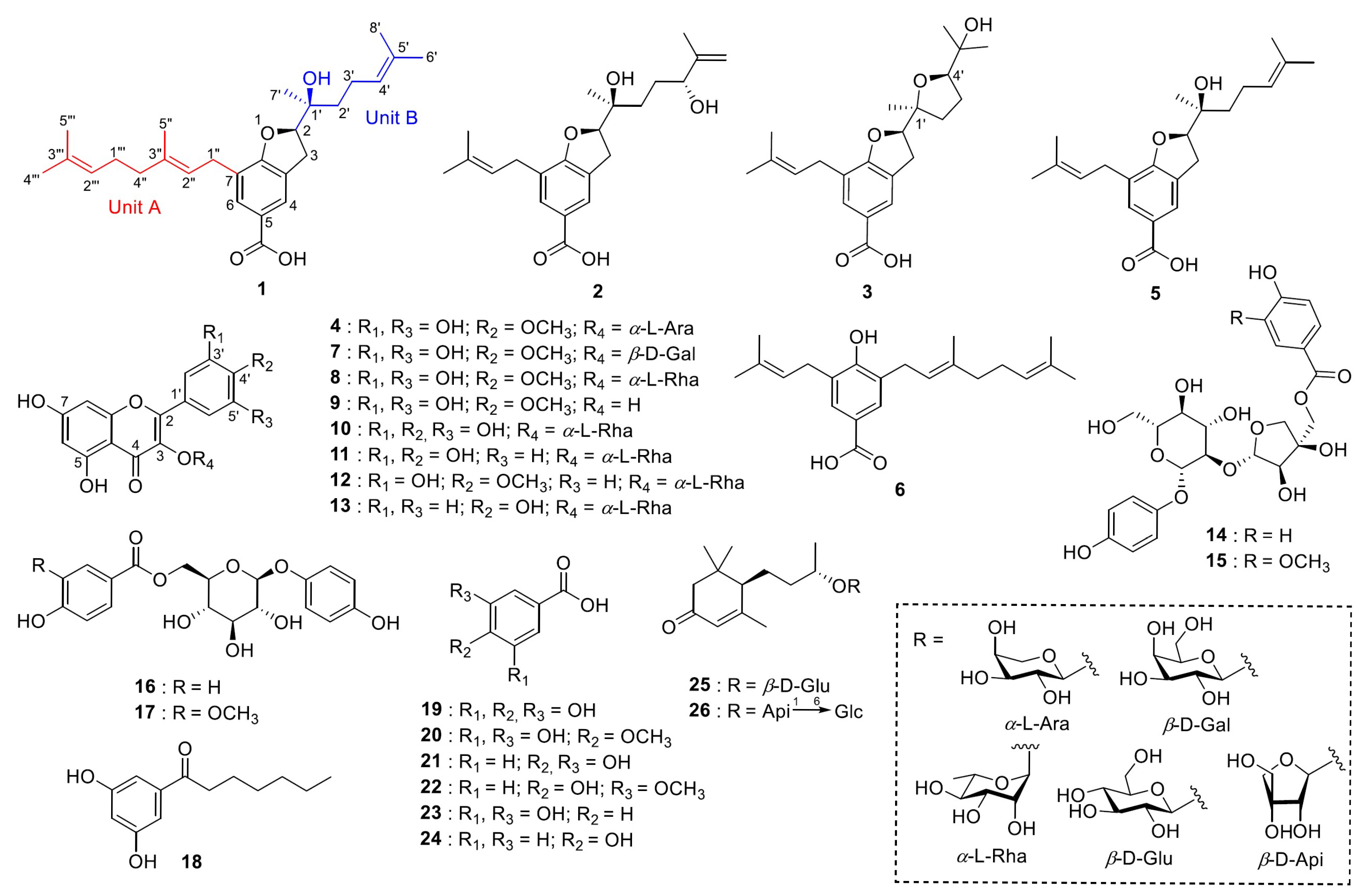
2. Results and Discussion
Chemical analysis of the methanol extract of the aerial parts of
M. seguinii led to the isolation and structural elucidation of three new terpeno-benzoic acid derivatives (
1–
3), a new flavonoid glycoside (
4), and 22 other known compounds. The known compounds were determined as myrsinoic acid B (
5), myrsinoic acid A (
6) [
8], myricetin 4′-methyl ether 3-
O-
b-D-galactopyranoside (
7) [
9], mearnsitrin (
8) [
10], mearnsetin (
9) [
10], myricitrin (
10) [
10], quercitrin (
11) [
2], tamarixetin 3-
O-
a-L-rhamnoside (
12) [
11], kaempferol 3-
O-
a-L-rhamnoside (
13) [
12], seguinoside D (
14) [
13], seguinoside E (
15) [
13], breynioside A (
16) [
14], 6′-
O-vanilloylarbutin (
17) [
15], vanillic acid 4-
O-
b-D-glucoside (
18) [
16], gallic acid (
19) [
17], 4-
O-methylgallic acid (
20) [
18], protocatechuic acid (
21) [
19], vanillic acid (
22) [
19], resorcylic acid (
23) [
20], 4-hydroxy-benzoic acid (
24) [
21], (6
R,9
S)-blumenol
C glucoside (
25) [
22], and byzantionoside B 6′-
O-
b-D-apiofuranoside (
26) [
23] (
Figure 1; SI
Figure S1.1). For myrsinoic acid B (
5), the previously assigned absolute configuration was incorrect and has been re-established in this paper.
Compound
1 was a yellow amorphous solid, and its molecular formula was determined to be C
27H
38O
4 by Electrospray Ionisation Time-of-Flight Mass Spectrometry (ESI-Q-TOF-MS), with a positive-ion peak at
m/
z 427.2818 [M + H]
+. The
1H and
13C NMR spectra exhibited signals for a tetrasubstituted benzene group [
δH 7.74 (2H, s, H-4, and H-6)/
δC 127.37 (C-3a), 125.22 (C-4), 121.94 (C-5), 131.66 (C-6), 123.37 (C-7), and 162.64 (C-7a)], an oxygenated methine [
δH 4.72 (1H, t,
J = 9.0 Hz, H-2)/
δC 89.77 (C-2)], a methylene [
δH 3.19 (2H, m, H-3)/
δC 30.11 (C-3)], and a carboxylic acid [
δC 171.96 (
COOH)] (
Table 1).
