Plasma-Assisted Synthesis of TiO2/ZnO Heterocomposite Microparticles: Phase Composition, Surface Chemistry, and Photocatalytic Performance
Abstract
1. Introduction
2. Results
2.1. Theoretical Calculations of Velocity and Heating Temperature of Particles in Plasma
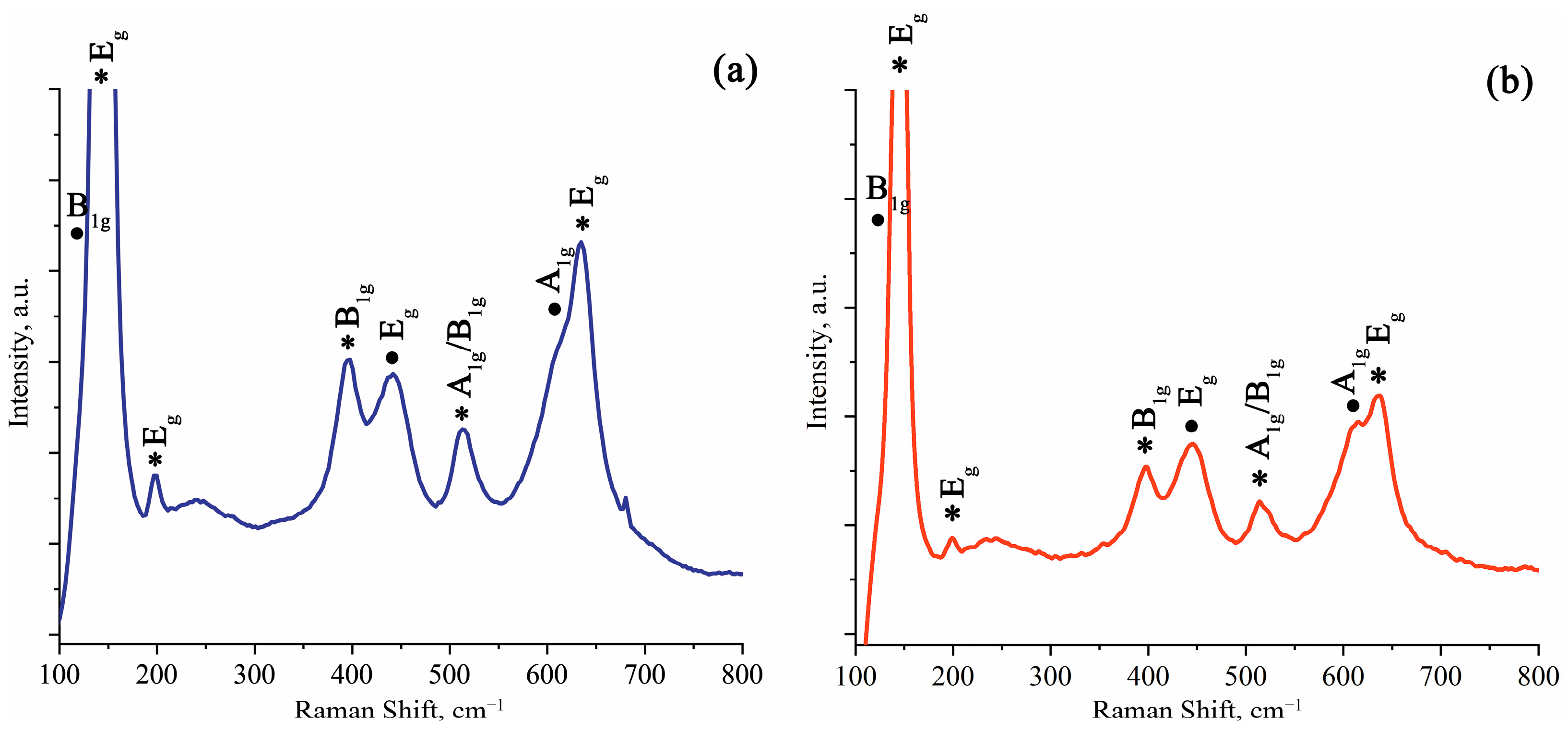
2.2. Study of Morphology, Elemental, and Structural-Phase Compositions of Microparticles
3. Discussion
- When using a catalyst mass of 5 mg, the MB degradation efficiency decreases to 86.5%, indicating a transition to a regime limited by the availability of the active sites. In this regime, the number of adsorption centers and photocatalytically active sites on the catalyst surface becomes insufficient. Reducing the catalyst mass proportionally decreases the specific surface area available for substrate adsorption and correspondingly lowers the concentration of photogenerated reactive oxygen species. Under these conditions, despite an adequate photon flux, the reaction rate is primarily controlled by the limited number of accessible catalytic centers, which prevents complete degradation of the organic dye;
- When the initial MB concentration is increased to 10 mg/L (using 20 mg of catalyst), the degradation efficiency drops to 61.5%. In this case, the limiting factor shifts to the competition for photon absorption between the dye molecules and the photocatalyst. A higher dye concentration significantly increases the optical absorption of the solution, which leads to the shielding of photocatalytic particles and a reduction in excitation efficiency. Additionally, at elevated substrate concentrations, the catalyst surface becomes saturated with adsorbed dye molecules, which further enhances competition for active sites and restricts the rate of the heterogeneous catalytic process.
