The Hypothalamic Nuclei Implicated in the Regulation of Polycystic Ovary Syndrome: A Review of Its Clinical, Metabolic, and Endocrine Aspects
Abstract
1. Introduction
2. Methodology
3. Clinical Features of PCOS
3.1. Hyperandrogenism
3.2. Polycystic Ovarian Morphology
3.3. Increases in Body Mass Index (BMI)
3.4. Metabolic Dysfunction–Associated Steatotic Liver Disease (MASLD)
4. Metabolic Alterations in PCOS
5. Endocrine Imbalance in PCOS
- Phenotype A (classic type I PCOS): characterized by hyperandrogenism, oligo/anovulation, and PCOM.
- Phenotype B (classic type II PCOS): with hyperandrogenism and oligo/anovulation, but without PCOM.
- Phenotype C (ovulatory PCOS): with hyperandrogenism and PCOM, but with preserved ovulatory cycles.
6. The Neuroendocrine Regulation of the Reproduction
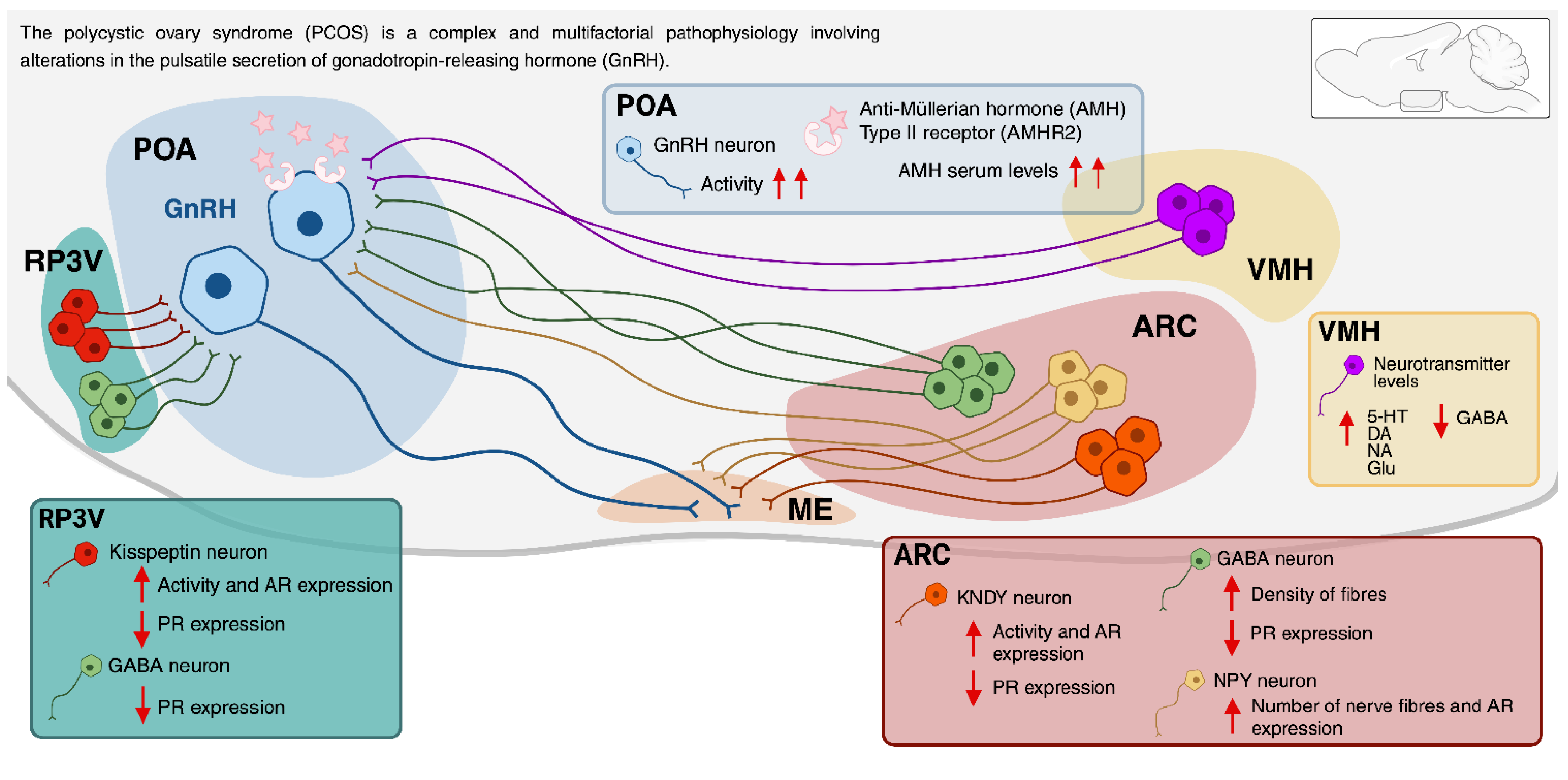
7. The Neuroendocrine Regulation of the PCOS
7.1. The Involvement of the Arcuate Nucleus (ARC) and the Rostral Periventricular Area of the Third Ventricle (RP3V) in PCOS
7.2. Expression of Steroid Hormone Receptors in the ARC and RP3V and Its Relationship with PCOS
7.3. The Role of Kisspeptin in PCOS
7.4. Alterations in the Kisspeptin System in Murine Models of PCOS
7.5. Evidence of Alterations in the Kisspeptin System in Women with PCOS
7.6. The Role of Other ARC Neurotransmitter Systems in PCOS
7.7. Role for Anti-Müllerian Hormone in the Regulation of GnRH and Gonadotrophins in PCOS
7.8. The Role of VMH in PCOS
8. Recent Therapeutic Strategies Employed in the Treatment of PCOS
9. Acupuncture as an Alternative Therapy for the Treatment of PCOS
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szydlarska, D.; Małgorzata, M.; Jakimiuk, A. History of discovery of polycystic ovary syndrome. Adv. Clin. Exp. Med. 2017, 26, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Rasquin, L.I.; Anastasopoulou, C. Polycystic Ovarian Syndrome. 2025 May 4. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations From the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Hutchison, S.K.; Norman, R.J.; Teede, H.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2011, 16, CD007506. [Google Scholar] [CrossRef] [PubMed]
- Cooney, L.G.; Lee, I.; Sammel, M.D.; Dokras, A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2017, 32, 1075–1091. [Google Scholar] [CrossRef]
- Karamali, M.; Gholizadeh, M. The effects of coenzyme Q10 supplementation on metabolic profiles and parameters of mental health in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2022, 38, 45–49. [Google Scholar] [CrossRef]
- Paoli, A.; Mancin, L.; Giacona, M.C.; Bianco, A.; Caprio, M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J. Transl. Med. 2020, 18, 104. [Google Scholar] [CrossRef]
- Borzan, V.; Riedl, R. Obermayer-Pietsch, B. Probiotic vs. placebo and metformin: Probiotic dietary intervention in polycystic ovary syndrome—A randomized controlled trial. BMC Endocr. Disord. 2023, 23, 82. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Teede, H.; Norman, R.J.; Legro, R.; Goodarzi, M.O.; Dokras, A.; Laven, J.; Hoeger, K.; Piltonen, T.T. Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2024, 10, 27. [Google Scholar] [CrossRef]
- Coutinho, E.A.; Kauffman, A.S. The role of the brain in the pathogenesis and physiology of polycystic ovary syndrome (PCOS). Med. Sci. 2019, 7, 84. [Google Scholar] [CrossRef]
- Chang, R.J.; Dumesic, D.A. Polycystic Ovary Syndrome and Hyperandrogenic States. Reprod. Endocrinol. 2020, 8, 520–555. [Google Scholar]
- Dewailly, D.; Lujan, M.E.; Carmina, E. Definition and significance of polycystic ovarian morphology: A task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum. Reprod. Update 2014, 20, 334. [Google Scholar] [CrossRef]