The data indicate that
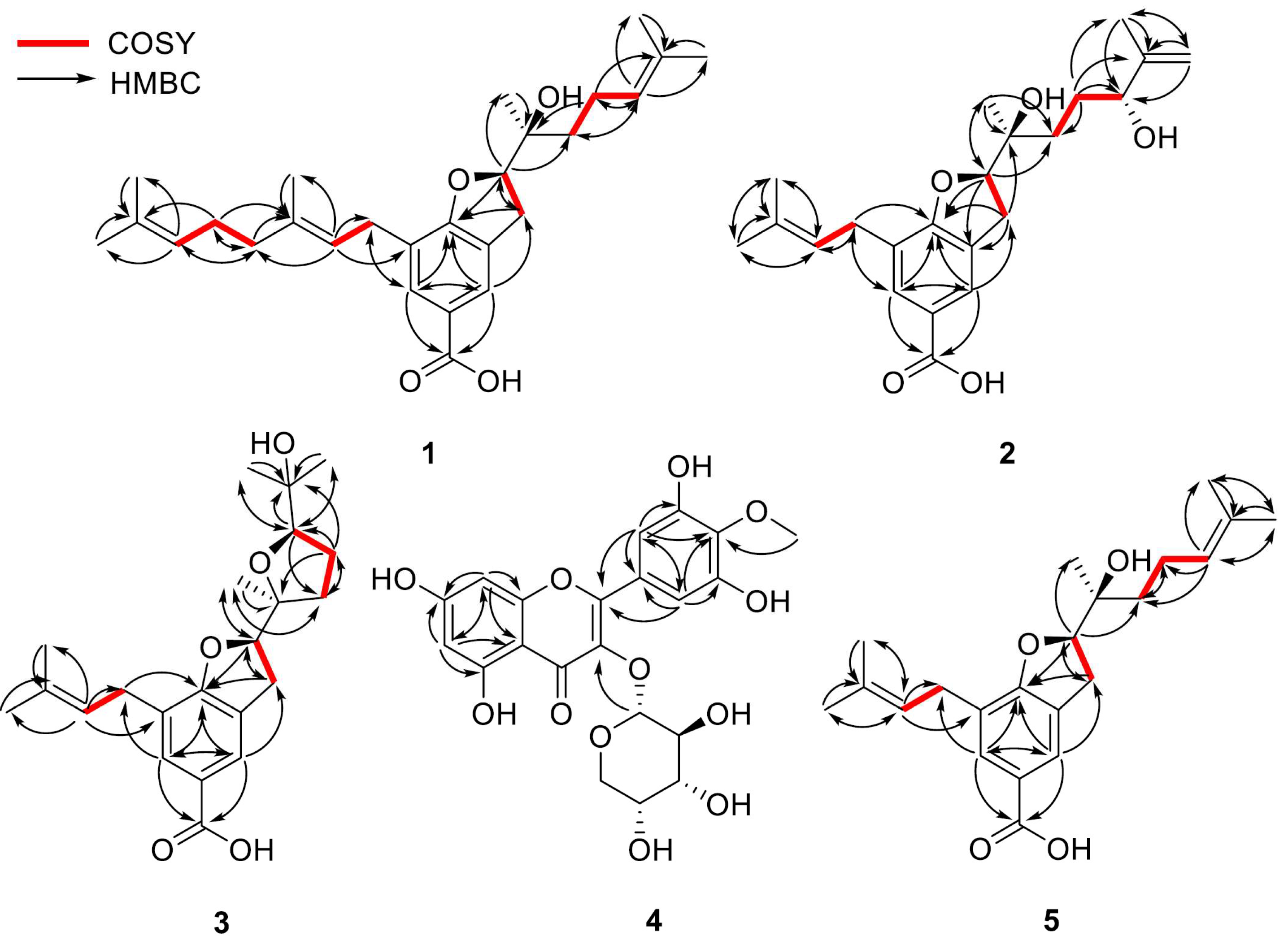
1 possesses a 2,3-dihydrobenzofuran-5-carboxylic acid skeleton, which is corroborated by the heteronuclear multiple-bond correlation (HMBC) (
Figure 2) between H-2 and C-7a. Further analysis of the remaining signals of
1 revealed the presence of two distinct side chains: units A and B. The 1D NMR (
1H and
13C NMR spectra) signals of unit A—
δH 1.57 (3H, s, H-5‴), 1.65 (3H, s, H-4‴), 1.70 (3H, s, H-5”), 2.01 (2H, m, H-4”), 2.08 (2H, m, H-1‴), 3.28 (2H, m, H-1”), 5.09 (1H, t,
J = 7.0 Hz, H-2‴), and 5.28 (1H, t,
J = 7.3 Hz, H-2”)/
δC 16.43 (C-5″), 17.89 (C-5‴), 25.87 (C-4‴), 26.87 (C-1‴), 28.38 (C-1″), 39.94 (C-4″), 121.37 (C-2″), 124.34 (C-2‴), 131.75 (C-3‴), and 137.07 (C-3″)—are consistent with a geranyl group. Unit B was identified as a 1′-hydroxy-1′,5′-dimethylhex-4′-enyl moiety, inferred from the following NMR data:
δH 1.28 (3H, s, H-7′), 1.51 (2H, m, H-2′), 1.61 (3H, s, H-8′), 1.67 (3H, s, H-6′), 2.11 (2H, m, H-3′), and 5.10 (1H, t,
J = 7.0 Hz, H-4′)/
δC 17.91 (C-8′), 22.18 (C-3′), 22.89 (C-7′), 25.90 (C-6′), 37.32 (C-2′), 73.96 (C-1′), 124.22 (C-4′), and 132.46 (C-5′). These assignments are further substantiated by 2D NMR data, including
1H-
1H correlated spectroscopy (COSY), correlations [unit A—H-1″/H-2″, H-4″/H-1‴, and H-1‴/H-2‴; unit B—H-2′/H-3′ and H-3′/H-4′] and HMBCs [unit A—H-2″/C-1″, C-4″, C-5″, H-4″/C-3″, C-1‴, C-2‴, H-5″/C-3″, H-1‴/C-3‴, H-2‴/C-4‴, C-5‴, H-4‴, H-5‴/C-3‴; unit B—H-3′/C-1′, C-4′, C-5′, H-4′/C-2′, C-6′, C-8′, H-6′, H-8′/C-5′, H-7′/C-1′] (
Figure 2).