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bisaria, K.; Sinha, S.; Singh, R.; Iqbal, H.M.N. Recent advances in structural modifications of photo-catalysts for organic pollutants degradation-A comprehensive review. Chemosphere 2021, 284, 131263. [Google Scholar] [CrossRef]
- Cao, S.; Wu, L.; Li, J.; Li, Y.; Da, K.; Chen, W.; Xue, R.; Yang, J.; Fan, X. Unraveling the generation mechanism of singlet oxygen in Bi2WxMo1−xO6 solid solution and roles in photocatalytic degradation of gaseous toluene under visible-light irradiation. J. Environ. Chem. Eng. 2024, 12, 112321. [Google Scholar] [CrossRef]
- Li, S.; Jiang, C.; Zhang, Y.; Tian, J.; Yang, H.; Wang, C.; Yan, J.; Li, X.; Lv, K.; Liu, Y. Synergistic effect of N doping and oxygen vacancies over TiO2 nanosheets with enhanced photocatalytic removal of tetracycline. Catal. Today 2024, 440, 114830. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, K.; Chang, L.; Yan, R.; Zhang, J.; Zhang, M.; Wang, L.; Chen, W.; Huang, G.-B. Construction of S-scheme MIL-101(Fe)/Bi2MoO6 heterostructures for enhanced catalytic activities towards tetracycline hydrochloride photodegradation and nitrogen photofixation. Sol. Energy 2023, 264, 112042. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, Z.-C.; Yang, Y.-P.; Zhang, Z.; Chen, W.; Yan, R.-Q.; Jin, Y.; Zhang, J. Defective WO3 nanoplates controllably decorated with MIL-101(Fe) nanoparticles to efficiently remove tetracycline hydrochloride by S-scheme mechanism. Sep. Purif. Technol. 2022, 300, 121846–121856. [Google Scholar] [CrossRef]
- Ortiz-Román, M.I.; Casiano-Muñiz, I.M.; Román-Velázquez, F.R. Ecotoxicological Effects of TiO2 P25 Nanoparticles Aqueous Suspensions on Zebrafish (Danio rerio) Eleutheroembryos. Nanomaterials 2024, 14, 373. [Google Scholar] [CrossRef]
- Zhou, X.-T.; Ji, H.-B.; Huang, X.-J. Photocatalytic Degradation of Methyl Orange over Metalloporphyrins Supported on TiO2 Degussa P25. Molecules 2012, 17, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Heo, M.B.; Kwak, M.; An, K.S.; Kim, H.J.; Ryu, H.Y.; Lee, S.M.; Lee, T.G. Oral toxicity of titanium dioxide P25 at repeated dose 28-day and 90-day in rats. Part. Fibre Toxicol. 2020, 17, 1–22. [Google Scholar] [CrossRef]
- Reis, A.T.; Costa, C.; Fraga, S. Editorial of Special Issue: The Toxicity of Nanomaterials and Legacy Contaminants: Risks to the Environment and Human Health. Int. J. Mol. Sci. 2023, 24, 11723. [Google Scholar] [CrossRef] [PubMed]
- Orudzhev, F.F.; Ramazanov, S.M.; Isaev, A.B.; Alikhanov, N.R.; Sobola, D.; Presniakov, M.Y.; Kaviyarasu, K. Self-organization of layered perovskites on TiO2 nanotubes surface by atomic layer deposition. Mater. Today Proc. 2021, 36, 364–367. [Google Scholar] [CrossRef]
