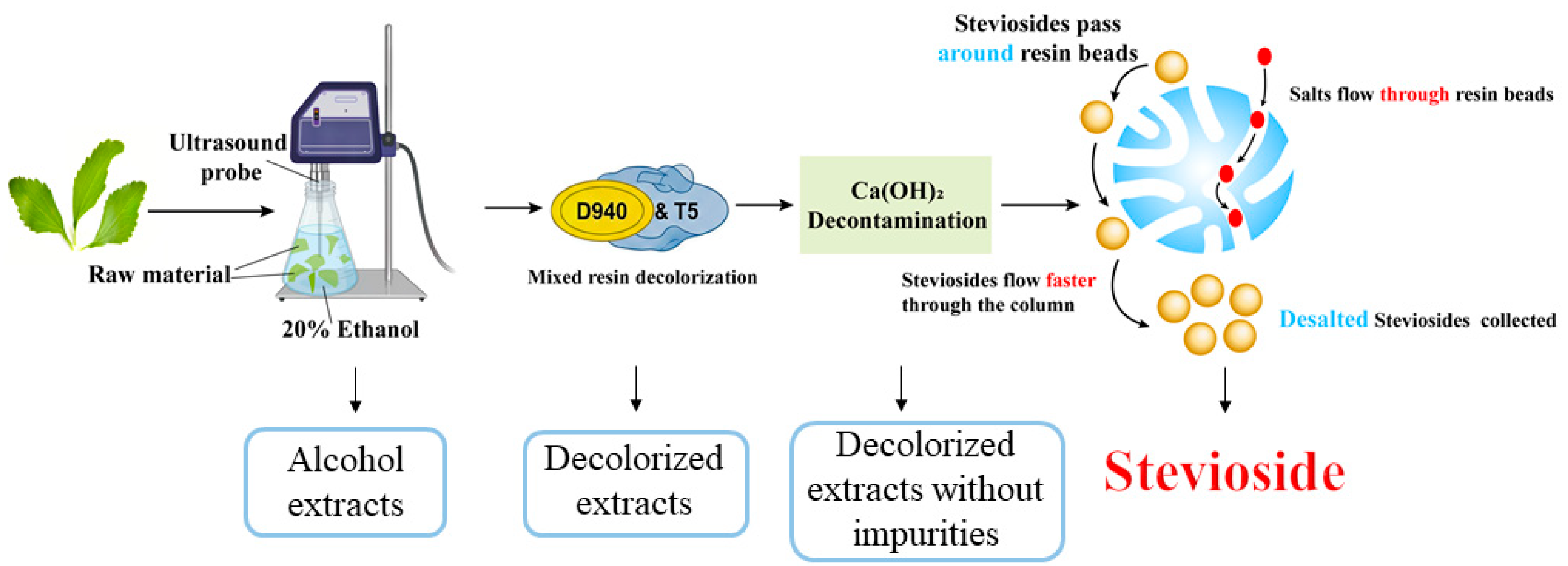
Despite notable progress in steviol glycoside extraction technologies, challenges such as low extraction efficiency, high energy consumption, elevated operational costs, environmental impact, and inconsistent product quality persisted, necessitating further optimization. This study systematically addressed these limitations by optimizing the extraction solvent, temperature, and material-to-liquid ratio, followed by decolorization, decontamination, and desalting, to enhance the yield and purity of steviol glycoside from Stevia rebaudiana leaves.
2.1. Optimization of Stevia Leaf Extraction
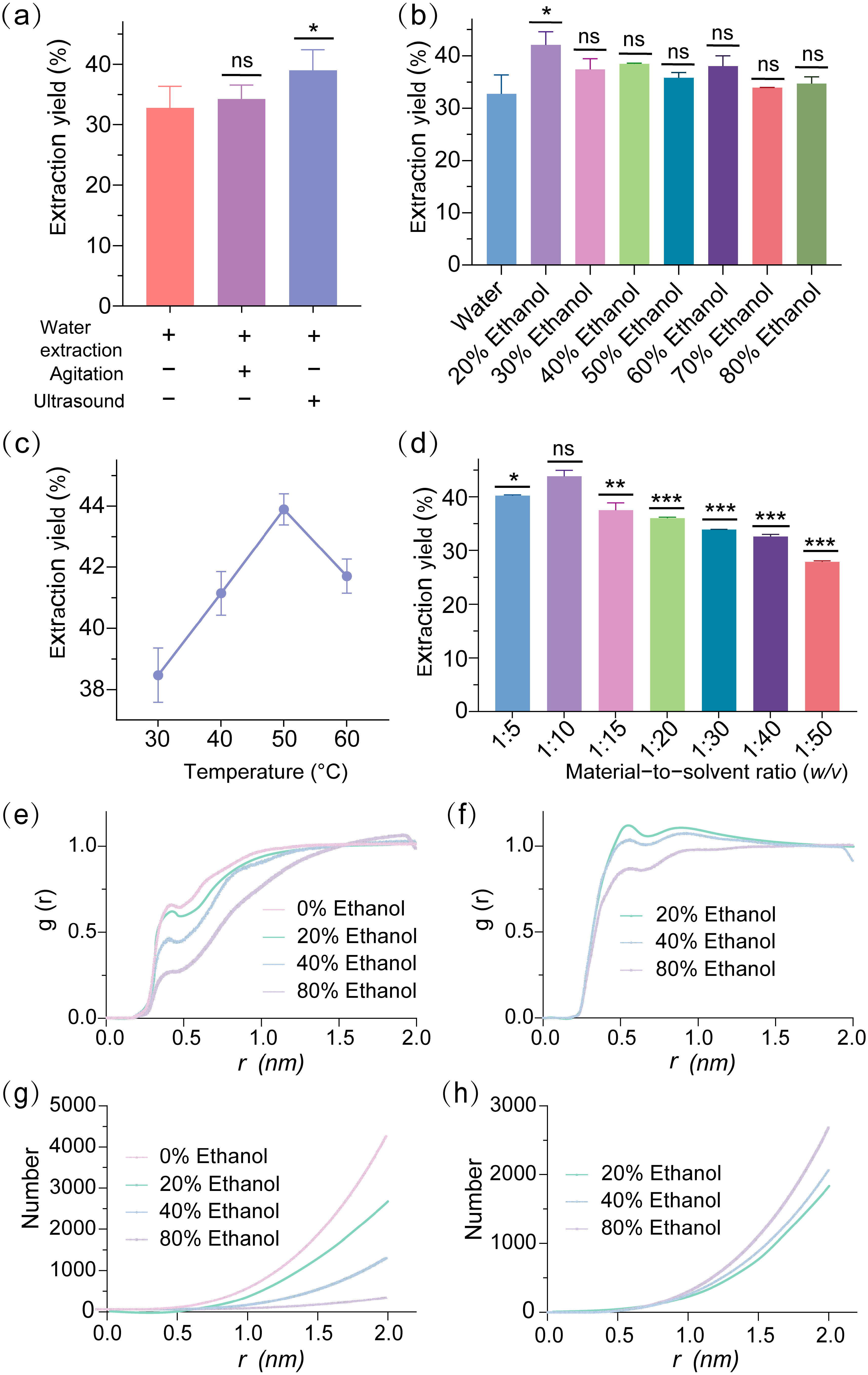
Comparison of water extraction with and without ultrasound (
Figure 1a) revealed a modest yield increase from 32.7% to 39.0% with ultrasonic assistance, highlighting its potential to improve efficiency. Effect of ethanol concentration on extraction yield (
Figure 1b) identified 20% ethanol as the optimal solvent, achieving the highest yield of 42.1%, significantly outperforming other concentrations (e.g., 50% at 35.8%, 80% at 34.7%), suggesting an optimal balance of solvent polarity and solubility. Optimization of extraction temperature using 20% ethanol with ultrasonic assistance (
Figure 1c) peaked at 50 °C with a yield of 43.9%, compared to 38.5% at 30 °C, indicating a temperature-driven enhancement in mass transfer and solubility. Evaluation of material-to-liquid ratio (
Figure 1d) established 1:10 as optimal, yielding 43.8% compared to 40.2% at 1:5, underscoring the importance of sufficient solvent volume for effective extraction. Additionally, the ability to remove proteins to some extent with 20% ethanol assisted by ultrasound suggests that the flocculation step can be bypassed in industrial processes, thereby minimizing the loss of steviol glycosides during purification. Comparison of extraction yield before and after optimization confirmed that the optimized process increased the yield in a 200 mL system from 32.0% to 49.1%, a 53.4% improvement, validating the efficacy of the integrated approach. Compared to Wen et al. [
4], who reported UAE yields of 35–40% with water, our 49.1% yield with 20% ethanol reflects enhanced solvent–stevioside interactions. Unlike MAE [
5], which requires high energy and equipment costs, UAE is more scalable. PHWE [
6] achieved similar yields but used higher temperatures, increasing energy consumption. Our method balances efficiency and sustainability.
To further elucidate solvent effects, molecular dynamics (MD) simulations and radial distribution function (RDF) analyses were conducted to investigate the interactions between steviol glycosides and solvents in 0%, 20%, 40%, and 80% ethanol–water mixtures. The RDF profiles for water–steviol glycoside interactions (
Figure 1e) revealed that within the first solvation shell (
r ranged from 0.3 to 0.6 nm), the 0% ethanol condition exhibited the highest g(r) peak value of 0.647, indicating the formation of a robust and densely packed hydrogen bonding network between water molecules and steviol glycosides, reflective of elevated local density under pure water conditions. The 20% ethanol condition displayed a slightly lower peak value of 0.565, characterized by a sharper and narrower peak, suggesting a more concentrated distribution of water molecules and an enhanced hydrogen bonding interaction, which aligns with experimental observations of 20% ethanol as the optimal extraction condition, likely due to its provision of an ideal hydrophobic–hydrophilic balance that facilitates the dissolution and