- Di Michele, S.; Fulghesu, A.M.; Pittui, E.; Cordella, M.; Sicilia, G.; Mandurino, G.; D’Alterio, M.N.; Vitale, S.G.; Angioni, S. Ultrasound Assessment in Polycystic Ovary Syndrome Diagnosis: From Origins to Future Perspectives—A Comprehensive Review. Biomedicines 2025, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Davies, M.J.; Norman, R.J.; Moran, L.J. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2012, 18, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Shen, Z.; Gu, W.; Lyu, Z.; Qi, X.; Mu, Y.; Ning, Y. Prevalence of obesity and associated complications in China: A cross-sectional, real-world study in 15.8 million adults. Diabetes Obes. Metab. 2023, 25, 3390–3399. [Google Scholar] [CrossRef] [PubMed]
- Fauser, B.C.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.; et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97, 28–38. [Google Scholar] [CrossRef]
- Ezeh, U.; Pall, M.; Mathur, R.; Azziz, R. Association of fat to lean mass ratio with metabolic dysfunction in women with polycystic ovary syndrome. Hum. Reprod. Open 2014, 29, 1508–1517. [Google Scholar] [CrossRef]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, J.; Lu, Y.; Wu, L. Association of metabolic-dysfunction associated steatotic liver disease with polycystic ovary syndrome. iScience 2024, 27, 108783. [Google Scholar] [CrossRef]
- Fan, J.G.; Kim, S.U.; Wong, V.W. New trends on obesity and NAFLD in Asia. J. Hepatol. 2017, 67, 862–873. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Wang, J.; Yang, R.; Miao, Y.; Zhang, X.; Paillard-Borg, S.; Fang, Z.; Xu, W. Metabolic Dysfunction-Associated Steatotic Liver Disease Is Associated with Accelerated Brain Ageing: A Population-Based Study. Liver Int. 2025, 45, e70109. [Google Scholar] [CrossRef]
- Michaliszyn, S.F.; Lee, S.; Tfayli, H.; Arslanian, S. Polycystic ovary syndrome and nonalcoholic fatty liver in obese adolescents: Association with metabolic risk profile. Fertil. Steril. Rep. 2013, 100, 1745–1751. [Google Scholar] [CrossRef]
- Elkattawy, H.A.; Alsemeh, A.E.; Ali, L.S.; Ahmed, M.M.; Eltaweel, A.M.; Shaikh, F.M.; Behiry, A.; Hassan, A.E.; Sabir, D.K.; Elsherbini, D.M.A.; et al. Decorin alleviates non-alcoholic fatty liver disease in rats with polycystic ovary syndrome. Tissue Cell 2025, 93, 102689. [Google Scholar] [CrossRef]
- Rocha, A.L.L.; Faria, L.C.; Guimarães, T.C.M. Non-alcoholic fatty liver disease in women with polycystic ovary syndrome: Systematic review and meta-analysis. J. Endocrinol. Investig. 2017, 40, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.; DeFronzo, R.A. Insulin Resistance and Hyperinsulinemia: The Egg and the Chicken. J. Clin. Endocrinol. Metab. 2021, 106, 1897–1899. [Google Scholar] [CrossRef]
- Armanini, D.; Boscaro, M.; Bordin, L.; Sabbadin, C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. Int. J. Mol. Sci. 2022, 23, 4110. [Google Scholar] [CrossRef]
- Qiao, J.; Feng, H.L. Extra-and intra-ovarian factors in polycystic ovary syndrome: Impact on oocyte maturation and embryo developmental competence. Hum. Reprod. Update 2011, 17, 17–33. [Google Scholar] [CrossRef]
- De Leo, V.; Musacchio, M.C.; Cappelli, V.; Massaro, M.G.; Morgante, G.; Petraglia, F. Genetic, hormonal and metabolic aspects of PCOS: An update. Reprod. Biol. Endocrinol. 2016, 14, 38. [Google Scholar] [CrossRef]
- Mehrabian, F.; Afghahi, M. Can Sex-hormone Binding Globulin Considered as a Predictor of Response to Pharmacological Treatment in Women with Polycystic Ovary Syndrome? J. Prev. Med. 2013, 4, 1169–1174. [Google Scholar]
- Jin, Y.; Sun, F.; Yang, A.; Yu, X.; Li, Y.; Liang, S.; Jing, X.; Wang, K.; Zhang, L.; Xiao, S.; et al. Insulin-like growth factor binding protein-1 and insulin in polycystic ovary syndrome: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1279717. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Bulsara, J.; Patel, P.; Soni, A.; Acharya, S.A. Review: Brief insight into polycystic ovarian syndrome. Endocr. Metab. Sci. 2021, 3, 100085. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Ricchieri, F.; Lanzoni, C. Use of metformin in the treatment of polycystic ovary syndrome. Women’s Health 2010, 6, 577–593. [Google Scholar] [CrossRef]
