Evaluation of the Anticancer Properties of Lamellar Alkaloid Drivatives Extracted from the Tunicate Didemnum abradatum (Moucha Island Sea, Djibouti): Pharmacological and Computational Approach
Abstract
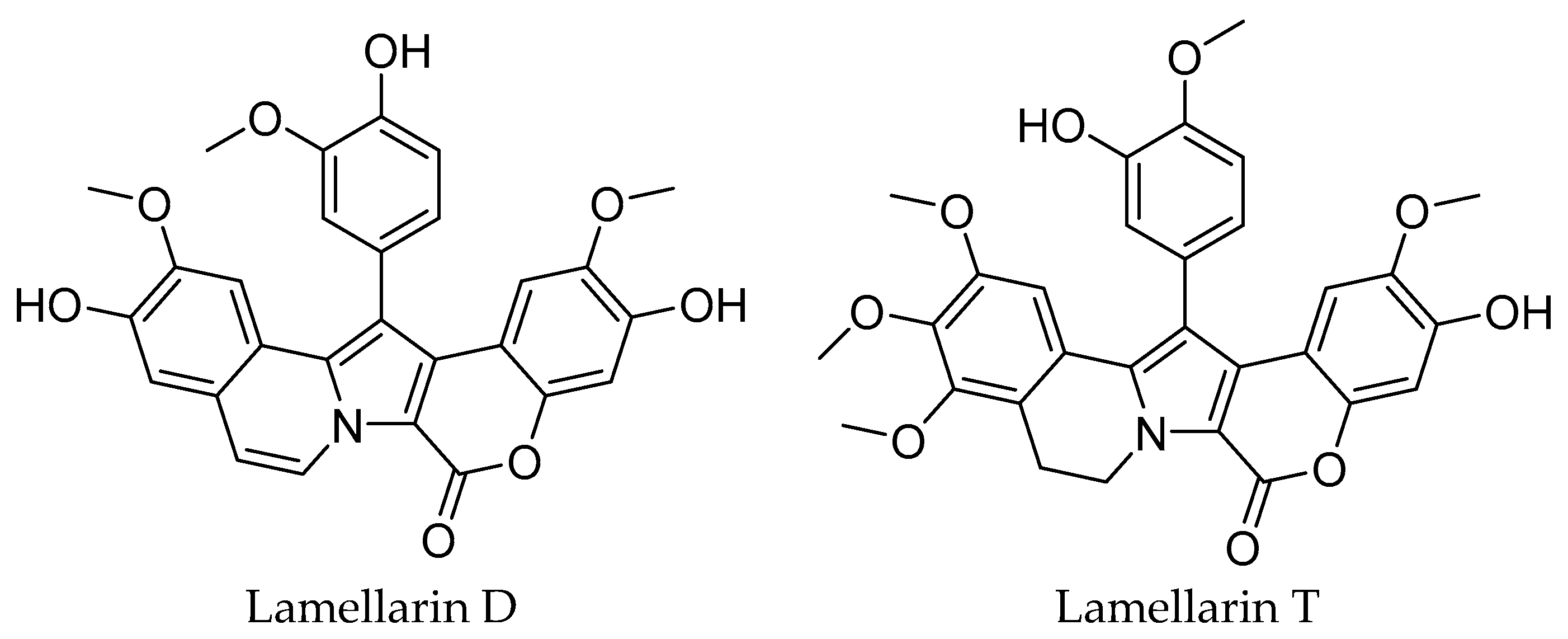
1. Introduction
2. Results
2.1. Extraction
2.2. Cell Viability Assessment
2.3. Integrated Biomolecular Profiling and Mechanism Evaluation (IBProME)
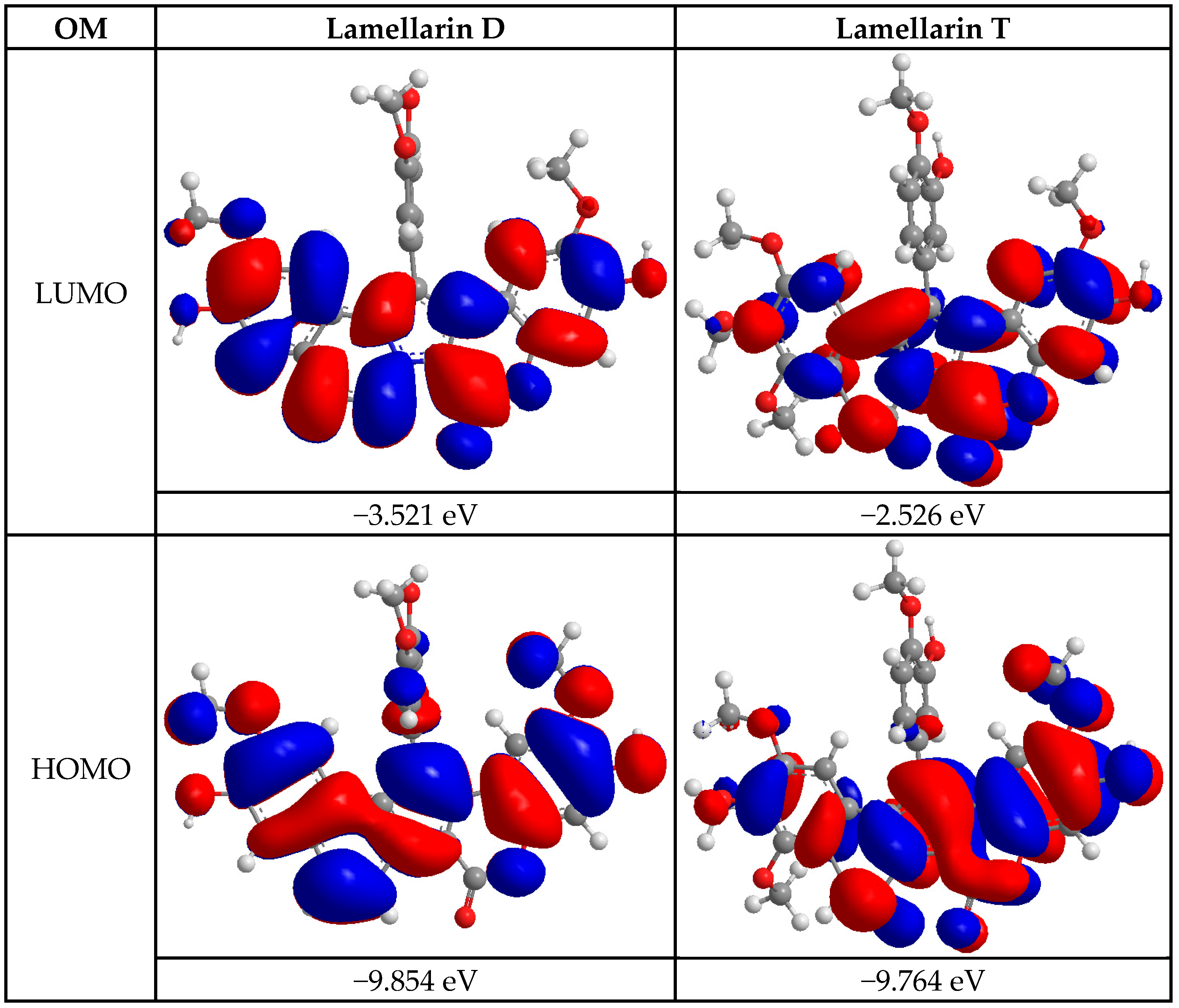
2.3.1. Computational Studies
2.3.2. Toxicity Study and Prediction
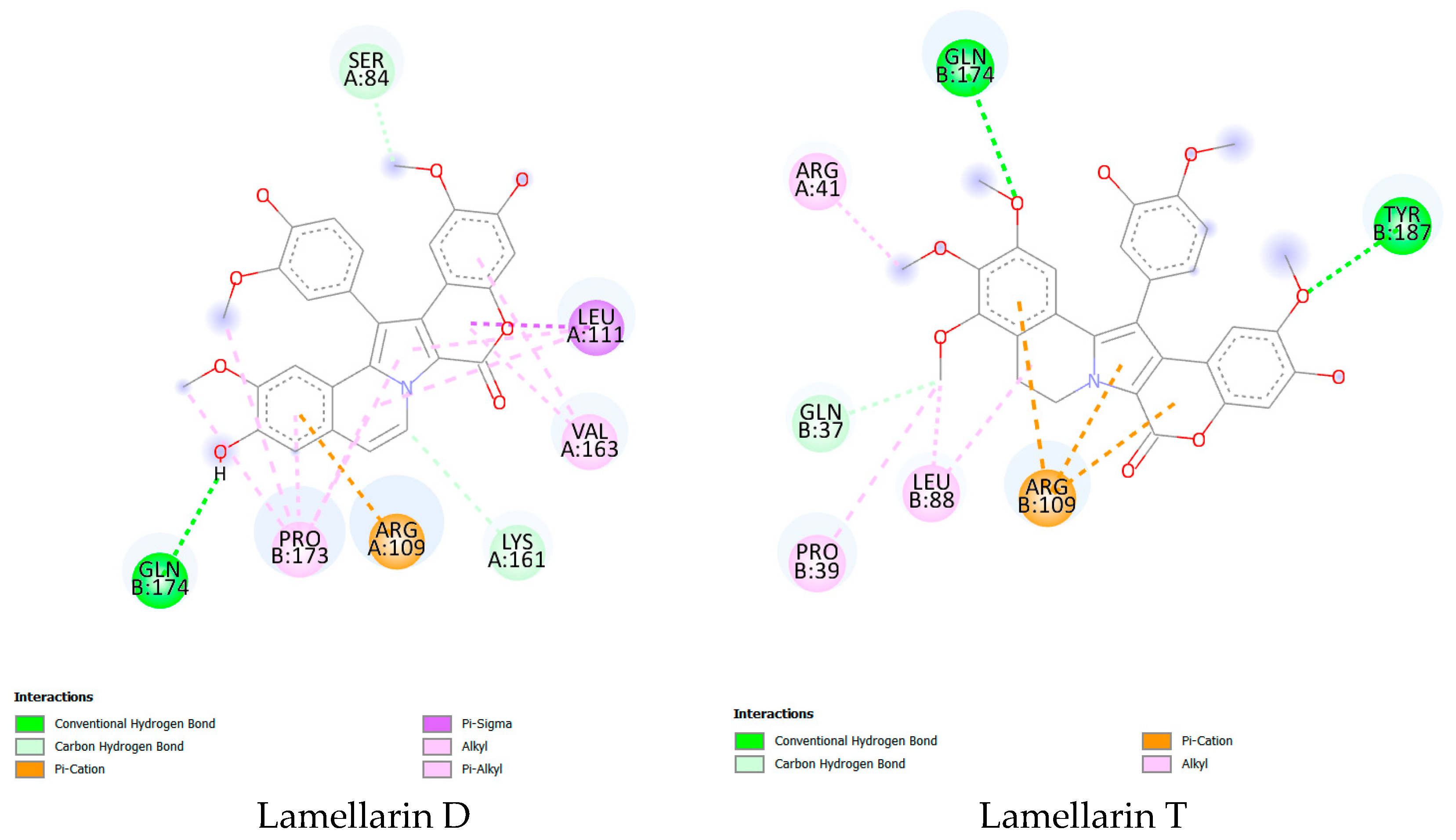
2.3.3. Molecular Docking
2.3.4. Pharmacokinetic Properties
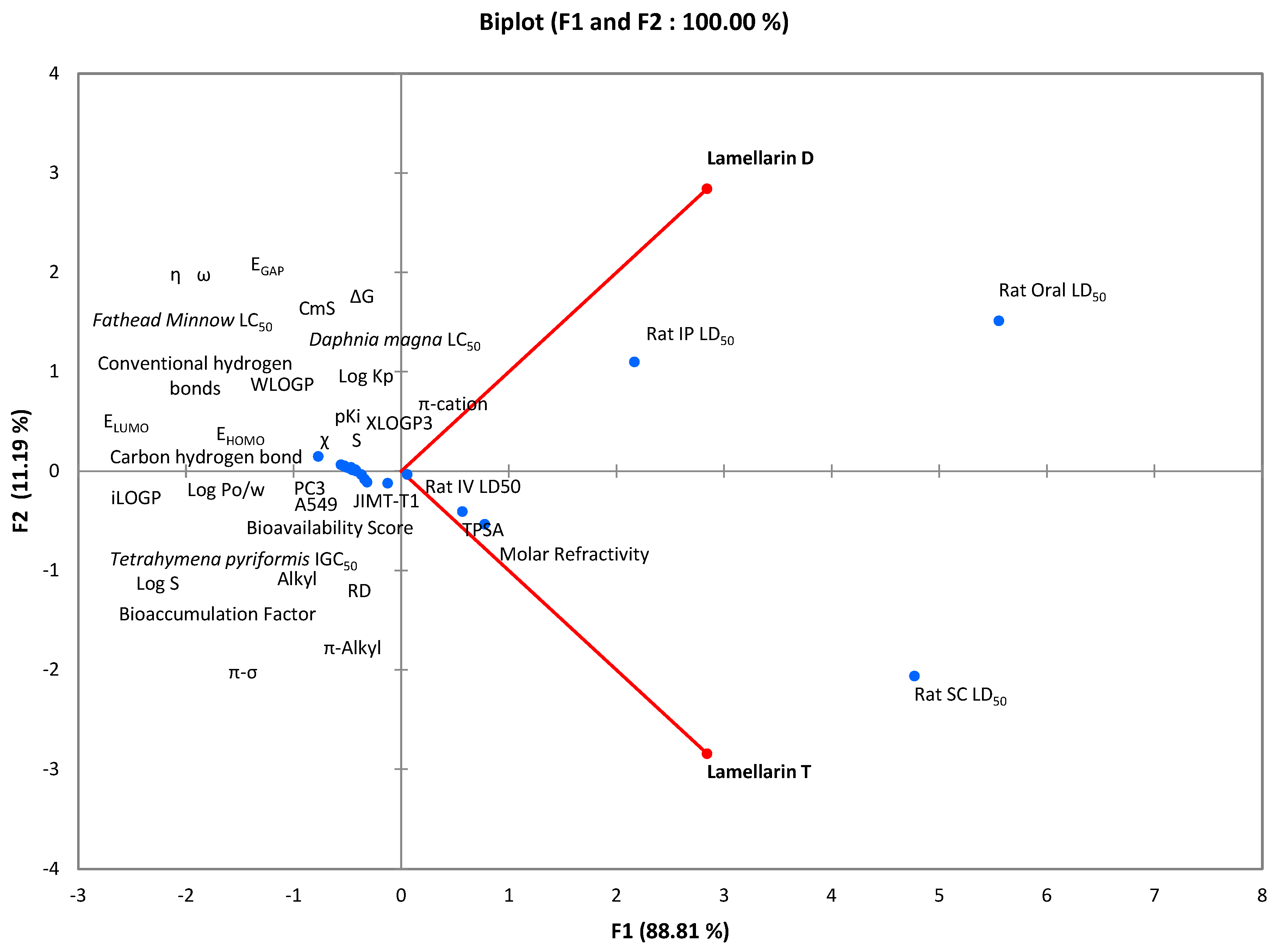
2.4. Multiple Correlation Study
3. Discussion
4. Material and Methods
4.1. Analytical Apparatus Technique
4.2. Biomass
4.3. Extraction and Purification
4.4. Cell Viability Assay
4.5. Integrated Biomolecular Profiling and Mechanism Evaluation (IBProME)
4.5.1. Quantum Profiling of Molecules
4.5.2. Toxicity Prediction
4.5.3. Molecular Docking Modeling
4.5.4. Pharmacokinetic Properties
4.6. Statistical Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, E.Y.; Danpanichkul, P.; Yong, J.N.; Yu, Z.; Tan, D.J.; Lim, W.H.; Koh, B.; Lim, R.Y.; Tham, E.K.; Mitra, K.; et al. Liver cancer in 2021: Global burden of disease study. J. Hepatol. 2025, 82, 851–860. [Google Scholar] [CrossRef]
- Saklani, S. Recent Patterns of Cancer Incidence and Mortality: Global and Indian Scenario. In Nanoparticles Cancer Theranostics; CRC Press: Boca Raton, FL, USA, 2024; pp. 40–52. [Google Scholar]
- Gona, P.; Gona, C.; Ballout, S.; Mapoma, C.; Rao, S.; Mokdad, A.; (GBD 2019 SADC BMI). Trends in the burden of most common obesity-related cancers in 16 Southern Africa development community countries, 1990–2019. Findings from the global burden of disease study. Obes. Sci. Pract. 2024, 10, e715. [Google Scholar] [CrossRef]
- Xu, P.; Chen, J.; Li, D.; Shen, L.; Zhang, Y.; Peng, R.; He, Y. Cirsiliol suppresses the proliferation of human oral cancer cells by targeting topoisomerase I. 3 Biotech 2025, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, S.; Singh, A.K.; Varshney, M.; Roy, S. Exploring Marine Alkaloids: A Natural Approach to Cancer Treatment. Curr. Pharm. Biotechnol. 2025, 26, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Chen, J.; Song, Y.; Monserrat, J.P.; Zhang, Y.; Shen, L. Progress on synthesis and structure-activity relationships of lamellarins over the past decade. Eur. J. Med. Chem. 2024, 269, 116294. [Google Scholar] [CrossRef]
- Morii, K.; Yasuda, Y.; Morikawa, D.; Mori, A.; Okano, K. Total Synthesis of Lamellarins G, J, L, and Z Using One-Pot Halogen Dance/Negishi Coupling. J. Org. Chem. 2021, 86, 13388–13401. [Google Scholar] [CrossRef]