extraction efficiency of steviol glycosides. In contrast, the 40% ethanol condition showed a significantly reduced peak value of 0.452, with a gradual curve ascent, indicating a weakened hydrogen bonding interaction between water and steviol glycosides. The 80% ethanol condition presented a peak value of 0.263, suggesting a progressive weakening of the water–steviol glycoside hydrogen bonding and the introduction of a competitive solvent effect with increasing ethanol concentration, thereby reducing extraction efficiency. The RDF profiles for ethanol–steviol glycoside interactions (
Figure 1f) demonstrated that at
r ranged from 0.3 to 0.5 nm, the 20% ethanol condition exhibited the highest g(r) peak value of 1.135, indicating limited direct interaction between ethanol molecules and steviol glycosides at low concentrations. However, this characteristic may indirectly optimize the extraction process by enhancing the dominant role of water molecules. The 40% ethanol condition displayed a modestly lower peak value of 1.029, with a steeper rise, suggesting an increased involvement of ethanol molecules in the interaction, though this did not translate into improved extraction efficiency. The 80% ethanol condition showed a peak value of 0.871, lower than that of 40%, reflecting a constrained interaction strength but an extended distribution range, potentially attributable to competitive dynamics and spatial repulsion among ethanol molecules at higher concentrations, which likely contributed to a decline in extraction performance.
The coordination number (CN) profiles for water–steviol glycoside interactions (
Figure 1g) indicated that the 0% ethanol condition stabilized at a CN of approximately 4000 at r = 2.0 nm, with a smooth ascending trend, suggesting the formation of an extensive solvation layer by water molecules around steviol glycosides in a pure water system. However, this broad distribution may not have achieved optimal extraction efficiency. The 20% ethanol condition exhibited a CN of about 2500, with a modest reduction and a relatively smooth curve, reflecting a moderate decrease in water molecule numbers yet an orderly distribution, a feature consistent with the highest extraction yield observed at 20% ethanol (
Figure 1b), highlighting its optimized solvent environment. The 40% ethanol condition declined to a CN of approximately 1000, with a slower rise, indicating a significant reduction in water molecule numbers, while the 80% ethanol condition further decreased to a CN of about 300, with an almost flat curve, underscoring a substantial diminution in water molecule coordination capacity and an unfavorable shift in the solvent environment due to increased ethanol content. CN profiles for ethanol–steviol glycoside interactions (
Figure 1h) revealed that the 80% ethanol condition stabilized at a CN of approximately 2500 at r = 2.0 nm, with a rapid initial rise followed by a plateau, suggesting the formation of a robust coordination layer by ethanol molecules at high concentrations, though this did not enhance extraction efficiency. The 40% ethanol condition showed a CN of about 2000, with a slightly slower rise, indicating a moderate coordination capacity at intermediate ethanol levels. The 20% ethanol condition displayed the lowest CN of approximately 1800, with a gradual increase, reflecting a limited coordination role of ethanol molecules at low concentrations; however, this limitation was compensated by the synergistic action of water molecules, achieving optimal extraction efficiency, consistent with the RDF observations in
Figure 1f.