- Orudzhev, F.F.; Gasanova, F.G.; Aliev, Z.M.; Isaev, A.B. Photoelectrocatalytic oxidation of phenol on platinum-modified TiO2 nanotubes. Nanotechnologies Russ. 2012, 7, 482–485. [Google Scholar] [CrossRef]
- Yang, L.; Ying, J.; Liu, Z.; He, G.; Xu, L.; Liu, M.; Xu, X.; Chen, G.; Guan, M. Synthesis of core/shell cobalt-doped rutile TiO2 nanorods for MB degradation under visible light. RSC Adv. 2025, 15, 10144–10149. [Google Scholar] [CrossRef]
- Kumar, R.; El-Shishtawy, R.M.; Barakat, M.A. Synthesis and Characterization of Ag-Ag2O/TiO2@polypyrrole Heterojunction for Enhanced Photocatalytic Degradation of Methylene Blue. Catalysts 2016, 6, 76. [Google Scholar] [CrossRef]
- Gadzhiev, M.K.; Orudzhev, F.F.; Muslimov, A.È. Electric arc synthesis of photoactive composite microparticles ZnO/TiO2/Nb2O5. Pisma V Zhurnal Tekhnicheskoi Fiz. 2023, 49, 3–7. [Google Scholar] [CrossRef]
- Muslimov, A.; Orudzhev, F.; Gadzhiev, M.; Selimov, D.; Tyuftyaev, A.; Kanevsky, V. Facile Synthesis of Ti/TiN/TiON/TiO2 Composite Particles for Plasmon-Enhanced Solar Photocatalytic Decomposition of Methylene Blue. Coatings 2022, 12, 1741. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Q.; Li, M.; Sun, S.; Zhao, H.; Jin, S.; Wang, J. An overview of low-temperature plasma surface modification of carbon materials for removal of pollutants from liquid and gas phases. Plasma Process. Polym. 2021, 18, 3. [Google Scholar] [CrossRef]
- Muslimov, A.E.; Gadzhiev, M.K.; Kanevsky, V.M. Synthesis of Superhydrophobic Barium Hexaferrite Coatings with Low Magnetic Hardness. Materials 2022, 15, 7865. [Google Scholar] [CrossRef]
- Zhu, X.; Qin, F.; He, L.; Jiao, Y.; Feng, W. Enhanced Photocatalytic Activity of Anatase/Rutile Heterojunctions by Lanthanum and Tin Co-Doping. Int. J. Mol. Sci. 2022, 23, 11339. [Google Scholar] [CrossRef]
- Maqbool, Q.; Favoni, O.; Wicht, T.; Lasemi, N.; Sabbatini, S.; Stöger-Pollach, M.; Ruello, M.L.; Tittarelli, F.; Rupprechter, G. Highly Stable Self-Cleaning Paints Based on Waste-Valorized PNC-Doped TiO2 Nanoparticles. ACS Catal. 2024, 14, 4820–4834. [Google Scholar] [CrossRef]
- Cölfen, H.; Antonietti, M. Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. Int. Ed. 2005, 44, 5576–5591. [Google Scholar] [CrossRef]
- Boytsova, O.; Zhukova, I.; Tatarenko, A.; Shatalova, T.; Beiltiukov, A.; Eliseev, A.; Sadovnikov, A. The Anatase-to-Rutile Phase Transition in Highly Oriented Nanoparticles Array of Titania with Photocatalytic Response Changes. Nanomaterials 2022, 12, 4418. [Google Scholar] [CrossRef]
