Pyrolysis of Polypropylene and Nitrile PPE Waste: Insights into Oil Composition, Kinetics, and Steam Cracker Integration
Abstract
1. Introduction
2. Results
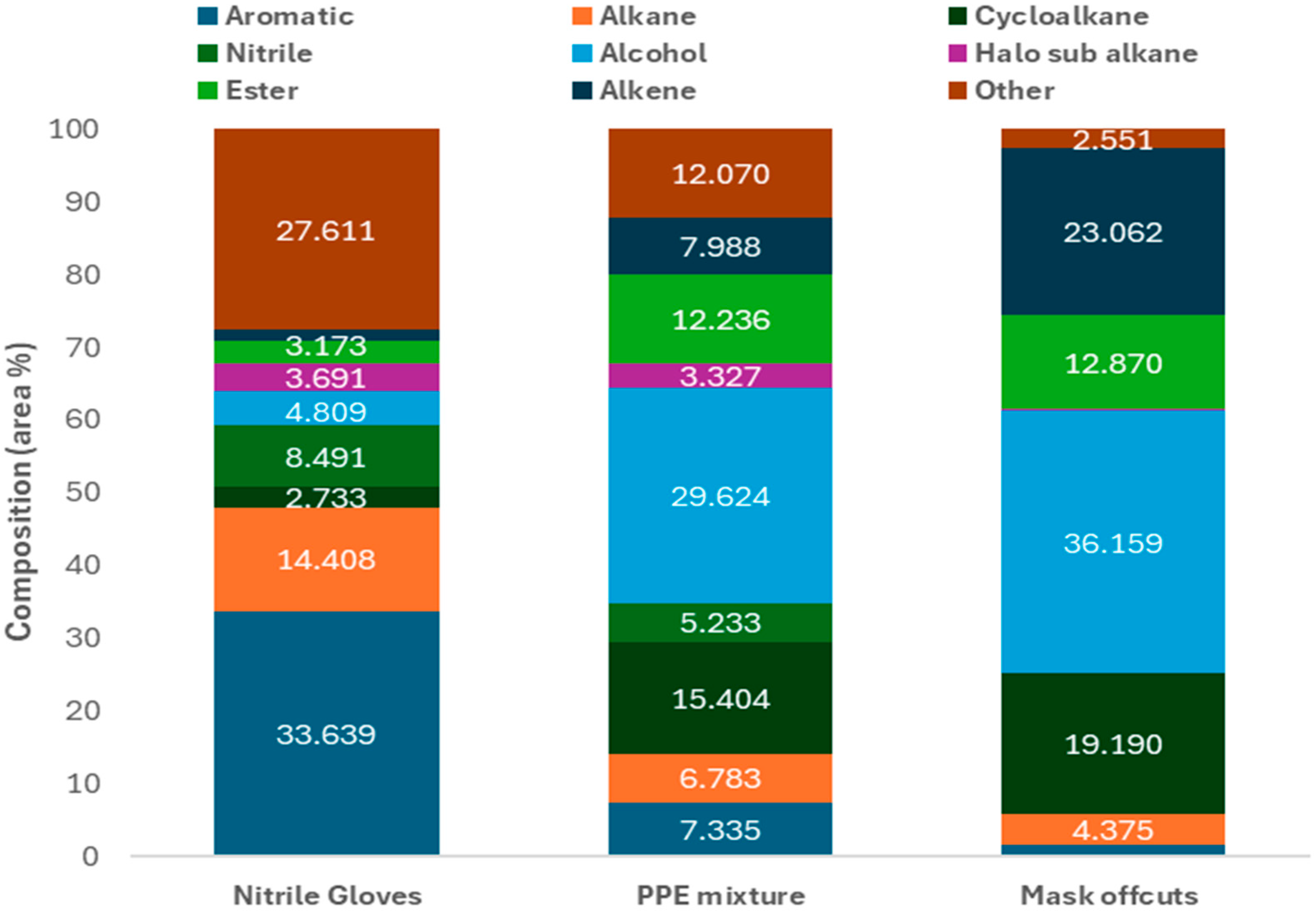
2.1. Feedstock Characterisation
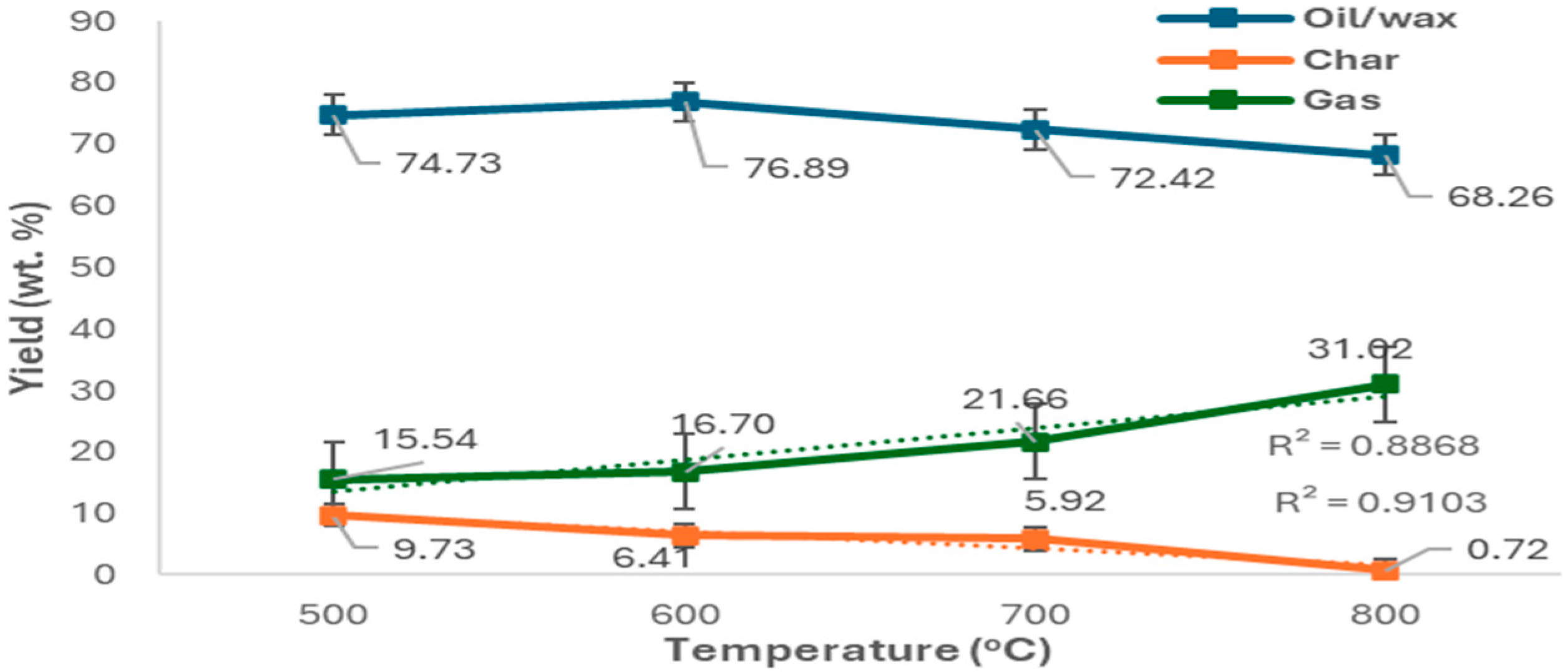
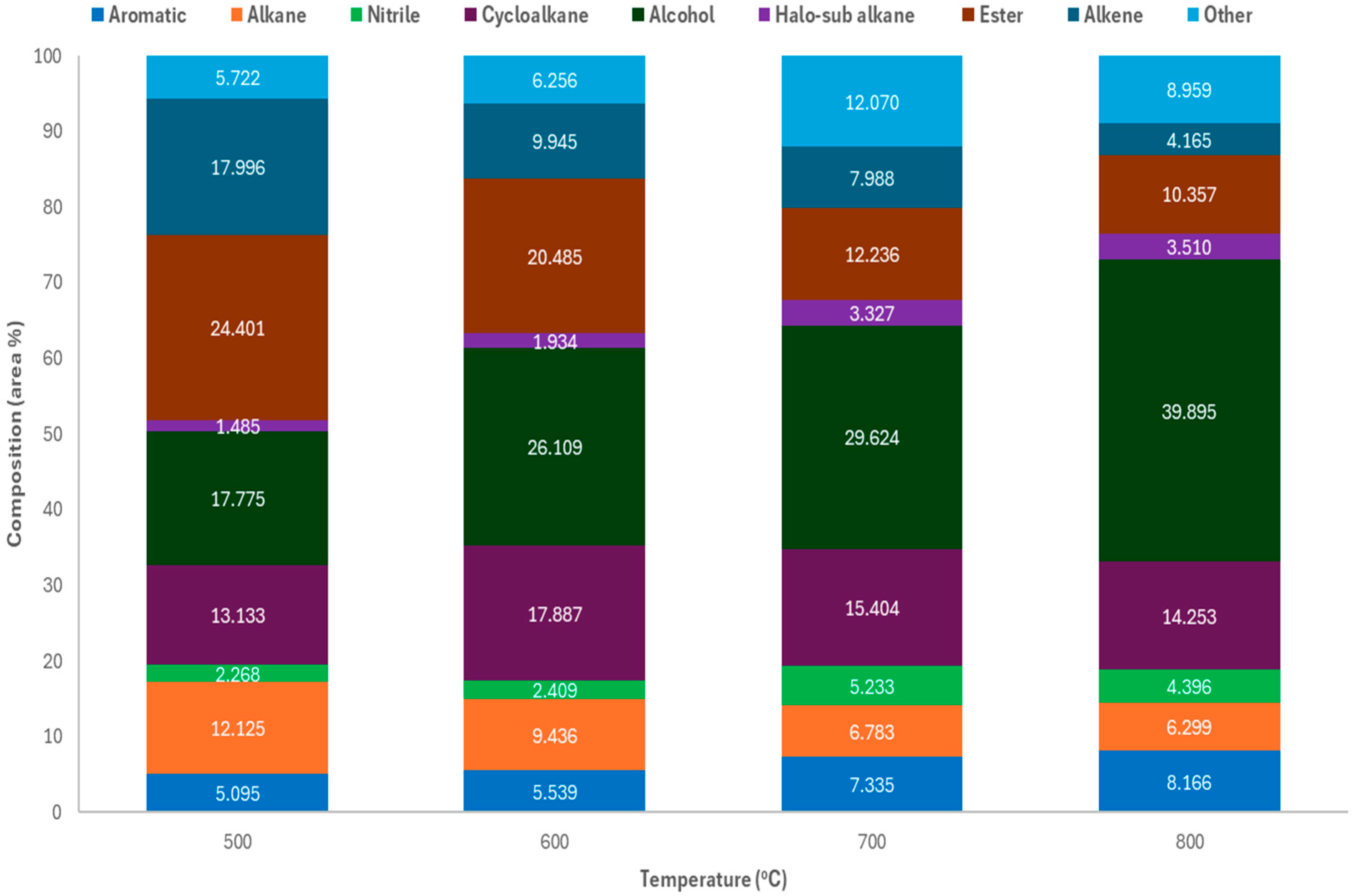
2.2. Thermal Pyrolysis of Mask Offcuts and Nitrile Gloves
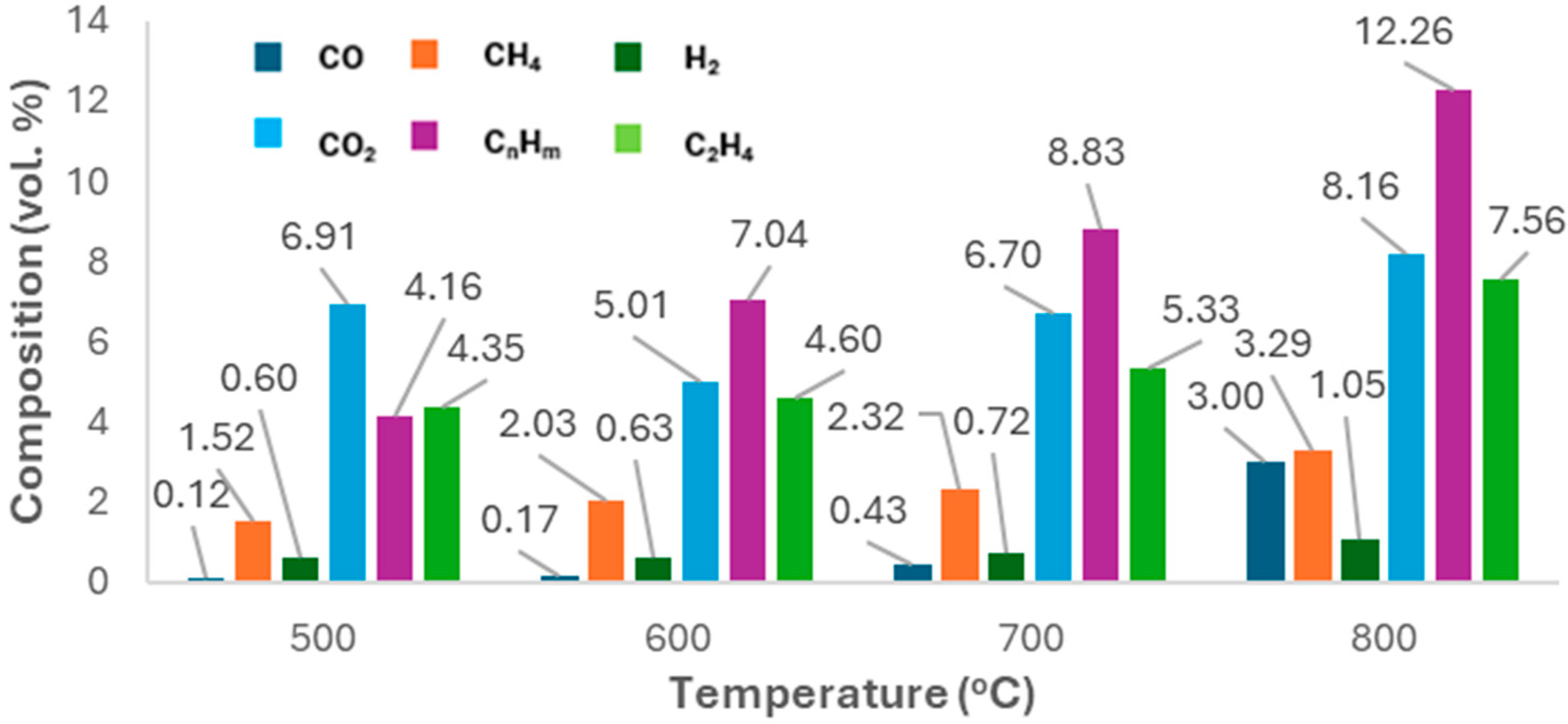
2.2.1. Gas Composition
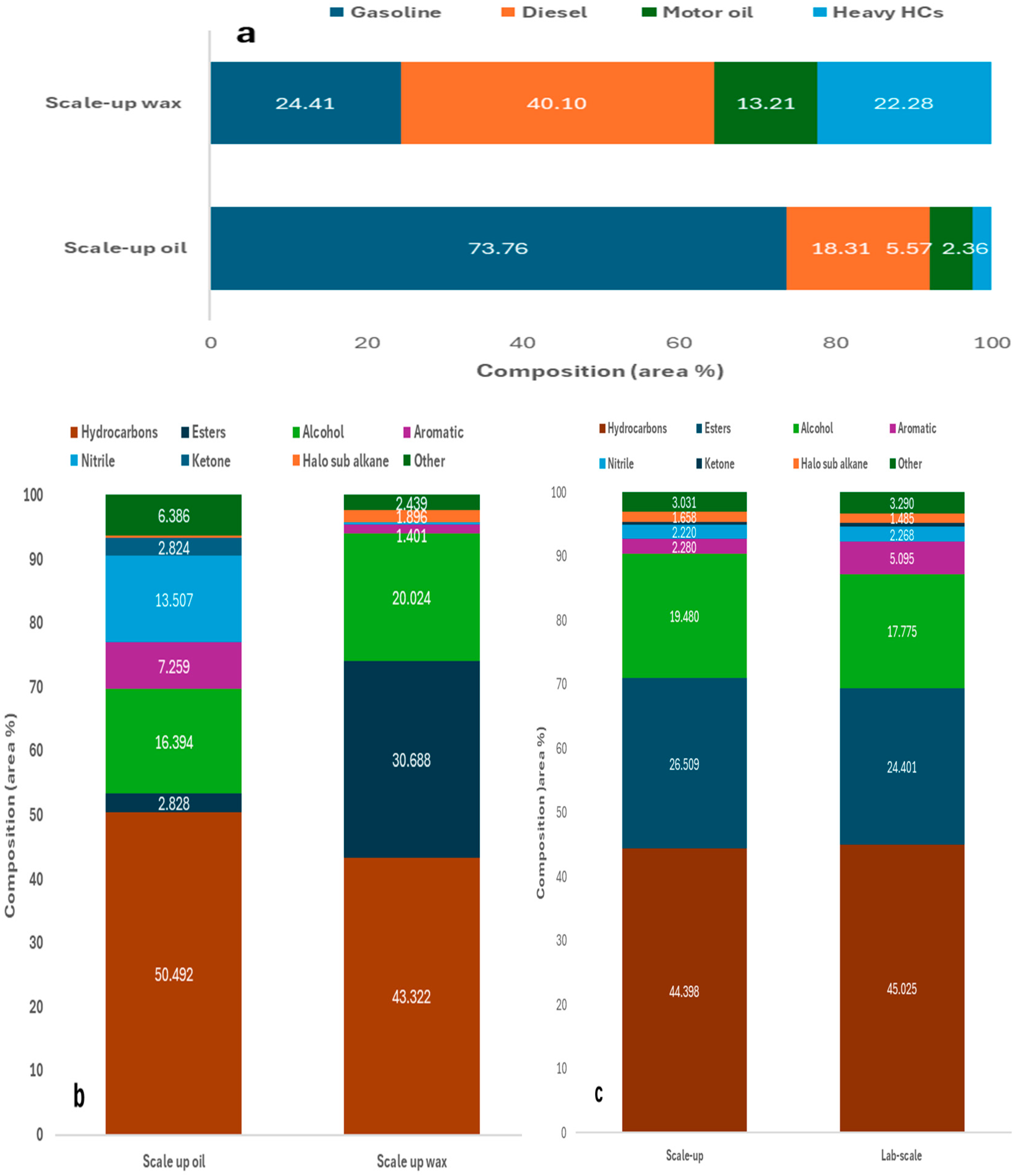
2.2.2. Scale-Up Auger Reactor Test
2.3. Analysis of Contaminants Present in PPE Oil and Associated Challenges for Oil Refinery Entry
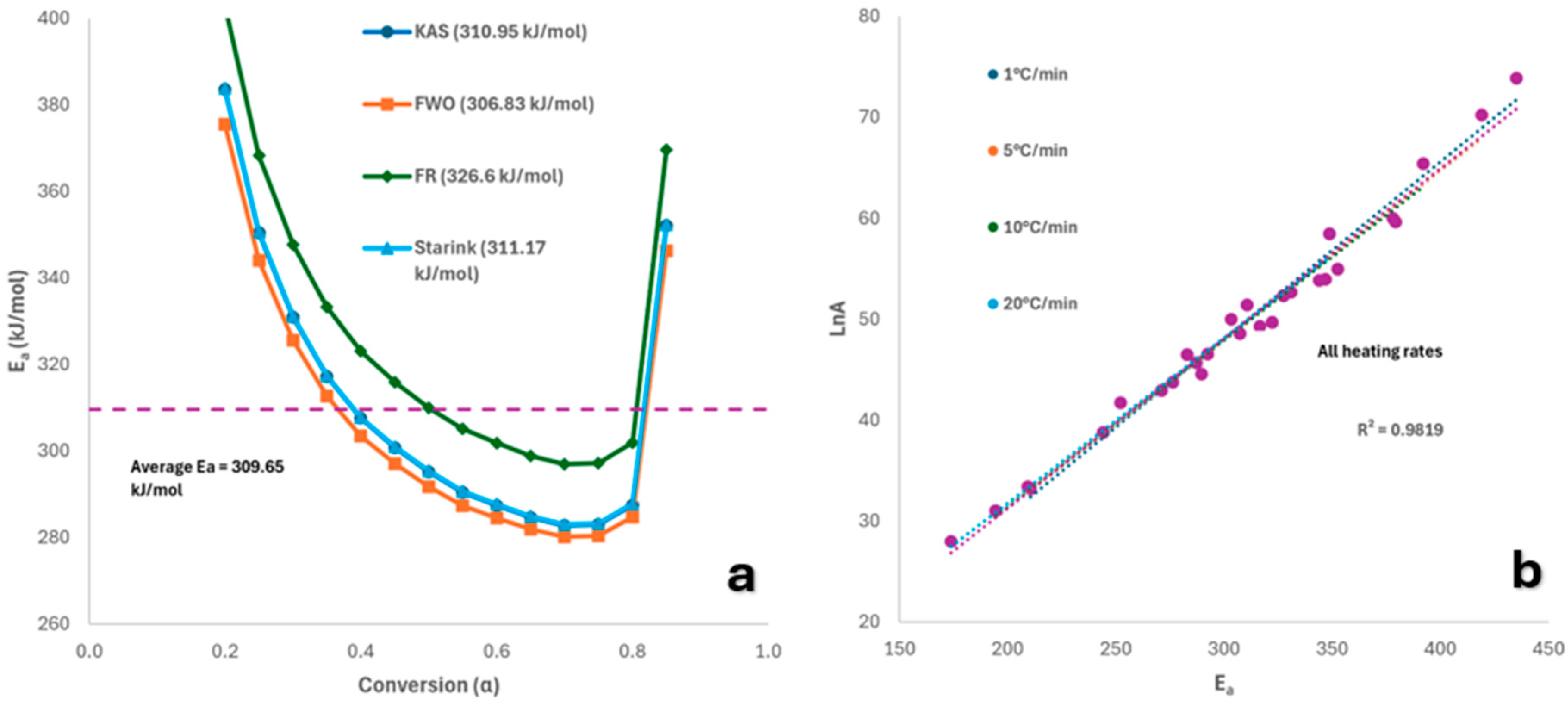
2.4. Kinetic Analysis
2.4.1. Model-Free Methods
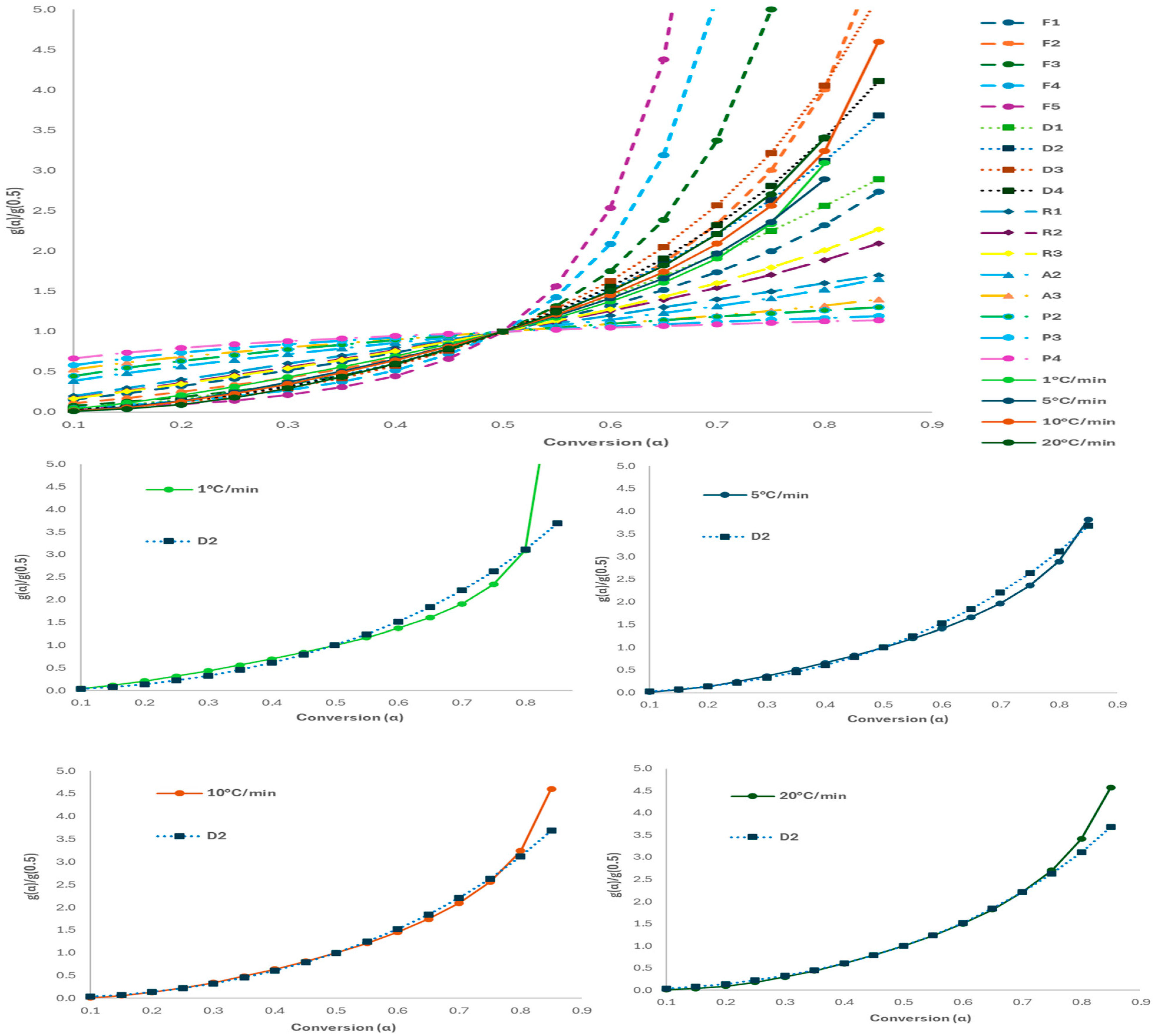
2.4.2. Determination of Reaction Order by the CR Method
3. Materials and Methods
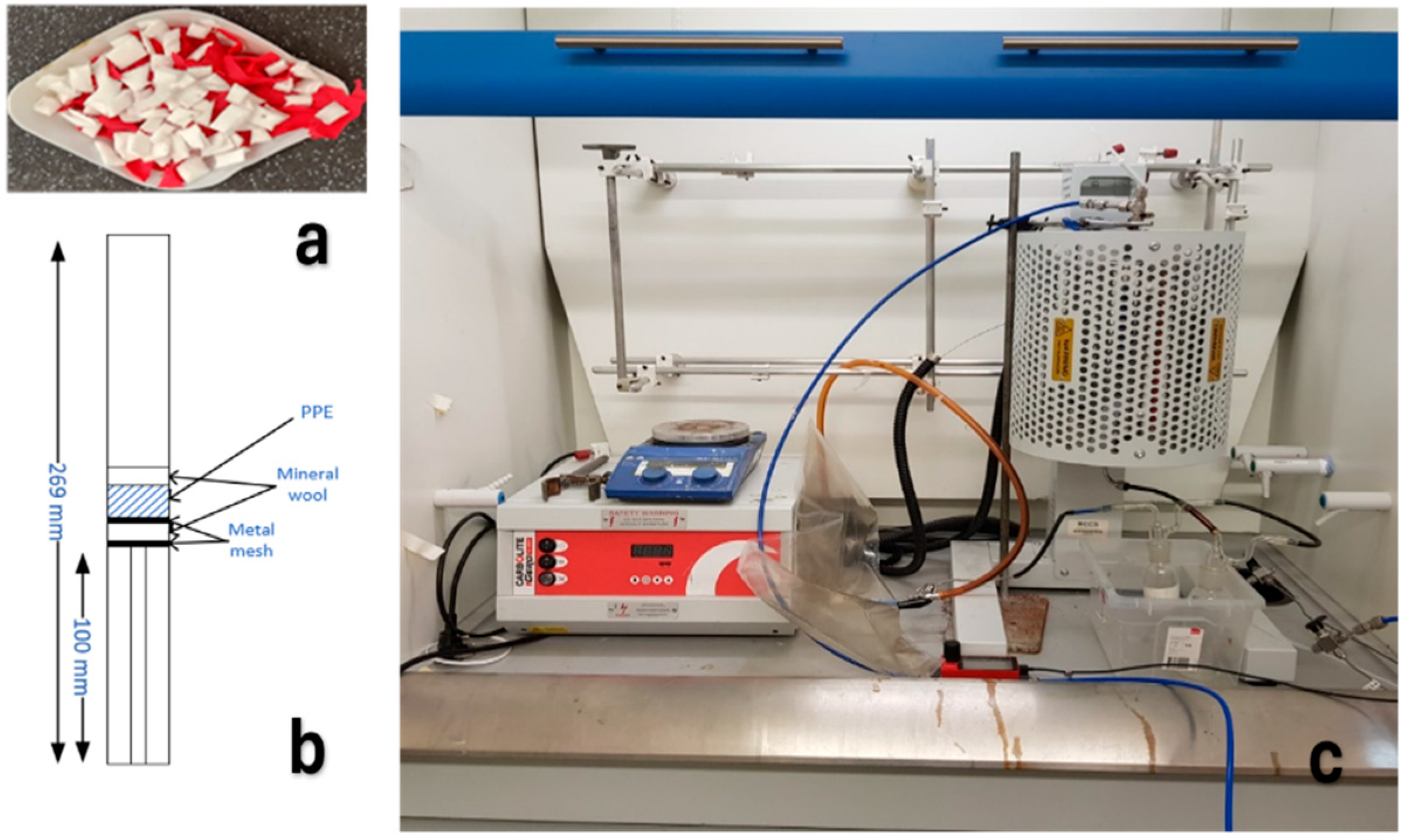
3.1. Materials
3.2. Pyrolysis Experimental Set-Up and Method
3.3. Materials Characterisation
3.4. Auger Reactor Scale-Up Test
3.5. Kinetic Triplet Determination
3.6. Model-Free Kinetic Methods
3.6.1. Kissinger–Akahira–Sunose (KAS)
3.6.2. Flynn–Wall–Ozawa (FWO)
3.6.3. Friedman (FR)
3.6.4. Starink
3.7. Model-Fitting Methods
3.7.1. Criado (Master Plots)