Additionally, key HMBCs of H-1″ with C-6 and H-2 with C-2′ and C-7′ revealed that A and B units are substituted at C-7 and C-2, respectively, of the 2,3-dihydrobenzofuran-5-carboxylic acid core. A planar structure of
1 was elucidated, as shown in
Figure 1. Comparison of the NMR data with those of myrosinoic acid B (
5) revealed that they possess an identical 2,3-dihydrobenzofuran-5-carboxylic acid scaffold. The only difference is that the 3,3-dimethylallyl moiety in
5 is replaced by a geranyl group corresponding to unit A in
1. Through HMBC peaks at
δH 2.08 (H-1‴)/
δC 137.07 (C-3″) and 39.94 (C-4″), attachment of an additional 3,3-dimethylallyl moiety at the C-4″ position was confirmed. Compound
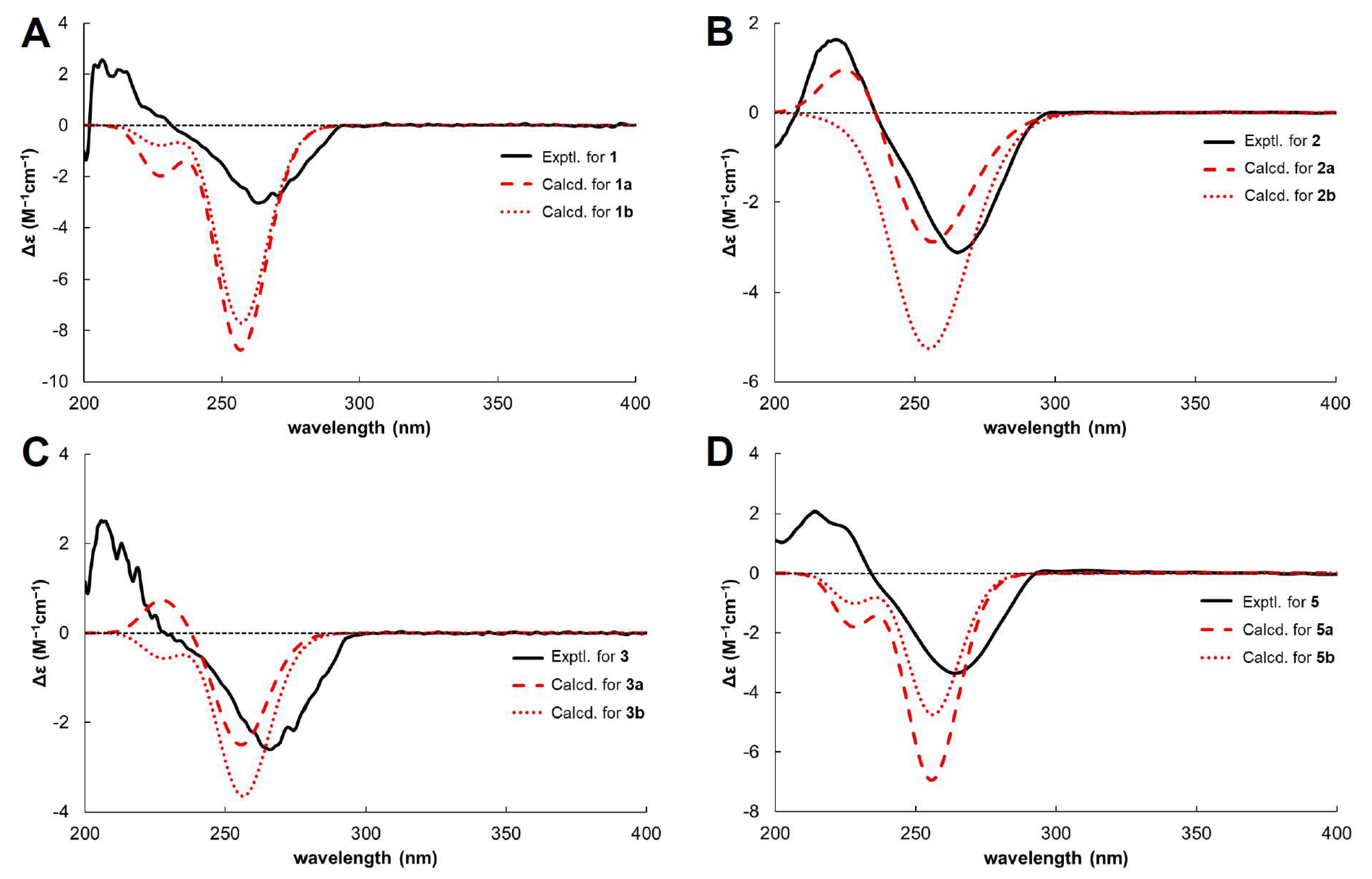
1 has chiral centres at C-2 and C-1′. The absolute configuration of C-2 was determined by comparing its circular dichroism (CD) spectrum (
Figure 3) with that of the dihydrobenzofuran derivative [
24]. The experimental CD spectrum of
1 exhibited a positive Cotton effect (CE) at 206.6 nm and a negative CE at 263.2 nm, suggesting C-2’s absolute configuration as
R. The NOESY correlation between H-2 and H-7′ was not suitable for determining the relative configuration due to the free rotation of the chain. Thus,
1 is proposed to have two possible isomers, (2
R,1′
S)-
1 (
1a) and (2
R,1′
R)-
1 (
1b). The C-1′ configuration was assigned based on a comparison of the carbon chemical shifts corresponding to C-2′ and C-7′ of
1 with those of the known compound bonannione B, sharing the identical dihydrobenzofuran with
1 [
25]. The experimental chemical values of C-2′ (
δC 37.32) and C-7′ (
δC 22.89) in
1 closely match the experimental and calculated values of 2
R,1′
S-bonannione B (
δC 36.6, 22.4 and
δC 37.3, 23.1, respectively). In contrast, the calculated shifts for 2
R,1′
R-bonannione B are distinct (e.g.,
δC 40.6 and 19.7), as generated by density functional theory (DFT)–NMR analysis [
25]. These results support the assignment of the 2
R,1′
S configuration for
1. To confirm the accurate configurational assignment of
1, the NMR chemical shifts of
1a and
1b were calculated using the Gauge-Including Atomic Orbital (GIAO) method and analysed using DP4+. The results revealed that the calculated
1H and
13C NMR data for
1a are in good agreement with the experimental data, with a higher linear correlation coefficient (
R2) and lower Mean Absolute Error (MAE) and Corrected Mean Absolute Error (CMAE) values than those of
1b. Additionally, the DP4+ analysis indicated that
1a is the most likely structure with a 100% probability (
Table 2). Finally, because of the similar ECD patterns observed for
1a and
1b, it was not possible to obtain additional differentiation through ECD experiments (
Figure 3). Based on the above evidence (
Figures S1.1–S1.6 and
Tables S1.1–S1.7), the absolute configuration of
1 was determined to be 2
R,1′
S and was named myrsinoic acid I [(
R)-7-((
E)-3,7-dimethylocta-2,6-dien-1-yl)-2-((
S)-2-hydroxy-6-methylhept-5-en-2-yl)-2,3-dihydrobenzofuran-5-carboxylic acid].
