Metabolomic Analysis of Plant Hormone-Related Metabolites in Medicago sativa Under Low-Temperature Stress
Abstract
1. Introduction
2. Results
2.1. Overall Sample Quality Control and PCA Analysis
2.2. OPLS-DA, and Permutation Test of Different Treatment Groups
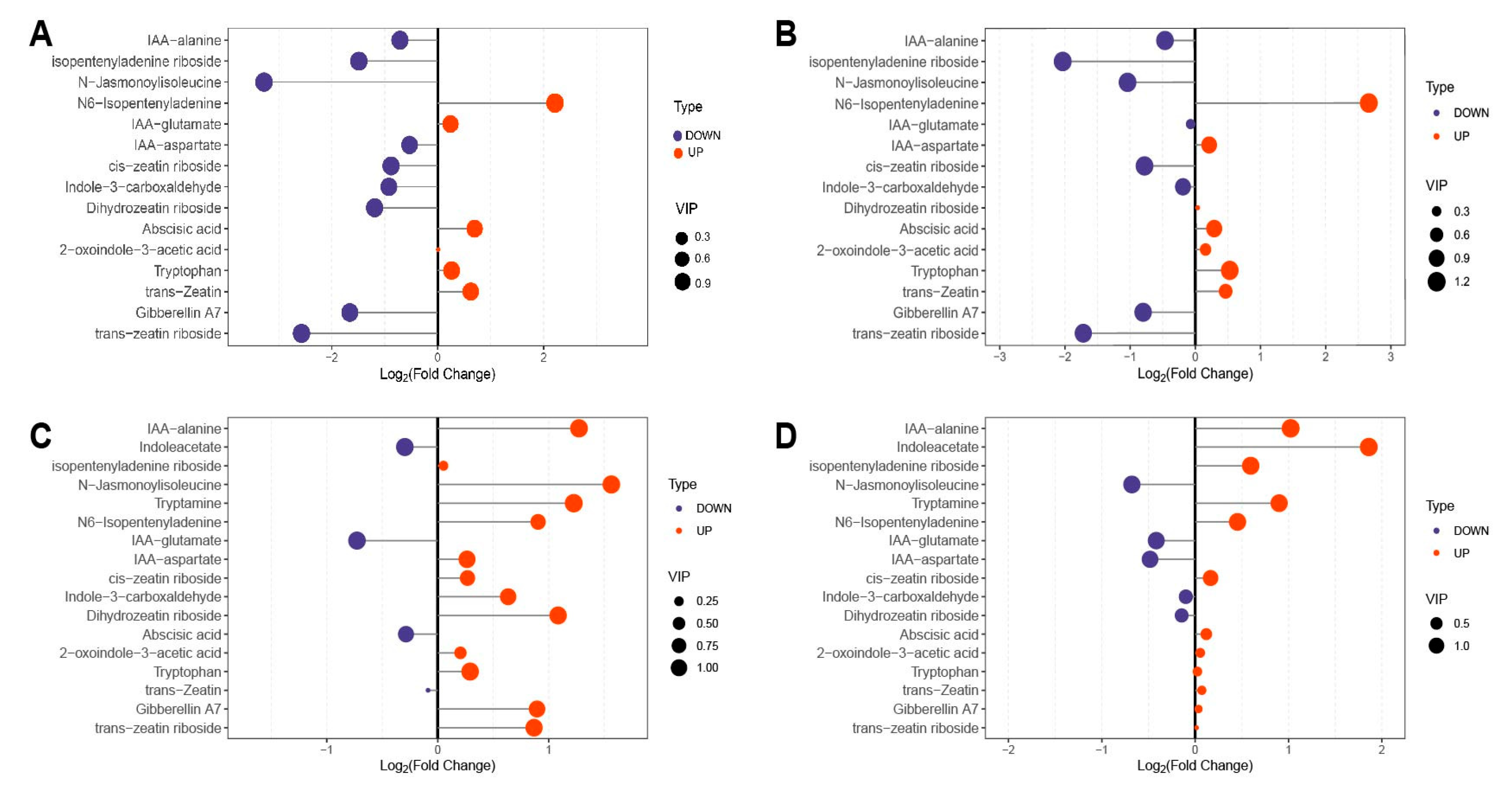
2.3. Screening of Differential Metabolites
2.4. Importance Analysis of Differential Metabolites
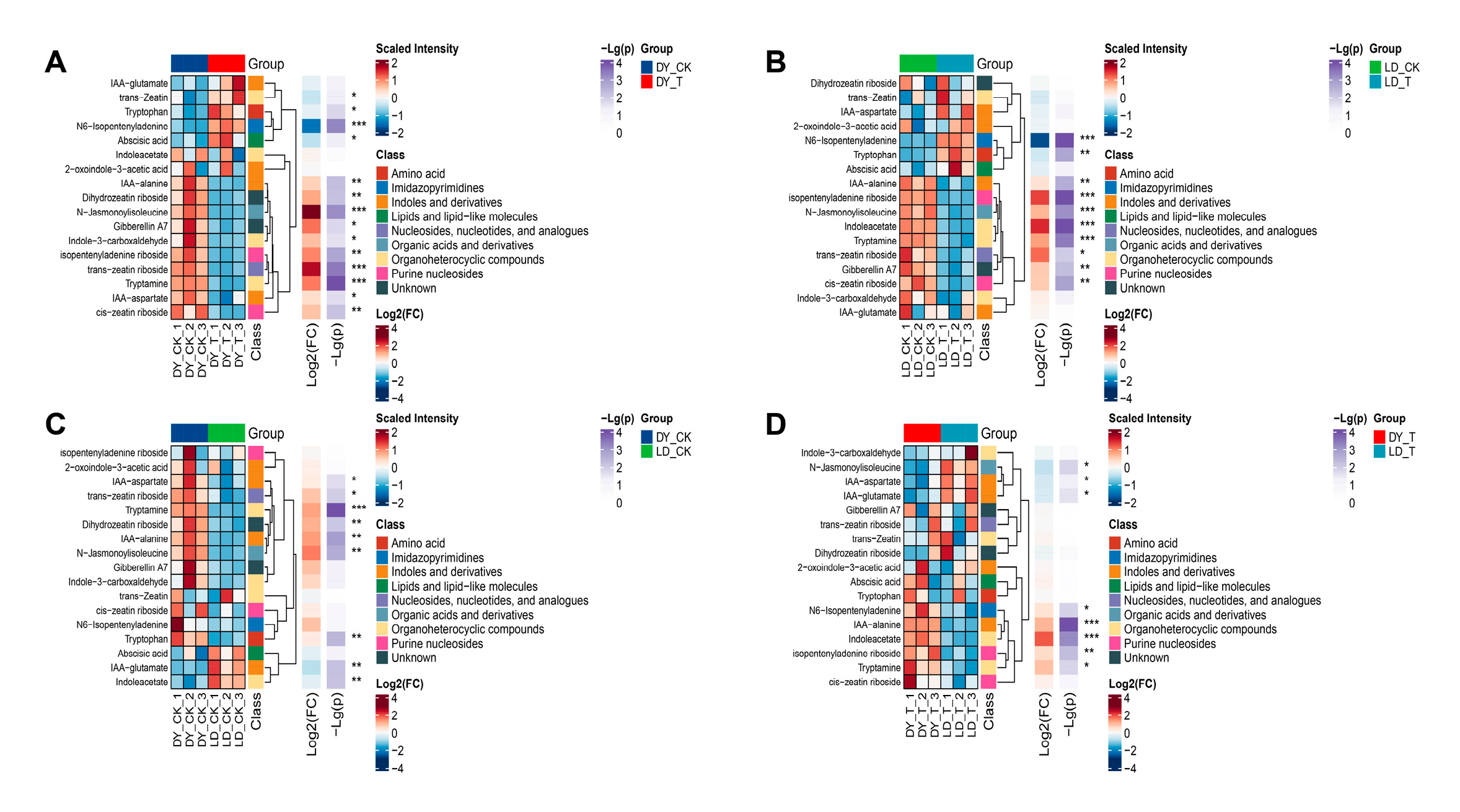
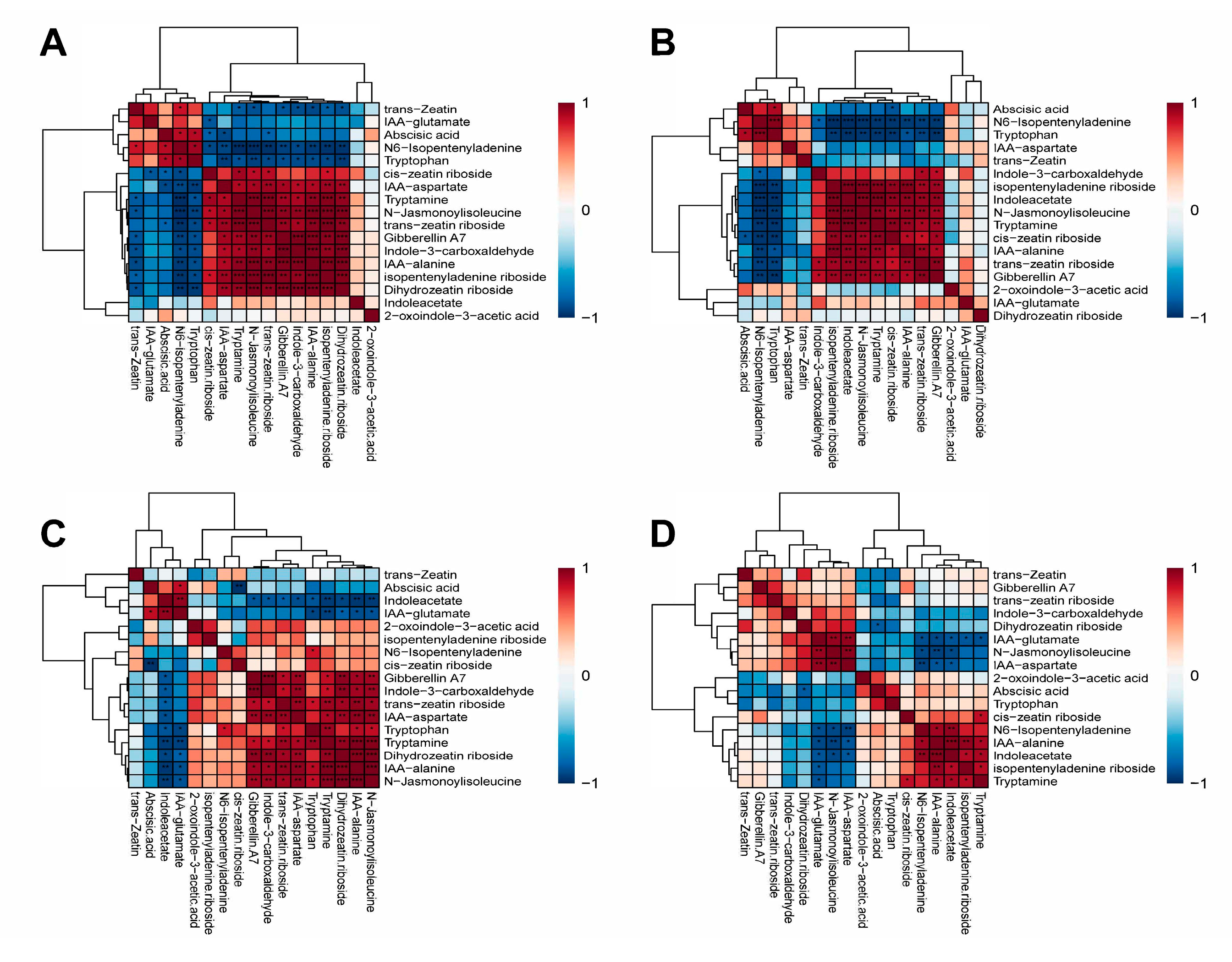
2.5. Cluster Analysis
2.6. Correlation Analysis
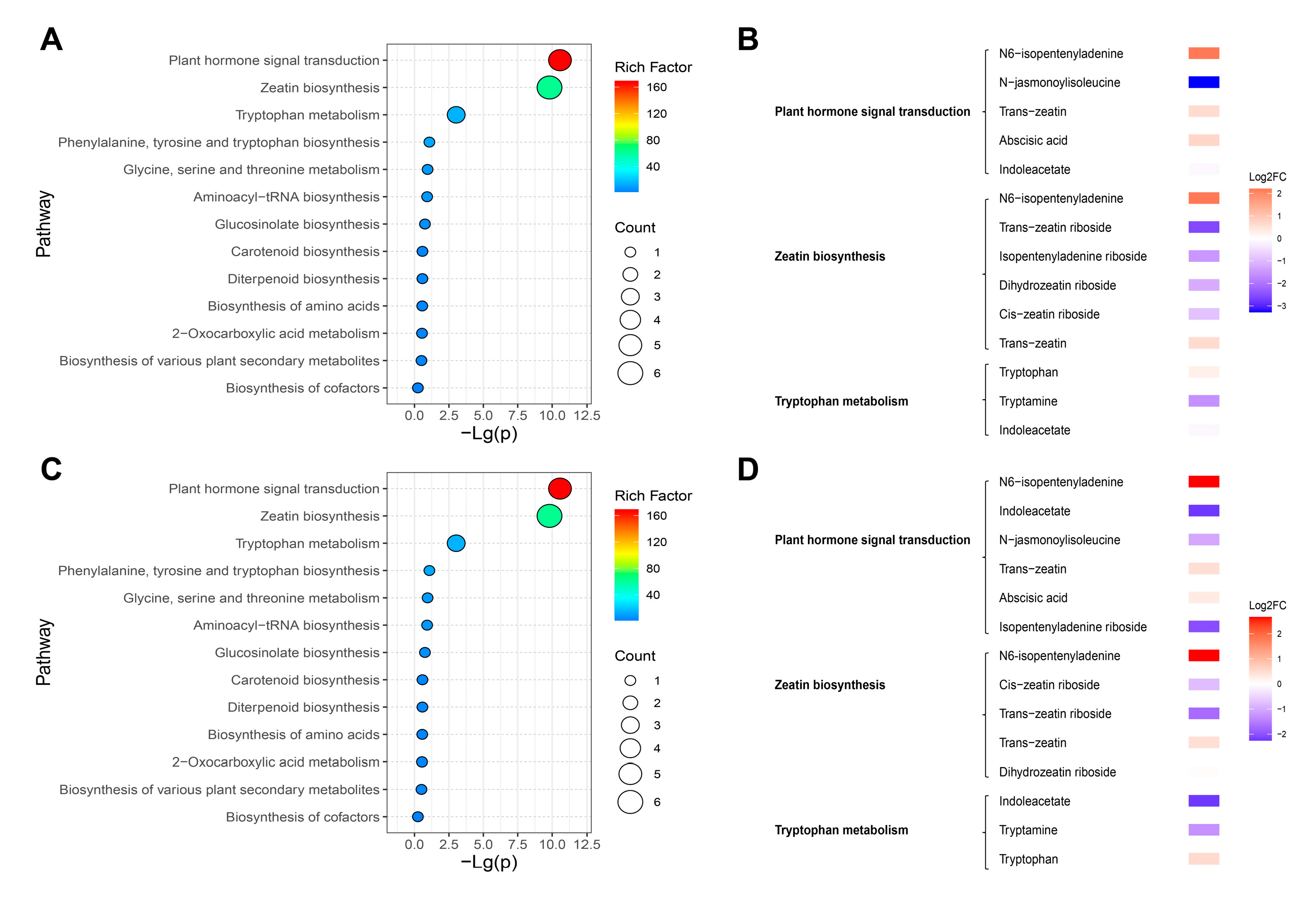
2.7. KEGG Analysis
3. Discussion
4. Materials and Methods
4.1. Test Materials and Cold Treatment
4.2. Metabolite Extraction
4.3. LC-MS/MS Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lorenzo, C.D.; Garcia-Gagliardi, P.; Antonietti, M.S.; Sanchez-Lamas, M.; Mancini, E.; Dezar, C.A.; Vazquez, M.; Watson, G.; Yanovsky, M.J.; Cerdan, P.D. Improvement of alfalfa forage quality and management through the down-regulation of MsFTa1. Plant Biotechnol. J. 2020, 18, 944–954. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L. Advances in basic biology of alfalfa (Medicago sativa L.): A comprehensive overview. Hortic. Res. 2025, 12, uhaf081. [Google Scholar] [CrossRef]
- Peng, W.; Cai, W.; Pan, J.; Su, X.; Dou, L. Molecular Mechanisms of Alfalfa Response to Abiotic Stresses. Plants 2025, 14, 487. [Google Scholar] [CrossRef]