3.7.2. Coats–Redfern (CR)
3.8. Determination of the Pre-Exponential Factor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Przygoda-Kuś, P.; Kosiorowska, K.E.; Urbanek, A.K.; Mirończuk, A.M. Current Approaches to Microplastics Detection and Plastic Biodegradation. Molecules 2025, 30, 2462. [Google Scholar] [CrossRef]
- Aragaw, T.; Mekonnen, B. Current plastics pollution threats due to COVID-19 and its possible mitigation techniques: A waste-to-energy conversion via Pyrolysis. Environ. Syst. Res. 2021, 10, 8–11. [Google Scholar] [CrossRef]
- OECD. Global Plastics Outlook: Policy Scenarios to 2060; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Kawale, S.V.; Mete, S.S.; Kundu, D. Remediation of plastic waste generated during the COVID-19 pandemic: A conceptual process design of pyrolysis using polyethylene, polypropylene, polystyrene based polymers. J. Environ. Chem. Eng. 2024, 12, 114042. [Google Scholar] [CrossRef]
- OECD (2023)—Processed by Our World in Data. Plastic Polution. 2023. Available online: https://ourworldindata.org/plastic-pollution (accessed on 31 October 2024).
- Xiu, F.R.; Zhan, L.S.; Qi, Y.Y.; Wu, T.; Ju, Y. Upcycling of waste disposable medical masks to high value-added gasoline fuel and surfactants products by sub/supercritical water degradation and partial oxidation. J. Hazard. Mater. 2024, 476, 134950. [Google Scholar] [CrossRef]
- Arjharnwong, O.; Vitidsant, T.; Permpoonwiwat, A.; Phowan, N.; Charusiri, W. Optimization of Kerosene-like Fuels Produced via Catalytic Pyrolysis of Packaging Plastic Waste via Central Composite Design and Response Surface Methodology: Performance of Iron-Doped Dolomite and Activated Carbon. Molecules 2025, 30, 2884. [Google Scholar] [CrossRef]
- Yang, Y.; Jalalah, M.; Alsareii, S.A.; Harraz, F.A.; Thakur, N.; Zheng, Y.; Koutb, M.; Yoon, Y.; Salama, E.S. Plastic wastes (PWs) and microplastics (MPs) formation: Management, migration, and environmental impact. J. Environ. Chem. Eng. 2024, 12, 112926. [Google Scholar] [CrossRef]
- de Souza, A.S.; Ferreira, P.G.; de Jesus, I.S.; de Oliveira, R.P.; de Carvalho, A.S.; Futuro, D.O.; Ferreira, V.F. Recent Progress in Polyolefin Plastic: Polyethylene and Polypropylene Transformation and Depolymerization Techniques. Molecules 2025, 30, 87. [Google Scholar] [CrossRef]
