Synthesis and Properties of Silver Nanoparticles Functionalized with β-Cyclodextrin and Their Loading with Lupinine and Its Acetyl Derivatives
Abstract
1. Introduction
2. Results and Discussion
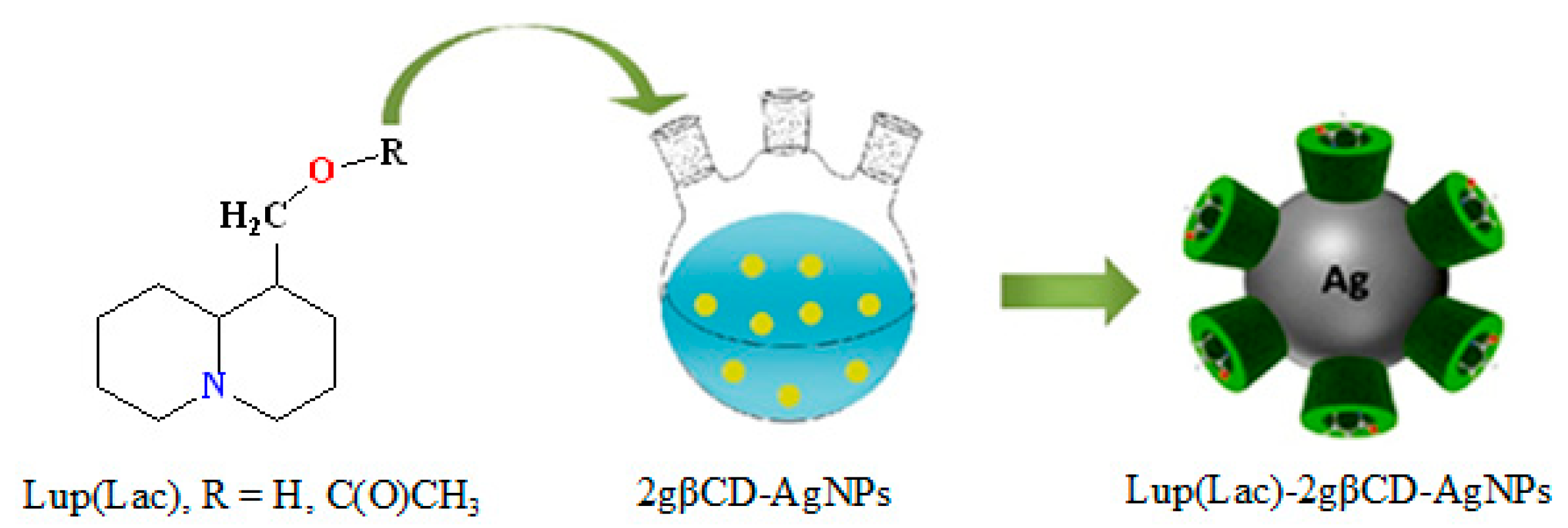
2.1. Structural Characteristics of Lup(Lac)-2gβCD-AgNPs Nanocomposites
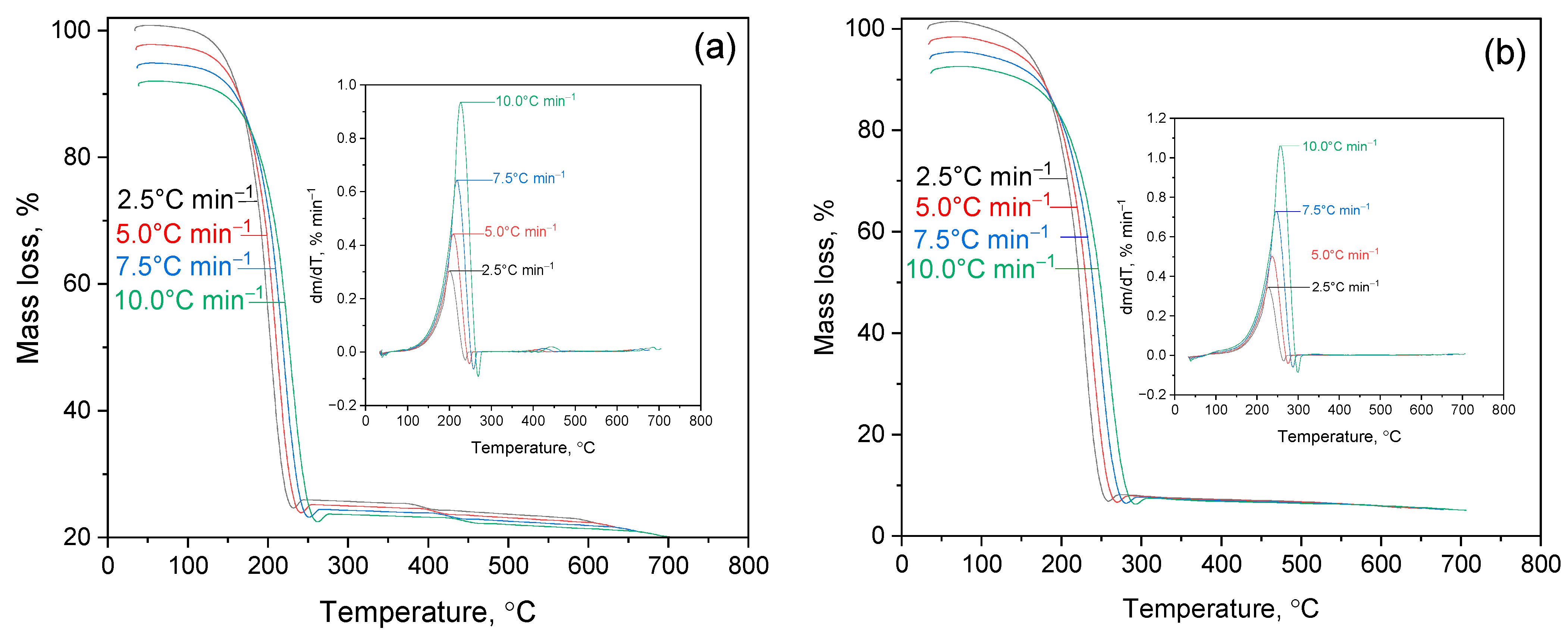
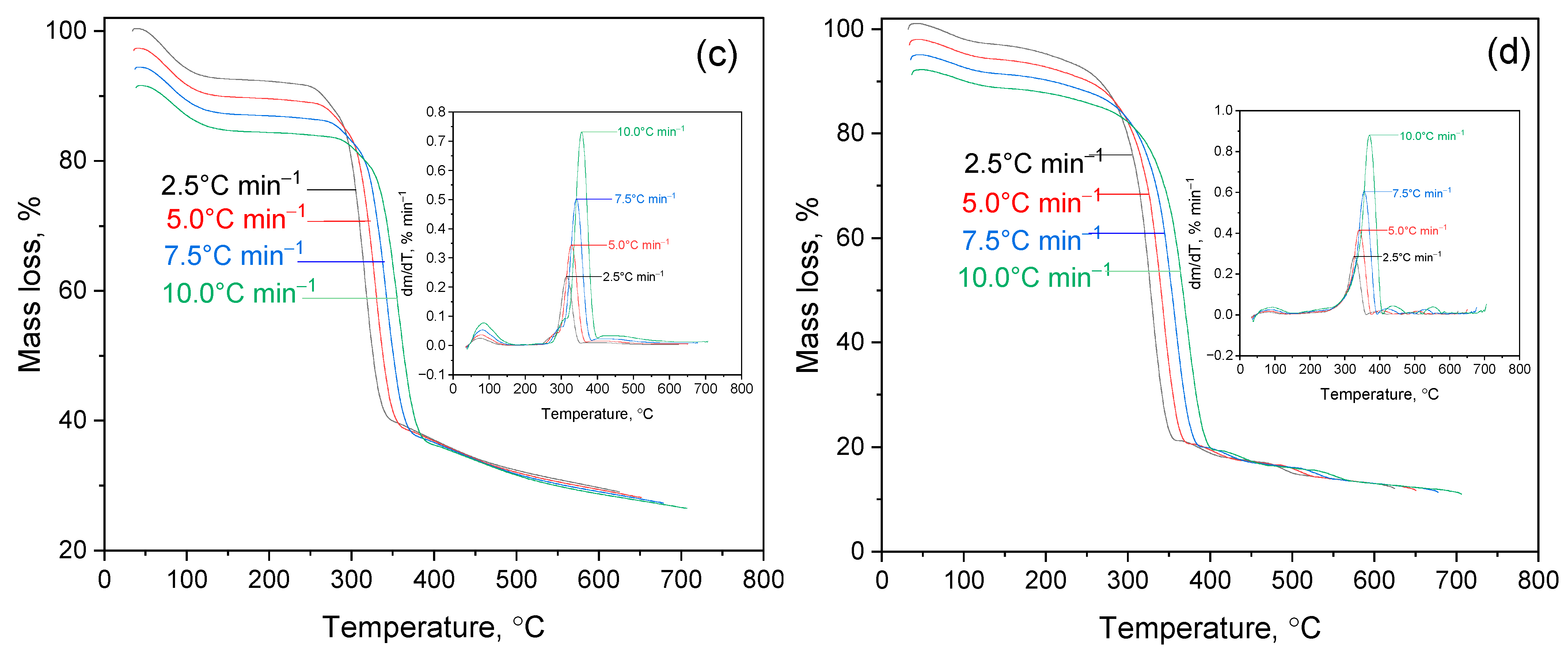
2.2. Thermogravimetric Analysis of 2gßCD-Lup(Lac)-AgNPs
2.2.1. Thermal Decomposition of Lup and Its Derivatives
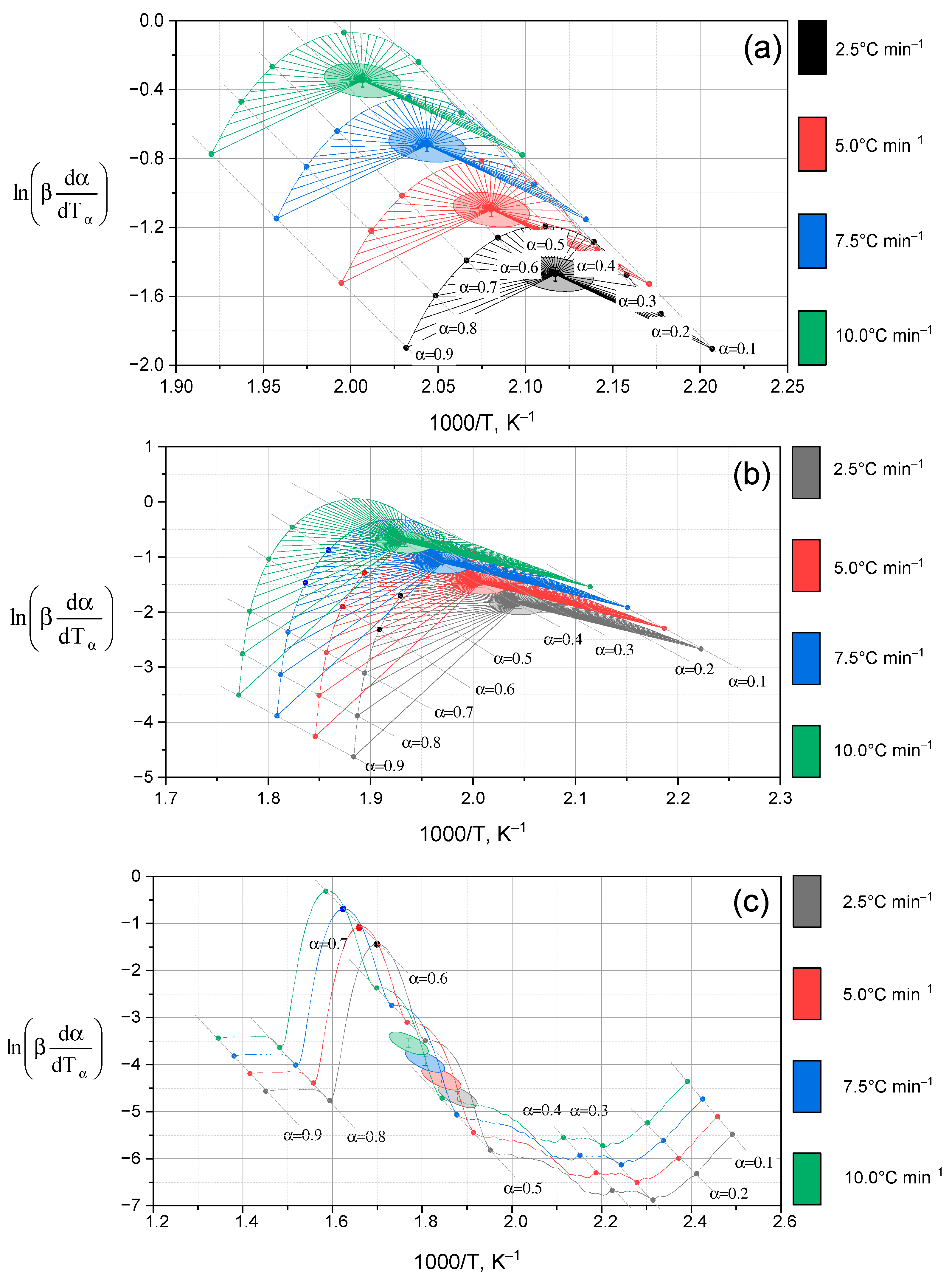
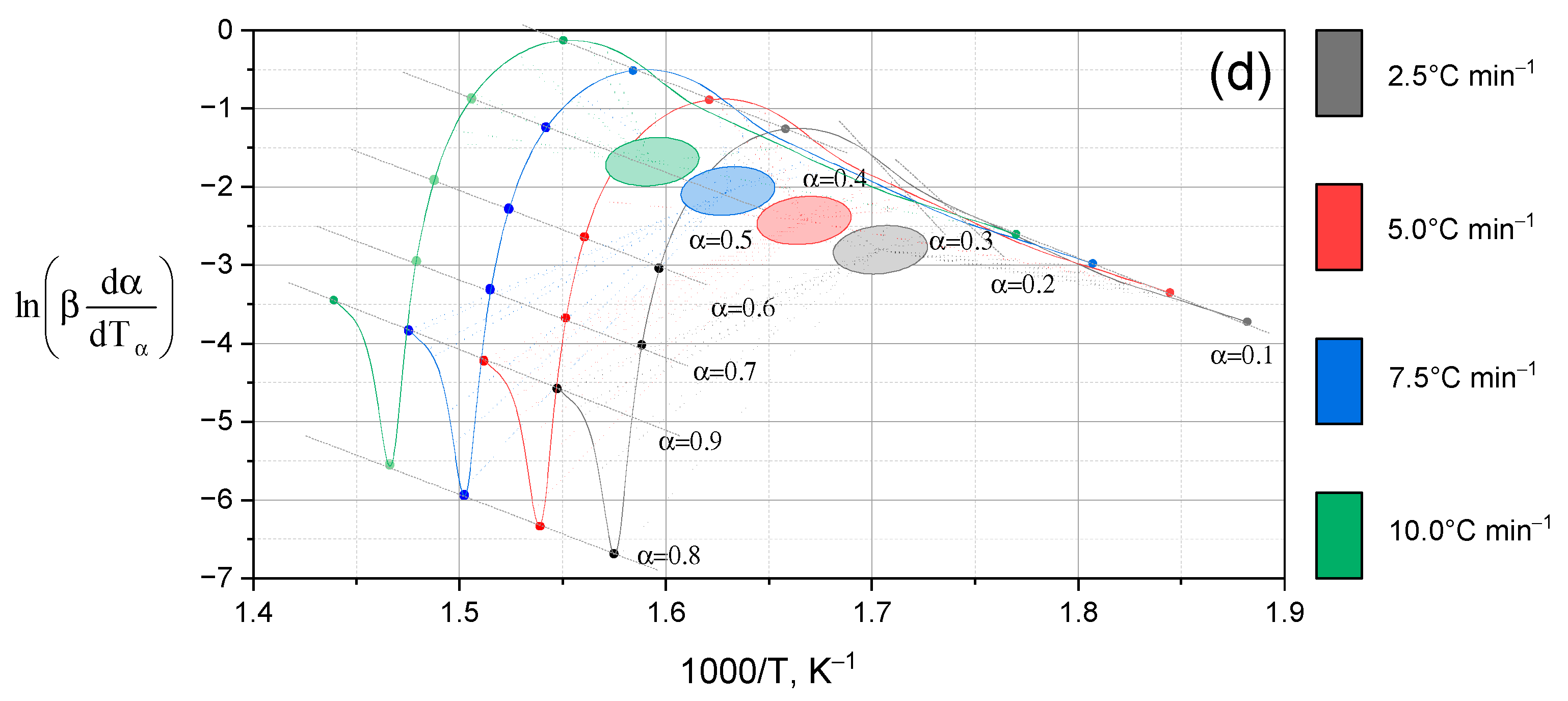
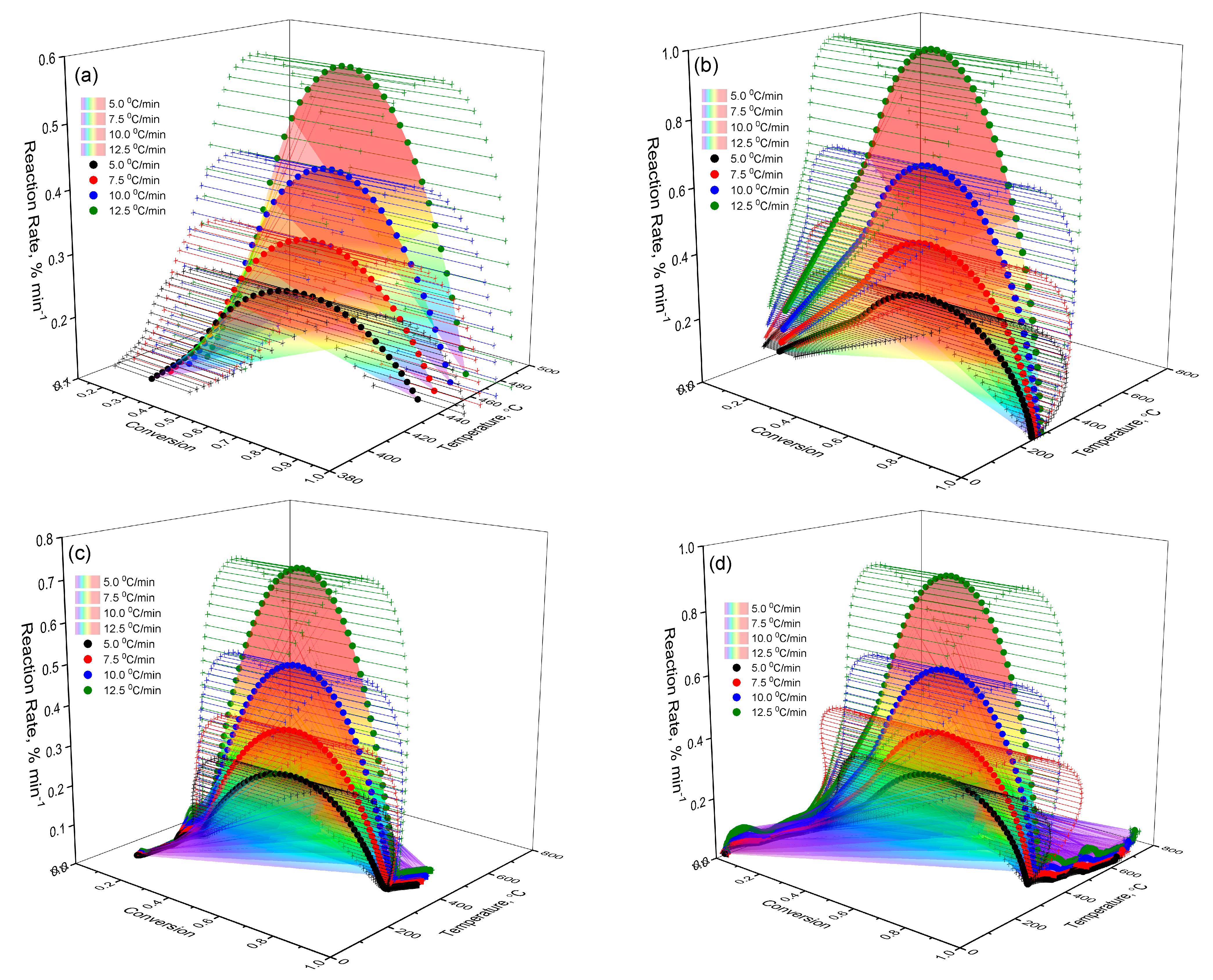
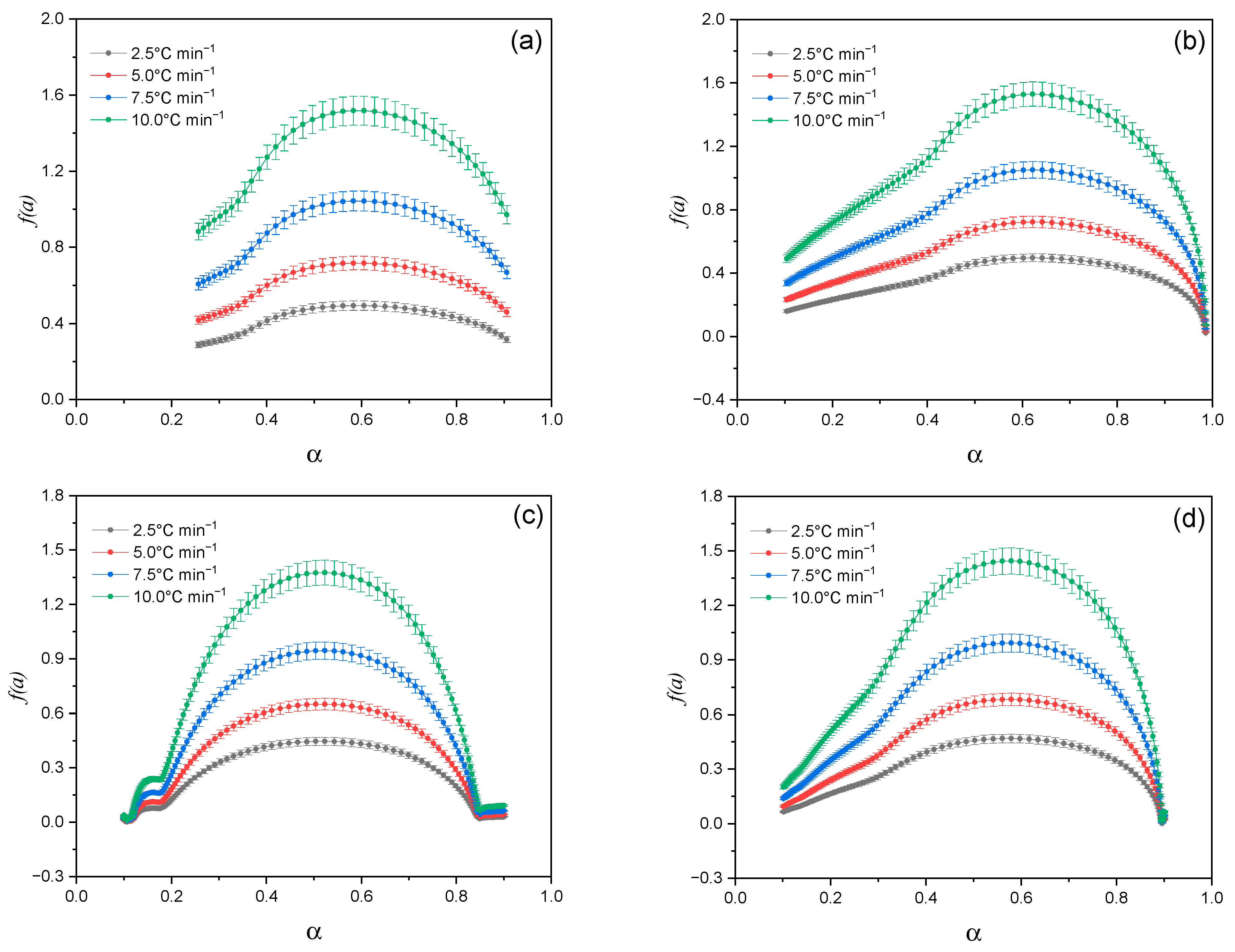
2.2.2. Kinetic Analysis of Thermal Decomposition of Lup Derivatives
- (a)
- Lup. As follows from the data in Figure 7a, when heating Lup, a typical increase in the reaction rate is observed with increasing temperature, reaching a maximum at the degree of transformation amax ≈ 0.5. After reaching this limit, the reaction rate begins to decrease. This decrease is associated with the depletion of active components and the formation of thermodynamically stable intermediates. The surface geometry is oblique and clearly reflects a kinetically controlled regime. In this regime, the reaction is most intense during the initial phase but slows down significantly in the later stages of the transformation.
- (b)
- Lac. According to Figure 7b, the general nature of the sample surface demonstrates a typical kinetic dependence: with increasing temperature, the reaction rate increases to a certain limit, after which it begins to decrease as the reagent is depleted. The reaction rate peaks are reached in the range of conversion degrees amax ≈0.6, which indicates the active stage of the destruction of the Lac molecule. A comparison of the curves at different heating rates (β) shows that increasing the heating rate causes the reaction peaks to shift toward higher temperatures. This shift is a characteristic feature of kinetically controlled processes. The graph surface for Lac exhibits a more pronounced maximum and steeper gradients compared to native Lup. This indicates more intense thermal decomposition and likely lower heat resistance, which may be attributed to the presence of an acetyl group.