- Maza, L.J.G.; Salgado, A.M.; Kouznetsov, V.V.; Meléndez, C.M. Pyrrolo [2, 1-a] isoquinoline scaffolds for developing anti-cancer agents. RSC Adv. 2024, 14, 1710–1728. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.B.; de Azevedo Teoténio Cavalcanti, M.; de Medeiros e Silva, Y.M.S.; dos Santos Nascimento, I.J.; de Moura, R.O. Overview of the new bioactive heterocycles as targeting topoisomerase inhibitors useful against colon cancer. Anti-Cancer Agents Med. Chem.-Anti-Cancer Agents 2024, 24, 236–262. [Google Scholar] [CrossRef]
- Mitsiou, V.P.M.; Antonaki, A.M.N.; Douka, M.D.; Litinas, K.E. An overview on the synthesis of lamellarins and related compounds with biological interest. Molecules 2024, 29, 4032. [Google Scholar] [CrossRef]
- Imbri, D.; Tauber, J.; Opatz, T. Synthetic approaches to the lamellarins—A comprehensive review. Mar. Drugs 2014, 12, 6142–6177. [Google Scholar] [CrossRef]
- Segura-Quezada, L.A.; Hernández-Velázquez, E.D.; Corrales-Escobosa, A.R.; de León-Solis, C.; Solorio-Alvarado, C.R. Ningalins, Pyrrole-Bearing Metabolites Isolated from Didemnum spp. Synthesis and MDR-Reversion Activity in Cancer Therapy. Chem. Biodivers. 2024, 21, e202300883. [Google Scholar] [CrossRef]
- Okuno, D.; Sakamoto, N.; Hayashi, H.; Fukuda, T.; Akiyama, Y.; Iketani, C.; Murakami, R.; Tokito, T.; Miyamura, T.; Yura, H.; et al. Lamellarin D Acts as an Inhibitor of Type I Collagen Production. ChemMedChem 2025, 20, e202401001. [Google Scholar] [CrossRef] [PubMed]
- Bisht, A.; Avinash, D.; Sahu, K.K.; Patel, P.; Das Gupta, G.; Kurmi, B.D. A comprehensive review on doxorubicin: Mechanisms, toxicity, clinical trials, combination therapies and nanoformulations in breast cancer. Drug Deliv. Transl. Res. 2025, 15, 102–133. [Google Scholar] [CrossRef]
- Rosales-López, A.; López-Castillo, G.N.; Sandoval-Ramírez, J.; Terán, J.L.; Carrasco-Carballo, A. Correlation between Molecular Docking and the Stabilizing Interaction of HOMO-LUMO: Spirostans in CHK1 and CHK2, an In Silico Cancer Approach. Int. J. Mol. Sci. 2024, 25, 8588. [Google Scholar] [CrossRef] [PubMed]
- Paneru, T.R.; Chaudhary, M.K.; Tandon, P.; Chaudhary, T.; Joshi, B.D. Theoretical study on molecular stability, reactivity, and drug potential of cirsilineol from DFT and molecular docking methods. Chem. Phys. Impact 2024, 8, 100641. [Google Scholar] [CrossRef]
- Chagaleti, B.K.; Kumar, B.S.; Rajagopal, R.; Alfarhan, A.; Arockiaraj, J.; Kumaradoss, K.M.; Namasivayam, S.K.R. Targeting cyclin-dependent kinase 2 CDK2: Insights from Molecular Docking and Dynamics Simulation-A systematic computational approach to discover novel cancer therapeutics. Comput. Biol. Chem. 2024, 112, 108134. [Google Scholar] [CrossRef]
- Rezvan, V.H. Molecular structure, HOMO–LUMO, and NLO studies of some quinoxaline 1, 4-dioxide derivatives: Computational (HF and DFT) analysis. Results Chem. 2024, 7, 101437. [Google Scholar] [CrossRef]