- Ajay, S.; Prathish, K. Dioxins emissions from bio-medical waste incineration: A systematic review on emission factors, inventories, trends and health risk studies. J. Hazard. Mater. 2024, 465, 133384. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, T.; Dai, J.; Zhang, Y.; Guang, M.; Li, B. Pyrolysis of plastic species: A review of resources and products. J. Anal. Appl. Pyrolysis 2021, 159, 105295. [Google Scholar] [CrossRef]
- Jung, S.; Lee, S.; Dou, X.M.; Kwon, E.E. Valorization of disposable COVID-19 mask through the thermo-chemical process. Chem. Eng. J. 2021, 405, 126658. [Google Scholar] [CrossRef] [PubMed]
- Vlassa, M.; Filip, M.; Beldean-Galea, S.; Thiébaut, D.; Vial, J.; Petean, I. Investigation of Liquid Oils Obtained by Thermo-Catalytic Degradation of Plastic Wastes in Energy Recovery. Molecules 2025, 30, 1959. [Google Scholar] [CrossRef]
- Miskolczi, N.; Gao, N.; Quan, C. Transformation of biomass and waste plastic mixtures into hydrocarbon oils and gases by pyrolysis using different reactor temperatures and pressures. J. Anal. Appl. Pyrolysis 2024, 180, 106520. [Google Scholar] [CrossRef]
- Arena, U.; Ardolino, F. Technical and environmental performances of alternative treatments for challenging plastics waste. Resour. Conserv. Recycl. 2022, 183, 106379. [Google Scholar] [CrossRef]