Compound
2 was isolated as a white amorphous solid and was confirmed to have the molecular formula C
22H
30O
5 based on the positive-ion peak at 375.2169 [M + H]
+. Analyses of the
1H and
13C NMR spectra predict that the overall structure of
2 is similar to that of
5 (
Table 1). Notable changes are observed in the
1H and
13C NMR spectra. The signals corresponding to the methyl protons and olefinic proton of the terminal 3,3-dimethylallyl group in unit B of
1 are replaced by signals for a methylene group [
δH 4.96 (1H, s, H-6′α), 4.85 (1H, s, H-6′β)/
δC 147.4 (C-5′) and 111.4 (C-6′)] and an oxygenated methine proton [
δH 4.10 (1H, dd,
J = 7.3, 4.6 Hz, H-4′)/
δC 76.0 (C-4′)], respectively. A comprehensive interpretation of the above 1D-NMR analysis (
Table 1) reveals that the terminal 3,3-dimethylallyl moiety connected to C-2′ in
5 is substituted with a 3-methyl-3-buten-2-ol moiety owing to the transformation, as supported by the COSY correlations [H-2′/H-3′ and H-3′/H-4′] and HMBCs [H-3′/C-2′, C-4′, C-5′, C-8′, H-6′/C-4′, C-5′, C-8′, H-7′/C-1′, C-2′, H-8′/C-4′, C-5′] (
Figure 2). Further additional HMBC analysis [H-3/C-1′ and H-2/C-2′] reveals the attachment of the 3-methyl-3-buten-2-ol moiety group to position C-2′ (
Figure 2). Based on the above evidence, the planar structure of
2 was established as depicted in
Figure 1.
In compound
2, three stereocentres are present at C-2, C-1′, and C-4′. As previously mentioned for
1, the stereochemical assignments at C-2 and C-1′ were determined using the same analytical procedure. The CD spectrum indicates that the absolute configuration of C-2 is
R, as evidenced by the positive CE at 222 nm and the negative CE at 265 nm. The configuration of C-1′ is assigned as
S according to the experimental carbon chemical shifts at C-2′ and C-7′ at
δC 33.13 and 22.83 ppm (
Table 1). To determine the stereochemistry of C-4′, NOESY cross peaks were considered; however, the conformational flexibility of the linear side chain makes a definitive assignment difficult [
26]. Accordingly, the absolute configuration of C-4′ was reconfirmed via computational analysis. GIAO NMR chemical shift calculations were conducted for two possible isomers, (2
R,1′
S,4′
S)-
2 (
2a) and (2
R,1′
S,4′
R)-
2 (
2b), followed by DP4+ probability analysis. The results demonstrate that the parameters from calculated NMR data exhibit a better agreement with the experimental
1H NMR spectrum for
2a, whereas the
13C NMR data show higher consistency with
2b (
Table 2). This discrepancy indicates that the NMR chemical shift calculations are inadequate for clearly distinguishing between the two isomers. Therefore, instead of comparing all chemical shifts, we evaluated the
1H NMR chemical shifts in H-2 and H-7′, where significant differences are observed between the two stereoisomers. As shown in
Table 3, the calculated
1H NMR chemical shifts for
2a exhibit greater consistency with the experimental data. These results are further supported by DP4+ analysis (
Table 2). This was further corroborated by comparing the experimental and calculated ECD spectra (
Figure 3). Finally, the absolute configuration of
2 was established as 2
R,1′
S,4′
S and named myrsinoic acid J [(
R)-2-((2
S,5
R)-2,5-dihydroxy-6-methylhept-6-en-2-yl)-7-(3-methylbut-2-en-1-yl)-2,3-dihydrobenzofuran-5-carboxylic acid] (
Figures S2.1–S2.6 and
Tables S2.1–S2.7).
