Gold Nanoparticles-Functionalized Ultrathin Graphitic Carbon Nitride Nanosheets for Boosting Solar Hydrogen Production: The Role of Plasmon-Induced Interfacial Electric Fields
Abstract
1. Introduction
2. Results and Discussion
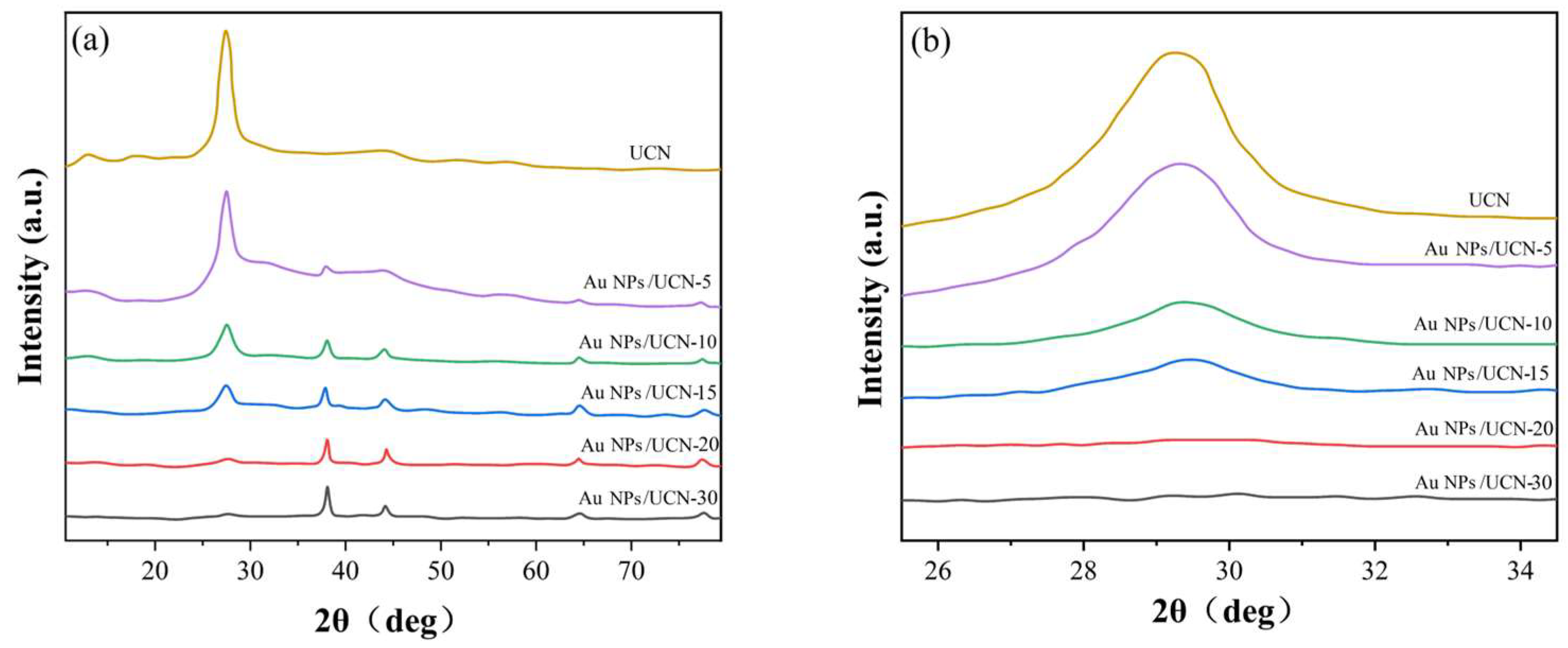
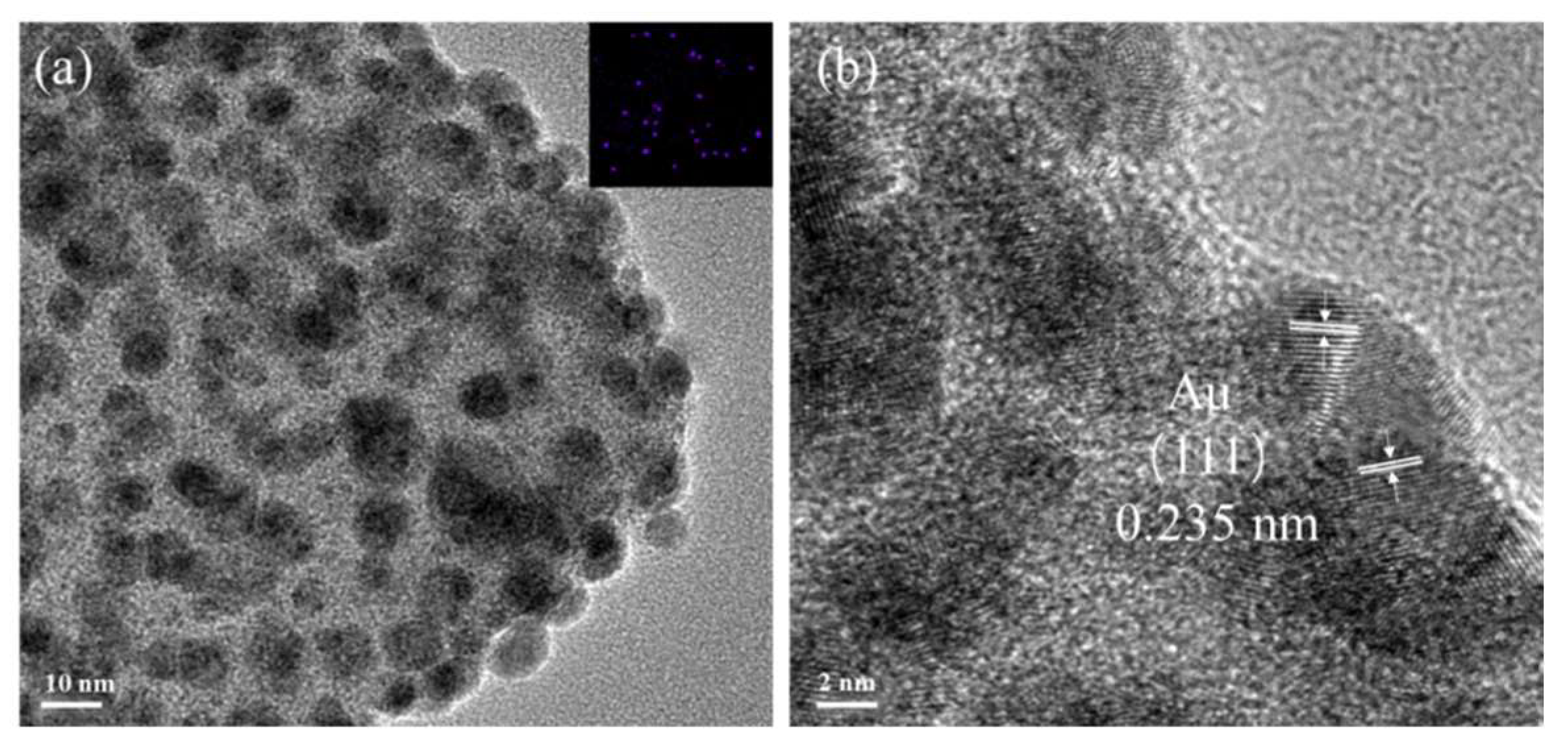
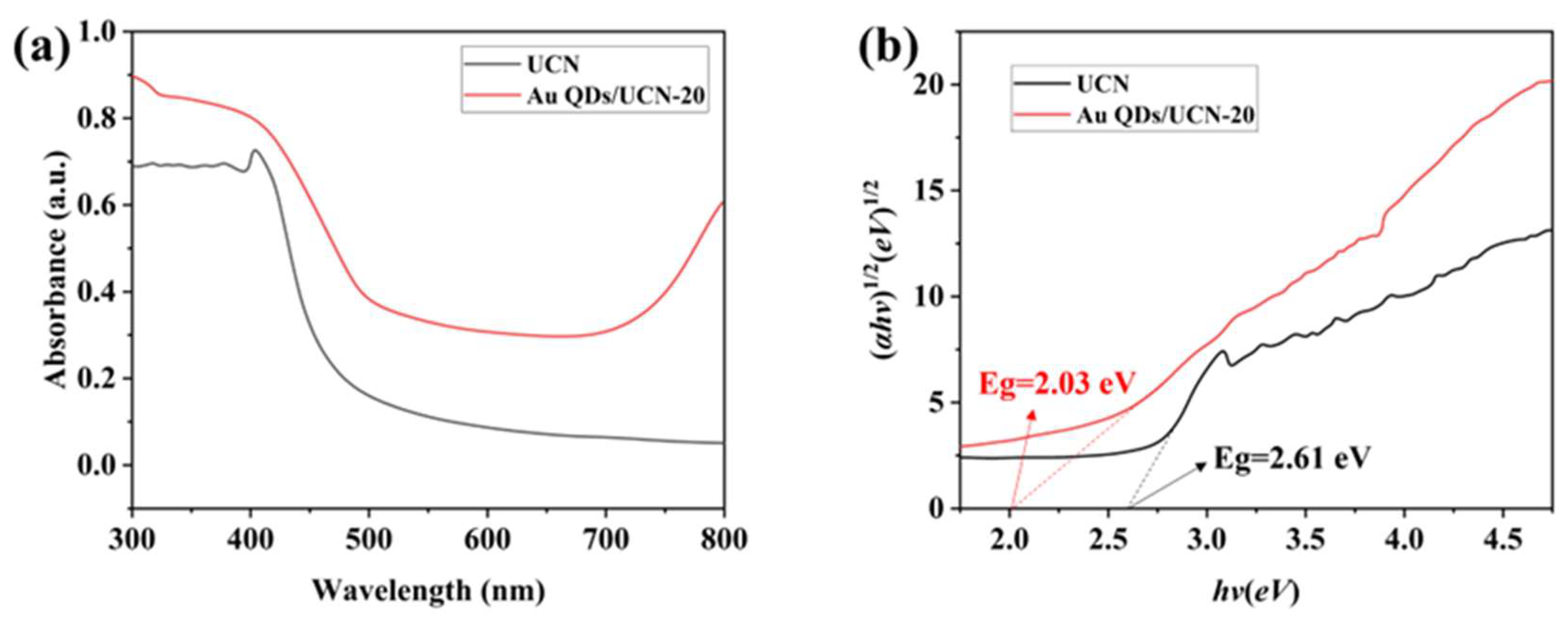
2.1. Structural Characterization
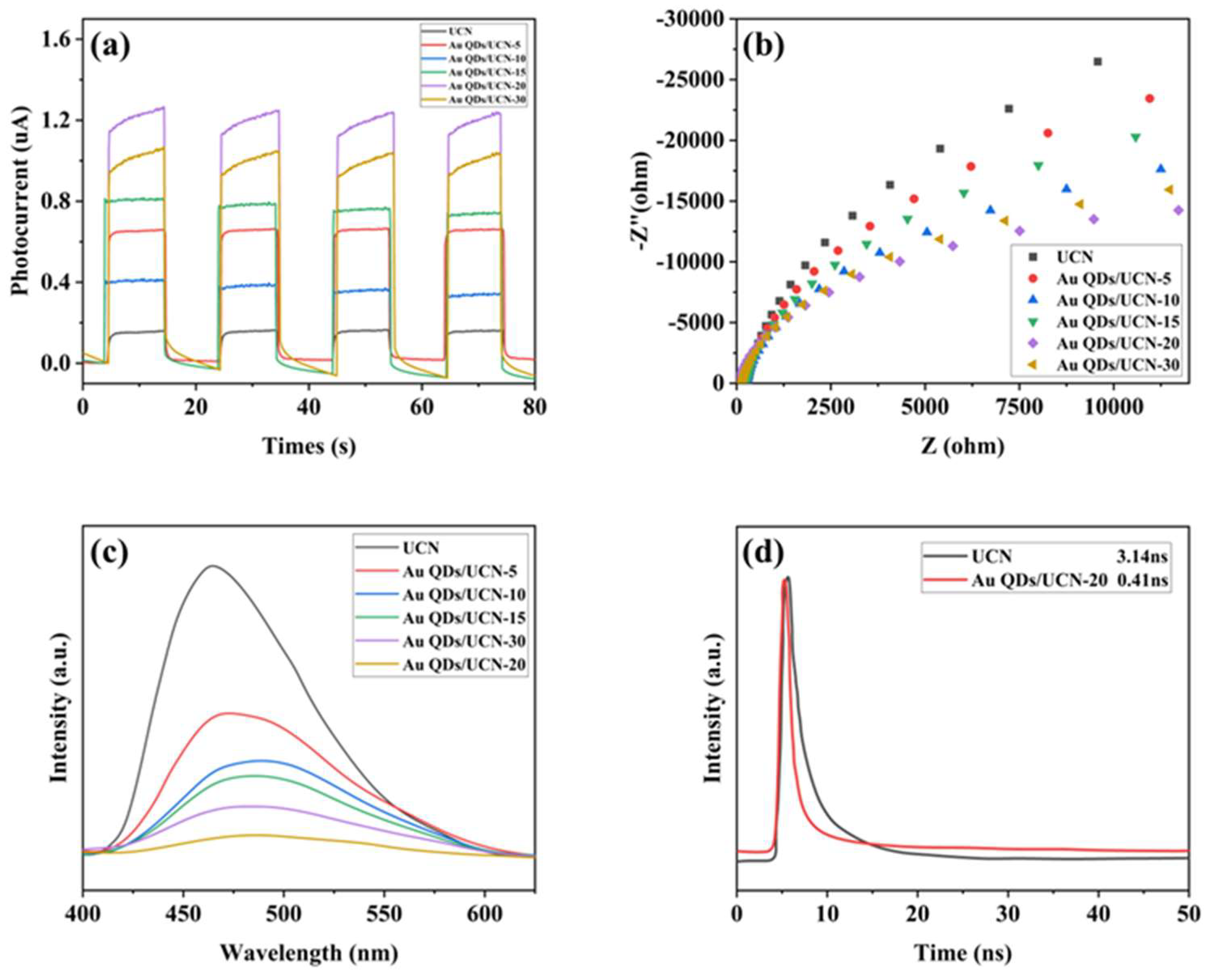
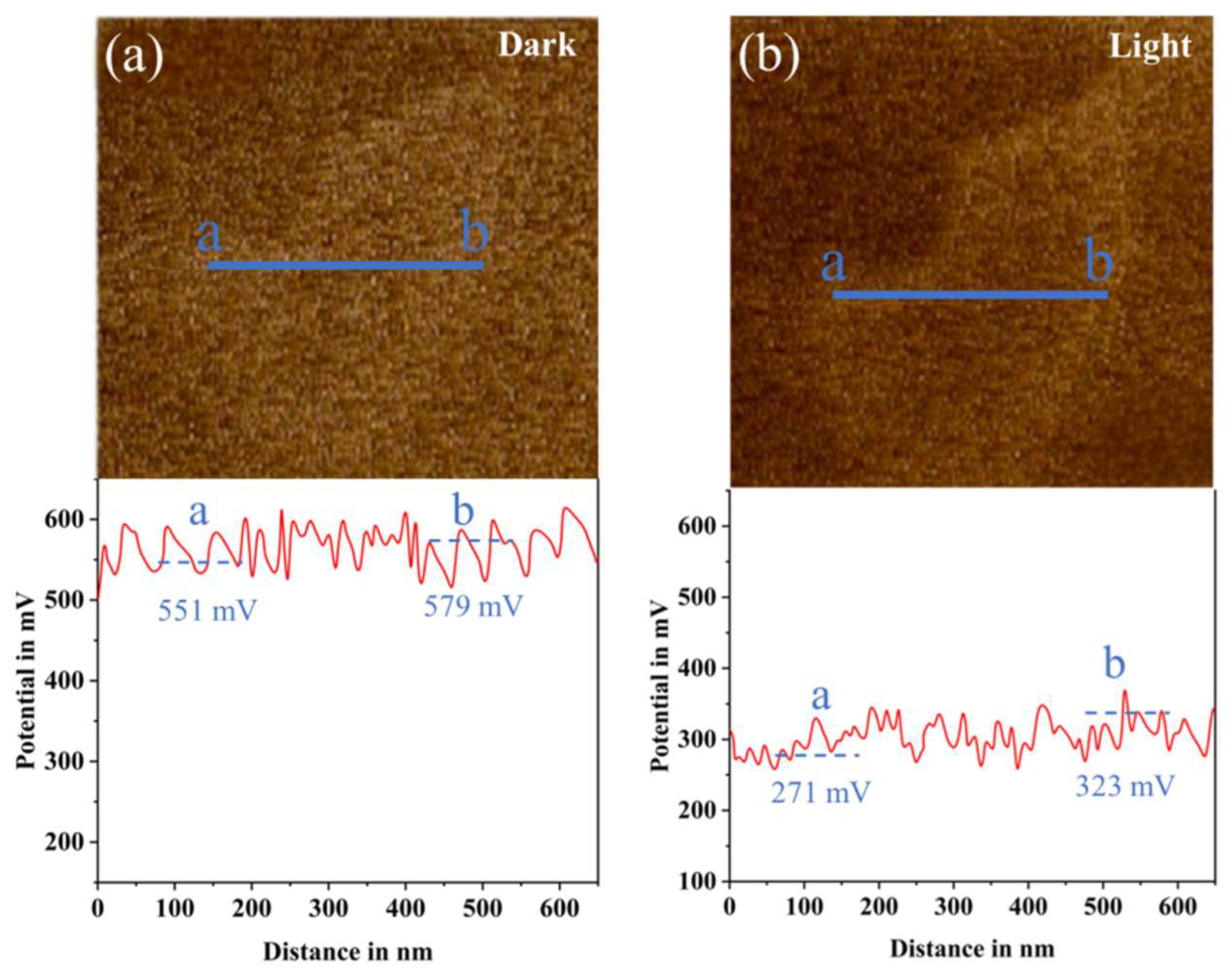
2.2. Photoelectrochemical Performance
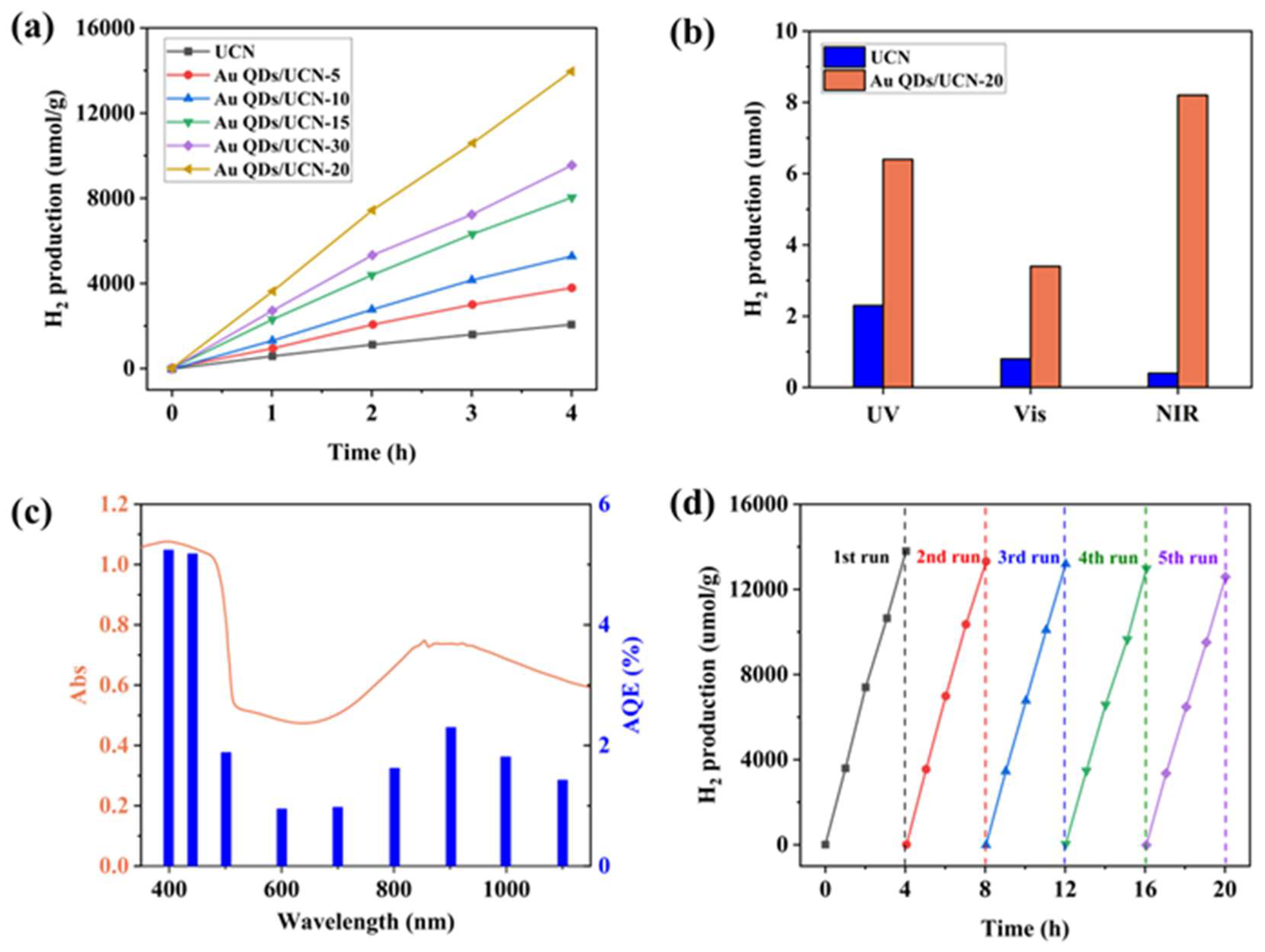
2.3. Photocatalytic Hydrogen Evolution
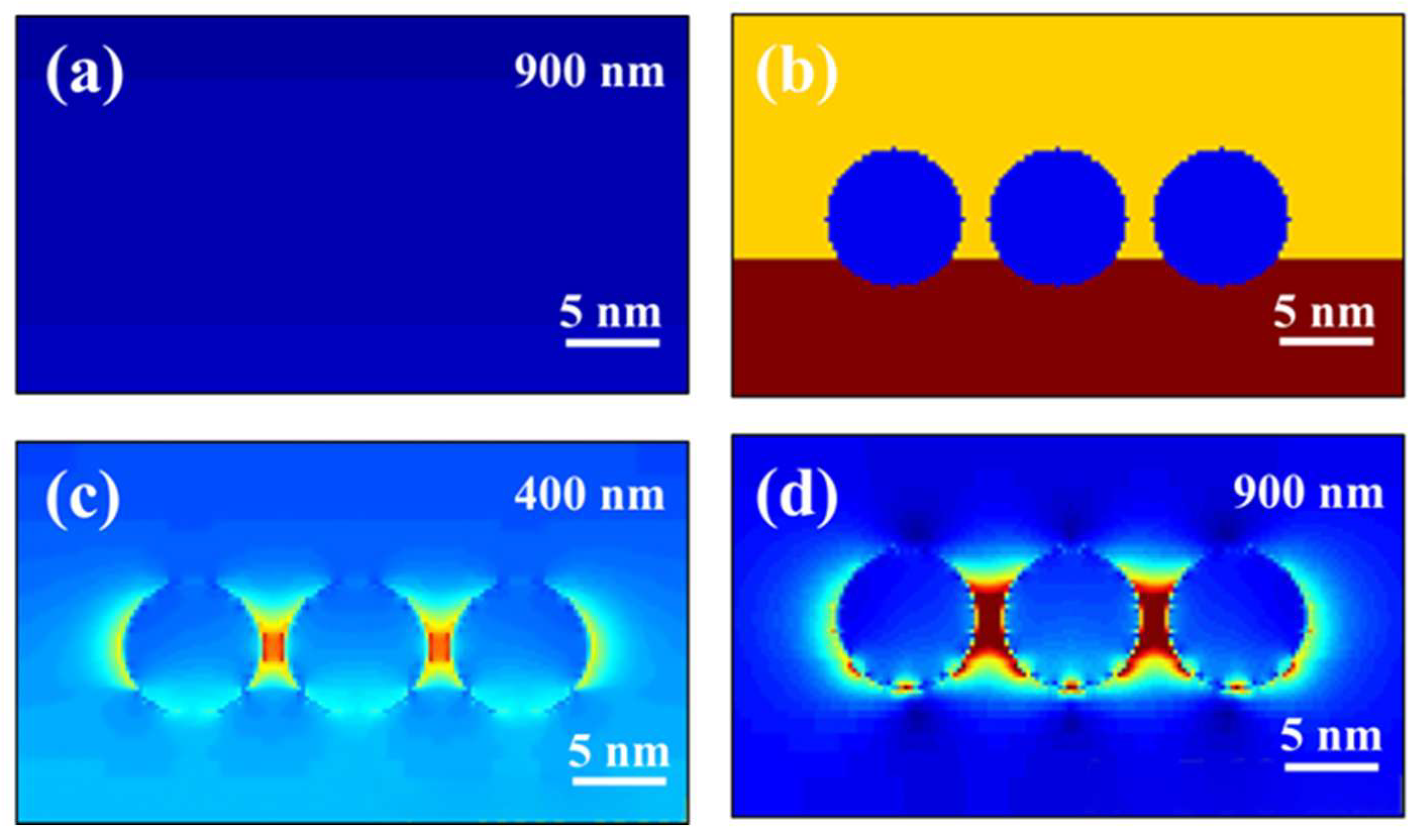
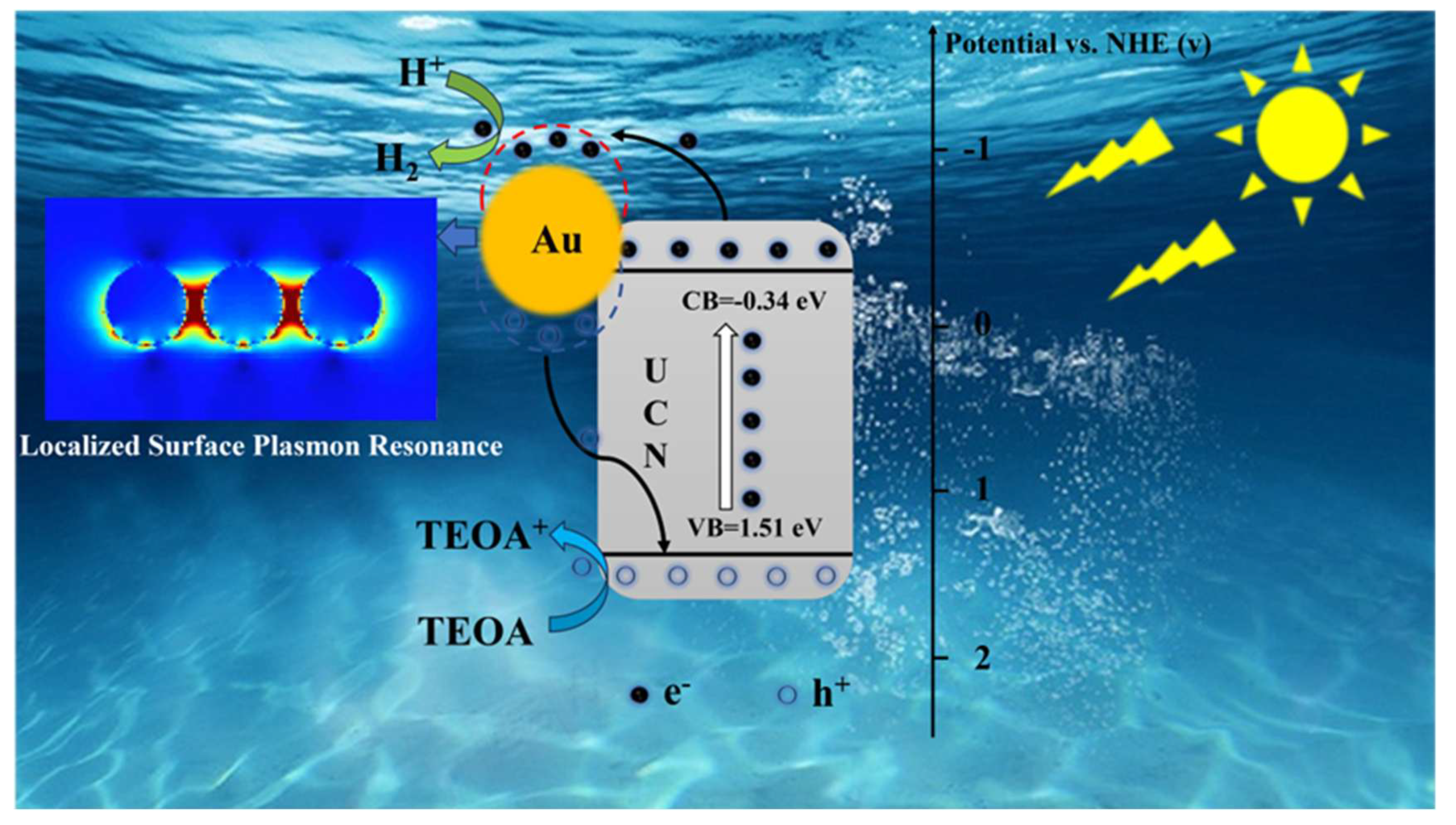
2.4. Analysis of the Photocatalytic Mechanism
3. Materials and Methods
3.1. Synthesis of Au/UCN
3.2. Photocatalytic and Photoelectrochemical Measurement
3.3. Samples Characterizations
3.4. FDTD Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Muzaffar, A.; Ahamed, M.B.; Hussain, C.M. Green supercapacitors: Latest developments and perspectives in the pursuit of sustainability. Renew. Sustain. Energy Rev. 2024, 195, 114324. [Google Scholar] [CrossRef]
- Imran, M.; Zaman, K.; Nassani, A.A.; Dincă, G.; Khan, H.U.R.; Haffar, M. Does nuclear energy reduce carbon emissions despite using fuels and chemicals? Transition to clean energy and finance for green solutions. Geosci. Front. 2023, 15, 101608. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, Y.; Feng, X.; Yu, X.; Gao, M.; Yu, S. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zheng, J.; Guo, M. La-doped NiFe-LDH coupled with hierarchical vertically aligned MXene frameworks for efficient overall water splitting. J. Energy Chem. 2022, 70, 472–479. [Google Scholar] [CrossRef]