2.2. Optimization of Decolorization Process
Stevioside extracts typically contained a substantial amount of impurities, including chlorophyll, flavonoids, and pigments, which compromised the quality and purity of steviosides, necessitating effective decolorization methods to achieve high-purity steviol glycosides [
22,
23]. Activated carbon, a porous material known for its large specific surface area and strong adsorption capacity, has been widely utilized in the decolorization of natural plant extracts such as grape seed extract, tea polyphenols, and
Poria cocos extract [
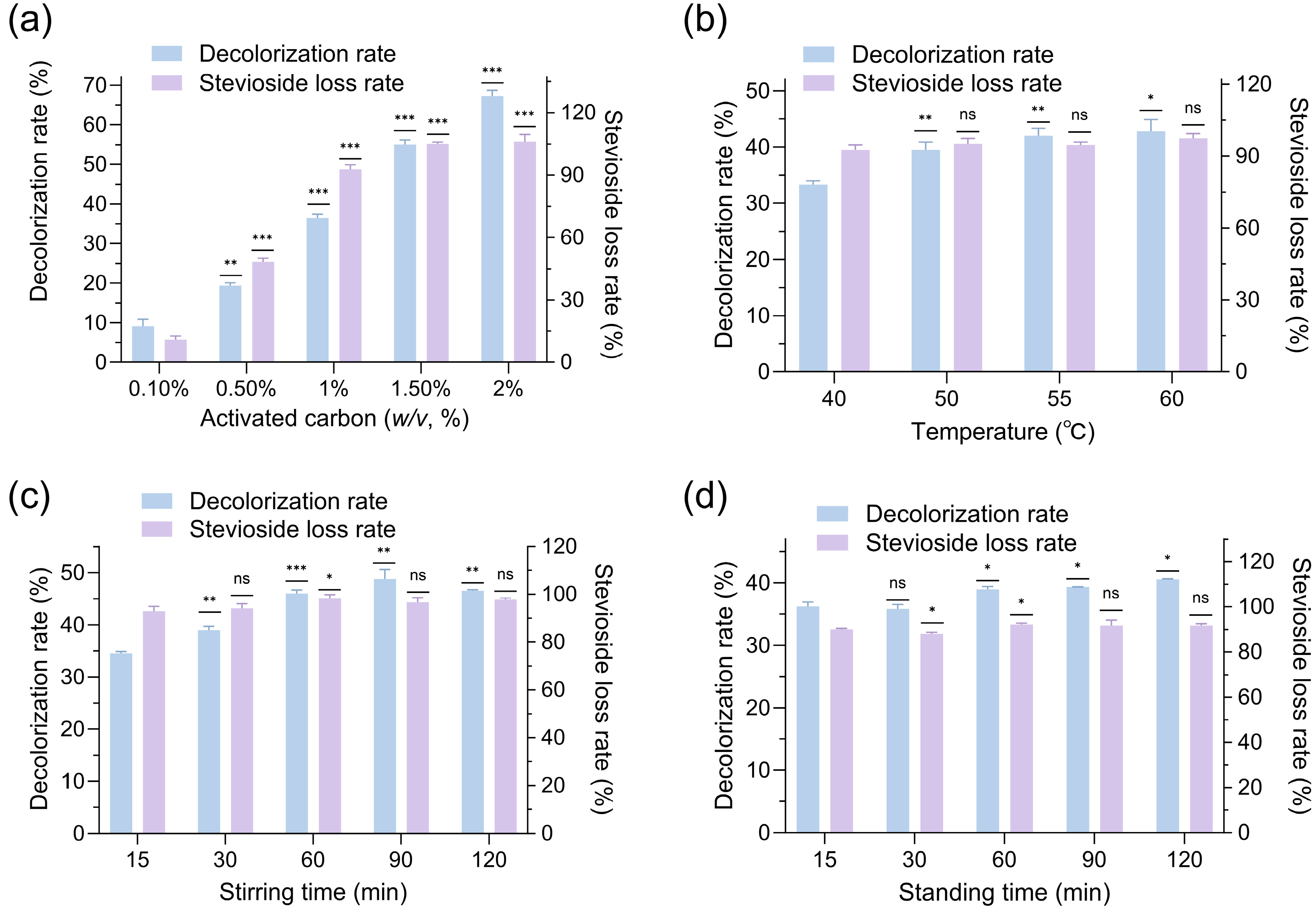
24]. In this study, activated carbon was employed to decolorize stevioside extracts, but the results revealed significant drawbacks. Effect of activated carbon dosage on decolorization and stevioside loss rates (
Figure 2a) showed that at 1.5–2.0% activated carbon, the decolorization rate ranged from 55.0% to 67.2%, while steviosides were almost entirely adsorbed, indicating that activated carbon non-selectively adsorbed steviosides alongside pigments. Influence of decolorization temperature on activated carbon performance (
Figure 2b) demonstrated that the stevioside loss rate remained consistently high at 92.6–97.4% across temperatures (40–60 °C), suggesting temperature had minimal impact on reducing stevioside adsorption. Effect of stirring time on activated carbon decolorization (
Figure 2c) revealed that stevioside loss rates stayed above 93% for stirring durations of 15–120 min, further confirming the strong, non-specific adsorption behavior of activated carbon. Impact of standing time on activated carbon decolorization (
Figure 2d) indicated that the decolorization rate reached a maximum value of 40.5% with 30 min of stirring and 120 min of standing at 50 °C, while stevioside loss remained unacceptably high at 91.7%. These findings underscored that activated carbon was unsuitable for stevioside decolorization due to its excessive adsorption of the target compound, prompting the exploration of alternative decolorizing agents.