- Craido, J.; Real, C. Mechanism of the inhibiting effect of phosphate on the anatase → rutile transformation induced by thermal and mechanical treatment of TiO2. J. Chem. Soc. Faraday Trans. 1983, 79, 2765. [Google Scholar] [CrossRef]
- Jeon, B.; Kwon, H.; Yoo, Y.W.; Kim, D.H.; Park, Y.; Kang, Y.-j.; Murphy, A.B.; Park, H. Computational Modeling of the Effect of Nitrogen on the Plasma Spray Process with Ar–H2–N2 Mixtures. Processes 2025, 13, 1155. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Kubiak, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO Binary Oxide Systems: Comprehensive Characterization and Tests of Photocatalytic Activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef]
- Delsouz Khaki, M.R.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Evaluating the efficiency of nano-sized Cu doped TiO2/ZnO photocatalyst under visible light irradiation. J. Mol. Liq. 2018, 258, 354–365. [Google Scholar] [CrossRef]
- Etafo, N.O.; Bamidele, M.O.; Bamisaye, A.; Alli, Y.A. Revolutionizing photocatalysis: Unveiling efficient alternatives to titanium (IV) oxide and zinc oxide for comprehensive environmental remediation. J. Water Proc. Eng. 2024, 62, 105369. [Google Scholar] [CrossRef]
- Ai, Y.; Hu, J.; Xiong, X.; Carabineiro, S.; Li, Y.; Sirotkin, N.; Agafonov, A.; Lv, K. Synergistic interfacial engineering of a S-scheme ZnO/In2S3 photocatalyst with S−O covalent bonds: A dual-functional advancement for tetracycline hydrochloride degradation and H2 evolution. Appl. Catal. B Environ. Energy. 2024, 353, 124098. [Google Scholar] [CrossRef]
- Liang, P.; Yang, W.; Peng, H.; Zhao, S. Efficient Degradation of Methylene Blue in Industrial Wastewater and High Cycling Stability of Nano ZnO. Molecules 2024, 29, 5584. [Google Scholar] [CrossRef] [PubMed]
- Trakulmututa, J.; Chuaicham, C.; Shenoy, S.; Srikhaow, A.; Sasaki, K.; Smith, S.M. Effect of Transformation Temperature toward Optical Properties of Derived CuO/ZnO Composite from Cu–Zn Hydroxide Nitrate for Photocatalytic Ciprofloxacin Degradation. Opt. Mater. 2022, 133, 112941. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukrullah, S.; Hussain, H.; Naz, M.Y.; Alsaif, F.K.; Alsulamy, S.; Khan, Y. Robust S-scheme ZnO-TiO2-Ag with efficient charge separations for highly active hydrogen evolution performance and photocatalytic mechanism insight. Appl. Catal. A Gen. 2023, 662, 119259. [Google Scholar] [CrossRef]
- Yasmeen, S.; Burratti, L.; Duranti, L.; Sgreccia, E.; Prosposito, P. Photocatalytic Degradation of Organic Pollutants—Nile Blue, Methylene Blue, and Bentazon Herbicide—Using NiO-ZnO Nanocomposite. Nanomaterials 2024, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Gadzhiev, M.K.; Muslimov, A.E.; Yusupov, D.I.; Il’ichev, M.V.; Kulikov, Y.M.; Chistolinov, A.V.; Venevtsev, I.D.; Volchkov, I.S.; Kanevsky, V.M.; Tyuftyaev, A.S. Gas-Thermal Spraying Synthesis of β-Ga2O3 Luminescent Ceramics. Materials 2024, 17, 6078. [Google Scholar] [CrossRef]