- Van Thuan, D.; Ngo, H.L.; Thi, H.P.; Chu, T.T.H. Photodegradation of hazardous organic pollutants using titanium oxides -based photocatalytic: A review. Environ. Res. 2023, 229, 116000. [Google Scholar] [CrossRef]
- Kumar, P.; Maia, G.; Praserthdam, S.; Praserthdam, P. Renovated FeCoP-NC nanospheres wrapped by CoP-NC nanopetals: As a tremendously effectual and robust MOF-assisted electrocatalyst for hydrogen energy production. Environ. Res. 2024, 246, 118153. [Google Scholar] [CrossRef]
- Kakavandi, B.; Moradi, M.; Hasanvandian, F.; Bahadoran, A.; Mohebolkhames, E.; Golshan, M.; Ganachari, S.; Aminabhavi, T.M. Visible light-assisted S-scheme p- and n-type semiconductors anchored onto graphene for increased photocatalytic H2 production via water splitting. Chem. Eng. J. 2024, 487, 150399. [Google Scholar] [CrossRef]
- Zhou, X.; Xian, Y.; Li, Z.; Yujie, C.; Luo, J.; Ning, X.; Fan, X.; Zhong, Y.; Zhou, X. All-solid-state red phosphorus/RGO/WO3 Z-scheme heterostructure for photocatalytic overall water splitting. Sep. Purif. Technol. 2025, 357, 130117. [Google Scholar] [CrossRef]
- Lee, D.-E.; Danish, M.; Alam, U.; Jo, W.-K. Review on inorganic and polymeric materials-coordinated metal-organic-framework photocatalysts for green hydrogen evolution. J. Energy Chem. 2024, 92, 322–356. [Google Scholar] [CrossRef]
- Liu, J.; Yang, X.; Guo, X.; Jin, Z. Flowered molybdenum base trimetallic oxide decorated by CdS nanorod construct S-scheme heterojunctions for efficient photocatalytic hydrogen evolution. J. Mater. Sci. Technol. 2024, 196, 112–124. [Google Scholar] [CrossRef]