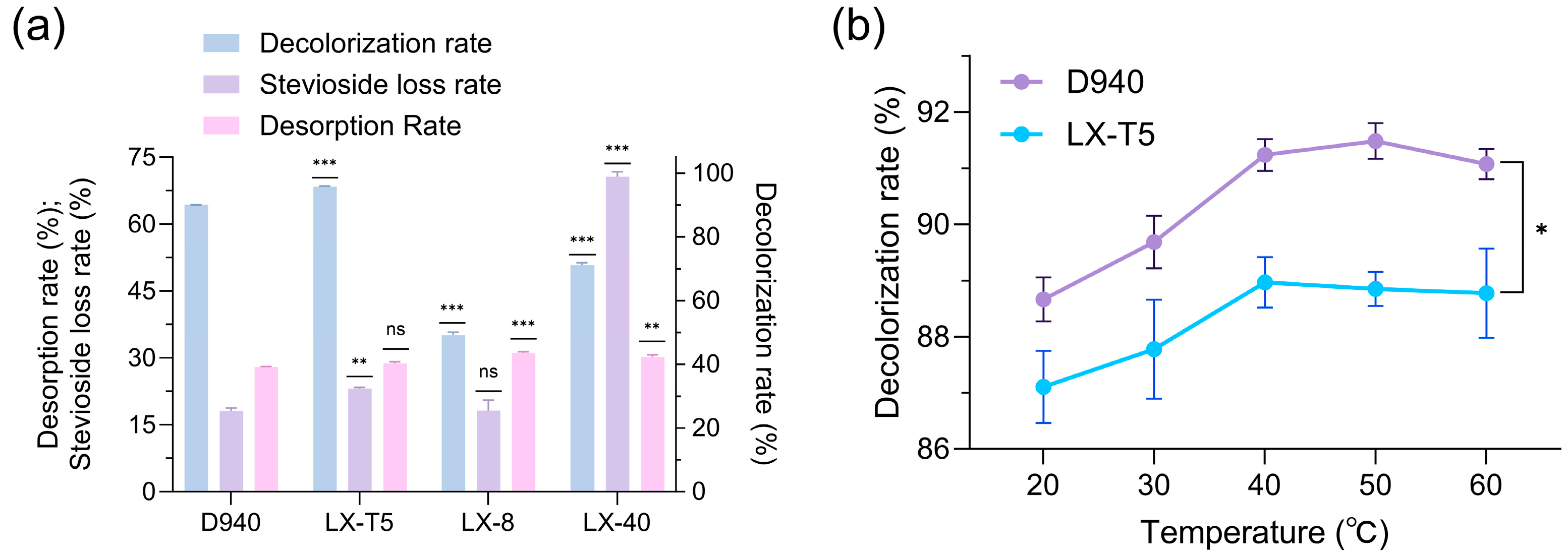
Given the limitations of activated carbon, decolorizing resins were investigated for their potential to preserve stevioside quality without causing physical or chemical damage during the decolorization process. Four decolorizing resins, namely D940, LX-T5, LX-8, and LX-40, were screened for their efficacy. Comparison of decolorization performance of resins D940, LX-T5, LX-8, and LX-40 (
Figure 3a) revealed that D940 and LX-T5 exhibited complementary decolorization effects, with LX-T5 achieving a decolorization rate of 95.7%, which was 5.6% higher than D940′s 90.1%, though its stevioside loss rate was also higher at 23.1% compared to D940′s 18.1%. Optimization of decolorization temperature for resins D940 and LX-T5 (
Figure 3b) identified 40–50 °C as the optimal range, where decolorization rates improved to 91.3% for D940 while the decolorization rate of LX-T5 was stabilized at 88.8%, which indicated a temperature-dependent enhancement in pigment removal efficiency.
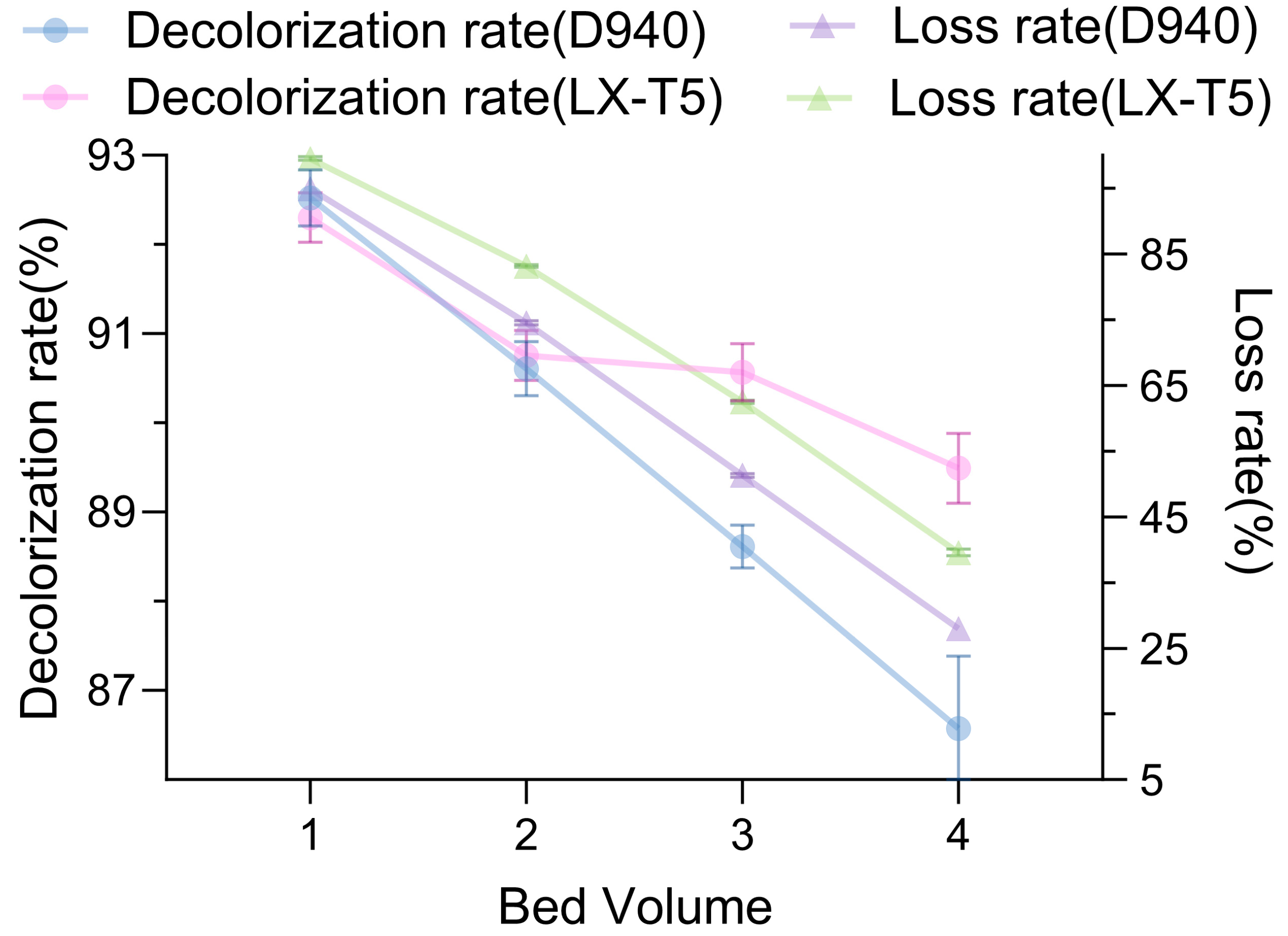
Comparison of decolorization rates of LX-T5 and D940 for large-scale fluid treatment (
Figure 4) demonstrated a widening gap in decolorization rates as fluid volume increased, with LX-T5 achieving a rate of 89.5% compared to D940 achieving a rate of 87.4%, suggesting LX-T5’s superior suitability for large-scale applications. However, LX-T5’s stevioside loss rate of 39% was approximately 12% higher than D940’s 27%, highlighting a trade-off between decolorization efficiency and product retention. In order to balance these factors, we propose a combined approach in which D940 and LX-T5 are proportionally added to the column to take advantage of their complementary roles to achieve high decolorization rates while reducing steviol glycoside loss. Resin-based decolorization, particularly with a proposed hybrid D940/LX-T5 column, could provide a sustainable and efficient alternative to activated carbon, warranting further studies on resin combinations and column configurations.