- Güleryüz, C.; Hassan, A.U.; Güleryüz, H.; Kyhoiesh, H.A.; Mahmoud, M.H. A machine learning assisted designing and chemical space generation of benzophenone based organic semiconductors with low lying LUMO energies. Mater. Sci. Eng. B 2025, 317, 118212. [Google Scholar] [CrossRef]
- Bhatia, M. An overview of conceptual-DFT based insights into global chemical reactivity of volatile sulfur compounds (VSCs). Comput. Toxicol. 2024, 29, 100295. [Google Scholar] [CrossRef]
- Sharma, P.; Ranjan, P.; Chakraborty, T. Applications of conceptual density functional theory in reference to quantitative structure–activity/property relationship. Mol. Phys. 2024, 122, e2331620. [Google Scholar] [CrossRef]
- Rakini Chanderasekaran, J.H.; Devi, D.; Meenakshi, R. Molecular structure, spectroscopic investigation, frontier molecular orbital and global reactivity descriptors analysis of 2-(2-Nitrovinyl) thiophene for anti-corrosion and DSSC applications. Chem. Pap. 2024, 78, 3273–3296. [Google Scholar] [CrossRef]
- Mohamed Abdoul-Latif, F.; Ainane, A.; Merito, A.; Houmed Aboubaker, I.; Mohamed, H.; Cherroud, S.; Ainane, T. The effects of khat chewing among Djiboutians: Dental chemical studies, gingival histopathological analyses and bioinformatics approaches. Bioengineering 2024, 11, 716. [Google Scholar] [CrossRef] [PubMed]
- Dzedulionytė, K.; Fuxreiter, N.; Schreiber-Brynzak, E.; Žukauskaitė, A.; Pichler, V.; Arbačiauskienė, E. From natural products to synthetic analogues: Pyrrole-pyrazole exchange in lamellarin O. In Chemistry and Chemical Technology: International Conference CCT-2023, March 10, 2023; Conference Book; Vilnius University Press: Vilnius, Lithuania, 2023. [Google Scholar]
- Stahl, A.; Heider, J.; Wüst, R.; Fallgatter, A.J.; Schenke-Layland, K.; Volkmer, H.; Templin, M.F. Patient iPSC-derived neural progenitor cells display aberrant cell cycle control, p53, and DNA damage response protein expression in schizophrenia. BMC Psychiatry 2024, 24, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.Q.J.; Adler, L.; Hajaj, E.; Soria, L.R.; Perry, R.B.T.; Darzi, N.; Brody, R.; Furth, N.; Lichtenstein, M.; Bab-Dinitz, E.; et al. ASS1 metabolically contributes to the nuclear and cytosolic p53-mediated DNA damage response. Nat. Metab. 2024, 6, 1294–1309. [Google Scholar] [CrossRef] [PubMed]
- Abdoul-Latif, F.M.; Ainane, A.; Mohamed, H.; Ali, A.M.; Cacciatore, S.; Ainane, T. Advanced Formulation of Ecological Bioinsecticides Based on Citrus limonum in Clayey Matrices: Optimization of Diffusive Dynamics. Sustainability 2025, 17, 785. [Google Scholar] [CrossRef]
- Abdoul-Latif, F.M.; El Mhamdi, M.I.; Ainane, A.; Ali, A.M.; Oumaskour, K.; Cherroud, S.; Cacciatore, S.; Ainane, T. Development and Perfection of Marine-Based Insecticide Biofilm for Pea Seed Protection: Experimental and Computational Approaches. Molecules 2025, 30, 1621. [Google Scholar] [CrossRef]