- Mao, L.; Zhai, B.; Shi, J.; Kang, X.; Lu, B.; Liu, Y.; Cheng, C.; Jin, H.; Lichtfouse, E.; Guo, L. Supercritical CH3OH-Triggered Isotype Heterojunction and Groups in g-C3N4 for Enhanced Photocatalytic H2 Evolution. ACS Nano 2024, 18, 13939–13949. [Google Scholar] [CrossRef]
- Shahid, M.U.; Najam, T.; Helal, M.H.; Hossain, I.; El-Bahy, S.M.; El-Bahy, Z.M.; Rehman, A.U.; Shah, S.S.A.; Nazir, M.A. Transition metal chalcogenides and phosphides for photocatalytic H2 generation via water splitting: A critical review. Int. J. Hydrogen Energy 2024, 62, 1113–1138. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, X.; Peng, L.; Luo, J.; Ning, X.; Fan, X.; Zhou, X.; Zhou, X. Pd(II) coordination molecule modified g-C3N4 for boosting photocatalytic hydrogen production. J. Colloid Interface Sci. 2024, 671, 134–144. [Google Scholar] [CrossRef]
- Liu, D.; Jiang, L.; Chen, D.; Hao, Z.; Deng, B.; Sun, Y.; Liu, X.; Jia, B.; Chen, L.; Liu, H. Twin S-Scheme g-C3N4/CuFe2O4/ZnIn2S4 Heterojunction with a Self-Supporting Three-Phase System for Photocatalytic CO2 Reduction: Mechanism Insight and DFT Calculations. ACS Catal. 2024, 14, 5326–5343. [Google Scholar] [CrossRef]
- Rezaei, M.; Nezamzadeh-Ejhieh, A.; Massah, A.R. A Comprehensive Review on the Boosted Effects of Anion Vacancies in Photocatalytic Solar Water Splitting: Focus on g-C3N4 with Carbon and Nitrogen Vacancies. Energy Fuels 2024, 38, 8406–8436. [Google Scholar] [CrossRef]
- Zhang, Q.; Yue, M.; Chen, P.; Ren, Q.; Kong, W.; Jia, C.; Lu, Q.; Wu, J.; Li, Y.; Liu, W.; et al. Accelerating photocatalytic hydrogen production by anchoring Pt single atoms on few-layer g-C3N4 nanosheets with Pt–N coordination. J. Mater. Chem. C 2024, 12, 3437–3449. [Google Scholar] [CrossRef]