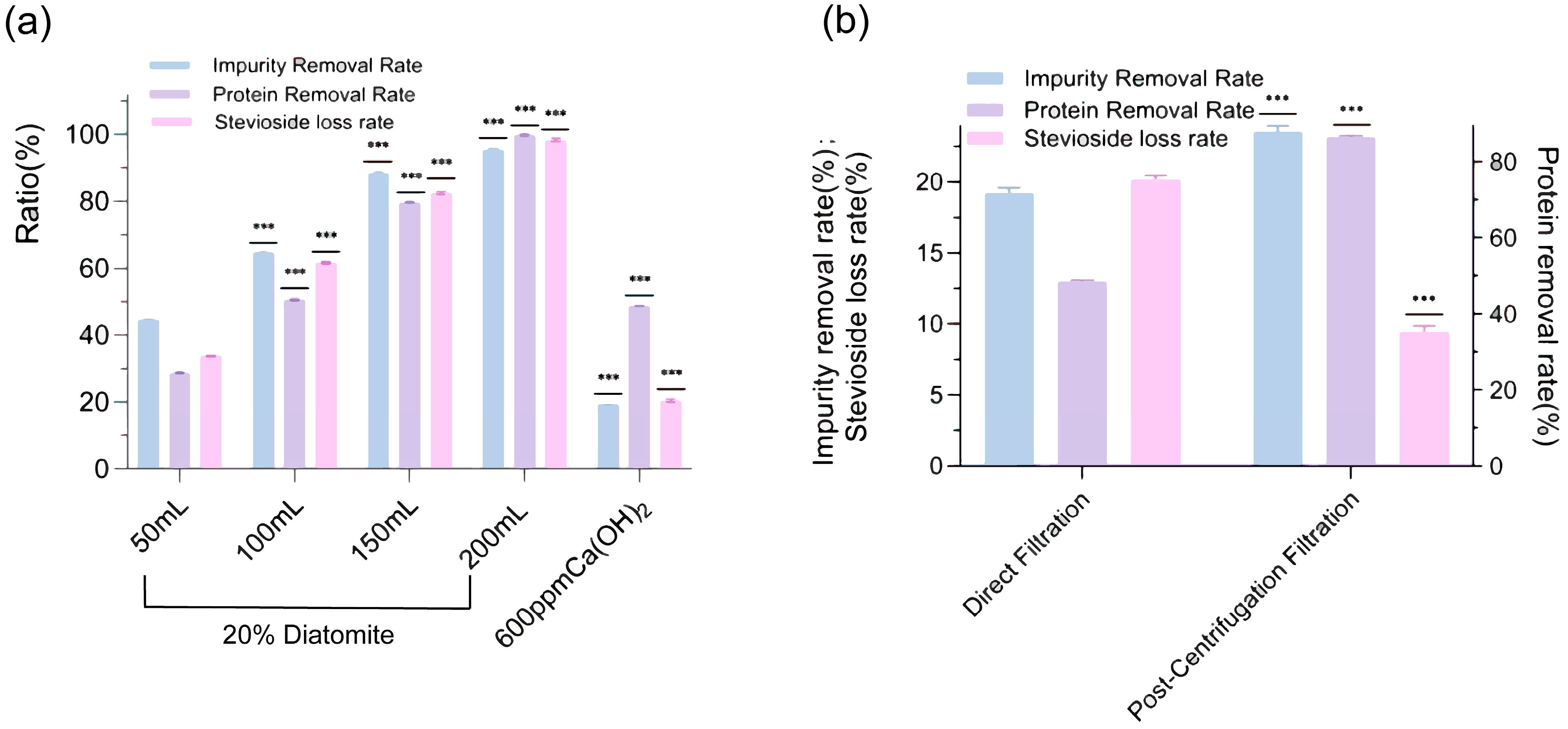
2.3. Comparison of Diatomaceous Earth Decontamination and Ca(OH)2 Decontamination
The presence of substantial impurities, such as chlorophyll, flavonoids, and proteins, in
Stevia rebaudiana leaf extracts posed significant challenges to achieving high-purity steviosides, necessitating effective decontamination strategies to enhance product quality. This study evaluated the efficacy of two de-impurity agents, diatomaceous earth and calcium hydroxide (Ca(OH)
2), in removing these contaminants while minimizing stevioside loss. Effect of diatomaceous earth and Ca(OH)
2 dosage on impurity removal and stevioside loss rates (
Figure 5a) demonstrated that diatomaceous earth exhibited superior impurity removal, achieving rates of up to 95% at 200 mL slurry volume, but this came at the cost of an excessively high stevioside loss rate, reaching nearly 100% when filtered through a diatom cake, indicating a non-selective adsorption mechanism. In contrast, Ca(OH)
2 achieved a lower impurity removal rate of 60% at 600 ppm, but its stevioside loss rate remained significantly lower at approximately 20%, with 13% of this loss attributed to filtration through a Büchner funnel, suggesting a more selective purification process. The analysis further revealed that the protein removal rate with Ca(OH)
2 reached 48% at 600 ppm, compared to 99% with diatomaceous earth at 200 mL, highlighting a trade-off between impurity removal efficiency and stevioside retention. These findings underscore the need for alternative filtration strategies to optimize the decontamination process.