The molecular formula of
3, a yellow, amorphous solid, was estimated to be C
22H
30O
5 based on the positive-ion ESI-Q-TOF-MS spectrum, which displayed an ion peak at
m/
z 375.2176 [M + H]
+. Compounds
2 and
3 had identical chemical formulas and similar
1H and
13C NMR spectra (
Table 1). A notable difference is the chemical shifts corresponding to unit B, attributable to the presence of two methylene groups [δ
H 2.02 (1H, dt,
J = 12.1, 9.0 Hz, H-2′α), 1.74 (1H, m, H-2′β), and 1.88 (2H, m, H-3′)/δ
C 33.76 (C-2′) and 26.61 (C-3′)], an oxygenated methine [δ
H 3.91 (2H, t,
J = 7.6 Hz, H-4′)/δ
C 87.45 (C-4′)], three methyl groups [δ
H 1.22 (3H, s, H-6′), 1.19 (3H, s, H-7′), 1.13 (3H, s, H-8′)/δ
C 27.78 (C-6′), 22.82 (C-7′), 24.23 (C-8′)], and two oxygenated sp
3 carbons [δ
C 84.68 (C-1′) and 71.03 (C-5′)]. Considering the 1D (
Table 1) and HSQC data (
Figure 2), and the molecular formula confirmed by the ESI-Q-TOF-MS spectrum of
3, it can be inferred that the terminal 3-methyl-3-buten-2-ol group in
2 is replaced by a tetrahydrofuran structure, as shown in
Figure 1. This conclusion was supported by
1H–
1H COSY correlations [H-2′/H-3′ and H-3′/H-4′] and HMBCs [H-2′/C-3′, H-3′/C-1′, C-4′, H-4′/C-2′, C-5′, C-6′, C-8′, H-6′, H-8′/C-5′, H-7′/C-1′] (
Figure 2).
Like
2, compound
3 possesses three stereocentres at C-2, C-1′, and C-4′. The configurations at C-2 and C-1′ were determined to be
R and
S, respectively, based on the positive CE at 205.7 nm and the negative CE at 266.2 nm in the CD spectrum (
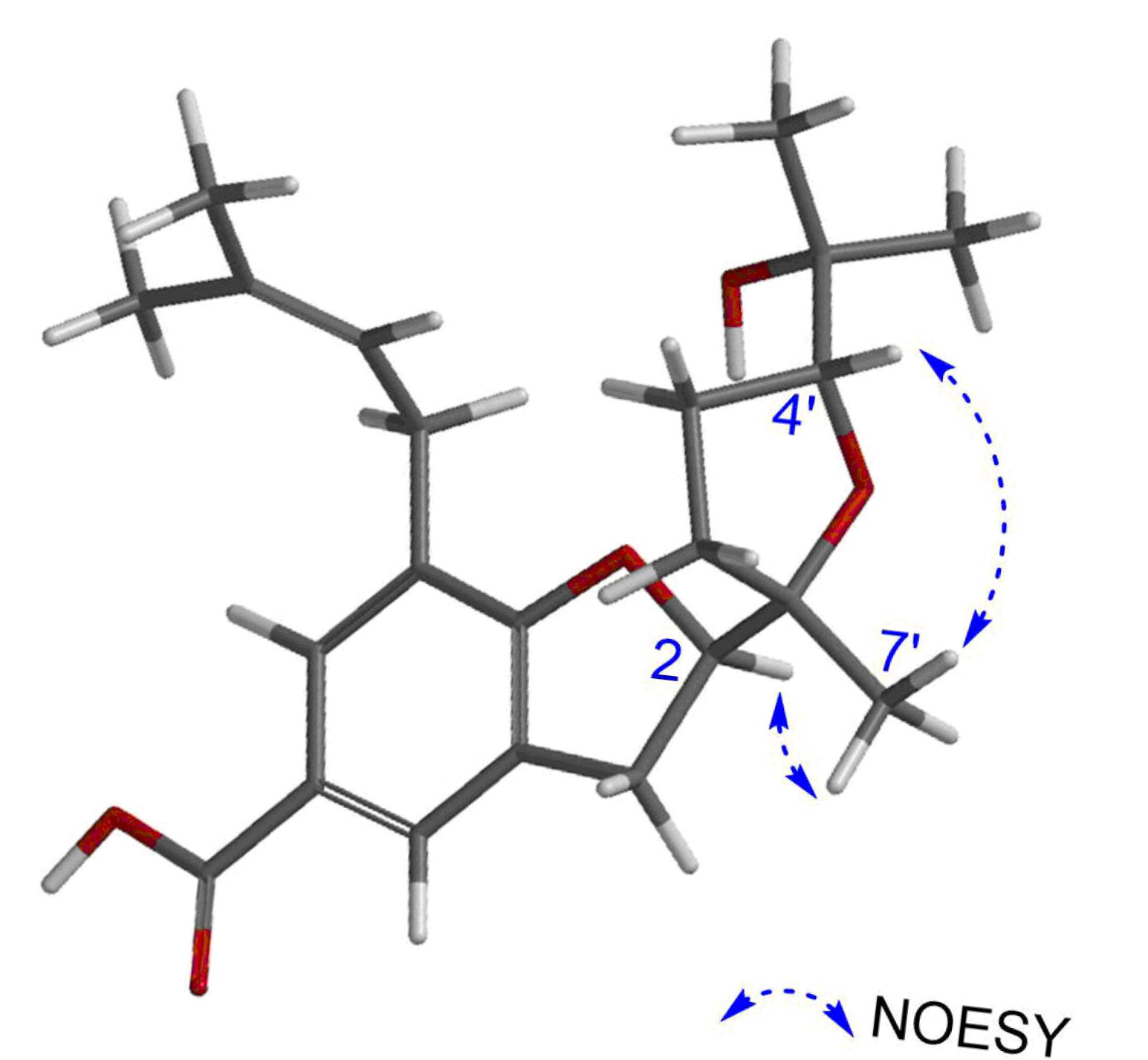
Figure 3), NOESY correlation peaks [
δH 4.83 (1H, m, H-2)/
δH 1.19 (3H, s, H-7′)] (
Figure 4), and the chemical shifts at C-2′ and C-7′ (
δC 33.76 and 22.82 ppm, respectively) (
Table 1). Additionally, the configuration of C-4′ was provisionally assigned as
R, supported by the NOESY correlation peak [
δH 3.91 (1H, t,
J = 7.6 Hz, H-4′)/1.19 (3H, s, H-7′)]. To clarify the stereochemistry of
3, NMR calculations and DP4+ analysis were performed on the two isomers, (2
R,1′
S,4′
R)-
3 (
3a) and (2
R,1′
S,4′
S)-
3 (
3b). The results showed that
3a matches the experimental data more closely than
3b, with higher
R2 and lower MAE and CMAE values. Moreover, the DP4+ calculations also afford a probability of 100% (
Table 2). Based on ECD calculations (
Figure 3), the absolute configuration of
3 was identified as 2
R,1′
S,4′
R and called myrsinoic acid K [(
R)-2-((2
S,5
R)-5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)-7-(3-methylbut-2-en-1-yl)-2,3-dihydrobenzofuran-5-carboxylic acid] (
Figures S3.1–3.6 and
Tables S3.1–S3.7).