- Sun, N.; Si, X.; He, L.; Zhang, J.; Sun, Y. Strategies for enhancing the photocatalytic activity of semiconductors. Int. J. Hydrogen Energy 2024, 58, 1249–1265. [Google Scholar] [CrossRef]
- Li, R.; Gao, T.; Wang, Y.; Chen, Y.; Luo, W.; Wu, Y.; Xie, Y.; Wang, Y.; Zhang, Y. Engineering of bimetallic Au–Pd alloyed particles on nitrogen defects riched g-C3N4 for efficient photocatalytic hydrogen production. Int. J. Hydrogen Energy 2024, 63, 1116–1127. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Zhao, P.; Zhang, L.; Dai, B.; Xu, J.; Huang, H.; He, Y.; Leung, D.Y.C. Novel Z-scheme Ag-C3N4/SnS2 plasmonic heterojunction photocatalyst for degradation of tetracycline and H2 production. Chem. Eng. J. 2021, 405, 126555. [Google Scholar] [CrossRef]
- Song, T.; Zhang, X.; Xie, C.; Yang, P. N-doped carbon nanotubes enhanced charge transport between Ni nanoparticles and g-C3N4 nanosheets for photocatalytic H2 generation and 4-nitrophenol removal. Carbon 2023, 210, 118052. [Google Scholar] [CrossRef]
- Khan, A.W.; Lali, N.S.; Sabei, F.Y.; Irfan, M.I.; Naeem-ul-Hassan, M.; Sher, M.; Safhi, A.Y.; Alsalhi, A.; Albariqi, A.H.; Kamli, F.; et al. Sunlight-assisted green synthesis of gold nanocubes using horsetail leaf extract: A highly se-lective colorimetric sensor for Pb2+, photocatalytic and antimicrobial agent. J. Environ. Chem. Eng. 2024, 12, 112576. [Google Scholar] [CrossRef]
- Kwon, K.C.; Suh, J.M.; Varma, R.S.; Shokouhimehr, M.; Jang, H.W. Electrocatalytic Water Splitting and CO2 Reduction: Sustainable Solutions via Single-Atom Catalysts Supported on 2D Materials. Small Methods 2019, 3, 1800492. [Google Scholar] [CrossRef]