- (c)
- Lup inclusion complex with 2gβCD and silver nanoparticles (Lup-2gβ-CD-AgNPs). The surface of Figure 7c is characterized by a more complex shape, including several local maxima, which indicates the multi-stage decomposition process of the complex. The main maximum of the reaction rate is observed in the range α = 0.4–0.6 and a temperature of about 270–300 °C, which indicates the active destruction of both Lup and 2gβCD. The peak shift with increasing β indicates the kinetic influence of temperature and is typical for reactions involving several components. Comparison with Figure 7a,b shows that, in the case of the Lup-2gβCD-AgNPs inclusion complex, the reaction proceeds at a higher decomposition rate. This increase may be attributed to the catalytic effect of silver nanoparticles. Additionally, changes in thermal stability due to the formation of the inclusion complex could contribute to this behavior. Steeper alpha responses may also indicate a sharper transition between stages, characteristic of cooperative effects in complex compounds.
- (d)
- Lup-2gβCD-AgNPs/acetone (inclusion complex obtained in an acetone solution). The three-dimensional surface shown in Figure 7d demonstrates the most pronounced characteristics among all the studied samples. Several intense zones of increased reaction rate are observed. This observation indicates the multi-stage nature of the thermal decomposition of the complex. The main peak of the reaction is located in the temperature range of 260–310 °C and corresponds to the degrees of transformation α ≈ 0.5–0.7.
2.3. Results of the Study of Cytotoxic, Antibacterial and Analgesic Activity of New Lup Derivatives
2.3.1. Antimicrobial Activity of the Samples
2.3.2. Cytotoxic Activity of the Samples
2.3.3. Results of the Study of the Analgesic Activity of the Samples
3. Materials and Methods
3.1. Determination of the Structural Characteristics of the Lup(Lac)-2gβCD-AgNPs Nanocomposite
3.2. Study of the Thermal Behavior and Kinetics of Decomposition of Lup and Its Modified Form
3.3. Investigation of Biological Properties of Lup Derivatives
