3) | −2.57 (7) | 10.7 (2) |
| RSD% interval (n = 3) | 0.9–4.8 % | 0.7–6.8 % | ||||||
| E-Cigarette Sample (20 mg/mL) | BI | BR | BT | CT | LB | |
|---|---|---|---|---|---|---|
| RP | conc. (μg/mL) | 185.91 | 193.29 | 187.22 | 181.99 | 163.90 |
| Bias % | −7.0% | −3.4% | −6.4% | −9.0% | −18.1% | |
| HILIC | conc. (μg/mL) | 195.20 | 204.69 | 199.95 | 191.09 | 175.68 |
| Bias % | −2.4% | 2.3% | −0.025% | −4.5% | −12.2% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moisi, R.; Comănescu, M.-A.; Medvedovici, A.-V. Reversed-Phase (RP) and Hydrophilic Interaction (HILIC) Separation Mechanisms for the Assay of Nicotine and E-Cigarette Liquids. Molecules 2025, 30, 3443. https://doi.org/10.3390/molecules30163443
Moisi R, Comănescu M-A, Medvedovici A-V. Reversed-Phase (RP) and Hydrophilic Interaction (HILIC) Separation Mechanisms for the Assay of Nicotine and E-Cigarette Liquids. Molecules. 2025; 30(16):3443. https://doi.org/10.3390/molecules30163443
Chicago/Turabian StyleMoisi, Răzvan, Mircea-Alexandru Comănescu, and Andrei-Valentin Medvedovici. 2025. "Reversed-Phase (RP) and Hydrophilic Interaction (HILIC) Separation Mechanisms for the Assay of Nicotine and E-Cigarette Liquids" Molecules 30, no. 16: 3443. https://doi.org/10.3390/molecules30163443
APA StyleMoisi, R., Comănescu, M.-A., & Medvedovici, A.-V. (2025). Reversed-Phase (RP) and Hydrophilic Interaction (HILIC) Separation Mechanisms for the Assay of Nicotine and E-Cigarette Liquids. Molecules, 30(16), 3443. https://doi.org/10.3390/molecules30163443