- Kuang, P.; Zheng, P.; Liu, Z.; Lei, J.; Wu, H.; Li, N.; Ma, T. Embedding Au Quantum Dots in Rimous Cadmium Sulfide Nanospheres for Enhanced Photocatalytic Hydrogen Evolution. Small 2016, 12, 6735–6744. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Song, G.; Liu, Q.; Zhang, W.; Gu, J.; Su, Y.; Su, H.; Guo, C.; Zhang, D. Enhanced photocatalytic hydrogen production on three-dimensional gold butterfly wing scales/CdS nanoparticles. Appl. Surf. Sci. 2018, 427, 807–812. [Google Scholar] [CrossRef]
- Gangadharan, D.T.; Xu, Z.; Liu, Y.; Izquierdo, R.; Ma, D. Recent advancements in plasmon-enhanced promising third-generation solar cells. Nanophotonics 2016, 6, 153–175. [Google Scholar] [CrossRef]
- Sun, X.; Chen, Z.; Shen, Y.; Qin, H.; Yuan, H.; Lu, J.; Guo, F.; Li, C.; Shi, W. Efficient photothermal-assisted photocatalytic H2 production using carbon dots-infused g-C3N4 nanoreactors synthesized via one-step template-free thermal polymerization. Chem. Eng. J. 2024, 488, 151041. [Google Scholar] [CrossRef]
- George, J.K.; Bhagat, A.; Bhaduri, B.; Verma, N. Carbon Nanofiber-Bridged Carbon Nitride-Fe2O3 Photocatalyst: Hydrogen Generation and Degradation of Aqueous Organics. Catal. Lett. 2022, 153, 419–431. [Google Scholar] [CrossRef]
- Al-Ansari, S.H.; Gomaa, H.; Abdel-Rahim, R.D.; Ali, G.A.M.; Nagiub, A.M. Recycled gold-reduced graphene oxide nanocomposite for efficient adsorption and photocatalytic degradation of crystal violet. Sci. Rep. 2024, 14, 4379. [Google Scholar] [CrossRef]
- Li, Z.; Kong, C.; Lu, G. Visible Photocatalytic Water Splitting and Photocatalytic Two-Electron Oxygen Formation over Cu- and Fe-Doped g-C3N4. J. Phys. Chem. C 2016, 120, 56–63. [Google Scholar] [CrossRef]