- Murphy, A.B. Transport coefficients of plasmas in mixtures of nitrogen and hydrogen. Chem. Phys. 2012, 398, 64–72. [Google Scholar] [CrossRef]
- Chen, W.L.T.; Heberlein, J.; Pfender, E. Critical analysis of viscosity data of thermal argon plasmas at atmospheric pressure. Plasma Chem. Plasma Process 1996, 16, 635–650. [Google Scholar] [CrossRef]
- Chen, X.; Pfender, E. Heat transfer to a single particle exposed to a thermal plasma. Plasma Chem. Plasma Process 1982, 2, 185–212. [Google Scholar] [CrossRef]
- Ye, J.; Liu, W.; Cai, J.; Chen, S.; Zhao, X.; Zhou, H.; Qi, L. Nanoporous Anatase TiO2 Mesocrystals: Additive-Free Synthesis, Remarkable Crystalline-Phase Stability, and Improved Lithium Insertion Behavior. J. Am. Chem. Soc. 2011, 133, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.O.; Gonalves, R.H.; Stroppa, D.G.; Ramirez, A.J.; Leite, E.R. Synthesis of Recrystallized Anatase TiO2 Mesocrystals with Wulff Shape Assisted by Oriented Attachment. Nanoscale 2011, 3, 1910–1916. [Google Scholar] [CrossRef]
- Gordillo, G.; Florez, J.M.; Hernandez, L.C. Preparation and characterization of CdTe thin films deposited by CSS. Sol. Energy Mater. Sol. Cells 1995, 37, 273–281. [Google Scholar] [CrossRef]
- Mote, D.V.; Purushotham, Y.P.; Dole, B.N. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 2012, 6, 1–8. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Norton, M.G. X-Rays and Diffraction. In X-Ray Diffraction; Springer: Boston, MA, USA, 1998; pp. 3–19. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Taudul, B.; Tielens, F.; Calatayud, M. On the Origin of Raman Activity in Anatase TiO2 (Nano)Materials: An Ab. Initio Investigation of Surface and Size Effects. Nanomaterials 2023, 13, 1856. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, Y.; Zhou, J.; Lu, Y.; Liu, Y.; Lv, X. Photocatalytic degradation of methylene blue using a ZnO/TiO2 heterojunction nanomesh electrode. Surf. Interfaces 2021, 22, 100889. [Google Scholar] [CrossRef]
- Ramos, P.G.; Flores, E.; Sánchez, L.A.; Candal, R.J.; Hojamberdiev, M.; Estrada, W.; Rodriguez, J. Enhanced photoelectrochemical performance and photocatalytic activity of ZnO/TiO2 nanostructures fabricated by an electrostatically modified electrospinning. Appl. Surf. Sci. 2017, 426, 844–851. [Google Scholar] [CrossRef]