Compound
4 was isolated as a yellow amorphous powder, and its molecular formula was determined to be C
21H
20O
12 based on the positive ESI-Q-TOF-MS spectrum, which exhibited an ion peak at
m/
z 487.0852 [M + Na]
+. The presence of distinctive peaks at 209.4, 264.2, and 349.8 nm in the UV spectrum indicates that the compound is a flavonoid (
Figure S4.4). In the
1H-NMR spectrum, the typical signals corresponding to the mearnsetin (myricetin 4′-methyl ether) scaffold are displayed at
δH 7.11 (2H, s, H-2′, 6′), 6.38 (1H, d,
J = 2.0 Hz, H-8), 6.21 (1H, d,
J = 2.0 Hz, H-6), and 3.75 (3H, s, 4′-OC
H3) (
Table 4) [
27]. Additionally, an anomeric proton signal arising from a sugar moiety occurs at
δH 5.22 (1H, d,
J = 5.5 Hz, H-1″), which suggests that
4 is a mearnsetin glycoside.
The
13C-NMR spectrum exhibits sixteen carbon signals corresponding to mearnsetin and another five signals for a sugar unit at
δC 101.32 (C-1″), 71.46 (C-2″), 70.33 (C-3″), 65.94 (C-4″), and 64.26 (C-5″). From the anomeric proton signal [
δH 5.22 (1H, d,
J = 5.5 Hz, H-1″)] and carbon resonances, the sugar unit was determined to be
α-arabinopyranoside. The presence of the L-arabinose unit was confirmed by acidic hydrolysis analysis of
4 (
Figure S4.4). Finally, the linkage between mearnsetin and the sugar moiety was determined through the HMBC spectrum, showing a correlation peak at
δH 5.22 (H-1″)/
δC 134.18 (C-3) (
Figure 2). Therefore, the chemical structure of
4 was elucidated to be mearnsetin 3-
O-
α-L-arabinopyranoside (
Figures S4.1–S4.4).
Compound
5 was isolated as a yellow amorphous solid, and its molecular formula was deduced to be C
22H
30O
4 based on the ion peak at
m/
z 359.2221 [M + H]
+ in the ESI-Q-TOF-MS spectrum. The
1H and
13C NMR spectra show similar patterns to those of
1; the difference observed in the
1H NMR spectrum is the replacement of the geranyl group (unit A) of
1 with a 3,3-dimethylallyl moiety (
Table 1). These detailed structural features are supported by 2D NMR and MS data (
Figure 2). By comparison with the reported literature data [
28], the planar structure
5 was identified as the known compound, myrosinoic acid B (
Figure 1).
Although myrsinoic acid B has chiral centres at the C-2 and C-1′ positions, some studies [
28,
29,
30] have presented only a planar structure, lacking stereochemical information. Amaro–Luis et al. assigned the absolute configuration as 2
R,1′
R; however, a review of their publication and the related literature provided no supporting evidence for this assignment [
4,
31]. Compounds
1 and
5 possess an identical 2,3-dihydrobenzofuran-5-carboxylic acid core and side chain unit B; therefore, the stereochemistry of
5 was determined using an approach identical to that of
1. In the CD spectrum (
Figure 3),
5 shows a CE pattern (positive at 214 nm and negative at 264 nm) similar to that of
1, suggesting that the configuration of
5 could be either (2
R,1′
S)-
5 (
5a) or (2
R,1′
R)-
5 (
5b). Based on the chemical shifts at C-2′ (
δC 37.10) and C-7′ (
δC 22.84), the absolute configuration of
5 was assigned as
5a. Similar to
1, the absolute configuration of
5 could not be clearly assigned because of the similarity between the calculated ECD spectra of
5a and
5b (
Figure 3). Furthermore, the configuration of
5 was confirmed by GIAO NMR chemical shift calculations and DP4+ probability analyses of the two isomers. As shown in
Table 2, the parameters obtained from the
13C NMR data were highly consistent with the calculated results for
5a; however, the
1H NMR data indicated that
5b exhibited higher
R2 and MAE values, leading to ambiguity in the interpretation. These results revealed that the NMR calculation data alone were insufficient to unambiguously determine the structure of
5. Therefore, a DP4+ probability analysis was performed, and
5a was identified as the correct configuration with a probability of 100 %. These results support the assignment of the 2
R,1′
S configuration for
5 (
Figures S5.1–S5.6 and Tables S4.1–S4.7). Therefore, the structure of myrsinoic acid B is re-established as (
R)-2-((
S)-2-hydroxy-6-methylhept-5-en-2-yl)-7-(3-methylbut-2-en-1-yl)-2,3-dihydrobenzofuran-5-carboxylic acid.
3. Materials and Methods
3.1. General
HPLC analyses were carried out on a Waters Alliance HPLC system (2695 separation module, Milford, MA, USA) equipped with a Luna C18 column (4.6 × 250 mm I.D., 5 mm; Phenomenex, Torrance, CA, USA). A Gilson preparative HPLC system (Middleton, WI, USA) consisting of a binary pump, manual injector, and UV/VIS detector was employed to separate the compounds. The preparative HPLC column was a Luna C18(2) column (21.2 × 250 mm, 5 mm; Torrance, CA, USA). The column chromatography (CC) was performed using silica gel 60 F-254 (40–63 mm; Merck, Darmstadt, Germany), ZEOprep 90 C18 (40–63 mm; Zeochem, Uetikon, Switzerland), and Diaion HP-20 (Mitsubishi Chemical, Tokyo, Japan). The structures of the isolated compounds were elucidated using 1D- and 2D-NMR data acquired using an AVANCE 500 spectrometer (Bruker, Karlsruhe, Germany). ESI-Q-TOF-MS spectra were obtained using a 6460 Q-TOF-MS spectrometer (Agilent Technologies, Santa Clara, CA, USA). Optical rotation and CD data were recorded using a P-2000 polarimeter and a J-815 CD spectrophotometer, respectively (Jasco, Tokyo, Japan). Organic solvents for extraction and column chromatography were purchased from Daejung Chemical and Metals Co., Ltd. (Kyunggido, Republic of Korea), and HPLC-grade methanol and acetonitrile were obtained from J.T. Baker Chemical Co. (Phillipsburg, NJ, USA). Deionised water was obtained using a Milli-Q water purification system (Millipore, Burlington, MA, USA).