- Demirbas, A. Biorefineries: Current activities and future developments. Energy Convers. Manag. 2009, 50, 2782–2801. [Google Scholar] [CrossRef]
- Li, Y.; Kang, S.; Han, W.; Yin, F. Synergistic Effects Between Mixed Plastics and Their Impact on Pyrolysis Behavior and Pyrolysis Products. Molecules 2024, 29, 6059. [Google Scholar] [CrossRef]
- Khaskhachikh, V.V.; Gerasimov, G.Y.; Kornil’eva, V.F. Thermal Decomposition of Medical Wastes in a Fixed-Bed Pyrolysis Reactor. High Temp. 2022, 60, 104–112. [Google Scholar] [CrossRef]
- Gerasimov, G.; Khaskhachikh, V.; Kornilieva, V.; Tarasov, G. Study of pyrolysis of components and mixture of medical waste. Chem. Eng. Trans. 2019, 76, 1423–1428. [Google Scholar]
- Kusenberg, M.; Eschenbacher, A.; Djokic, M.R.; Zayoud, A.; Ragaert, K.; De Meester, S.; Van Geem, K.M. Opportunities and challenges for the application of post-consumer plastic waste pyrolysis oils as steam cracker feedstocks: To decontaminate or not to decontaminate? Waste Manag. 2022, 138, 83–115. [Google Scholar] [CrossRef] [PubMed]
- Folić, M. Feedstocks for Steam Crackers: Key Considerations. Topsoe, 30 September 2022. Available online: https://www.topsoe.com/blog/feedstocks-for-steam-crackers (accessed on 28 January 2025).