- Mikhael, S.; Punjala-Patel, A.; Gavrilova-Jordan, L. Hypothalamic-Pituitary-Ovarian Axis Disorders Impacting Female Fertility. Biomedicines 2019, 7, 5. [Google Scholar] [CrossRef]
- Rudnicka, E.; Kunicki, M.; Calik-Ksepka, A.; Suchta, K.; Duszewska, A.; Smolarczyk, K.; Smolarczyk, R. Anti-Müllerian Hormone in Pathogenesis, Diagnostic and Treatment of PCOS. J. Mol. Sci. 2021, 22, 12507. [Google Scholar] [CrossRef]
- Chen, M.; Guo, X.; Zhong, Y.; Liu, Y.; Cai, B.; Wu, R.; Huang, C.; Zhou, C. AMH inhibits androgen production in human theca cells. J. Steroid Biochem. Mol. Biol. 2023, 226, 106216. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Cacciapuoti, N.; Lonardo, M.S.; Nasti, G.; Gautiero, C.; Belfiore, A.; Guida, B.; Chiurazzi, M. Pathophysiology and Nutritional Approaches in Polycystic Ovary Syndrome (PCOS): A Comprehensive Review. Curr. Nutr. Rep. 2023, 12, 527–544. [Google Scholar] [CrossRef]
- Siddiqui, S.; Mateen, S.; Ahmad, R.; Moin, S. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J. Assist. Reprod. Genet. 2022, 39, 2439–2473. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Chen, Y. Androgen excess: A hallmark of polycystic ovary syndrome. Front. Endocrinol. 2023, 14, 1273542. [Google Scholar] [CrossRef]
- Dumitrescu, R.; Mehedintu, C.; Briceag, I.; Purcarea, V.L.; Hudita, D. The polycystic ovary syndrome: An update on metabolic and hormonal mechanisms. J. Med. Life 2015, 8, 142–145. [Google Scholar]
- Chun, S. Relationship between early follicular serum estrone level and other hormonal or ultrasonographic parameters in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2020, 36, 143–147. [Google Scholar] [CrossRef]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 2016, 22, 709–724. [Google Scholar] [CrossRef]
- Xu, X.L.; Huang, Z.Y.; Yu, K.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Biosynthesis and Signal Transduction in Ovarian Disease. Front. Endocrinol. 2022, 13, 827032. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Wang, R.; Xue, S.; Ying, Q.; Jin, L. Roles of estrogen and its receptors in polycystic ovary syndrome. Front. Cell Dev. Biol. 2024, 12, 1395331. [Google Scholar] [CrossRef]
- Guastella, E.; Longo, R.A.; Carmina, E. Clinical and endocrine characteristics of the main polycystic ovary syndrome phenotypes. Fertil. Steril. 2010, 94, 2197–2201. [Google Scholar] [CrossRef]
- Azziz, R. Polycystic Ovary Syndrome. Obs. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef]
- van Keizerswaard, J.; Dietz de Loos, A.L.P.; Louwers, Y.V.; Laven, J.S.E. Changes in individual polycystic ovary syndrome phenotypical characteristics over time: A long-term follow-up study. Fertil. Steril. 2022, 117, 1059–1066. [Google Scholar] [CrossRef]
- Duan, C.; Allard, J. Gonadotropin-releasing hormone neuron development in vertebrates. Gen. Comp. Endocrinol. 2020, 292, 113465. [Google Scholar] [CrossRef]
- Vastagh, C.; Farkas, I.; Csillag, V.; Watanabe, M.; Kalló, I.; Liposits, Z. Cholinergic Control of GnRH Neuron Physiology and Luteinizing Hormone Secretion in Male Mice: Involvement of ACh/GABA Cotransmission. J. Neurosci. 2024, 44, e1780232024. [Google Scholar] [CrossRef]
- Casoni, F.; Malone, S.A.; Belle, M.; Luzzati, F.; Collier, F.; Allet, C.; Hrabovszky, E.; Rasika, S.; Prevot, V.; Chédotal, A.; et al. Development of the neurons controlling fertility in humans: New insights from 3D imaging and transparent fetal brains. Development 2016, 143, 3969–3981. [Google Scholar] [CrossRef]