3.3.1. Antibacterial Activity of the Samples (In Vitro)
3.3.2. Cytotoxic Activity
3.3.3. Analgesic Activity of the Samples
3.4. Preparation of the Lac-2gßCD-AgNPs Inclusion Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michael, J.P. Indolizidine and quinolizidine alkaloids. Nat. Prod. Rep. 2007, 24, 191–222. [Google Scholar] [CrossRef]
- Tasso, B.; Mattioli, L.B.; Tonelli, M.; Boido, V.; Chiarini, A.; Sparatore, F.; Budriesi, R. Further quinolizidine derivatives as antiarrhythmic agents-3. Molecules 2023, 28, 6916. [Google Scholar] [CrossRef]
- Takao, K.; Munakata, R.; Tadano, K. Recent advances in natural product synthesis by using intramolecular Diels-Alder reactions. Chem. Rev. 2005, 105, 4779–4807. [Google Scholar] [CrossRef] [PubMed]
- Nurmaganbetov, Z.S.; Nurkenov, O.A.; Khlebnikov, A.I.; Fazylov, S.D.; Seidakhmetova, R.B.; Tukhmetova, Z.K.; Takibayeva, A.T.; Khabdolda, G.; Rakhimberlinova, Z.B.; Kaldybayeva, A.K.; et al. Antiviral Activity of (1S,9aR)-1-[(1,2,3-Triazol-1-yl)-methyl]-octahydro-1H-quinolizines from the Alkaloid Lupinine. Molecules 2024, 29, 5742. [Google Scholar] [CrossRef]
- Magalhaes, S.; Fernandes, F.; Cabrita, A.R.J.; Fonseca, A.J.M.; Valentao, P.; Andrade, P.B. Alkaloids in the valorization of European Lupinus spp. seeds crop. Ind. Crops Prod. 2016, 95, 286–295. [Google Scholar] [CrossRef]
- Konrath, E.L.; Passos, C.D.S.; Klein-Junior, L.C.; Henriques, A.T. Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2013, 65, 1701–1725. [Google Scholar] [CrossRef] [PubMed]
- Tlegenov, R.T.; Dalimov, D.N.; Haitbaev, H.H.; Abduvahabov, A.A.; Uteniyazov, K.U. Synthesis and antikholinesterase activites of a number of the alkaloids lupinine. Chem. Nat. Compound. 1990, 26, 434–436. [Google Scholar] [CrossRef]
- Turdybekov, K.M.; Nurkenov, O.A.; Nurmaganbetov, Z.S.; Satpaeva, Z.B.; Turdybekov, D.M.; Makhmutova, A.S.; Fazylov, S.D. Synthesis, crystal structure, and stability of N-lupinylphthalimide conformers. J. Struct. Chem. 2020, 61, 1823–1826. [Google Scholar] [CrossRef]
- Frik, K.M.; Kamphuis, L.G.; Siddique, K.M.; Singh, K.B.; Foley, R.C. Quinolizidine alkaloid Biosynthesis in Lupins and Prospects for Grain Quality Improvement. Front. Plant Sci. 2017, 31, 87. [Google Scholar] [CrossRef]