- Wang, S.Y.; Sun, L.; Jiang, H.; Yang, G.L.; He, Z.N.; Jing, Y.Y.; Gao, F.Q. Effects of compound lactic acid bacteria on the quality and microbial diversity of alfalfa silage in saline-alkali soils. Front. Microbiol. 2024, 15, 1524296. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, Y.; Zhao, M.; Shen, H.; Xu, L.; Luo, Y.; Liu, Y.; Xing, A.; Kang, J.; Jing, H.; et al. Yield and quality properties of alfalfa (Medicago sativa L.) and their influencing factors in China. Eur. J. Agron. 2022, 141, 126637. [Google Scholar] [CrossRef]
- Yao, X.; Qian, L.; Changhui, L.; Yi, S. Effects of altitude and varieties on overwintering adaptability and cold resistance mechanism of alfalfa roots in the Qinghai-Tibet Plateau. J. Sci. Food Agric. 2023, 103, 2446–2458. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Geng, J.C.; Sha, X.Y.; Zhao, Y.X.; Hu, T.M.; Yang, P.Z. Effect of rhizobium symbiosis on low temperature tolerance and antioxidant response in alfalfa (Medicago sativa L.). Front. Plant Sci. 2019, 10, 538. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, L.; Hassan, M.A. Mitigating low-temperature stress in alfalfa by postponing phosphorus application and remodeling of antioxidant activities and carbon-nitrogen metabolism. Front. Plant Sci. 2025, 16, 1550026. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Mehrotra, S.; Verma, S.; Kumar, S.; Kumari, S.; Mishra, B.N. Transcriptional regulation and signalling of cold stress response in plants: An overview of current understanding. Environ. Exp. Bot. 2020, 180, 104243. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Ashraf, M.A.; Cheng, Y.; Wang, B.; Qin, Y. Plant Low-Temperature Stress: Signaling and Response. Agronomy 2022, 12, 702. [Google Scholar] [CrossRef]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of Basal ABA in Plant Growth and Development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, S.; Zhang, Q.; Cui, M.; Zhao, M.; Li, N.; Wang, S.; Wu, R.; Zhang, L.; Cao, Y.; et al. The interaction of ABA and ROS in plant growth and stress resistances. Front. Plant Sci. 2022, 13, 1050132. [Google Scholar] [CrossRef]
- Aili, R.; Deng, Y.; Yang, R.; Zhang, X.; Huang, Y.; Li, H.; Jia, S.; Yu, L.; Zhang, T. Molecular Mechanisms of Alfalfa (Medicago sativa L.) in Response to Combined Drought and Cold Stresses. Agronomy 2023, 13, 3002. [Google Scholar] [CrossRef]
- An, J.P.; Xu, R.R.; Liu, X.; Su, L.; Yang, K.; Wang, X.F.; Wang, G.L.; You, C.X. Abscisic acid insensitive 4 interacts with ICE1 and JAZ proteins to regulate ABA signaling-mediated cold tolerance in apple. J. Exp. Bot. 2022, 73, 980–997. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shi, H.; Hu, Z.; Liu, A.; Amombo, E.; Chen, L.; Fu, J. ABA Is involved in regulation of cold stress response in bermudagrass. Front. Plant Sci. 2017, 8, 1613. [Google Scholar] [CrossRef]
- Jing, H.; Wilkinson, E.G.; Sageman-Furnas, K.; Strader, L.C. Auxin and abiotic stress responses. J. Exp. Bot. 2023, 74, 7000–7014. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of jasmonic acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Q.; Wang, B.; Yuan, F. Roles of phytohormones and their signaling pathways in leaf development and stress responses. J. Agric. Food Chem. 2021, 69, 3566–3584. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Luo, D.; Chai, X.; Wu, Y.; Wang, Y.; Nan, Z.; Yang, Q.; Liu, W.; Liu, Z. Multiple regulatory networks are activated during cold stress in Medicago sativa L. Int. J. Mol. Sci. 2018, 19, 3169. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Fu, Y.; Xu, C.; Li, X.; Wang, W.; De, K.; Wei, X.; Yao, X. Transcriptomic analyses provide molecular insight into the cold stress response of cold-tolerant alfalfa. BMC Plant Biol. 2024, 24, 741. [Google Scholar] [CrossRef]
- Nxumalo, W.; Elateeq, A.A.; Sun, Y. Can Cordyceps cicadae be used as an alternative to Cordyceps militaris and Cordyceps sinensis?—A review. J. Ethnopharmacol. 2020, 257, 112879. [Google Scholar] [CrossRef]