- Jansen, H.T.; Hershey, J.; Mytinger, A.; Foster, D.L.; Padmanabhan, V. Developmental programming: Reproductive endocrinopathies in the adult female sheep after prenatal testosterone treatment are reflected in altered ontogeny of GnRH afferents. Endocrinology 2011, 152, 4288–4297. [Google Scholar] [CrossRef]
- Hu, K.L.; Chen, Z.; Li, X.; Cai, E.; Yang, H.; Chen, Y.; Wang, C.; Ju, L.; Deng, W.; Mu, L. Advances in clinical applications of kisspeptin-GnRH pathway in female reproduction. Reprod. Biol. Endocrinol. 2022, 20, 81. [Google Scholar] [CrossRef]
- Cimino, I.; Casoni, F.; Liu, X.; Messina, A.; Parkash, J.; Jamin, S.P.; Catteau-Jonard, S.; Collier, F.; Baroncini, M.; Dewailly, D.; et al. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat. Commun. 2016, 7, 10055. [Google Scholar] [CrossRef]
- Herbison, A.E. Chapter 11—Physiology of the Adult Gonadotropin-Releasing Hormone Neuronal Network. In Knobil and Neill’s Physiology of Reproduction; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Moore, A.M.; Campbell, R.E. The neuroendocrine genesis of polycystic ovary syndrome: A role for arcuate nucleus GABA neurons. J. Steroid Biochem. Mol. Biol. 2016, 160, 106–117. [Google Scholar] [CrossRef]
- Zeng, X.; Xie, Y.J.; Liu, Y.T.; Long, S.L.; Mo, Z.C. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin. Chim. Acta 2020, 502, 214–221. [Google Scholar] [CrossRef]
- Long, B.Y.; Liao, X.; Liang, X. The Hypothalamus and Pituitary Gland Regulate Reproduction and Are Involved in the Development of Polycystic Ovary Syndrome. J. Neuroendocrinol. 2025, 115, 315–334. [Google Scholar] [CrossRef]
- Sucquart, I.E.; Coyle, C.; Rodriguez Paris, V.; Prescott, M.; Glendining, K.A.; Potapov, K.; Begg, D.P.; Gilchrist, R.B.; Walters, K.A.; Campbell, R.E. Investigating GABA Neuron-Specific Androgen Receptor Knockout in two Hyperandrogenic Models of PCOS. Endocrinology 2024, 165, bqae060. [Google Scholar] [CrossRef]
- Coutinho, E.A.; Prescott, M.; Hessler, S.; Marshall, C.J.; Herbison, A.E.; Campbell, R.E. Activation of a Classic Hunger Circuit Slows Luteinizing Hormone Pulsatility. J. Neuroendocrinol. 2020, 110, 671–687. [Google Scholar] [CrossRef]
- Esparza, L.A.; Schafer, D.; Ho, B.S.; Thackray, V.G.; Kauffman, A.S. Hyperactive LH Pulses and Elevated Kisspeptin and NKB Gene Expression in the Arcuate Nucleus of a PCOS Mouse Model. Endocrinology 2020, 161, bqaa018. [Google Scholar] [CrossRef]
- Mohr, M.A.; Esparza, L.A.; Steffen, P.; Micevych, P.E.; Kauffman, A.S. Progesterone Receptors in AVPV Kisspeptin Neurons Are Sufficient for Positive Feedback Induction of the LH Surge. Endocrinology. 2021, 162, bqab161. [Google Scholar] [CrossRef]
- Moore, A.M. Neuroendocrine mechanisms responsible for elevated gonadotrophin-releasing hormone and luteinising hormone pulses in polycystic ovary syndrome. J. Neuroendocrinol. 2025, 37, e70028. [Google Scholar] [CrossRef]
- Moore, A.M.; Lohr, D.B.; Coolen, L.M.; Lehman, M.N. Prenatal Androgen Exposure Alters KNDy Neurons and Their Afferent Network in a Model of Polycystic Ovarian Syndrome. Endocrinology 2021, 162, bqab158. [Google Scholar] [CrossRef]
- Moore, A.M.; Campbell, R.E. Polycystic ovary syndrome: Understanding the role of the brain. Front. Neuroendocrinol. 2017, 46, 1–14. [Google Scholar] [CrossRef]
- Xie, Q.; Kang, Y.; Zhang, C.; Xie, Y.; Wang, C.; Liu, J.; Yu, C.; Zhao, H.; Huang, D. The Role of Kisspeptin in the Control of the Hypothalamic-Pituitary-Gonadal Axis and Reproduction. Front. Endocrinol. 2022, 13, 925206. [Google Scholar] [CrossRef]
- Clarkson, J.; Han, S.Y.; Piet, R.; McLennan, T.; Kane, G.M.; Ng, J.; Porteous, R.W.; Kim, J.S.; Colledge, W.H.; Iremonger, K.J.; et al. Definition of the Hypothalamic GnRH Pulse Generator in Mice. Proc. Natl. Acad. Sci. USA 2017, 114, E10216–E10223. [Google Scholar] [CrossRef]