- Gusarova, N.K.; Malysheva, S.F.; Oparina, L.A.; Belogorlova, N.A.; Tantsyrev, A.P.; Parshina, L.N.; Sukhov, B.G.; Tlegenov, R.T.; Trofimov, B.A. Synthesis of novel alkaloid derivatives from vinyl ether of lupinine and PH-addends. Arkivoc 2009, 7, 260–267. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Nurmaganbetov, Z.S.; Fazylov, S.D.; Nurkenov, O.A.; Khlebnikov, A.I.; Seilkhanov, T.M.; Kishkentaeva, A.S.; Shults, E.E.; Quinn, M.T. Inhibition of Acetylcholinesterase by Novel Lupinine Derivatives. Molecules 2023, 28, 3357. [Google Scholar] [CrossRef]
- Su, D.; Wang, X.; Shao, C.; Xu, J.; Zhu, R.; Hu, Y. Total Synthesis of (+)-Epilupinine via an intramolecular nitrile oxide-alkene cycloaddition. J. Org. Chem. 2011, 76, 188–194. [Google Scholar] [CrossRef]
- Semenov, V.E.; Zueva, I.V.; Mukhamedyarov, M.A.; Lushchekina, S.V.; Kharlamova, A.D. 6-Methyluracil derivatives as bifunctional acetyl-cholinesterase inhibitors for the treatment of Alzheimer’s Disease. Chem. Med. Chem. 2015, 10, 1863–1874. [Google Scholar] [CrossRef]
- Nurkenov, O.A.; Nurmaganbetov, Z.S.; Fazylov, S.D.; Seidakhmetova, R.B.; Shulgau, Z.T.; Muldakhmetov, Z.M. Synthesis, structure and biological activity of (1S,9aR)-1H-1,2,3-triazol-1-yl)methyl)octahydro-1H-quinolizine derivatives of lupinine. Arch. Razi Inst. 2022, 77, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial affects of silver nanomaterials and potential implications for human health and the enironment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Zargar, M.; Yunus, W.M.Z.W.; Ibrahim, N.A.; Shabanzadeh, P.; Moghaddam, M.G. Synthesis and characterization of silver/montmorillonite/chitosan bionanocomposites by chemical reduction method and their antibacterial activity. Int. J. Nanomed. 2011, 6, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, J.; Chi, H.; Wei, G.; Zhang, J.; Dai, L. Preparation and antibacterial activity of polyvinyl alcohol/regenerated silk fibroin composite fibers containing Ag nanoparticles. J. Appl. Polym. Sci. 2012, 123, 20–25. [Google Scholar] [CrossRef]