- Senthil, R.; Thamizhvel, R.; Sudagar, S. A comparative analysis of pyrolytic oil from medical waste and its suitability as fuel in a Compression Ignition engine. Energy Sources Part A Recovery Util. Environ. Eff. 2024, 46, 2040–2058. [Google Scholar]
- Mishra, R.K.; Mohanty, K. Co-pyrolysis of waste biomass and waste plastics (polystyrene and waste nitrile gloves) into renewable fuel and value-added chemicals. Carbon Resour. Convers. 2020, 3, 145–155. [Google Scholar]
- Polat, K.; Bursalı, E.A. A promising strategy for the utilization of waste nitrile gloves: Cost-effective adsorbent synthesis. J. Mater. Cycles Waste Manag. 2019, 21, 659–665. [Google Scholar] [CrossRef]
- Prabowo, J.N.; Pratama, I.; Chalid, M. The effect of modified ijuk fibers to crystallinity of polypropylene composite. Conf. Ser. Mater. Sci. Eng. 2017, 223, 012020. [Google Scholar] [CrossRef]
- Nawaz, A.; Kumar, P. Thermal degradation of hazardous 3-layered COVID-19 face mask through pyrolysis: Kinetic, thermodynamic, prediction modelling using ANN and volatile product characterization. J. Taiwan Inst. Chem. Eng. 2022, 139, 104538. [Google Scholar] [CrossRef] [PubMed]
- Kusch, P. Identification of organic additives in nitrile rubber materials by Pyrolysis-GC-MS. LCGC N. Am. 2013, 31, 248. [Google Scholar]
- Lee, S.B.; Lee, J.; Tsang, Y.F.; Tsang, Y.F.; Kim, Y.-M.; Jae, J.; Jung, S.-C.; Park, Y.-K. Production of value-added aromatics from wasted COVID-19 mask via catalytic pyrolysis. Environ. Pollut. 2021, 283, 117060. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.F.; Ge, H.C.; Liu, Y.; Wei, Q.; Gao, T.; Gu, H.; Fu, H.; Li, F.; Lin, H.; Miao, L.; et al. Self-assembly kieselguhr-based ZSM-5 for catalytic pyrolysis of mask waste to produce high value-added aromatics. Fuel 2024, 358, 130127. [Google Scholar] [CrossRef]
- Muensterman, D.J.; Cahuas, L.; Titaley, I.A.; Schmokel, C.; De la Cruz, F.B.; Barlaz, M.A.; Carignan, C.C.; Peaslee, G.F.; Field, J.A. Per- and Polyfluoroalkyl Substances (PFAS) in Facemasks: Potential Source of Human Exposure to PFAS with Implications for Disposal to Landfills. Environ. Sci. Technol. Lett. 2022, 9, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Z.; Shi, L.; Liu, Q. Pyrolysis of COVID-19 disposable masks and catalytic cracking of the volatiles. J. Anal. Appl. Pyrolysis 2022, 163, 105481. [Google Scholar] [CrossRef]
- Luda, M.P.; Dall’Anese, R. On the microstructure of polypropylenes by pyrolysis GC-MS. Polym. Degrad. Stab. 2014, 110, 35–43. [Google Scholar] [CrossRef]
- Idris, A.; Nisamaneenate, J.; Atong, D.; Sricharoenchaikul, V. A comprehensive study of facemasks pyrolysis using Py-GC/MS, kinetic analysis and ANN modeling. Arab. J. Chem. 2024, 17, 105605. [Google Scholar] [CrossRef]
- Choudhary, R.; Mukhija, A.; Sharma, S.; Choudhary, R.; Chand, A.; Dewangan, A.K.; Gaurav, G.K.; Klemeš, J.J. Energy-saving COVID–19 biomedical plastic waste treatment using the thermal—Catalytic pyrolysis. Energy 2022, 264, 126096. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Fu, Z.G.; Wang, W.; Ji, G.; Zhao, M.; Li, A. Kinetics, Product Evolution, and Mechanism for the Pyrolysis of Typical Plastic Waste. ACS Sustain. Chem. Eng. 2021, 10, 91–103. [Google Scholar] [CrossRef]
- Wang, C.X.; Zou, R.G.; Lei, H.W.; Qian, M.; Lin, X.; Mateo, W.; Wang, L.; Zhang, X.; Ruan, R. Biochar-advanced thermocatalytic salvaging of the waste disposable mask with the production of hydrogen and mono-aromatic hydrocarbons. J. Hazard. Mater. 2022, 426, 128080. [Google Scholar] [CrossRef]
- Jeon, W.; Choi, S.-A.; Kim, Y.-D.; Lee, K.-H. Low-temperature dechlorination methods for pyrolysis oil of municipal plastic waste. Fuel 2024, 381, 133286. [Google Scholar] [CrossRef]
- Johns, J. Evaluating Purchased Feedstocks for Hydrocrackers and Hydrotreaters. Becht. 19 June 2023. Available online: https://becht.com/becht-blog/entry/evaluating-purchased-feedstocks-for-hydrocrackers-and-hydrotreaters/ (accessed on 28 January 2025).
- Martínez-Narro, G.; Royston, N.J.; Billsborough, K.L.; Phan, A.N. Kinetic modelling of mixed plastic waste pyrolysis. Chem. Thermodyn. Therm. Anal. 2023, 9, 100105. [Google Scholar] [CrossRef]
- Das, P. Pyrolysis study of a waste plastic mixture through different kinetic models using isothermal and nonisothermal mechanism. RSC Adv. 2024, 14, 25599–25618. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yuan, Y.; Chen, R.; Xu, X.; Zhang, D. Kinetic, thermodynamic and chemical reaction analyses of typical surgical face mask waste pyrolysis. Therm. Sci. Eng. Prog. 2021, 26, 101135. [Google Scholar] [CrossRef]