- Piet, R.; de Croft, S.; Liu, X.; Herbison, A.E. Electrical properties of kisspeptin neurons and their regulation of GnRH neurons. Front. Neuroendocrinol. 2015, 36, 15–27. [Google Scholar] [CrossRef]
- Sobrino, V.; Avendaño, M.S.; Perdices-López, C.; Jimenez-Puyer, M.; Tena-Sempere, M. Kisspeptins and the neuroendocrine control of reproduction: Recent progress and new frontiers in kisspeptin research. Front. Neuroendocrinol. 2022, 65, 100977. [Google Scholar] [CrossRef]
- Aasif, A.; Alam, R.; Ahsan, H.; Khan, M.M.; Khan, A.; Khan, S. The role of kisspeptin in the pathogenesis of a polycystic ovary syndrome. Endocr. Regul. 2023, 57, 292–303. [Google Scholar] [CrossRef]
- Coutinho, E.A.; Esparza, L.A.; Rodriguez, J.; Yang, J.; Schafer, D.; Kauffman, A.S. Targeted inhibition of kisspeptin neurons reverses hyperandrogenemia and abnormal hyperactive LH secretion in a preclinical mouse model of polycystic ovary syndrome. Hum. Reprod. 2024, 39, 2089–2103. [Google Scholar] [CrossRef]
- Umayal, B.; Jayakody, S.N.; Chandrasekharan, N.V.; Wijesundera, W.S.; Wijeyaratne, C.N. Polycystic ovary syndrome (PCOS) and kisspeptin—A Sri Lankan study. J. Postgrad. Med. 2019, 65, 18–23. [Google Scholar] [CrossRef]
- Abbara, A.; Abbara, A.; Jayasena, C.N.; Christopoulos, G.; Narayanaswamy, S.; Izzi-Engbeaya, C.; Nijher, G.M.; Comninos, A.N.; Peters, D.; Buckley, A.; et al. Efficacy of kisspeptin-54 to trigger oocyte maturation in women at high risk of ovarian hyperstimulation syndrome (OHSS) during in vitro fertilization (IVF) therapy. J. Clin. Endocrinol. Metab. 2015, 100, 3322–3331. [Google Scholar] [CrossRef]
- Romero-Ruiz, A.; Skorupskaite, K.; Gaytan, F.; Torres, E.; Perdices-Lopez, C.; Mannaerts, B.M.; Qi, S.; Leon, S.; Manfredi-Lozano, M.; Lopez-Rodriguez, C.; et al. Kisspeptin treatment induces gonadotropic responses and rescues ovulation in a subset of preclinical models and women with polycystic ovary syndrome. Hum. Reprod. 2019, 34, 2495–2512. [Google Scholar] [CrossRef]
- George, J.T.; Kakkar, R.; Marshall, J.; Scott, M.L.; Finkelman, R.D.; Ho, T.W.; Veldhuis, J.; Skorupskaite, K.; Anderson, R.A.; McIntosh, S.; et al. Receptor Antagonism in Women with Polycystic Ovary Syndrome: A Randomized, Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2016, 101, 4313–4321. [Google Scholar] [CrossRef]
- Skorupskaite, K.; George, J.T.; Veldhuis, J.D.; Millar, R.P.; Anderson, R.A. Kisspeptin and neurokinin B interactions in modulating gonadotropin secretion in women with polycystic ovary syndrome. Hum. Reprod. 2020, 35, 1421–1431. [Google Scholar] [CrossRef]
- Akad, M.; Socolov, D.; Akad, F.; Covali, R.; Crauciuc, E.; Stan, C.; Stan, C.; Socolov, R. Treatments in Patients with Polycystic Ovary Syndrome and Effects on Kisspeptin Serum Levels. Maedica 2022, 17, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.M.; Prescott, M.; Marshall, C.J.; Yip, S.H.; Campbell, R.E. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Moenter, S.M.; DeFazio, R.A. Endogenous gamma-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology 2005, 146, 5374–5379. [Google Scholar] [CrossRef] [PubMed]
- Herbison, A.E.; Moenter, S.M. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: Towards an emerging consensus. J. Neuroendocr. 2011, 23, 557–569. [Google Scholar] [CrossRef]
- Chen, W.H.; Shi, Y.C.; Huang, Q.Y.; Chen, J.M.; Wang, Z.Y.; Lin, S.; Shi, Q.Y. Potential for NPY receptor-related therapies for polycystic ovary syndrome: An updated review. Hormones 2023, 22, 441–451. [Google Scholar] [CrossRef]
- Terasawa, E. Mechanism of pulsatile GnRH release in primates: Unresolved questions. Mol. Cell. Endocrinol. 2019, 498, 110578. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.J.; Prescott, M.; Campbell, R.E. Investigating the NPY/AgRP/GABA to GnRH Neuron Circuit in Prenatally Androgenized PCOS-Like Mice. J. Endocr. Soc. 2020, 4, bvaa129. [Google Scholar] [CrossRef] [PubMed]