- Caro, C.; Castillo, P.M.; Klippstein, R.; Pozo, D.; Zaderenko, A.P. Silver nanoparticles: Sensing and imaging applications. In Silver Nanoparticles; IntechOpen: London, UK, 2010. [Google Scholar] [CrossRef]
- Prozorova, G.F.; Pozdnyakov, A.S.; Kuznetsova, N.P.; Korzhova, S.A.; Emel’yanov, A.I.; Ermakova, T.G.; Fadeeva, T.V.; Sosedova, L.M. Green Synthesis of Water-Soluble Non-Toxic Polymeric Nanocomposites Containing Silver Nanoparticles. Int. J. Nanomed. 2014, 16, 1883–1889. [Google Scholar] [CrossRef]
- Elliott, C. The effects of silver sulfadiazine on chronic and burns wound healing. Br. J. Nurs. 2010, 19, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Rupp, M.E.; Fitzgerald, T.; Marion, N.; Helget, V.; Puumala, S.; Anderson, J.R.; Fey, P.D. Effect of silver-coated urinary catheters: Efficacy, cost-effectiveness, and antimicrobial resistance. Am. J. Infect. Control 2004, 32, 445–450. [Google Scholar] [CrossRef]
- Cho, K.H.; Park, J.E.; Osaka, T.; Park, S.G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta. 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Chou, W.L.; Yu, D.G.; Yang, M.C. The preparation and characterization of silver-loading cellulose acetate hollow fiber membrane for water treatment. Polym. Adv. Technol. 2005, 16, 600–607. [Google Scholar] [CrossRef]
- Sun, R.W.; Chen, R.; Chung, N.P.; Ho, C.M.; Lin, C.L.; Che, C.M. Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chem. Commun. 2005, 40, 5059–5061. [Google Scholar] [CrossRef]
- Luo, C.C.; Zhang, Y.H.; Zeng, X.W.; Zeng, Y.W.; Wang, Y.G. The role of poly(ethylene glycol) in the formation of silver nanoparticles. J. Colloid Interface Sci. 2005, 288, 444–448. [Google Scholar] [CrossRef]
- Valencia, G.A.; Vercik, L.C.O.; Cilla, T.; Vercik, A. A Simple and Green Method for Syhthesis of Ag and Au Nanoparticles using Biopolymers and Sugar as Reducing Agent. Mater. Res. Soc. Symp. Proc. 2012, 1386, 1103. [Google Scholar] [CrossRef]
- Husen, A.; Siddiqi, K.S. Phytosynthesis of nanoparticles: Concept, controversy and application. Nano Res. Lett. 2014, 9, 229. [Google Scholar] [CrossRef]