- Adhikari, L.; Baral, R.; Paudel, D.; Min, D.; Makaju, S.O.; Poudel, H.P.; Acharya, J.P.; Missaoui, A.M. Cold stress in plants: Strategies to improve cold tolerance in forage species. Plant Stress 2022, 4, 100081. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Najafi-Kakavand, S.; Abbas, S.; Shoaib, Y.; Anwar, S.; Sharifi, S.; Lu, G.; Siddique, K.H. Role of phytohormones in regulating cold stress tolerance: Physiological and molecular approaches for developing cold-smart crop plants. Plant Stress 2023, 8, 100152. [Google Scholar] [CrossRef]
- Kim, J.S.; Kidokoro, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulatory networks in plant responses to drought and cold stress. Plant Physiol. 2024, 195, 170–189. [Google Scholar] [CrossRef]
- Wang, K.; Nan, L.L.; Xia, J.; Wu, S.W.; Yang, L.L. Metabolomics reveal root differential metabolites of different root-type alfalfa under drought stress. Front. Plant Sci. 2024, 15, 1341826. [Google Scholar] [CrossRef]
- Tian, J.; Ma, Y.; Chen, Y.; Chen, X.; Wei, A. Plant Hormone Response to Low-Temperature Stress in Cold-Tolerant and Cold-Sensitive Varieties of Zanthoxylum bungeanum Maxim. Front. Plant Sci. 2022, 13, 847202. [Google Scholar] [CrossRef]