- Chaudhary, V.; Chaturvedi, S.; Wadhwa, A.; Verma, P.; Gautam, D.; Sharma, D.; Garg, A.; Singh, V.; Kumar, R.; Mishra, A.K. Homology modeling, molecular docking and MD simulations study of 6, 7-dimethoxy-1, 2, 3, 4-tetrahydroisoquinoline derivatives as sigma-2 receptor ligands. J. Biomol. Struct. Dyn. 2025, 7, 1–15. [Google Scholar] [CrossRef]
- Abdoul-Latif, F.M.; Mohamed, H.; Houmed Aboubaker, I.; Saoudi, O.; Ainane, A.; Ali, A.M.; Cacciatore, S.; Zerbini, L.F.; Abourriche, A.; Ainane, T. Cynthichlorine Extracted from Ascidian Cynthia savignyi from Djibouti: Optimization of Extraction, In Vitro Anticancer Profiling, and In Silico Approach. Mar. Drugs 2025, 23, 172. [Google Scholar] [CrossRef]
- Nevskaya, A.A.; Purgatorio, R.; Borisova, T.N.; Varlamov, A.V.; Anikina, L.V.; Obydennik, A.Y.; Nevskaya, E.Y.; Niso, M.; Colabufo, N.A.; Carrieri, A.; et al. Nature-inspired 1-phenylpyrrolo [2, 1-a] isoquinoline scaffold for novel antiproliferative agents circumventing P-glycoprotein-dependent multidrug resistance. Pharmaceuticals 2024, 17, 539. [Google Scholar] [CrossRef]
- Aly, O.; Mekky, R.H.; Pereira, F.; Diab, Y.M.; Tammam, M.A.; El-Demerdash, A. Deciphering the potential of Cymbopogon citratus (DC.) Stapf as an anti-obesity agent: Phytochemical profiling, in vivo evaluations and molecular docking studies. Food Funct. 2024, 15, 12146–12168. [Google Scholar] [CrossRef]
- Klimoszek, D.; Jeleń, M.; Dołowy, M.; Morak-Młodawska, B. Study of the lipophilicity and ADMET parameters of new anticancer diquinothiazines with pharmacophore substituents. Pharmaceuticals 2024, 17, 725. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, K.; Chandran, K.; Zochedh, A.; Ansar, S.; Sultan, A.B.; Kumar, Y.A.; Kathiresan, T. Pharmacoinformatics and quantum chemicals-based analysis of aromatic molecule decanal as a potent drug against breast cancer. Int. J. Quantum Chem. 2024, 124, e27451. [Google Scholar] [CrossRef]
- Reddy, S.M.; Srinivasulu, M.; Satyanarayana, N.; Kondapi, A.K.; Venkateswarlu, Y. New potent cytotoxic lamellarin alkaloids from Indian ascidian Didemnum obscurum. Tetrahedron 2005, 61, 9242–9247. [Google Scholar] [CrossRef]
- Islam, F.; Dehbia, Z.; Zehravi, M.; Das, R.; Sivakumar, M.; Krishnan, K.; Billah, A.A.; Bose, B.; Ghosh, A.; Paul, S.; et al. Indole alkaloids from marine resources: Understandings from therapeutic point of view to treat cancers. Chem.-Biol. Interact. 2023, 383, 110682. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Lamellarins, from A to Z: A family of anticancer marine pyrrole alkaloids. Curr. Med. Chem.-Anti-Cancer Agents 2004, 4, 363–378. [Google Scholar] [CrossRef]