- Bahavarnia, F.; Hasanzadeh, M.; Bahavarnia, P.; Shadjou, N. Advancements in application of chitosan and cyclodextrins in biomedicine and pharmaceutics: Resent progress and future trends. RVS Adv. 2024, 14, 13384. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Junfeng, L.; Laichun, L.; Meilin, L.; Tanfang, X.; Junfen, Z. Synthesis of cationic b-cyclodextrin functionalized silver nanoparticles and their drug-loading applications. RSC Adv. 2023, 13, 7250. [Google Scholar] [CrossRef]
- Fazylov, S.D.; Nurkenov, O.A.; Nurmaganbetov, Z.S.; Sarsenbekova, A.Z.; Bakirova, R.Y.; Seilkhanov, O.T.; Sviderskiy, A.K.; Syzdykov, A.K.; Mendibayeva, A.Z. Synthesis of β-Cyclodextrin-Functionalized Silver Nanoparticles and Their Application for Loading Cytisine and Its Phosphorus Derivative. Molecules 2025, 30, 1337. [Google Scholar] [CrossRef] [PubMed]
- Šesták, J.; Kratochvíl, J. Rational approach to thermodynamic rrocesses and constitutive equations in isothermal and non-isothermal kinetics. J. Therm. Anal. 1973, 5, 193. [Google Scholar] [CrossRef]
- Serra, R.; Nomen, R.; Sempere, J. The non-parametric kinetics a new method for the kinetic study of thermoanalytical data. J. Therm. Anal. Calorim. 1998, 52, 933. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of Thermal Degradation of Char-Forming Plastics from Thermogravimetry. Application to a Phenolic Plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183. [Google Scholar] [CrossRef]
- Ozawa, T.A. New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881. [Google Scholar] [CrossRef]
- Wenzler, E.; Maximos, M.; Asempa, T.E.; Biehle, L.; Schuetz, A.N.; Hirsch, E.B. Antimicrobial susceptibility testing: An updated primer for clinicians in the era of antimicrobial resistance: Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2023, 43, 264–278. [Google Scholar] [CrossRef]
- Barrot, M. Tests and models of nociception and pain in rodents. Neuroscience 2012, 1, 39–50. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982, 45, 31. [Google Scholar] [CrossRef]
- McLaughlin, J.L. Crown-gall tumours in potato discs and brine shrimp lethality: Two simple bioassays for higher plant screening and fractionation. In Methods in Plant Biochemistry; Hostettmann, K., Ed.; London Academic Press: London, UK, 1991; Part 6; pp. 1–31. [Google Scholar]