- Yang, X.; Liu, C.; Li, M.; Li, Y.; Yan, Z.; Feng, G.; Liu, D. Integrated transcriptomics and metabolomics analysis reveals key regulatory network that response to cold stress in common Bean (Phaseolus vulgaris L.). BMC Plant Biol. 2023, 23, 85. [Google Scholar] [CrossRef]
- Sun, R.Z.; Wang, Y.Y.; Chen, X.X.; Deng, X. Transcriptomic and Metabolomic Evidence Reveal the Vital Role of Lactose in the Acquisition of Rapid Desiccation Tolerance in Boea hygrometrica. Plant Cell Environ. 2025, 48, 4564–4584. [Google Scholar] [CrossRef]
- Prerostova, S.; Cerny, M.; Dobrev, P.I.; Motyka, V.; Hluskova, L.; Zupkova, B.; Gaudinova, A.; Knirsch, V.; Janda, T.; Brzobohaty, B.; et al. Light Regulates the Cytokinin-Dependent Cold Stress Responses in Arabidopsis. Front. Plant Sci. 2020, 11, 608711. [Google Scholar] [CrossRef] [PubMed]
- Vedenicheva, N.; Shcherbatyuk, M.; Kosakivska, I. Effect of low-temperature stress on the growth of plants of Secale cereale (Poaceae) and endogenous cytokinin content in roots and shoots. Ukr. Bot. J. 2022, 79, 184–192. [Google Scholar] [CrossRef]
- Kong, Y.; Hou, X.; Liu, Z.; Li, Y. Cold-stress induced metabolomic and transcriptomic changes in leaves of three mango varieties with different cold tolerance. BMC Plant Biol. 2024, 24, 266. [Google Scholar] [CrossRef] [PubMed]