- Lemago, H.H.; Tolezani, L.; Igricz, T.; Hessz, D.; Pál, P.; Cserháti, C.; Vecsei, G.; Sárközi, B.; Baradács, E.M.; Erdélyi, Z.; et al. Enhanced photocatalysis via inverse opal structures: Synthesis and characterization of TiO2/ZnO and ZnO/TiO2 composites using plasma-enhanced ALD. Ceram. Int. 2025, 51, 339–352. [Google Scholar] [CrossRef]
- Ansari, A.T.; Mukherji, S.; Mukherji, S. Enhanced Visible-Light Photocatalysis of a Senary Mixture of Antibiotics Using a Low-Dose of TiO2–ZnO Nanocomposite. Environ. Res. 2025, 282, 122083. [Google Scholar] [CrossRef]
- Qin, R.; Meng, F.; Khan, M.W.; Yu, B.; Li, H.; Fan, Z.; Gong, J. Fabrication and enhanced photocatalytic property of TiO2-ZnO composite photocatalysts. Mater. Lett. 2019, 240, 84–87. [Google Scholar] [CrossRef]
- Akhter, P.; Nawaz, S.; Shafiq, I.; Nazir, A.; Shafique, S.; Jamil, F.; Park, Y.-K.; Hussain, M. Efficient visible light assisted photocatalysis using ZnO/TiO2 nanocomposites. Mol. Catal. 2023, 535, 112896. [Google Scholar] [CrossRef]
- Araújo, E.S.; da Costa, B.P.; Oliveira, R.A.; Libardi, J.; Faia, P.M.; de Oliveira, H.P. TiO2/ZnO hierarchical heteronanostructures: Synthesis, characterization and application as photocatalysts. J. Environ. Chem. Eng. 2016, 4, 2820–2829. [Google Scholar] [CrossRef]
- Ani, I.J.; Akpan, U.G.; Olutoye, M.A.; Hameed, B.H.; Egbosiuba, T.C. Adsorption–photocatalysis synergy of reusable mesoporous TiO2–ZnO for photocatalytic degradation of doxycycline antibiotic. Heliyon 2024, 10, e30531. [Google Scholar] [CrossRef]
- Prasannalakshmi, P.; Shanmugam, N. Fabrication of TiO2/ZnO nanocomposites for solar energy driven photocatalysis. Mater. Sci. Semicond. Process. 2017, 61, 114–124. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Y.; Yu, Y.; Shen, K.; Hu, K.; Zhou, J.; Wang, Z. Effect of synthesis process steps on the structure and photocatalytic performance of ZnO–TiO2 composites. Ceram. Int. 2024, 50, 3168–3175. [Google Scholar] [CrossRef]
- Dombrovskii, L.A.; Isakaev, E.H.; Senchenko, V.N.; Chinnov, V.F.; Scherbakov, V.V. Efficiency of particle acceleration, heating, and melting in high-enthalpy plasma jets. High Temp. 2012, 50, 145–153. [Google Scholar] [CrossRef]



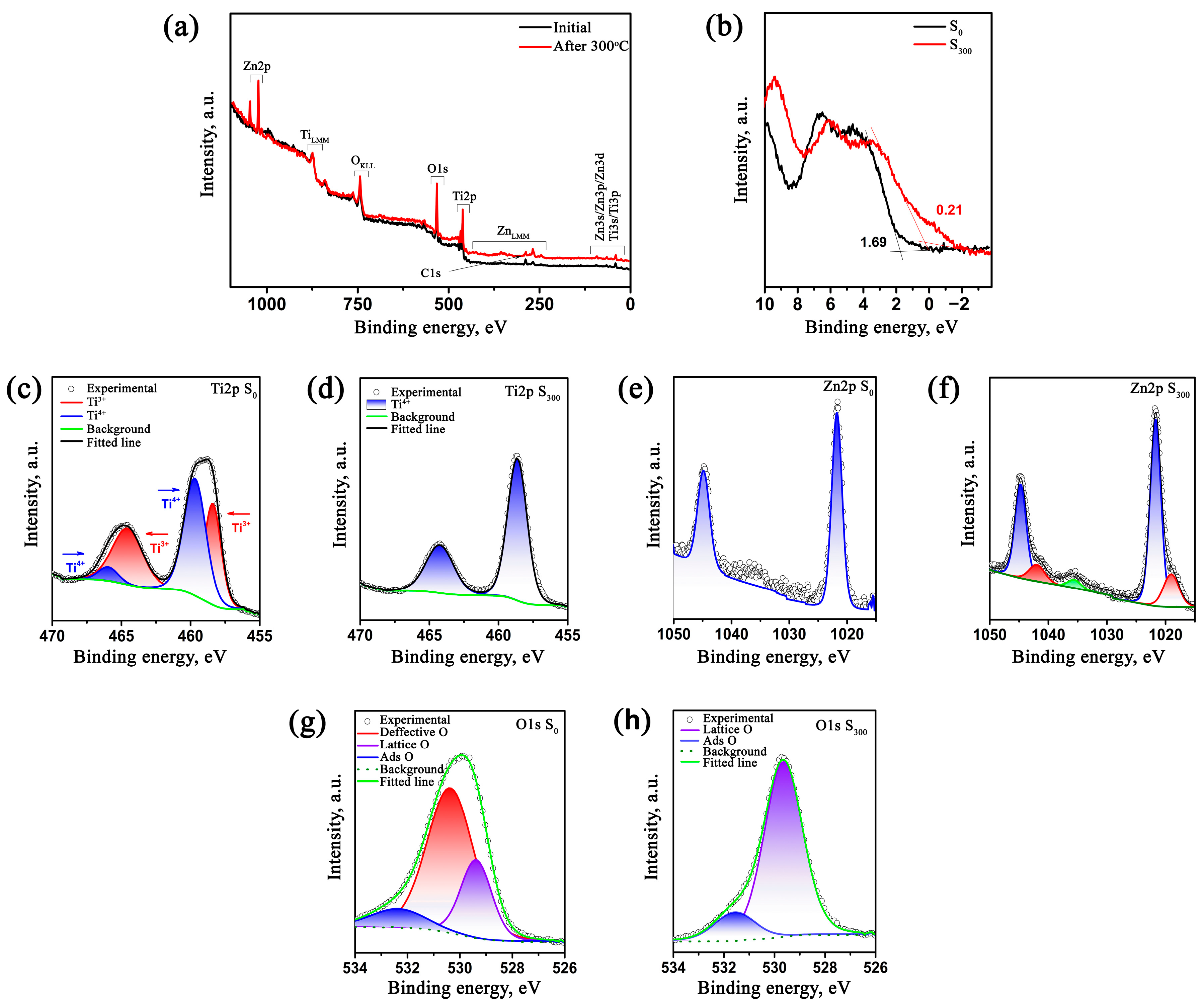



| Type of Sample | Element | At. % |
|---|---|---|
| S0 | Ti | 31.41 |
| O | 68.38 | |
| Zn | 0.21 | |
| S300 | Ti | 30.56 |
| O | 69.26 | |
| Zn | 0.18 |
| Type of Sample | Anatase, % | Rutile, % | Brookite, % |
|---|---|---|---|
| S0 | 36 | 60 | 4 |
| S300 | 38 | 62 | 0 |
| Phase | Anatase | Rutile | ||
|---|---|---|---|---|
| Lattice Parameter, Å | a | c | a | c |
| S0 | 3.787 | 9.520 | 4.596 | 2.958 |
| S300 | 3.787 | 9.559 | 4.599 | 2.963 |
| Reference | 3.7892 | 9.5370 | 4.5933 | 2.9580 |
| Phase | Anatase | Rutile | ||