- Council, N.R. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; p. 220. [Google Scholar]
- Adekenov, S.M.; Zhanimkhanova, P.Z.; Nurmaganbetov, Z.S.; Amanzhan, A.; Chernov, S.V.; Turmukhambetov, A.Z.; Bagryanskaya, I.Y.; Gatilov, Y.V.; Shults, E.E. Synthetic modifications of carboline alkaloid harmine: Synthesis of 8-substituted derivatives. Chem. Heterocycl. Compd. 2019, 55, 135–141. [Google Scholar] [CrossRef]
- Nurmaganbetov, Z.S.; Fazylov, S.D.; Nurkenov, O.A.; Sarsenbekova, A.Z.; Pustolaikina, I.A.; Seilkhanov, O.T.; Sviderskiy, A.K.; Minayeva, Y.V. Combined Computational and Experimental Study of Inclusion Complexes of Lupinine and its 1,2,3-triazole Derivative with β-cyclodextrin. Eurasian Chem.-Technol. J. 2025, 27, 127–138. [Google Scholar] [CrossRef]
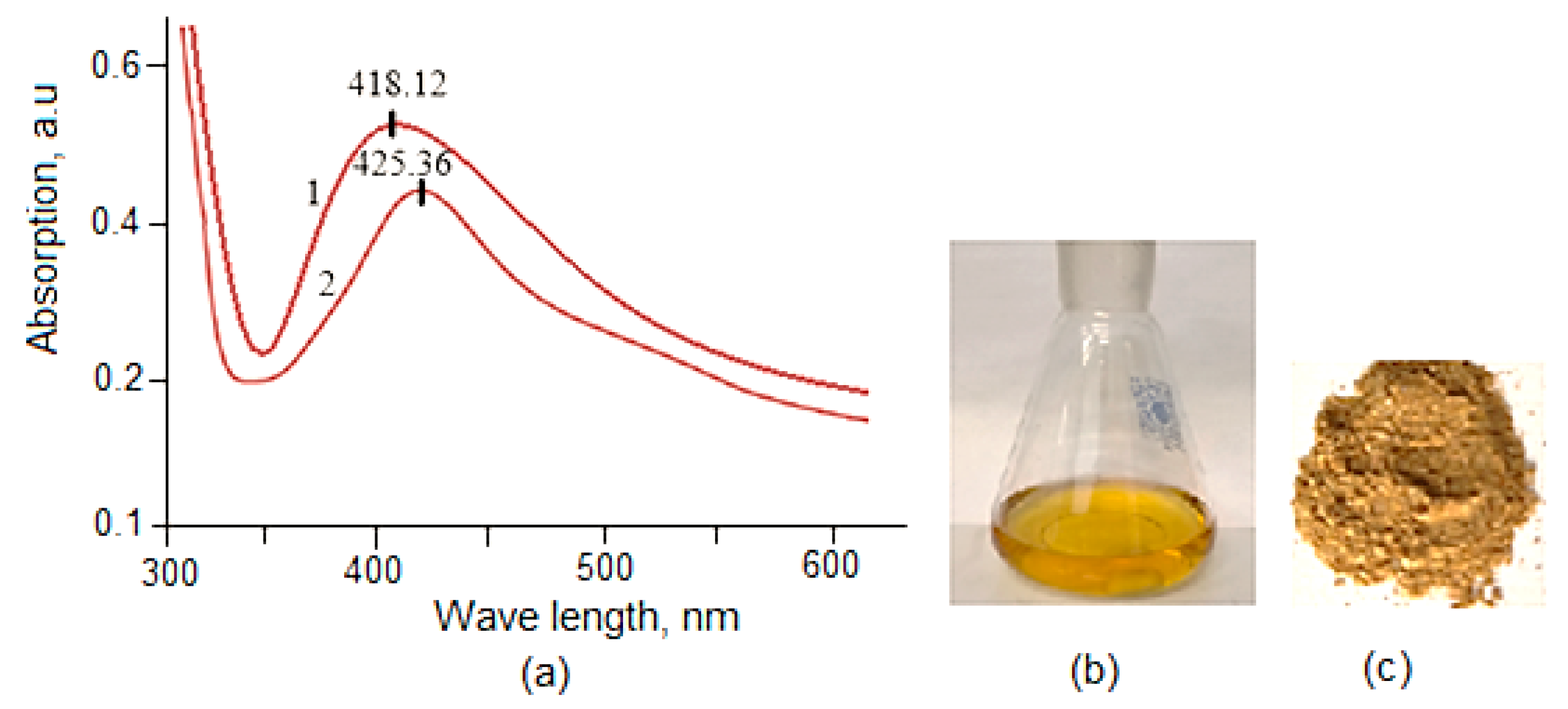
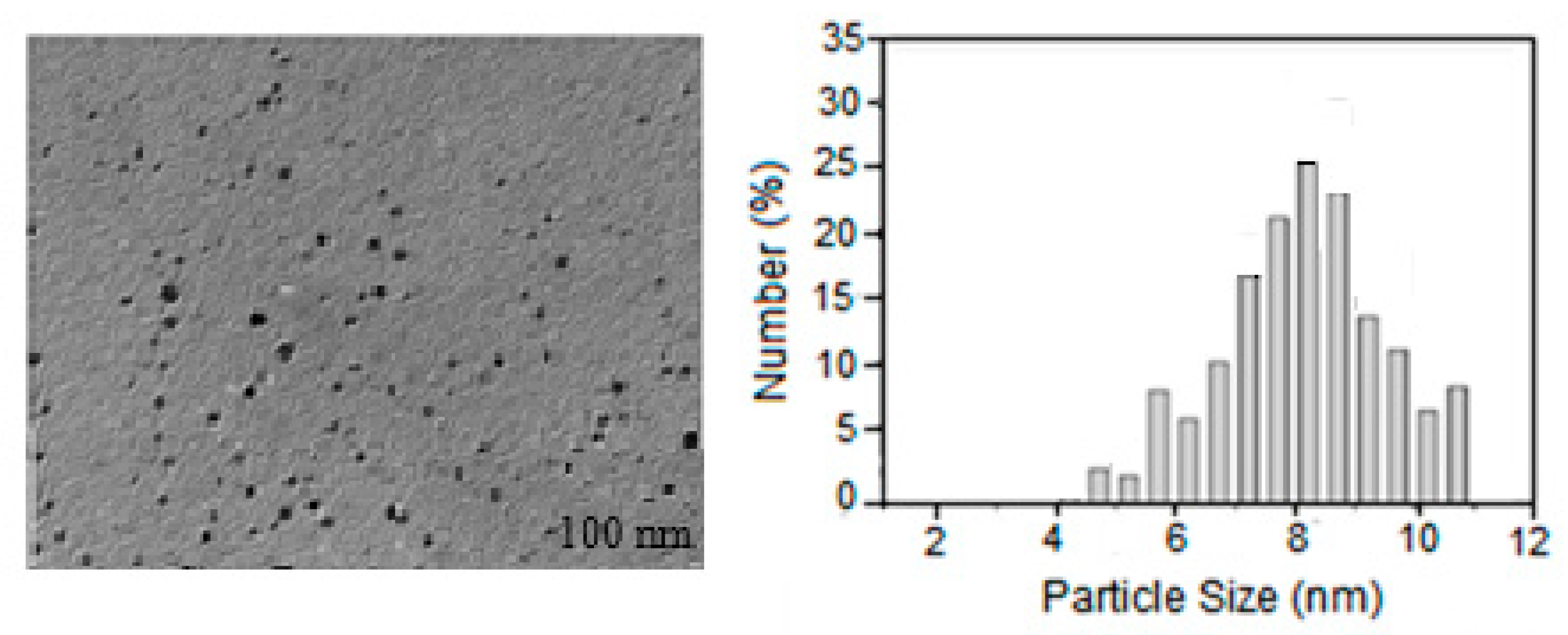
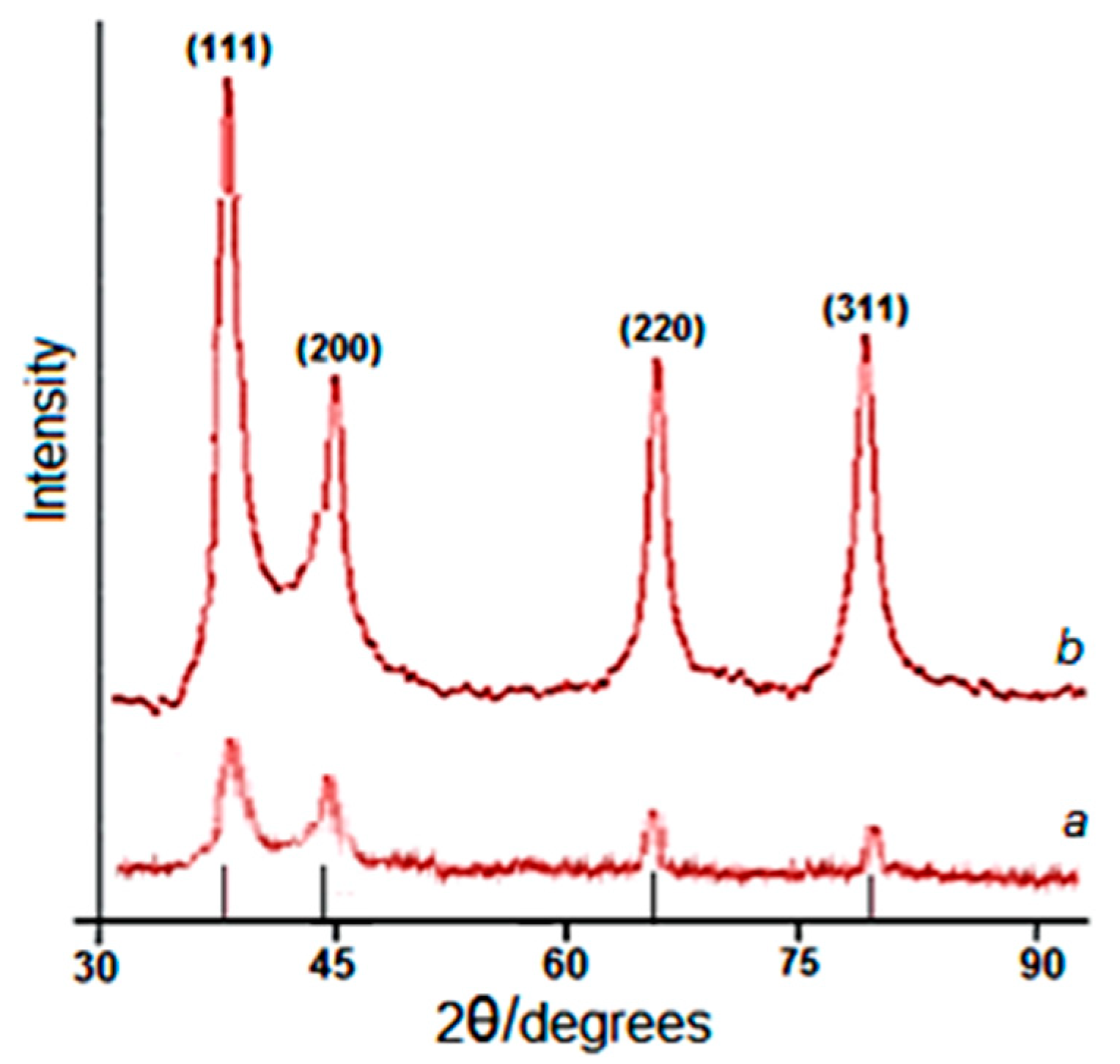
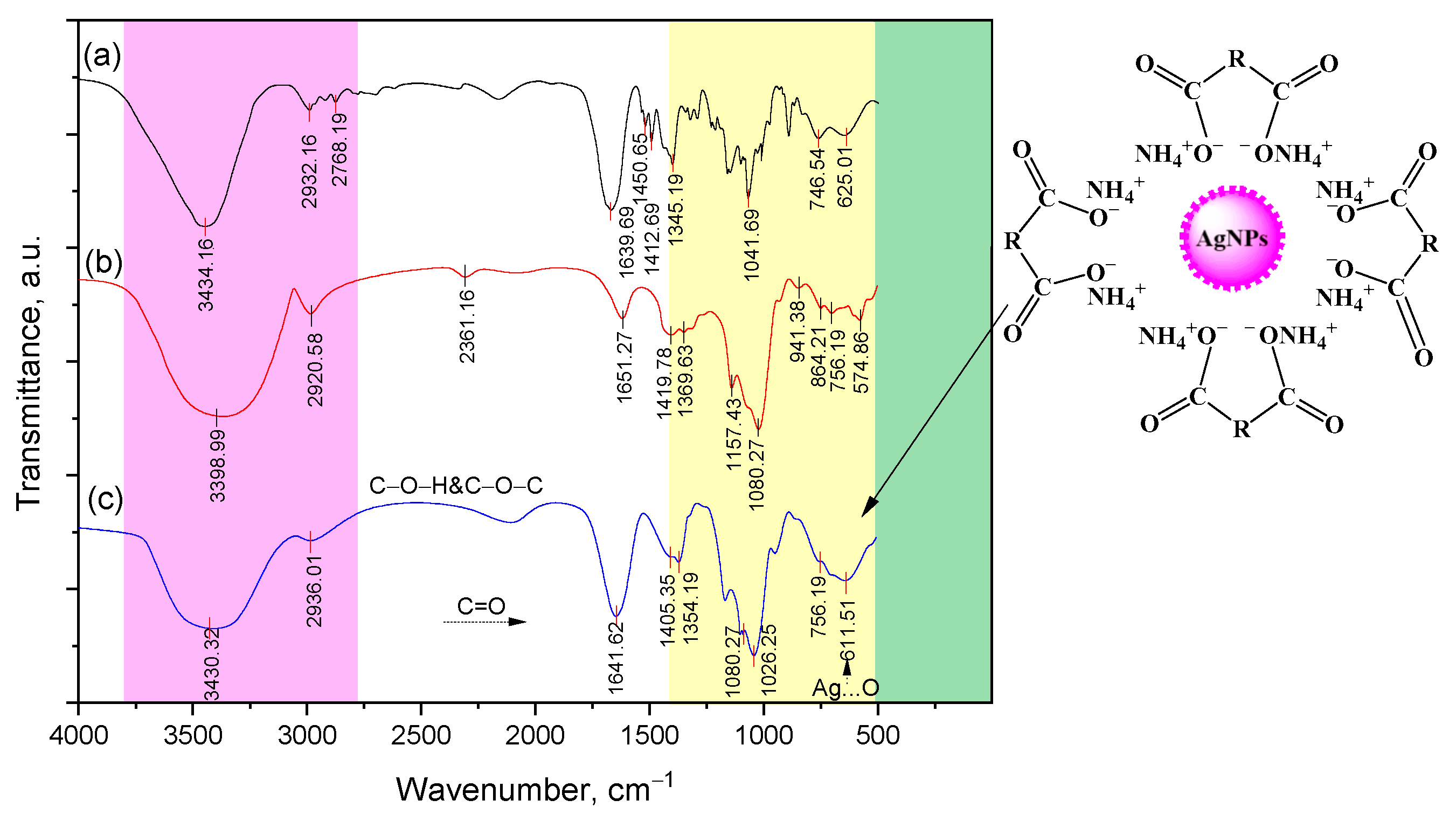

| Sample/α | Method | 0.1 | 0.3 | 0.5 | 0.7 | 0.9 |
|---|---|---|---|---|---|---|
| (a) Lup | Friedman | 88.35 ± 1.20 | 85.75 ± 1.20 | 84.87 ± 1.12 | 84.40 ± 0.90 | 83.89 ± 1.32 |
| OFW | 90.26 ± 1.00 | 87.77 ± 1.41 | 86.80 ± 1.13 | 85.3 ± 1.71 | 85.52 ± 1.21 | |
| (b) Lac | Friedman | 86.36 ± 1.30 | 84.42 ± 1.23 | 83.90 ± 1.02 | 83.65 ± 1.54 | 83.49 ± 1.70 |
| OFW | 88.61 ± 1.41 | 86.22 ± 1.31 | 85.68 ± 1.41 | 85.48 ± 1.90 | 85.01 ± 1.50 | |
| (c) Lup-2gβCD-AgNPs | Friedman | 93.86 ± 1.10 | 83.98 ± 1.00 | 84.28 ± 1.20 | 84.64 ± 1.10 | 88.36 ± 1.00 |
| OFW | 95.03 ± 1.51 | 85.85 ± 1.30 | 86.71 ± 1.20 | 86.18 ± 1.70 | 90.57 ± 2.00 | |
| (d) Lup-2gβCD-AgNPs/acetone | Friedman | 86.37 ± 1.00 | 84.92 ± 1.10 | 84.59 ± 0.90 | 84.16 ± 1.00 | 83.43 ± 1.10 |
| OFW | 88.98 ± 1.10 | 86.01 ± 1.21 | 86.68 ± 1.11 | 86.27 ± 1.30 | 85.03 ± 1.21 |
| Composition | F-Statistic | p-Value (ANOVA) | t-Statistic | p-Value (t-Test) |
|---|---|---|---|---|
| (a) Lup | 0.2839 | 0.6086 | −1.0484 | 0.3536 |
| (b) Lac | 4.9978 | 0.0558 | −15.6370 | 0.0001 |
| (c) Lup-2gβCD-AgNPs | 0.5119 | 0.4947 | −8.1465 | 0.0012 |
| (d) Lup-2gβCD-AgNPs/acetone | 5.4098 | 0.0485 | −7.3667 | 0.0018 |