3.2. Plant Materials
Aerial parts of Myrsine seguinii were collected from Popa Mountain National Park, Mandalay, Myanmar, in August 2015 and identified by Khin Myo Htwe (staff officer, Popa Mountain National Park, Mandalay, Myanmar). The dried and powdered aerial parts of M. seguinii were stored in a freezer at −80 °C before use. A voucher specimen (#M-MS-20150811) was deposited in the herbarium of the College of Pharmacy at the Catholic University of Korea.
3.3. Extraction and Isolation
Aerial parts of dried and powdered M. seguinii (1.2 kg) were extracted using methanol (MeOH) at room temperature (6 L × 90 min × 3 times) in an ultrasonic bath (Bransonic, Model 5510, 42 kHz, 185 W), and the solvent was evaporated under reduced pressure at 40 °C. The MeOH extract (104.88 g) was suspended in 90% aqueous MeOH (1.5 L) and partitioned with n-hexane (n-Hex, 1.5 L × 3 times) to give an n-Hex-soluble extract (9.26 g). Subsequently, the 90% MeOH layer was evaporated under reduced pressure and resuspended in water (1.5 L), followed by consecutive partitioning using organic solvents to yield ethyl acetate (EtOAc, 32.89 g)- and n-butanol (n-BuOH, 13.56 g)-soluble extracts.
The EtOAc-soluble extract (32.89 g) was subjected to silica gel column chromatography (CC) [n-Hex:EtOAc (5:1→2:1, v/v), CH2Cl2:MeOH (10:1→5:1→2:1, v/v), and MeOH] to afford 20 sub-fractions (E1–E20). Fraction E5 (234.3 mg) was purified by reverse-phase (RP) HPLC and eluted with acetonitrile (MeCN)–water (H2O) (85:15, v/v) to afford 6 (myrsinoic acid A, 10 mg, tR = 40.8 min). Fraction E7 (2.5 g) was subjected to RP CC (MeOH-H2O step gradient elution from 5:5 to 9:1, v/v) to yield nine sub-fractions (E7.1–E7.9), and 5 (myrsinoic acid B, 20.9 mg, tR = 38.9 min) and 18 (1-(3,5-dihydroxyphenyl)heptan-1-one, 7.0 mg, tR = 31.8 min) were obtained from fractions E7.8 (1.17 g) and E7.3 (7.7 mg), respectively. Fraction E7.9 (73 mg) was purified by RP-HPLC using MeCN-H2O (90:10, v/v) to obtain 1 (myrsinoic acid I, 15.8 mg, tR = 45.3 min). Compound 2 (myrsinoic acid J, 2.9 mg, tR = 31.4 min) was isolated by RP-HPLC [MeCN-H2O (90:10, v/v)] from fraction E7.4 (24.8 mg). Fraction E9 (500 mg) was subjected to RP medium-pressure liquid chromatography (MPLC) (MeOH-H2O step gradient elution from 3:7 to 8:2, v/v) to obtain 16 sub-fractions (E9.1–E9.16). Fraction E9.1 (45.3 mg) was resolved by RP-HPLC eluted with aqueous MeCN (17%) to yield 22 (vanillic acid, 15 mg, tR = 16.9 min) and 24 (4-hydroxy-benzoic acid, 5.3 mg, tR = 16.3 min). Fraction E9.15 (42.6 mg) was subjected to RP-HPLC using MeCN-H2O (55:45, v/v) to yield 3 (myrsinoic acid K, 20.8 mg, tR = 33.8 min). Fraction E11 (1.81 g) was subjected to RP-MPLC (MeOH-H2O, 2:8 to 8:2, v/v) to yield 13 sub-fractions (E11.1–E11.13). Fraction E11.1 (55.3 mg) was subjected to RP-HPLC using 12% aqueous MeCN to afford compounds 20 (4-O-methylgallic acid, 10.5 mg, tR = 14.1 min), 21 (protocatechuic acid, 6.6 mg, tR = 13.0 min), and 23 (resorcylic acid, 1.0 mg, tR = 13.1 min). Fraction E11.5 (48 mg) was separated by RP-HPLC using 35% aqueous MeCN to yield compound 9 (mearnsetin, 7.7 mg, tR = 22.4 min). Fraction E12 (2.69 g) was separated using silica gel CC with CH2Cl2-MeOH (12.5:1→10:1→7.5:1, v/v) to afford six sub-fractions (E12.1–E12.6). Fraction E12.5 (316 mg) was subjected to RP-HPLC with MeCN-H2O (21:79, v/v) to yield 4 (mearnsetin 3-O-a-L-arabinopyranoside, 5.3 mg, tR = 20.3 min), 12 (tamarixetin 3-O-α-L-rhamnoside, 10.7 mg, tR = 22.1 min), 13 (kaempferol 3-O-α-L-rhamnoside, 1.6 mg, tR = 22.0 min), and 19 (gallic acid, 4.9 mg, tR = 7.8 min). Fraction E12.6 (38.5 mg) was subjected to RP-HPLC with MeCN (16%) in H2O to give 16 (breynioside A, 3.2 mg, tR = 18.2 min) and 17 (6′-O-vanilloylarbutin, 10.3 mg, tR = 18.3 min). Fraction E13 (2 g) underwent RP-MPLC using MeOH (30%) in H2O, giving eight sub-fractions (E13.1–E13.8), and 8 (mearnsitrin, 249.8 mg, tR = 20.2 min) was obtained from Fraction E13.3. Compound 10 (myricitrin, 10.4 mg, tR = 19.1 min) was purified from fraction E17 (147.9 mg) by RP-HPLC using MeCN-H2O (30:70, v/v). Fraction E14 (1.36 g) was subjected to RP-MPLC using MeOH-H2O (step gradient elution from 1.5:8.5 to 4:6, v/v), resulting in the generation of nine sub-fractions (E14.1–E14.9), and 14 (seguinoside D, 4.7 mg, tR = 17.3 min) and 15 (seguinoside E, 6.0 mg, tR = 17.1 min) were generated from fractions E14.1 and E14.2, respectively. Fraction E14.6 (98.5 mg) was purified by RP-HPLC eluted with 50% aqueous MeOH to yield 7 (myricetin 4′-methyl ether 3-O-β-D-galactopyranoside, 2.9 mg, tR = 19.2 min) and 11 (quercitrin, 13.5 mg, tR = 20.9 min).