- Guzelkas, I.; Orbak, Z.; Doneray, H.; Ozturk, N.; Sagsoz, N. Serum kisspeptin, leptin, neuropeptide Y, and neurokinin B levels in adolescents with polycystic ovary syndrome. J. Pediatr. Endocrinol. Metab. 2022, 35, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Pastoor, H.; Both, S.; Laan, E.T.M.; Laven, J.S.E. Sexual dysfunction in women with PCOS: A case control study. Hum. Reprod. 2023, 38, 2230–2238. [Google Scholar] [CrossRef]
- Steinberg-Weiss, M.; Roe, A.H.; Allison, K.C.; Dodson, W.C.; Kris-Etherton, P.M.; Kunselman, A.R.; Stetter, C.M.; Williams, N.I.; Gnatuk, C.L.; Estes, S.J.; et al. Lifestyle modifications alone or combined with hormonal contraceptives improve sexual dysfunction in women with polycystic ovary syndrome. Fertil. Steril. 2021, 115, 474–482. [Google Scholar] [CrossRef]
- Pinto, C.A.; Fonseca, B.M.; Sá, S.I. Effects of chronic tamoxifen treatment in female rat sexual behaviour. Heliyon 2022, 8, e12362. [Google Scholar] [CrossRef]
- Silva, M.S.B.; Decoster, L.; Trova, S.; Mimouni, N.E.H.; Delli, V.; Chachlaki, K.; Yu, Q.; Boehm, U.; Prevot, V.; Giacobini, P. Female sexual behavior is disrupted in a preclinical mouse model of PCOS via an attenuated hypothalamic nitric oxide pathway. Proc. Natl. Acad. Sci. USA 2022, 119, e2203503119. [Google Scholar] [CrossRef]
- Kim, K.W.; Li, S.; Zhao, H.; Peng, B.; Tobet, S.A.; Elmquist, J.K.; Parker, K.L.; Zhao, L. CNS-specific ablation of steroidogenic factor 1 results in impaired female reproductive function. Mol. Endocrinol. 2010, 24, 1240–1250. [Google Scholar] [CrossRef]
- Sotomayor-Zárate, R.; Tiszavari, M.; Cruz, G.; Lara, H.E. Neonatal exposure to single doses of estradiol or testosterone programs ovarian follicular development-modified hypothalamic neurotransmitters and causes polycystic ovary during adulthood in the rat. Fertil. Steril. 2011, 96, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Li, J.; Tang, K. The Paraventricular Nucleus of the Hypothalamus: Development, Function, and Human Diseases. Endocrinology 2018, 159, 3458–3472. [Google Scholar] [CrossRef] [PubMed]
- Ressler, I.B.; Grayson, B.E.; Ulrich-Lai, Y.M.; Seeley, R.J. Diet-induced obesity exacerbates metabolic and behavioral effects of polycystic ovary syndrome in a rodent model. Am. J. Physiol.-Endocrinol. Metab. 2015, 308, E1076–E1084. [Google Scholar] [CrossRef]
- Vercruysse, P.; Vieau, D.; Blum, D.; Petersén, Å.; Dupuis, L. Hypothalamic Alterations in Neurodegenerative Diseases and Their Relation to Abnormal Energy Metabolism. Front. Mol. Neurosci. 2018, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Hutchison, S.K.; Van Ryswyk, E.; Norman, R.J.; Teede, H.J.; Moran, L.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019, 3, CD007506. [Google Scholar] [CrossRef]
- Cowan, S.; Lim, S.; Alycia, C.; Pirotta, S.; Thomson, R.; Gibson-Helm, M.; Blackmore, R.; Naderpoor, N.; Bennett, C.; Ee, C.; et al. Lifestyle management in polycystic ovary syndrome—beyond diet and physical activity. BMC Endocr. Disord. 2023, 23, 14. [Google Scholar] [CrossRef]
- Wasim, T.; Nasrin, T.; Zunair, J.; Irshad, S. Efficacy of Letrozole vs. Clomiphene Citrate for induction of ovulation in women with polycystic ovarian syndrome. Pak. J. Med. Sci. 2024, 40, 78–83. [Google Scholar] [CrossRef]
- Saadati, S.; Mason, T.; Godini, R.; Vanky, E.; Teede, H.; Mousa, A. Metformin use in women with polycystic ovary syndrome (PCOS): Opportunities, benefits, and clinical challenges. Diabetes Obes. Metab. 2025, 27, 31–47. [Google Scholar] [CrossRef]
- Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients 2019, 11, 2278. [Google Scholar] [CrossRef]
- Trop-Steinberg, S.; Heifetz, E.M.; Azar, Y.; Kafka, I.; Weintraub, A.; Gal, M. Omega-3 Intake Improves Clinical Pregnancy Rate in Polycystic Ovary Patients: A Double-Blind, Randomized Study. Isr. Med. Assoc. J. 2023, 25, 131–136. [Google Scholar]