- Guo, Z.; Dai, F.; Yin, H.; Zhang, M.; Xing, J.; Wang, L. The dual role of Au nanoparticles in the surface plasmon resonance enhanced photocatalyst Au/g-C3N4. Colloid Interface Sci. Commun. 2022, 48, 100615. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, N.; Lou, L.; Iwasaki, K.; Wu, H.; Luo, Y.; Li, D.; Nakata, K.; Fujishima, A.; Meng, Q. Localized Surface Plasmon Resonance Enhanced Photocatalytic Hydrogen Evolution via Pt@Au NRs/C3N4 Nanotubes under Visible-Light Irra-diation. Adv. Funct. Mater. 2018, 29, 1806774. [Google Scholar] [CrossRef]
- Tian, H.; Liu, X.; Liang, Z.; Qiu, P.; Qian, X.; Cui, H.; Tian, J. Gold nanorods/g-C3N4 heterostructures for plasmon-enhanced photocatalytic H2 evolution in visible and near-infrared light. J. Colloid Interface Sci. 2019, 557, 700–708. [Google Scholar] [CrossRef]
- Yassin, J.M.; Taddesse, A.M.; Sánchez-Sánchez, M. Sustainable synthesis of a new semiamorphous Ti-BDC MOF material and the photocatalytic performance of its ternary composites with Ag3PO4 and g-C3N4. Appl. Surf. Sci. 2022, 578, 151996. [Google Scholar] [CrossRef]
- Guo, Y.; Jia, H.; Yang, J.; Yin, H.; Yang, Z.; Wang, J.; Yang, B. Understanding the roles of plasmonic Au nanocrystal size, shape, aspect ratio and loading amount in Au/g-C3N4hybrid nanostructures for photocatalytic hydrogen generation. Phys. Chem. Chem. Phys. 2018, 20, 22296–22307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shao, M.; Ning, F.; Xu, S.; Li, Z.; Wei, M.; Evans, D.G.; Duan, X. Au nanoparticles sensitized ZnO nanorod@nanoplatelet core–shell arrays for enhanced photoelectrochemical water splitting. Nano Energy 2015, 12, 231–239. [Google Scholar] [CrossRef]