- Marco, E.; Laine, W.; Tardy, C.; Lansiaux, A.; Iwao, M.; Ishibashi, F.; Bailly, C.; Gago, F. Molecular determinants of topoisomerase I poisoning by Lamellarins: Comparison with Camptothecin and structure− activity relationships. J. Med. Chem. 2005, 48, 3796–3807. [Google Scholar] [CrossRef]
- Pla, D.; Marchal, A.; Olsen, C.A.; Francesch, A.; Cuevas, C.; Albericio, F.; Álvarez, M. Synthesis and structure− activity relationship study of potent cytotoxic analogues of the marine alkaloid lamellarin D. J. Med. Chem. 2006, 49, 3257–3268. [Google Scholar] [CrossRef]
- Rajesh, R.P.; Annappan, M. Anticancer effects of brominated indole alkaloid Eudistomin H from marine ascidian Eudistoma viride against cervical cancer cells (HeLa). Anticancer Res. 2015, 35, 283–293. [Google Scholar]
- Huang, X.C.; Xiao, X.; Zhang, Y.K.; Talele, T.T.; Salim, A.A.; Chen, Z.S.; Capon, R.J. Lamellarin O, a pyrrole alkaloid from an Australian marine sponge, Ianthella sp., reverses BCRP mediated drug resistance in cancer cells. Mar. Drugs 2014, 12, 3818–3837. [Google Scholar] [CrossRef]
- Baunbæk, D.; Trinkler, N.; Ferandin, Y.; Lozach, O.; Ploypradith, P.; Rucirawat, S.; Ishibashi, F.; Iwao, M.; Meijer, L. Anticancer alkaloid lamellarins inhibit protein kinases. Mar. Drugs 2008, 6, 514–527. [Google Scholar] [CrossRef]
- Parveen, B.; Narayanan, M. Enhancing Cancer Treatment with Marine Algae-Derived Bioactive Chemicals: A Review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 1, 1–13. [Google Scholar] [CrossRef]
- Rusanov, D.A.; Mutasim Alfadul, S.; Portnyagina, E.Y.; Silyanova, E.A.; Kuznetsov, N.A.; Podpovetny, K.E.; Samet, A.V.; Semenov, V.V.; Babak, M.V. Toward “E-Ring-Free” Lamellarin Analogues: Synthesis and Preliminary Biological Evaluation. ChemBioChem 2023, 24, e202300161. [Google Scholar] [CrossRef] [PubMed]
- Duc, D.X.; Quoc, N.V. Isolation, Bioactivities, and Synthesis of Lamellarin Alkaloids: A Review. Curr. Org. Chem. 2022, 26, 961–990. [Google Scholar] [CrossRef]
- Zheng, L.; Gao, T.; Ge, Z.; Ma, Z.; Xu, J.; Ding, W.; Shen, L. Design, synthesis and structure-activity relationship studies of glycosylated derivatives of marine natural product lamellarin D. Eur. J. Med. Chem. 2021, 214, 113226. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, N.; Fenical, W.; Van Duyne, G.D.; Clardy, J. New alkaloids of the lamellarin class from the marine ascidian Didemnum chartaceum (Sluiter, 1909). J. Org. Chem. 1988, 53, 4570–4574. [Google Scholar] [CrossRef]
- Ham, J.Y.; Kang, H.J. A novel cytotoxic alkaloid of lamellarin class from a marine ascidian Didemnum sp. Bull. Korean Chem. Soc. 2002, 23, 163–166. [Google Scholar]
- Davis, R.A.; Carroll, A.R.; Pierens, G.K.; Quinn, R.J. New Lamellarin Alkaloids from the Australian Ascidian, Didemnum chartaceum. J. Nat. Prod. 1999, 62, 419–424. [Google Scholar] [CrossRef]