- Jarošová, J.; Prerostova, S.; Černý, M.; Dobrev, P.; Gaudinova, A.; Knirsch, V.; Kobzová, E.; Müller, K.; Fiala, R.; Benczúr, K.; et al. Hormonal responses of rice to organ-targeted cold stress. Environ. Exp. Bot. 2024, 222, 105739. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; He, F.; Li, M.; Zi, Y.; Long, R.; Zhao, G.; Zhu, L.; Hong, L.; Wang, S.; et al. Multi-omics integrative analysis provided new insights into alkaline stress in alfalfa. Plant Physiol. Biochem. 2024, 215, 109048. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xu, X.; Xie, Y.; Huang, T.; Wang, W.; Zhao, L.; Ma, D. Comparative metabolomic analysis of the metabolism pathways under drought stress in alfalfa leaves. Environ. Exp. Bot. 2021, 183, 104329. [Google Scholar] [CrossRef]
- Zhang, J.; Sohail, H.; Xu, X.; Zhang, Y.; Zhang, Y.; Chen, Y. Unveiling tolerance mechanisms in pepper to combined low-temperature and low-light stress: A physiological and transcriptomic approach. BMC Plant Biol. 2025, 25, 171. [Google Scholar] [CrossRef]
- Wang, P.; Li, M.; Ma, X.; Zhao, B.; Jin, X.; Zhang, H.; Chen, S.; Wu, X.; Zhang, X. Integrative transcriptome and metabolome analysis revealed key mechanisms in two maize inbred lines. Preprient 2025. [Google Scholar] [CrossRef]
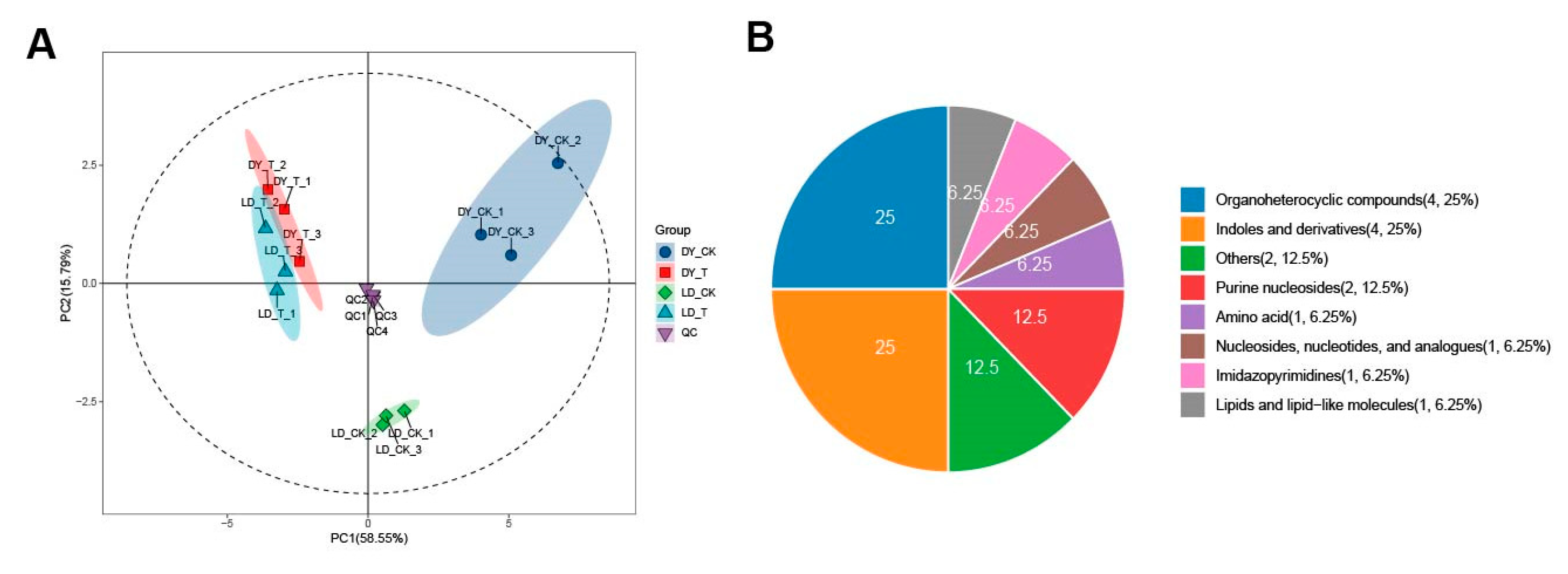
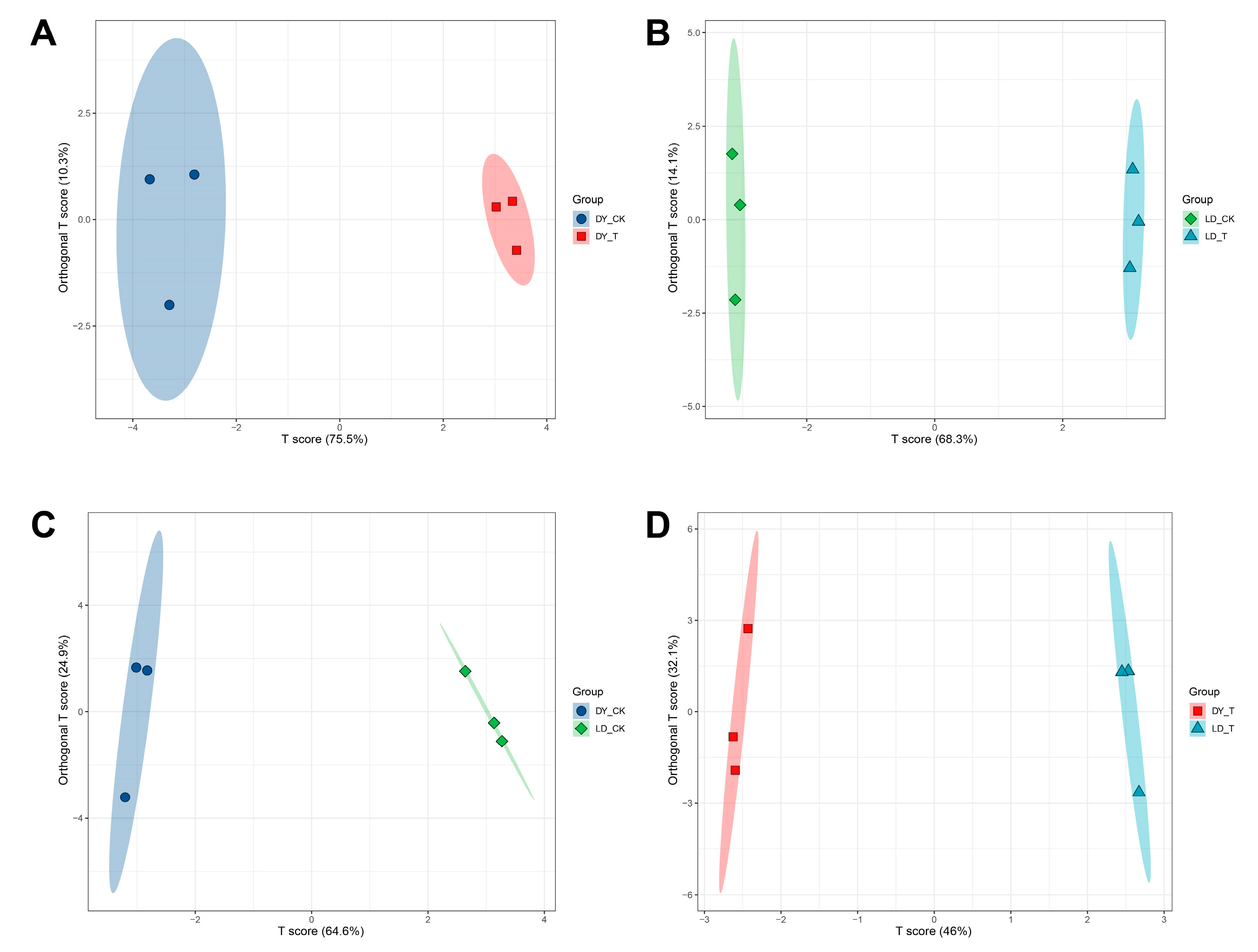
| Group | R2X (cum) | R2Y (cum) | Q2 (cum) |
|---|---|---|---|
| DY_CK. vs. DY_T | 0.858 | 0.993 | 0.974 |
| LD_CK. vs. LD_T | 0.944 | 1 | 0.997 |