To address the limitations of initial filtration methods, we investigated the impact of different purification techniques on decontamination efficiency. Comparison of direct filtration and post-centrifugal filtration on impurity, protein, and stevioside loss rates (
Figure 5b) showed that direct filtration achieved an impurity removal rate of 19%, a protein removal rate of 48%, and a stevioside loss rate of 20%, reflecting moderate purification with notable product loss. In contrast, post-centrifugal filtration significantly enhanced performance, increasing the impurity removal rate to 23%, the protein removal rate to 86%, and reducing the stevioside loss rate by approximately 10%. These results suggested that centrifugation effectively separated precipitated impurities and proteins, thereby minimizing stevioside entrapment during filtration. The enhanced protein removal rate of 86% with post-centrifugal filtration indicated a robust mechanism for targeting proteinaceous impurities, which was critical for improving stevioside purity. Additionally, the reduction in stevioside loss to 10% highlighted the potential of centrifugal filtration to preserve yield, offering a practical advantage over direct filtration methods.
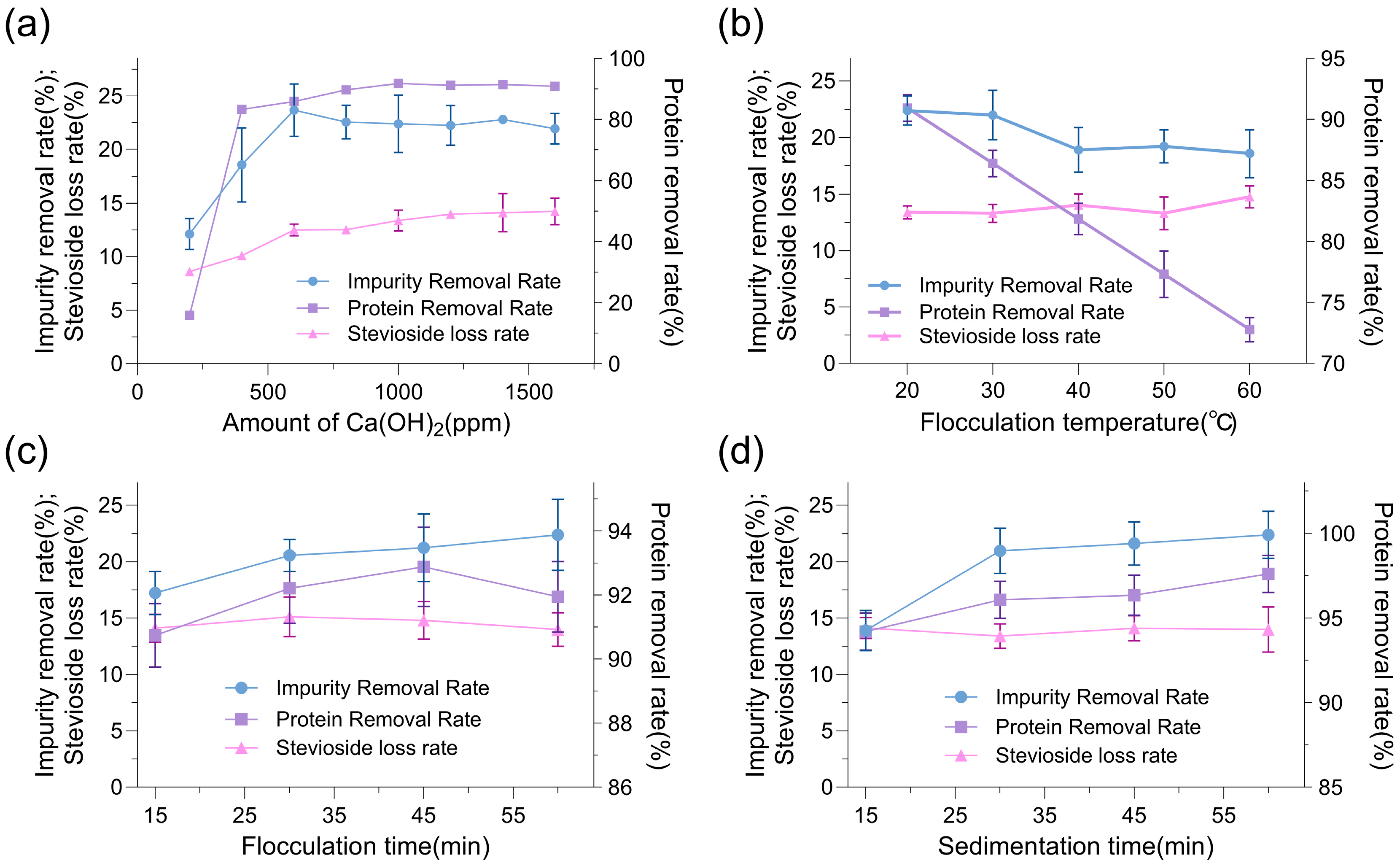
2.4. Optimization of Ca(OH)2 Decontamination Process
Given the superior performance of Ca(OH)
2 over diatomaceous earth in balancing impurity removal with minimal stevioside loss, we further optimized the Ca(OH)
2 decontamination process to maximize protein removal efficiency while preserving stevioside yield. The parameters investigated included Ca(OH)
2 addition, flocculation temperature, stirring time, and resting time, with the goal of enhancing the overall purity of
Stevia rebaudiana stevioside extracts. Effect of Ca(OH)
2 addition on protein removal and stevioside loss rates (
Figure 6a) revealed that increasing the Ca(OH)
2 concentration from 600 ppm to 1000 ppm improved the protein removal rate from 86% to 92%, while the stevioside loss rate remained relatively stable at 12–14%, indicating that 1000 ppm represented an optimal dosage for effective flocculation without compromising product yield. Influence of flocculation temperature on decontamination efficiency (
Figure 6b) demonstrated that a temperature of 20 °C yielded the highest protein removal rate of 90.9%, compared to 86.4% at 30 °C and 81.8% at 40 °C, suggesting that lower temperatures enhanced the flocculation of proteinaceous impurities, possibly due to reduced solubility and increased precipitation at 20 °C, while the stevioside loss rate remained consistent within 15% across the temperature range. Optimization of stirring time for Ca(OH)
2 flocculation (
Figure 6c) indicated that stirring for 30–45 min achieved the optimal protein removal rate of 92.8%, with no significant improvement beyond 45 min (e.g., 91.9% at 60 min), while the stevioside loss rate was maintained at 15%, highlighting that a stirring duration of 30–45 min was sufficient for effective mixing and flocculation without causing unnecessary shear stress that could lead to product loss. Impact of resting time on Ca(OH)
2 decontamination (
Figure 6d) showed that a resting period of 30–60 min after stirring resulted in a protein removal rate of 97%, while the stevioside loss rate remained at 14%, confirming that a 30–60 min resting period allowed optimal settling of impurities without affecting stevioside stability. Under these optimized conditions—Ca(OH)
2 addition of 1000 ppm, flocculation temperature of 20 °C, stirring for 30–45 min, and resting for 30–60 min—the protein removal rate increased from 86% to 97%, representing a 13% improvement in efficiency, while the stevioside loss rate was minimized to 10%. These findings underscored the effectiveness of the optimized Ca(OH)
2 flocculation process in achieving high-purity stevioside extracts by targeting proteinaceous impurities with minimal impact on the target compound. The consistency of the stevioside loss rate within 15% across all parameters suggested that the optimized conditions not only enhanced decontamination but also ensured product retention, making this process a promising candidate for industrial-scale purification of stevioside extracts.