| Sample | , kJ mol−1 ± SD | ·107, s−1 ± SD | Šesták–Berggren αm(1 − α)n | , kJ mol−1 ± SD | ·107, s−1 ± SD | |
|---|---|---|---|---|---|---|
| m | n | |||||
| (a) Lup | 84.54 ± 0.12 | 1.45 ± 0.05 | 0.41 | 0.30 | 84.95 ± 0.12 | 2.22 ± 0.18 |
| (b) Lac | 84.01 ± 0.15 | 1.78 ± 0.04 | 0.35 | 0.20 | 84.24 ± 0.15 | 0.80 ± 0.10 |
| (c) Lup-2gβCD-AgNPs | 85.76 ± 0.75 | 2.54 ± 0.54 | 0.47 | 0.43 | 85.82 ± 1.50 | 3.98 ± 1.20 |
| (d) Lup-2gβCD–AgNPs/acetone | 84.87 ± 0.41 | 2.01 ± 0.05 | 0.42 | 0.30 | 84.63 ± 1.10 | 1.39 ± 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazylov, S.D.; Nurmaganbetov, Z.S.; Nurkenov, O.A.; Sarsenbekova, A.Z.; Seilkhanov, O.T.; Seidakhmetova, R.B.; Mendibayeva, A.Z.; Bakirova, R.Y.; Muldakhmetov, Z.M. Synthesis and Properties of Silver Nanoparticles Functionalized with β-Cyclodextrin and Their Loading with Lupinine and Its Acetyl Derivatives. Molecules 2025, 30, 3354. https://doi.org/10.3390/molecules30163354
Fazylov SD, Nurmaganbetov ZS, Nurkenov OA, Sarsenbekova AZ, Seilkhanov OT, Seidakhmetova RB, Mendibayeva AZ, Bakirova RY, Muldakhmetov ZM. Synthesis and Properties of Silver Nanoparticles Functionalized with β-Cyclodextrin and Their Loading with Lupinine and Its Acetyl Derivatives. Molecules. 2025; 30(16):3354. https://doi.org/10.3390/molecules30163354
Chicago/Turabian StyleFazylov, Serik D., Zhangeldy S. Nurmaganbetov, Oralgazy A. Nurkenov, Akmaral Z. Sarsenbekova, Olzhas T. Seilkhanov, Roza B. Seidakhmetova, Anel Z. Mendibayeva, Ryszhan Y. Bakirova, and Zainulla M. Muldakhmetov. 2025. "Synthesis and Properties of Silver Nanoparticles Functionalized with β-Cyclodextrin and Their Loading with Lupinine and Its Acetyl Derivatives" Molecules 30, no. 16: 3354. https://doi.org/10.3390/molecules30163354
APA StyleFazylov, S. D., Nurmaganbetov, Z. S., Nurkenov, O. A., Sarsenbekova, A. Z., Seilkhanov, O. T., Seidakhmetova, R. B., Mendibayeva, A. Z., Bakirova, R. Y., & Muldakhmetov, Z. M. (2025). Synthesis and Properties of Silver Nanoparticles Functionalized with β-Cyclodextrin and Their Loading with Lupinine and Its Acetyl Derivatives. Molecules, 30(16), 3354. https://doi.org/10.3390/molecules30163354