- Mohamed Abdoul-Latif, F.; Ainane, A.; Houmed Aboubaker, I.; Merito Ali, A.; El Montassir, Z.; Kciuk, M.; Mohamed, J.; Ainane, T. Chemical composition of the essential oil of Catha edulis forsk from Djibouti and its toxicological investigations in vivo and in vitro. Processes 2023, 11, 1324. [Google Scholar] [CrossRef]
- Mohamed Abdoul-Latif, F.; Elmi, A.; Merito, A.; Nour, M.; Risler, A.; Ainane, A.; Bignon, J.; Ainane, T. Essential oils of Ocimum basilicum L. and Ocimum americanum L. from Djibouti: Chemical composition, antimicrobial and cytotoxicity evaluations. Processes 2022, 10, 1785. [Google Scholar] [CrossRef]
- Mohamed Abdoul-Latif, F.; Elmi, A.; Merito, A.; Nour, M.; Risler, A.; Ainane, A.; Bignon, J.; Ainane, T. Chemical Analysis of Essential Oils of Cymbopogon schoenanthus (L.) Spreng. and Nepeta azurea R. Br. ex Benth from Djbouti, In-Vitro Cytotoxicity against Cancer Cell Lines and Antibacterial Activities. Appl. Sci. 2022, 12, 8699. [Google Scholar] [CrossRef]
- Ainane, T.; Ainane, A. Integrated Biomolecular Profiling and Mechanism Evaluation (IBProME): A computational method for analyzing the biological properties and mechanisms of action of high-value molecules. J. Anal. Sci. Appl. Biotechnol. 2025, 7, 42–47. [Google Scholar]
- Flores-Holguín, N.; Salas-Leiva, J.S.; Glossman-Mitnik, D. Talarolide A and Talaropeptides A–D: Potential Marine-Derived Therapeutic Peptides with Interesting Chemistry and Biological Activity Studied through Density Functional Theory (DFT) and Conceptual DFT. Molecules 2023, 28, 6708. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.T.; da Silva Mendes, F.R.; Almeida-Neto, F.W.Q.; Marinho, E.S.; da Silva, L.; da Silva Julião, M.S.; Marinho, M.M.; Vidal, L.M.; Ayala, A.P.; Coutinho, H.D.; et al. Structural characterization, DFT calculations, ADMET studies, antibiotic potentiating activity, evaluation of efflux pump inhibition and molecular docking of heterocylcic chalcone (E)-1-(4-aminophenyl)-3-(thiophen-2-yl) prop-2-en-1-one. J. Mol. Struct. 2024, 1312, 138497. [Google Scholar] [CrossRef]
- Wu, D.; Gowathaman, R.; Pierce, B.G.; Mariuzza, R.A. T cell receptors employ diverse strategies to target a p53 cancer neoantigen. J. Biol. Chem. 2022, 298, 101684. [Google Scholar] [CrossRef]
- Hjouji, K.; El Barnossi, A.; Er-Rajy, M.; Atemni, I.; Grenha, A.; Yagoubi, M.; Ainane, T.; Taleb, M.; Rais, Z. Inhibitory potency of active metabolites from different polarities of Datura Stramonium seed extracts: GC-MS analysis, biological evaluations, and molecular docking studies. J. Med. Mycol. 2025, 35, 101521. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Jurowski, K.; Krośniak, A. ADME profile of AP-238-opioid designer drug (CAS: 140924-11-4): First application of multi-in silico approach methodology for comprehensive prediction of ADME profile (absorption, distribution, metabolism and excretion) important for clinical toxicology and forensic purposes. Chem.-Biol. Interact. 2025, 415, 111493. [Google Scholar]