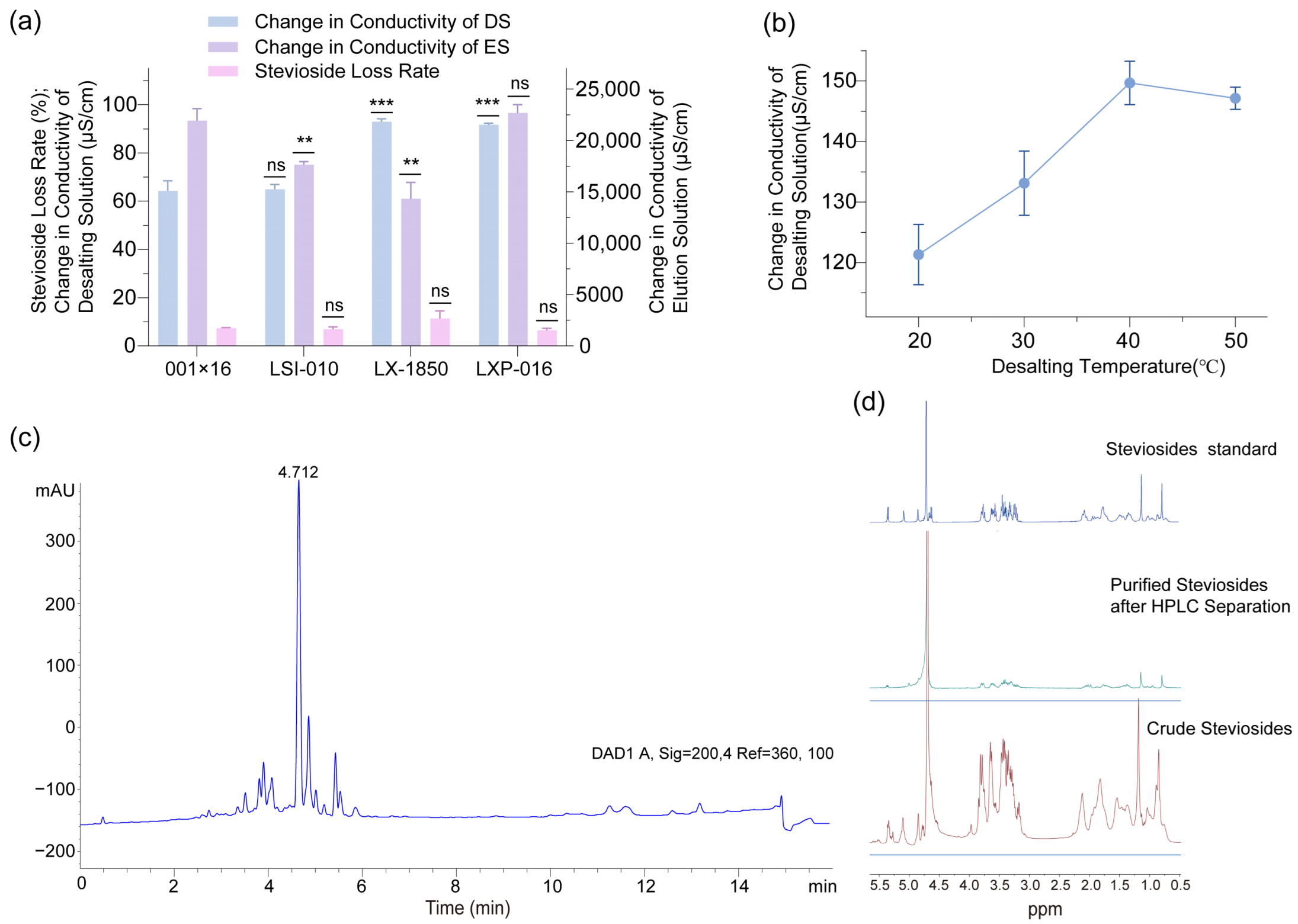
2.5. Optimization of Desalting Process
To further elevate the purity and quality of steviosides extracted from
Stevia rebaudiana leaves, this study employed desalting resins to reduce ionic impurities, a critical step in refining steviol glycoside products for commercial applications. Conductivity measurements, conducted using a conductivity meter, were utilized to assess the changes in conductivity of both the draw solution (DS) and extracted solution (ES) before and after desalting, as well as before and after desorption, to evaluate resin performance and stevioside retention. Comparison of conductivity changes and stevioside loss rates across different desalting resins (
Figure 7a) revealed that the change in DS conductivity was comparable between LX-1850 and LXP-016, reaching approximately 91.7 µS/cm. However, the amount of conductivity change of the desorbed solution after LX-1850 treatment was less than that of LXP-016, suggesting a higher efficiency in ionic removal by LXP-016 during desorption. Additionally, the stevioside loss rate with LX-1850 was approximately 11.4%, twice that of LXP-016 at 6.4%, indicating that LXP-016 preserved stevioside yield more effectively while maintaining comparable desalting efficiency. These findings identified LXP-016 as the optimal desalting resin due to its balanced performance in impurity removal and product retention. Effect of desalting temperature on LXP-016 performance (
Figure 7b) demonstrated that the change in DS conductivity increased from 121.3 µS/cm at 20 °C to a peak of 149.7 µS/cm at 40 °C, followed by a slight decline to 147.2 µS/cm at 50 °C, reflecting an optimal temperature range for ionic transport and resin efficiency. The stevioside loss rate remained stable at 10% across the temperature range, suggesting that temperature variations had minimal impact on product loss. Consequently, 40 °C emerged as the optimal desalting temperature for LXP-016, as it maximized conductivity change and maintained a low stevioside loss rate. The optimized conditions with LXP-016 at 40 °C not only enhanced the removal of ionic impurities, as evidenced by the peak conductivity change, but also ensured a stevioside retention rate of 90%, representing a significant improvement over the 80% retention observed with LX-1850. This optimization underscored the importance of resin selection and temperature control in the desalting process, offering a robust method to achieve high-purity stevioside extracts. The results also suggested that further investigation into the scalability of the LXP-016 resin system, including the evaluation of continuous flow desalting units and the impact of varying feed concentrations, could enhance its industrial applicability, potentially reducing processing costs and improving consistency in large-scale stevioside production.
The crude extract was subjected to high-performance liquid chromatography (HPLC) for separation. The HPLC results revealed that the characteristic retention time of stevioside was approximately 4.7 min (
Figure 7c), which is consistent with the typical retention profile of stevioside. The stevioside collected from HPLC purification was analyzed using nuclear magnetic resonance (NMR) and compared with standard and crude samples (
Figure 7d). The purified stevioside exhibited characteristic chemical shifts in the NMR spectrum that aligned with those of the standard, typically observed in the characteristic regions for glycosides and terpenoids, with sharper and clearer signals, fewer impurity peaks, hydrogen spectral integration ratios consistent with the standard, and coupling patterns similar to those of the standard, indicating the intact stereochemical structure of the sugar and terpenoid rings. In contrast, the crude sample spectrum likely showed additional broad or overlapping signals and abnormal integration due to impurity interference. These features collectively validate the effectiveness of the HPLC purification process and demonstrate the high purity of the purified stevioside.