- Jiang, F.; Zhou, X.; Lv, J.; Chen, J.; Chen, J.; Kongcharoen, H.; Zhang, Y.; Lee, P.S. Stretchable, Breathable, and Stable Lead-Free Perovskite/Polymer Nanofiber Composite for Hybrid Triboelectric and Piezoelectric Energy Harvesting. Adv. Mater. 2022, 34, 2200042. [Google Scholar] [CrossRef]
- Yu, G.; Wang, X.; Cao, J.; Wu, S.; Yan, W.; Liu, G. Plasmonic Au nanoparticles embedding enhances the activity and stability of CdS for photocatalytic hydrogen evolution. Chem. Commun. 2015, 52, 2394–2397. [Google Scholar] [CrossRef]
- Yu, H.; Cao, X.; Zhang, S.; Luo, S.; Feng, L.; An, X.; Jiang, H.; Yao, S. Nano-sized aggregate Ti3C2–TiO2 supported on the surface of Ag2NCN as a Z-scheme catalyst with enhanced visible light photocatalytic performance. Dalton Trans. 2023, 52, 14640–14648. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, W.; Shi, Z.; Ji, Y.; Lyu, J.; Pan, G.; Zhu, J.; Tian, Y.; Li, X.; Song, H. Plasmonic gold nanorods decorated Ti3C2 MXene quantum dots-interspersed nanosheet for full-spectrum photoelectrochemical water splitting. Chem. Eng. J. 2021, 426, 130818. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Qin, F.; Wan, J.; Wu, G.; Deng, L.; Sun, Q.; Wang, X.; Yao, D.; Kan, J. Flower-shaped S-scheme CdS-ZnO nanorods heterojunction assisted with SPR of low-content Au for accelerating photocatalytic hydrogen production. Int. J. Hydrogen Energy 2024, 58, 302–309. [Google Scholar] [CrossRef]
- Jian, A.; Feng, K.; Jia, H.; Zhang, Q.; Sang, S.; Zhang, X. Quantitative investigation of plasmonic hot-electron injection by KPFM. Appl. Surf. Sci. 2019, 492, 644–650. [Google Scholar] [CrossRef]