- Klimoszek, D.; Jeleń, M.; Morak-Młodawska, B.; Dołowy, M. Evaluation of the Lipophilicity of Angularly Condensed Diquino-and Quinonaphthothiazines as Potential Candidates for New Drugs. Molecules 2024, 29, 1683. [Google Scholar] [CrossRef]
- Jiang, S.; Li, X.; Xue, W.; Xia, S.; Wang, J.; Sai, Y.; Dai, G.; Su, W. Preclinical pharmacokinetic characterization of amdizalisib, a novel PI3Kδ inhibitor for the treatment of hematological malignancies. Front. Pharmacol. 2024, 15, 1392209. [Google Scholar] [CrossRef]
- Montassir, Z.E.; Abdoul-Latif, F.M.; Ainane, A.; Lachtioui, Y.; Bajjou, O.; Liba, A.; Ainane, T.; Giuffrè, A.M. Phytoremediation of hexavalent chromium by absinthe: Experimental approach and computational modeling of the transfer and production of some secondary metabolites. Plant Physiol. Biochem. 2025, 226, 110034. [Google Scholar] [CrossRef]
- Ainane, A.; Abdoul-Latif, F.M.; Mohamed, J.; Attahar, W.; Ouassil, M.; Shybat, Z.L.; Ainane, T. Behaviour desorption study of the essential oil of Cedrus atlantica in a porous clay versus insecticidal activity against Sitophilus granarius: Explanation of the phenomenon by statistical studies. Int. J. Metrol. Qual. Eng. 2021, 12, 12. [Google Scholar] [CrossRef]
| Cell Line | Lamellarin D | Lamellarin T | Doxorubicine |
|---|---|---|---|
| PC3 | 5.25 ± 0.05 | 20.22 ± 0.45 | 3.10 ± 0.05 |
| A549 | 8.64 ± 0.10 | 14.35 ± 0.35 | 55.22 ± 0.95 |
| JIMT-T1 | 40.78 ± 0.10 | 41.17 ± 0.05 | 89.87 ± 1.55 |
| Energetic Descriptors | Lamellarin D | Lamellarin T |
|---|---|---|
| EHOMO (eV) | −9.854 | −9.764 |
| ELUMO (eV) | −3.521 | −2.526 |
| EGAP (eV) | 6.333 | 7.238 |
| η (eV) | 3.167 | 3.619 |
| χ (eV) | 6.688 | 6.145 |
| S (eV−1) | 0.316 | 0.276 |
| ω (eV) | 7.062 | 5.217 |
| CmS (eV−1) | 0.158 | 0.138 |
| RD (eV) | 8.862 | 13.989 |
| Toxicity Type | Parameter | Lamellarin D | Lamellarin T |
|---|---|---|---|
| Acute Toxicity in Rats | Rat IP LD50 (g/kg) | 752.00 | 129.40 |
| Rat IV LD50 (g/kg) | 95.30 | 48.88 | |
| Rat Oral LD50 (g/kg) | 1521.00 | 374.10 | |
| Rat SC LD50 (g/kg) | 638.40 | 603.70 | |
| Environmental Toxicity | Bioaccumulation Factor (BCF) | 3.59 | 2.29 |
| Daphnia magna LC50 (mol/L) | 5.02 × 106 | 1.38 × 107 | |
| Fathead minnow LC50 (mmol/L) | 8.89 × 10−5 | 4.68 × 10−5 | |
| Tetrahymena pyriformis IGC50 (mol/L) | 4.04 | 24.58 |
| Parameter | Lamellarin D | Lamellarin T |
|---|---|---|
| ΔG (kJ/mol) | −35.564 | −33.990 |
| pKi | 5.820 | 6.000 |
| Conventional hydrogen bonds | 1 | 2 |
| Carbon hydrogen bond | 2 | 1 |
| π-cation | 1 | 3 |
| π-σ | 1 | 0 |
| Alkyl | 5 | 4 |
| π-Alkyl | 4 | 0 |
| ADME Step | Parameter | Lamellarin D | Lamellarin T |
|---|---|---|---|
| Absorption | GI absorption (Gastrointestinal absorption) | Low | Low |