- Weng, J.; Wang, X.; Cheng, Z.; Zhou, Z.; Liu, H.; Ren, H.; Wang, J.; Pan, J. Studies on co-pyrolysis of microalgae and polymeric waste (plastic/rubber): Thermal behavior, kinetics, and product characteristics. J. Anal. Appl. Pyrolysis 2025, 186, 106924. [Google Scholar] [CrossRef]
- George, S.; Varughese, K.T.; Thomas, S. Thermal and crystallisation behaviour of isotactic polypropylene/nitrile rubber blends. Polymer 2000, 41, 5485–5503. [Google Scholar] [CrossRef]
- Barrie, P.J. The mathematical origins of the kinetic compensation effect: 1. the effect of random experimental errors. Phys. Chem. Chem. Phys. 2014, 14, 318–326. [Google Scholar] [CrossRef]
- Krishna, J.; Korobeinichev, O.P.; Vinu, R. Isothermal fast pyrolysis kinetics of synthetic polymers using analytical Pyroprobe. J. Anal. Appl. Pyrolysis 2019, 139, 48–58. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, X.; Cai, J. Modified Friedman isoconversional kinetic method for effective activation energies of waste tires rubber pyrolysis. React. Kinet. Catal. Lett. 2024, 137, 1987–2001. [Google Scholar] [CrossRef]
- Cai, J.; Xu, D.; Dong, Z.; Yu, X.; Yang, Y.; Banks, S.W.; Bridgwater, A.V. Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: Case study of corn stalk. Renew. Sustain. Energy Rev. 2018, 82, 2705–2715. [Google Scholar] [CrossRef]
- Gebre, S.H.; Sendeku, M.G.; Bahri, M. Recent Trends in the Pyrolysis of Non-Degradable Waste Plastics. ChemistryOpen 2021, 10, 1202–1226. [Google Scholar] [CrossRef]
- Soni, V.K.; Singh, G.; Vijayan, B.K.; Chopra, A.; Kapur, G.S.; Ramakumar, S.S.V. Thermochemical Recycling of Waste Plastics by Pyrolysis: A Review. Energy Fuels 2021, 35, 12763–12808. [Google Scholar] [CrossRef]
- Guastaferro, M.; Vaccari, M.; Rossi, F.; Tognotti, L. How bubbles formation and growth affect plastic pyrolysis process: A phenomenological investigation through dimensionless numbers. Chem. Eng. J. 2025, 519, 165598. [Google Scholar] [CrossRef]
- AlMohamadi, H.; Aljabri, A.; Mahmoud, E.R.; Khan, S.Z.; Aljohani, M.S.; Shamsuddin, R. Catalytic Pyrolysis of Municipal Solid Waste: Effects of Pyrolysis Parameters. Bull. Chem. React. Eng. Catal. 2021, 16, 342–352. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Scaldaferri, C.A. Scaldaferri. Catalytic upgrading of intermediate pyrolysis bio-oil to hydrocarbon-rich liquid biofuel via a novel two-stage solvent-assisted process. Fuel 2023, 352, 129015. [Google Scholar] [CrossRef]
- Nardella, F.; Bellavia, S.; Mattonai, M.; Ribechini, E. Co-pyrolysis of biomass and plastic: Synergistic effects and estimation of elemental composition of pyrolysis oil by analytical pyrolysis–gas chromatography/mass spectrometry. Bioresour. Technol. 2022, 354, 127170. [Google Scholar] [CrossRef] [PubMed]
- Ware, R.L.; Rowland, S.M.; Lu, J.; Rodgers, R.P.; Marshall, A.G. Compositional and Structural Analysis of Silica Gel Fractions from Municipal Waste Pyrolysis Oils. Energy Fuels 2018, 32, 7752–7761. [Google Scholar] [CrossRef]
- Sangpatch, T.; Kanokkantapong, V.; Puasuwon, P.; Prasertsak, T.; Tuntiwiwattanapun, N. Development of a catalyst Co-pyrolysis process for producing pyrolysis oil and wax from cooking oil contaminated polypropylene plastic. Sci. Rep. 2025, 15, 27440. [Google Scholar] [CrossRef] [PubMed]
- Sanna, A.; Kalateh, R.; Sun, D. Oil, Method and Apparatus. UK Patent WO 2019/043411 Al, 7 March 2019. [Google Scholar]
- Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Al-Fatesh, A.S.; Harrison, J.; Rooney, D.W. Pyrolysis kinetic modelling of abundant plastic waste (PET) and in-situ emission monitoring. Environ. Sci. Eur. 2020, 32, 112. [Google Scholar] [CrossRef]
- Mortezaeikia, V.; Tavakoli, O.; Khodaparasti, M.S. A review on kinetic study approach for pyrolysis of plastic wastes using thermogravimetric analysis. J. Anal. Appl. Pyrolysis 2021, 160, 105340. [Google Scholar] [CrossRef]
- Doyle, C.D. Series Approximations to the Equation of Thermogravimetric Data. Nature 1965, 207, 290–291. [Google Scholar] [CrossRef]
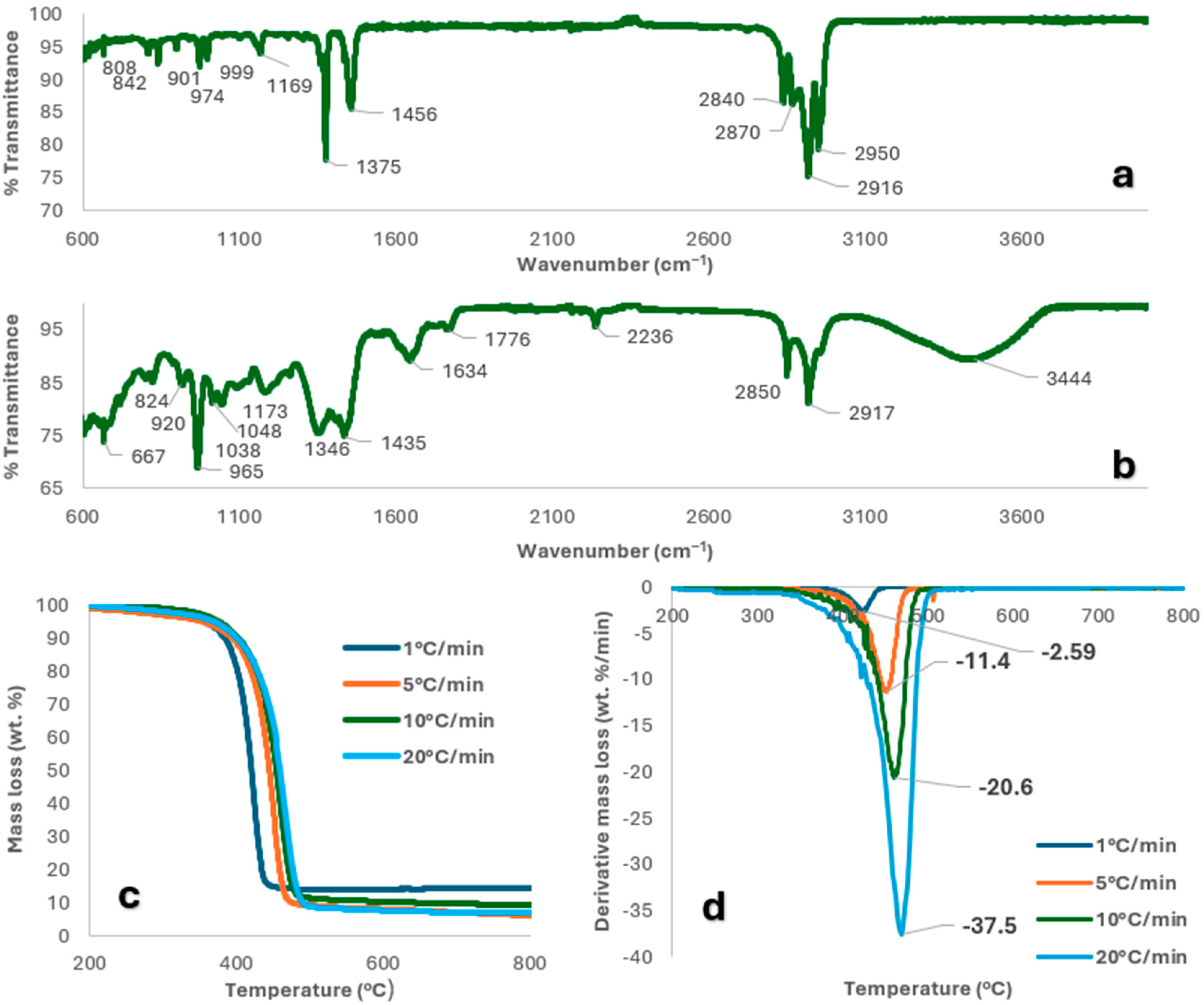
| Proximate Analysis (wt.%) | Mask Offcuts | Nitrile Gloves | Mixture |
| Moisture (MC) | 0.72 | 0.93 | 0.05 |
| Volatile Matter (VM) | 97.52 | 84.74 | 88.98 |
| Fixed Carbon (FC) | 1.35 | 3.72 | 2.03 |
| Solid Residue (SC) | 0.41 | 10.62 | 8.95 |
| Total | 100.00 | 100.00 | 100.00 |