|---|---|---|---|---|
| Parameter | D, nm | ε | D, nm | ε |
| S0 | 72.03 | 0.001017 | 72.13 | 0.001351 |
| S300 | 45.25 | 0.000388 | 64.79 | 0.001812 |
| Experiment No. | Catalyst Mass (mg) | MB Concentration (mg/L) | Degradation Efficiency (%) | Specific Activity (% per mg of Catalyst) |
|---|---|---|---|---|
| 1 | 20 | 2.5 | 99 | 4.9 |
| 2 | 10 | 2.5 | 94.5 | 9.5 |
| 3 | 5 | 2.5 | 86.5 | 17.3 |
| 4 | 20 | 5 | 92.9 | 4.7 |
| 5 | 20 | 10 | 61.5 | 3.1 |
| Ref. | Catalyst (Composition, Ratio) | Phases/Morphology/Size | Synthesis Method | Pollutant (Type, conc.) | Catalyst Loading (Dose, Morphology, Volume) | Light Source | Reaction Time | Solution Volume | Degradation (%) |
|---|---|---|---|---|---|---|---|---|---|
| [44] | ZnO/TiO2 heterojunction nanomesh | Anatase + rutile TiO2, ZnO (zinc blend), 3D mesh, tubes 200–400 nm, particles 20–80 nm | Anodization + Zn-acetate impregnation, calc. 600 °C | Methylene blue, 5 mg/L | Mesh (200/inch), immobilized | Xe lamp 500 W (vis/UV) | 90 min | 50 mL | 92% (ZnO/TiO2), 84% (TiO2) |
| [45] | ZnO/TiO2 nanofibers (various Zn:Ti ratios) | Hex. ZnO, anatase TiO2; fibers 134–228 nm; particles 31–52 nm | Electrospinning, calc. 600 °C | Methyl orange, 3 mg/L | Fibers on FTO, mass not stated | Xe, UV-A 220 W | 120 min | 50 mL | 96% (ZnO/TiO2), 87% (ZnO) |
| [46] | IO-TiO2, IO-ZnO, TiO2/ZnO, ZnO/TiO2 (inverse opals, composites) | Macroporous, pores ~290 nm, film 30–40 nm | PEALD on template, annealing 500 °C | 4-nitrophenol, Rh6G; conc. not specified | Coating on reactor wall | UV Osram 18 W + visible light | 240 min | – | 75–100% (details in text) |
| [47] | TiO2–ZnO nanocomposite (1:0.34), ~50–100 nm, polycrystal (anatase, rutile, ZnO, ZnTiO3, Zn2TiO4) | Anatase, rutile, ZnO, ZnTiO3, Zn2TiO4; 50–100 nm (TEM) | Modified sol–gel | 6 antibiotics (mixture), 0.6–60 mg/L each | 10 mg/L (0.01 g/L), 500 mL | Visible 125 W, UV | 240 min | 500 mL | >99% at 0.6 mg/L; 38–70% at 60 mg/L |
| [48] | TiO2-ZnO composite (hydrothermal), TiO2 nanotubes + ZnO nanoparticles | Anatase TiO2, hex. ZnO; nanotubes 4–5.5 µm, ZnO <100 nm | Hydrothermal | Rhodamine B, 10−5 M (4.8 mg/L) | Loading not specified (typically 0.5–1 g/L), 25 mL | Visible | 180 min | 25 mL | 89% (TiO2-ZnO), 77% (TiO2), 31% (ZnO) |
| [49] | ZnO/TiO2 nanocomposite, sol–gel | Anatase TiO2, ZnO, 20–60 nm (XRD) | Sol–gel | Methylene blue, 50 mg/L | 0.8 g/L, 50 mL | Visible, Xe 1000 W | 120 min | 50 mL | 96% |
| [50] | TiO2/ZnO hierarchical fibers | Anatase TiO2, ZnO rods on fibers, ~100 nm | Electrospinning, hydrothermal | Rhodamine B, ~10 mg/L | Membrane, mass not specified | Visible | 70 min | – | 90% |