- Hu, L.; Ma, L.; Xia, X.; Ying, T.; Zhou, M.; Zou, S.; Yu, H.; Yin, J. Efficacy of Bariatric Surgery in the Treatment of Women with Obesity and Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2022, 107, e3217–e3229. [Google Scholar] [CrossRef]
- Goldberg, A.; Graca, S.; Liu, J.; Rao, V.; Witchel, S.F.; Pena, A.; Li, R.; Mousa, A.; Tay, C.T.; Pattuwage, L.; et al. Anti-obesity pharmacological agents for polycystic ovary syndrome: A systematic review and meta-analysis to inform the 2023 international evidence-based guideline. Obes. Rev. 2024, 25, e13704. [Google Scholar] [CrossRef] [PubMed]
- Szeliga, A.; Rudnicka, E.; Maciejewska-Jeske, M.; Kucharski, M.; Kostrzak, A.; Hajbos, M.; Niwczyk, O.; Smolarczyk, R.; Meczekalski, B. Neuroendocrine Determinants of Polycystic Ovary Syndrome. Int. J. Environ. Res. Public Health 2022, 19, 3089. [Google Scholar] [CrossRef] [PubMed]
- Outeiriño-Iglesias, V.; Romaní-Pérez, M.; González-Matías, L.C.; Vigo, E.; Mallo, F. GLP-1 Increases Preovulatory LH Source and the Number of Mature Follicles, As Well As Synchronizing the Onset of Puberty in Female Rats. Endocrinology 2015, 156, 4226–4237. [Google Scholar] [CrossRef]
- Farkas, I.; Vastagh, C.; Farkas, E.; Bálint, F.; Skrapits, K.; Hrabovszky, E.; Fekete, C.; Liposits, Z. Glucagon-Like Peptide-1 Excites Firing and Increases GABAergic Miniature Postsynaptic Currents (mPSCs) in Gonadotropin-Releasing Hormone (GnRH) Neurons of the Male Mice via Activation of Nitric Oxide (NO) and Suppression of Endocannabinoid Signaling Pathways. Front. Cell. Neurosci. 2016, 10, 214. [Google Scholar]
- Vastagh, C.; Farkas, I.; Scott, M.M.; Liposits, Z. Networking of glucagon-like peptide-1 axons with GnRH neurons in the basal forebrain of male mice revealed by 3DISCO-based immunocytochemistry and optogenetics. Brain Struct. Funct. 2021, 226, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.M.; Clarke, I.J.; Scott, C.J.; Stephen, C.P.; Rao, A.; Gunn, A.J. The GLP-1 agonist, exendin-4, stimulates LH secretion in female sheep. J. Endocrinol. 2023, 259, e230105. [Google Scholar] [CrossRef]
- Heppner, K.M.; Baquero, A.F.; Bennett, C.M.; Lindsley, S.R.; Kirigiti, M.A.; Bennett, B.; Bosch, M.A.; Mercer, A.J.; Rønnekleiv, O.K.; True, C.; et al. GLP-1R Signaling Directly Activates Arcuate Nucleus Kisspeptin Action in Brain Slices but Does not Rescue Luteinizing Hormone Inhibition in Ovariectomized Mice During Negative Energy Balance. eNeuro 2017, 4, ENEURO.0198-16.2016. [Google Scholar] [CrossRef]
- Oride, A.; Kanasaki, H.; Mijiddorj, T.; Sukhbaatar, U.; Hara, T.; Tumurbaatar, T.; Kyo, S. GLP-1 increases Kiss-1 mRNA expression in kisspeptin-expressing neuronal cells. Biol. Reprod. 2017, 97, 240–248. [Google Scholar] [CrossRef]
- Ye, Y.; Zhou, C.C.; Hu, H.Q.; Fukuzawa, I.; Zhang, H.L. Underlying mechanisms of acupuncture therapy on polycystic ovary syndrome: Evidences from animal and clinical studies. Front. Endocrinol. 2022, 13, 1035929. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Zhang, H.; Li, R.; Friden, C.; Li, D.; Wang, W.; Wang, H.; Chang, C.; Li, S.; Huo, Z.; et al. Acupuncture or metformin to improve insulin resistance in women with polycystic ovary syndrome: Study protocol of a combined multinational cross sectional case-control study and a randomised controlled trial. BMJ Open 2019, 9, e024733. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, X.; Li, Z.; Hu, J.; Li, X.; Li, J.; Chen, Y. Effects of electroacupuncture on the kisspeptin-gonadotropin-releasing hormone (GnRH)/luteinizing hormone (LH) neural circuit abnormalities and androgen receptor expression of kisspeptin/neurokinin B/dynorphin neurons in PCOS rats. J. Ovarian Res. 2023, 16, 15. [Google Scholar] [CrossRef]