The n-BuOH-soluble extract (6.5 g) was subjected to Diaion HP-20 CC eluted with H2O, 50% aqueous MeOH, and MeOH to afford BW (1.5 g), B50M (1.5 g), and BM (1.2 g) fractions, respectively. The BM fraction was separated by silica gel CC with CH2Cl2-MeOH (step gradient elution from 40:1 to 5:1, v/v) to yield 14 sub-fractions (BM1-BM14). Fraction BM5 (21.9 mg) was purified using RP-HPLC [MeCN-H2O (25:75, v/v)] and subsequently re-chromatographed using RP-HPLC with MeOH (40%) in H2O, resulting in the isolation of 25 [(6R,9S)-blumenol C glucoside, 0.9 mg, tR = 20.0 min]. Fraction BM8 (86 mg) was purified by RP-HPLC eluted with MeCN-H2O (20:80, v/v) to afford 26 (byzantionoside B 6′-O-β-D-apiofuranoside 4.6 mg, tR = 19.1 min).
Myrsinoic acid I (
1): C
27H
38O
4; white amorphous solid;
= −62.57° (
c 0.02, MeOH); ESI-Q-TOF-MS:
m/
z 427.2818 [M + H]
+ (calcd for C
27H
38O
4, 427.2848); UV (
c 0.0033, MeOH) λ
max (log ε) 206.6 (4.42), 264.8 (4.07) nm;
1H-NMR (500 MHz, CDCl
3):
Table 1;
13C-NMR (125 MHz, CDCl
3):
Table 1.
Myrsinoic acid J (
2): C
22H
30O
5; white amorphous solid;
= −44.17° (
c 0.03, MeOH); ESI-Q-TOF-MS:
m/
z 375.2169 [M + H]
+ (calcd for C
22H
30O
5, 375.2171); UV (
c 0.0033, MeOH) λ
max (log ε) 208.4 (4.41), 264.8 (4.18) nm;
1H-NMR (500 MHz, CDCl
3):
Table 1;
13C-NMR (125 MHz, CDCl
3):
Table 1.
Myrsinoic acid K (
3): C
22H
30O
5; yellow amorphous solid;
= −56.38° (
c 0.37, MeOH); ESI-Q-TOF-MS:
m/
z 375.2176 [M + H]
+ (calcd for C
22H
30O
5, 375.2171); UV (
c 0.0033, MeOH) λ
max (log ε) 207.0 (4.36), 266.4 (4.10) nm;
1H-NMR (500 MHz, CDCl
3):
Table 1;
13C-NMR (125 MHz, CDCl
3):
Table 1.
Mearnsetin 3-
O-
a-L-arabinopyranoside (
4): C
21H
20O
12; yellow amorphous powder;
= −77.37° (
c 0.33, MeOH); ESI-Q-TOF-MS:
m/
z 487.0852 [M + Na]
+ (calcd for C
21H
20O
12, 487.0852); UV (c 0.0022, MeOH) λ
max (log ε) 209.4 (4.61), 264.2 (4.23), 349.8 (4.09) nm;
1H-NMR (500 MHz, DMSO-
d6):
Table 4;
13C-NMR (125 MHz, DMSO-
d6):
Table 4.
Myrsinoic acid B (
5): C
22H
30O
4; yellow amorphous solid;
= −42.81° (
c 0.524, MeOH); ESI-Q-TOF-MS:
m/
z 359.2221 [M + H]
+ (calcd for C
22H
30O
4, 359.2222);
1H-NMR (500 MHz, CDCl
3):
Table 1;
13C-NMR (125 MHz, CDCl
3):
Table 1.
3.4. Sugar Analysis
Compound 4 (1 mg) was subjected to acid hydrolysis in 1 M HCl (1.0 mL) at 80 °C for 2 h. Subsequently, the aqueous layer, presumed to contain sugar, was analysed by TLC to determine its composition. The TLC plate was developed in chloroform/methanol/water (30:20:4, v/v/v), and subsequently visualised using an aniline hydrogen phthalate reagent. The Rf value was identical to that of the standard L-arabinose, confirming that the sugar moiety of 4 corresponds to L-arabinose.
3.5. Computational Analysis
Conformational searches were performed using Merck Molecular Force Field (MMFF) 94 in Spartan’14 software (Spartan Software, San Francisco, CA, USA). All conformers within 21 kJ/mol of the lowest energy minimum were subjected to geometry optimisation and frequency calculations using density functional theory (DFT) at the B3LYP/6-31G(d) level of theory in the gas phase. NMR calculations were performed using the GIAO method at the B3LYP/6-31 + G(d,p) level in chloroform using Gaussian 09 software (Gaussian Inc., Wallingford, CT, USA). The shielding constants of tetramethylsilane (TMS) were obtained using the same level of theory. DP4+ probability analysis of each possible candidate was performed using an Excel spreadsheet provided by Grimblat et al. [
6]. The ECD calculations of all the optimised conformers were conducted using the time-dependent DFT (TDDFT) method at the CAM-B3LYP/6-31 + G(d,p) level using the CPCM model.