- Wang, K.L.; Yang, Y.; Farhan, S.; Wu, Y.; Lin, W.F. S-scheme p-n junction Na0.6 CoO2 /g-C3N4 heterostructure as an efficient photocatalyst for green hydrogen production: Fabrication, characterization and mechanisms. Chem. Eng. J. 2024, 490, 151408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Wei, Z.; Gao, Q.; Qu, P.; Wang, R.; Luo, X.; Sun, X.; Li, D.; Zhang, X.; Liu, J.; et al. Gold Nanoparticles-Functionalized Ultrathin Graphitic Carbon Nitride Nanosheets for Boosting Solar Hydrogen Production: The Role of Plasmon-Induced Interfacial Electric Fields. Molecules 2025, 30, 3406. https://doi.org/10.3390/molecules30163406
Yu H, Wei Z, Gao Q, Qu P, Wang R, Luo X, Sun X, Li D, Zhang X, Liu J, et al. Gold Nanoparticles-Functionalized Ultrathin Graphitic Carbon Nitride Nanosheets for Boosting Solar Hydrogen Production: The Role of Plasmon-Induced Interfacial Electric Fields. Molecules. 2025; 30(16):3406. https://doi.org/10.3390/molecules30163406
Chicago/Turabian StyleYu, Haidong, Ziqi Wei, Qiyue Gao, Ping Qu, Rui Wang, Xuehui Luo, Xiao Sun, Dong Li, Xiao Zhang, Jiufen Liu, and et al. 2025. "Gold Nanoparticles-Functionalized Ultrathin Graphitic Carbon Nitride Nanosheets for Boosting Solar Hydrogen Production: The Role of Plasmon-Induced Interfacial Electric Fields" Molecules 30, no. 16: 3406. https://doi.org/10.3390/molecules30163406
APA StyleYu, H., Wei, Z., Gao, Q., Qu, P., Wang, R., Luo, X., Sun, X., Li, D., Zhang, X., Liu, J., & Feng, L. (2025). Gold Nanoparticles-Functionalized Ultrathin Graphitic Carbon Nitride Nanosheets for Boosting Solar Hydrogen Production: The Role of Plasmon-Induced Interfacial Electric Fields. Molecules, 30(16), 3406. https://doi.org/10.3390/molecules30163406