The optimized extraction, decolorization, decontamination, and desalting processes significantly improved stevioside yield, purity, and sustainability, offering a scalable framework for industrial production.
The extraction phase benefited greatly from the integration of ultrasound-assisted techniques with 20% ethanol, which markedly enhanced stevioside yield to 49.1%, representing a 53.4% improvement over baseline methods. This enhancement likely stemmed from ultrasound’s ability to improve mass transfer and solvent penetration, coupled with the optimal solvent polarity of 20% ethanol, which balanced stevioside solubility and extraction efficiency. Molecular dynamics simulations further supported this approach by illustrating how ethanol concentration influenced solvent–stevioside interactions, with 20% ethanol emerging as a practical choice due to its cost-effectiveness and additional benefit of removing proteins. This dual role of the solvent in extraction and protein removal suggests a potential for process simplification, reducing the need for additional flocculation steps in industrial settings, which could lower both energy consumption and stevioside loss. These findings align with prior research emphasizing ultrasound’s role in enhancing plant matrix extraction [
25], and they highlight the importance of solvent selection in achieving sustainable production practices.
Decolorization posed another significant hurdle due to the presence of pigments like chlorophyll and flavonoids in the crude extract. While activated carbon initially seemed promising due to its adsorption capacity, its non-selective nature led to excessive stevioside loss, rendering it impractical. Decolorizing resins D940 and LX-T5 achieved decolorization rates of 90.1% and 95.7%, respectively, with stevioside loss of 18.1–23.1%. A proposed hybrid D940/LX-T5 column could leverage its complementary effects to balance efficiency and retention, warranting further investigation [
26].
Decontamination efforts targeting impurities like proteins and flavonoids revealed the limitations of diatomaceous earth, which, despite high impurity removal, caused near-total stevioside loss due to non-selective adsorption. Calcium hydroxide (Ca(OH)2), however, provided a more balanced alternative, achieving effective impurity removal while keeping stevioside loss at 15% after optimization. The incorporation of centrifugal filtration further enhanced protein removal efficiency to 98%, suggesting that process integration can significantly improve yield preservation. This approach highlights Ca(OH)2’s potential as a selective decontaminant, offering a sustainable option for industrial purification by minimizing product loss and targeting proteinaceous impurities effectively.
The desalting process addressed ionic impurities, critical for ensuring stevioside quality in food and pharmaceutical applications. LXP-016 proved to be the optimal resin, balancing ionic removal with a low stevioside loss rate of 6.4%, particularly at 40 °C, where it achieved a 90% retention rate. This optimization underscores the importance of resin selection and temperature control in achieving high-purity steviosides, providing a reliable method for industrial refinement that aligns with industry standards for sweetener purification [
27].
Overall, these optimized processes collectively tackled the primary challenges in stevioside production, improving extraction yield, purity, and retention while reducing environmental impact through streamlined steps and eco-friendly reagents. However, limitations such as the scalability of hybrid resin columns and centrifugal filtration systems remain, necessitating large-scale validation. Subsequent research endeavors should concentrate on continuous processing systems, economic viability, and the sensory characteristics of the final product to ascertain its appropriateness for a range of applications. These advancements lay a strong foundation for sustainable and efficient stevioside production, supporting its broader adoption in the food and pharmaceutical industries.