| Ultimate Analysis (wt.%) | Mask Offcuts | Nitrile Gloves | Mixture |
| C | 85.45 | 73.20 | 79.33 |
| H | 14.45 | 8.58 | 11.52 |
| O | 0.00 | 11.61 | 5.81 |
| N | 0.10 | 6.62 | 3.36 |
| Total | 100.00 | 100.00 | 100.00 |
| Higher heating value, daf (MJ kg−1) | 46.86 | 34.36 | 40.61 |
| Property (Units) | Limit | PPE Mixture | Reference |
|---|---|---|---|
| Total Oxygen (ppm) | >100 | 29,200 (est. from GC-MS) | [20] |
| S (wt.%) | >1.5 | <0.10 (est. from GC-MS) | [38] |
| 5% pt (°C) | >510 | 200.18 | [38] |
| 95% pt (°C) | >720 | 467.90 | [38] |
| FBP (°C) | >700 | 517.56 | [38] |
| N total (wt ppm) | >100 | 38,700 | [20] |
| Ca (ppm) | >0.5 | 2.00 | [20] |
| Na (ppm) | >1 | 3.41 | [20] |
| Ni (ppm) | >100 | 0.22 | [20] |
| Fe (ppm) | >2 | 2.71 | [38] |
| Cl (ppm) | >3 | 800 (est. from GC-MS) | [20] |
| Br (ppm) | >20 | 0.28 | [38] |
| F (ppm) | >2 | 15,600 (est. from GC-MS) | [20] |
| Dioxines (ppb) | Detectable | Not detectable | |
| Furanes (ppb) | Detectable | Not detectable |
| Conversion | KAS | FWO | FR | Starink | ||||
|---|---|---|---|---|---|---|---|---|
| α | Ea (kJ/mol) | lnA | Ea (kJ/mol) | lnA | Ea (kJ/mol) | lnA | Ea (kJ/mol) | lnA |
| 0.1 | 530.80 | 90.11 | 515.08 | 88.34 | 559.04 | 87.96 | 530.81 | 82.94 |
| 0.15 | 428.12 | 70.50 | 417.69 | 69.20 | 449.93 | 67.33 | 428.23 | 63.56 |
| 0.20 | 383.59 | 62.20 | 375.49 | 61.14 | 403.05 | 58.69 | 383.74 | 55.38 |
| 0.25 | 350.44 | 56.21 | 344.08 | 55.34 | 368.23 | 52.49 | 350.62 | 49.48 |
| 0.30 | 330.88 | 52.73 | 325.57 | 52.00 | 347.65 | 48.88 | 331.08 | 46.08 |
| 0.35 | 317.23 | 50.36 | 312.66 | 49.72 | 333.27 | 46.42 | 317.45 | 43.76 |
| 0.40 | 307.54 | 48.71 | 303.50 | 48.14 | 323.06 | 44.72 | 307.77 | 42.15 |
| 0.45 | 300.70 | 47.57 | 297.04 | 47.05 | 315.85 | 43.54 | 300.93 | 41.05 |
| 0.50 | 295.12 | 46.66 | 291.78 | 46.18 | 309.97 | 42.61 | 295.36 | 40.17 |
| 0.55 | 290.46 | 45.90 | 287.39 | 45.47 | 305.08 | 41.84 | 290.70 | 39.46 |
| 0.60 | 287.42 | 45.43 | 284.54 | 45.02 | 301.85 | 41.36 | 287.67 | 39.01 |
| 0.65 | 284.60 | 44.98 | 281.90 | 44.60 | 298.89 | 40.92 | 284.85 | 38.60 |
| 0.70 | 282.75 | 44.69 | 280.17 | 44.32 | 296.96 | 40.64 | 283.01 | 38.34 |
| 0.75 | 282.95 | 44.72 | 280.40 | 44.35 | 297.14 | 40.69 | 283.20 | 38.40 |
| 0.80 | 287.52 | 45.44 | 284.81 | 45.05 | 301.91 | 41.46 | 287.78 | 39.15 |
| 0.85 | 352.04 | 55.90 | 346.27 | 55.14 | 369.54 | 52.27 | 352.25 | 49.46 |
| Average (0.2–0.85) | 310.95 | 49.39 | 306.83 | 48.82 | 326.60 | 45.47 | 311.17 | 42.89 |
| Average (0.1–0.85) | 332.01 | 53.26 | 326.77 | 52.57 | 348.84 | 49.49 | 332.22 | 46.69 |
| Model | 1 °C/min | 5 °C/min | 10 °C/min | 20 °C/min | Average | R2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ea (kJ/mol) | lnA | Ea (kJ/mol) | lnA | Ea (kJ/mol) | lnA | Ea (kJ/mol) | lnA | Ea (kJ/mol) | lnA | ||
| F1 | 210.50 | 33.2 | 209.45 | 33.4 | 194.49 | 31.0 | 173.80 | 28.0 | 197.06 | 31.40 | 0.9968 |
| F2 | 310.75 | 51.4 | 303.31 | 50.0 | 282.88 | 46.5 | 252.17 | 41.7 | 287.28 | 47.40 | 0.9987 |
| F3 | 435.18 | 73.9 | 419.02 | 70.2 | 391.94 | 65.4 | 348.72 | 58.4 | 398.72 | 66.99 | 0.9960 |
| D1 | 287.25 | 45.7 | 292.46 | 46.5 | 271.07 | 42.9 | 244.06 | 38.8 | 273.71 | 43.47 | 0.9993 |
| D2 | 327.33 | 52.3 | 331.05 | 52.7 | 307.28 | 48.6 | 276.36 | 43.7 | 310.51 | 49.32 | 0.9982 |
| D3 | 378.05 | 60.0 | 379.23 | 59.6 | 352.56 | 55.0 | 316.64 | 49.3 | 356.62 | 55.96 | 0.9963 |
| D4 | 344.04 | 53.8 | 346.94 | 54.0 | 322.21 | 49.7 | 289.65 | 44.5 | 325.71 | 50.50 | 0.9976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baird, R.; Ocone, R.; Sanna, A. Pyrolysis of Polypropylene and Nitrile PPE Waste: Insights into Oil Composition, Kinetics, and Steam Cracker Integration. Molecules 2025, 30, 3351. https://doi.org/10.3390/molecules30163351
Baird R, Ocone R, Sanna A. Pyrolysis of Polypropylene and Nitrile PPE Waste: Insights into Oil Composition, Kinetics, and Steam Cracker Integration. Molecules. 2025; 30(16):3351. https://doi.org/10.3390/molecules30163351
Chicago/Turabian StyleBaird, Ross, Raffaella Ocone, and Aimaro Sanna. 2025. "Pyrolysis of Polypropylene and Nitrile PPE Waste: Insights into Oil Composition, Kinetics, and Steam Cracker Integration" Molecules 30, no. 16: 3351. https://doi.org/10.3390/molecules30163351
APA StyleBaird, R., Ocone, R., & Sanna, A. (2025). Pyrolysis of Polypropylene and Nitrile PPE Waste: Insights into Oil Composition, Kinetics, and Steam Cracker Integration. Molecules, 30(16), 3351. https://doi.org/10.3390/molecules30163351