| [51] | Mesoporous TiO2–ZnO (Ti:Zn = 3:1), 10–50 nm | Anatase, wurtzite, pores 10–20 nm | Microwave hydrothermal | Doxycycline, 50 mg/L | 1 g/L, 100 mL | UV 28 W (254 nm) | 30–100 min | 100 mL | 100% (50 ppm, 30 min); 100 ppm—100 min |
| [52] | TiO2/ZnO nanocomposites (0.25–1 M ZnO), TZO1–TZO4 | Anatase TiO2, ZnO (varied), rods 100–500 nm | Precipitation | Methylene blue, malachite green, 1 mg/L | 2 g/L, 100 mL | Solar (950 W/m2) | 60 min | 100 mL | MB: up to 98% (best at 0.25 M ZnO) |
| [53] | ZnO–TiO2 composites: Z@T, T@Z (core–shell) | Anatase TiO2, ZnO, 30–60 nm | Precipitation + calc. 600 °C | Methyl orange, ~10 mg/L | Not specified (std. 0.5–1 g/L), 100 mL | UV | 40 min | 100 mL | T@Z-600: 95%, Z@T-600: 54% |
| This work | TiO2/ZnO microparticles (10:1, micron-sized), anatase/rutile/ZnO | Anatase 36–38%, rutile 60–62%, nanoparticles 50–100 nm, microspheres ~10 μm | Plasma-assisted, annealed 300 °C | Methylene blue 2.5–10 mg/L, metronidazole 2.5 mg/L | 1 g/L (20 mg/20 mL), 20 mL | Hg 250 W (UV/Vis), solar 75 W | 30 min | 20 mL | MB: 99% (2.5 mg/L, 20 mg catalyst, 30 min); MNZ: 80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orudzhev, F.; Gadzhiev, M.; Abdulkerimov, M.; Muslimov, A.; Krasnova, V.; Il’ichev, M.; Kulikov, Y.; Chistolinov, A.; Volchkov, I.; Tyuftyaev, A.; et al. Plasma-Assisted Synthesis of TiO2/ZnO Heterocomposite Microparticles: Phase Composition, Surface Chemistry, and Photocatalytic Performance. Molecules 2025, 30, 3371. https://doi.org/10.3390/molecules30163371
Orudzhev F, Gadzhiev M, Abdulkerimov M, Muslimov A, Krasnova V, Il’ichev M, Kulikov Y, Chistolinov A, Volchkov I, Tyuftyaev A, et al. Plasma-Assisted Synthesis of TiO2/ZnO Heterocomposite Microparticles: Phase Composition, Surface Chemistry, and Photocatalytic Performance. Molecules. 2025; 30(16):3371. https://doi.org/10.3390/molecules30163371
Chicago/Turabian StyleOrudzhev, Farid, Makhach Gadzhiev, Magomed Abdulkerimov, Arsen Muslimov, Valeriya Krasnova, Maksim Il’ichev, Yury Kulikov, Andrey Chistolinov, Ivan Volchkov, Alexander Tyuftyaev, and et al. 2025. "Plasma-Assisted Synthesis of TiO2/ZnO Heterocomposite Microparticles: Phase Composition, Surface Chemistry, and Photocatalytic Performance" Molecules 30, no. 16: 3371. https://doi.org/10.3390/molecules30163371
APA StyleOrudzhev, F., Gadzhiev, M., Abdulkerimov, M., Muslimov, A., Krasnova, V., Il’ichev, M., Kulikov, Y., Chistolinov, A., Volchkov, I., Tyuftyaev, A., & Kanevsky, V. (2025). Plasma-Assisted Synthesis of TiO2/ZnO Heterocomposite Microparticles: Phase Composition, Surface Chemistry, and Photocatalytic Performance. Molecules, 30(16), 3371. https://doi.org/10.3390/molecules30163371