- Li, Y.; Zhi, W.; Dong, H.; Qing, W.; Ling, C.; Ping, Y.; Huang, D. Effects of electroacupuncture on the expression of hypothalamic neuropeptide Y and ghrelin in pubertal rats with polycystic ovary syndrome. PLoS ONE 2022, 17, e0259609. [Google Scholar] [CrossRef]
- Roa, J.; Herbison, A.E. Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology 2012, 11, 5587–5599. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, A.; Liu, J.; Wang, X.; Feng, J.; Chen, Y. Effects of Electroacupuncture on ovarian expression of the androgen receptor and Connexin 43 in rats with Letrozole-induced polycystic ovaries. Evid. Based Complement. Altern. Med. 2020, 2020, 3608062. [Google Scholar] [CrossRef] [PubMed]
- Budihastuti, U.R.; Melinawati, E.; Sulistyowati, S.; Nurwati, I. Electroacupuncture effect on polycystic ovary syndrome to improve oocytes’ growth. Med. Acupunct. 2019, 31, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Xia, M.F.; Song, J.Y.; Liu, D.Q.; Lian, F. Effect of electro-acupuncture on expression of IRS-1/PI3K/GLUT4 pathway in ovarian granulosa cells of infertile patients with polycystic ovary syndrome-insulin resistance of phlegm-dampness syndrome. Chin. J. Integr. Med. 2021, 27, 330–335. [Google Scholar] [CrossRef]
- Li, J.; Cui, W.; Sun, W.; Zhang, Q.Y.; Guan, Q. Effect of electroacupuncture on the spindle and oocytes quality in patients with PCOS. Chin. J. Integr. Tradit. West. Med. 2015, 35, 304–309. [Google Scholar]
- Pu, R.S.; Fang, X.L.; Jie, W.J.; Liu, D.L.; Quan, K.; Su, C.H.; Yang, C.G.; Zhang, Y.J. Characteristics of the analgesic effect of buccal needle therapy on rheumatoid arthritis with rabbits as a model. Chin. Gen. Pr. 2017, 20, 4183–4187. [Google Scholar]
- Yang, Y.; Jin, L.; Xu, S.; Ye, H.; Luo, X.; Li, R.; Yue, Y. Observation on efficacy and underlying mechanism of cheek acupuncture on ovulation induction for infertile women with PCOS: Case series. Medicine 2024, 103, e37370. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, H.; Cong, J.; Qu, Q. Comparative effects of acupuncture and metformin on insulin sensitivity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Front. Endocrinol. 2025, 16, 1553684. [Google Scholar] [CrossRef]
- Robert, F. Erectile Dysfunction as a Cardiovascular Impairment; Academic Press: Cambridge, MA, USA, 2014; pp. 203–230. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieyra, E.; Silva, C.-C.; Linares, R.; Rosas, G.; Espinoza, J.-A.; Chaparro, A.; Calderón, R.; de la Peña, B.; Morales-Ledesma, L. The Hypothalamic Nuclei Implicated in the Regulation of Polycystic Ovary Syndrome: A Review of Its Clinical, Metabolic, and Endocrine Aspects. Molecules 2025, 30, 3407. https://doi.org/10.3390/molecules30163407
Vieyra E, Silva C-C, Linares R, Rosas G, Espinoza J-A, Chaparro A, Calderón R, de la Peña B, Morales-Ledesma L. The Hypothalamic Nuclei Implicated in the Regulation of Polycystic Ovary Syndrome: A Review of Its Clinical, Metabolic, and Endocrine Aspects. Molecules. 2025; 30(16):3407. https://doi.org/10.3390/molecules30163407
Chicago/Turabian StyleVieyra, Elizabeth, Carlos-Camilo Silva, Rosa Linares, Gabriela Rosas, Julieta-Azucena Espinoza, Andrea Chaparro, Roberto Calderón, Belinda de la Peña, and Leticia Morales-Ledesma. 2025. "The Hypothalamic Nuclei Implicated in the Regulation of Polycystic Ovary Syndrome: A Review of Its Clinical, Metabolic, and Endocrine Aspects" Molecules 30, no. 16: 3407. https://doi.org/10.3390/molecules30163407
APA StyleVieyra, E., Silva, C.-C., Linares, R., Rosas, G., Espinoza, J.-A., Chaparro, A., Calderón, R., de la Peña, B., & Morales-Ledesma, L. (2025). The Hypothalamic Nuclei Implicated in the Regulation of Polycystic Ovary Syndrome: A Review of Its Clinical, Metabolic, and Endocrine Aspects. Molecules, 30(16), 3407. https://doi.org/10.3390/molecules30163407