| Log S (Water Solubility) | −6.78 | −6.13 | |
| Distribution | Log Po/w (Lipophilicity) | 4.12 | 4.09 |
| BBB permeant (Blood-brain barrier permeability) | No | No | |
| P-gp substrate (P-glycoprotein substrate) | No | Yes | |
| Metabolism | CYP2C19 inhibitor (CYP2C19 inhibitor) | Yes | Yes |
| CYP2C9 inhibitor (CYP2C9 inhibitor) | Yes | Yes | |
| CYP1A2 inhibitor (CYP1A2 inhibitor) | No | No | |
| CYP2D6 inhibitor (CYP2D6 inhibitor) | No | Yes | |
| CYP3A4 inhibitor (CYP3A4 inhibitor) | No | No | |
| Excretion | Log Kp (Skin permeation) | −5.32 cm/s | −6.43 cm/s |
| Pharmacokinetics | Bioavailability Score | 0.55 | 0.55 |
| Physicochemical Properties | TPSA (Topological Polar Surface Area) | 123.00 Å2 | 121.75 Å2 |
| Molar Refractivity | 139.43 | 149.47 | |
| Lipophilicity Properties | Log Po/w (iLOGP) | 3.73 | 4.19 |
| Log Po/w (XLOGP3) | 5.67 | 4.51 | |
| Log Po/w (WLOGP) | 5.16 | 5.09 |
| Descriptor | Symbol | Formula |
|---|---|---|
| Energy gap | EGAP | |
| Chemical hardness | η | |
| Electronegativity | χ | |
| Softness | S | |
| Pearson Reactivity Index | ω | |
| Chemical Stability | CmS | |
| Reactivity Density | RD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdoul-Latif, F.M.; Aboubaker, I.H.; Mohamed, H.; Ainane, A.; Chakrouni, M.; Ali, A.M.; Jutur, P.P.; Ainane, T. Evaluation of the Anticancer Properties of Lamellar Alkaloid Drivatives Extracted from the Tunicate Didemnum abradatum (Moucha Island Sea, Djibouti): Pharmacological and Computational Approach. Molecules 2025, 30, 3338. https://doi.org/10.3390/molecules30163338
Abdoul-Latif FM, Aboubaker IH, Mohamed H, Ainane A, Chakrouni M, Ali AM, Jutur PP, Ainane T. Evaluation of the Anticancer Properties of Lamellar Alkaloid Drivatives Extracted from the Tunicate Didemnum abradatum (Moucha Island Sea, Djibouti): Pharmacological and Computational Approach. Molecules. 2025; 30(16):3338. https://doi.org/10.3390/molecules30163338
Chicago/Turabian StyleAbdoul-Latif, Fatouma Mohamed, Ibrahim Houmed Aboubaker, Houda Mohamed, Ayoub Ainane, Mouhcine Chakrouni, Ali Merito Ali, Pannaga Pavan Jutur, and Tarik Ainane. 2025. "Evaluation of the Anticancer Properties of Lamellar Alkaloid Drivatives Extracted from the Tunicate Didemnum abradatum (Moucha Island Sea, Djibouti): Pharmacological and Computational Approach" Molecules 30, no. 16: 3338. https://doi.org/10.3390/molecules30163338
APA StyleAbdoul-Latif, F. M., Aboubaker, I. H., Mohamed, H., Ainane, A., Chakrouni, M., Ali, A. M., Jutur, P. P., & Ainane, T. (2025). Evaluation of the Anticancer Properties of Lamellar Alkaloid Drivatives Extracted from the Tunicate Didemnum abradatum (Moucha Island Sea, Djibouti): Pharmacological and Computational Approach. Molecules, 30(16), 3338. https://doi.org/10.3390/molecules30163338