| DY_CK. vs. LD_CK | 0.895 | 0.995 | 0.976 |
| DY_T. vs. LD_T | 0.881 | 0.999 | 0.965 |
| Group | Sig. | Increased | Decreased |
|---|---|---|---|
| DY_CK vs. DY_T | 12 | 3 | 9 |
| LD_CK vs. LD_T | 8 | 1 | 7 |
| DY_CK vs. LD_CK | 6 | 5 | 1 |
| DY_T vs. LD_T | 5 | 4 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, J.; Xu, C.; Zhao, Y.; Li, X.; Liu, J.; Pu, X. Metabolomic Analysis of Plant Hormone-Related Metabolites in Medicago sativa Under Low-Temperature Stress. Molecules 2025, 30, 3373. https://doi.org/10.3390/molecules30163373
Zhao Y, Wang J, Xu C, Zhao Y, Li X, Liu J, Pu X. Metabolomic Analysis of Plant Hormone-Related Metabolites in Medicago sativa Under Low-Temperature Stress. Molecules. 2025; 30(16):3373. https://doi.org/10.3390/molecules30163373
Chicago/Turabian StyleZhao, Yue, Jie Wang, Chengti Xu, Yuanyuan Zhao, Xiuzhang Li, Jing Liu, and Xiaojian Pu. 2025. "Metabolomic Analysis of Plant Hormone-Related Metabolites in Medicago sativa Under Low-Temperature Stress" Molecules 30, no. 16: 3373. https://doi.org/10.3390/molecules30163373
APA StyleZhao, Y., Wang, J., Xu, C., Zhao, Y., Li, X., Liu, J., & Pu, X. (2025). Metabolomic Analysis of Plant Hormone-Related Metabolites in Medicago sativa Under Low-Temperature Stress. Molecules, 30(16), 3373. https://doi.org/10.3390/molecules30163373







