Advances in Hydrodechlorination Technologies for Diclofenac Removal from Aqueous Systems
Abstract
1. Introduction
2. Current Studies Covering Aqueous-Phase HDC of DFC
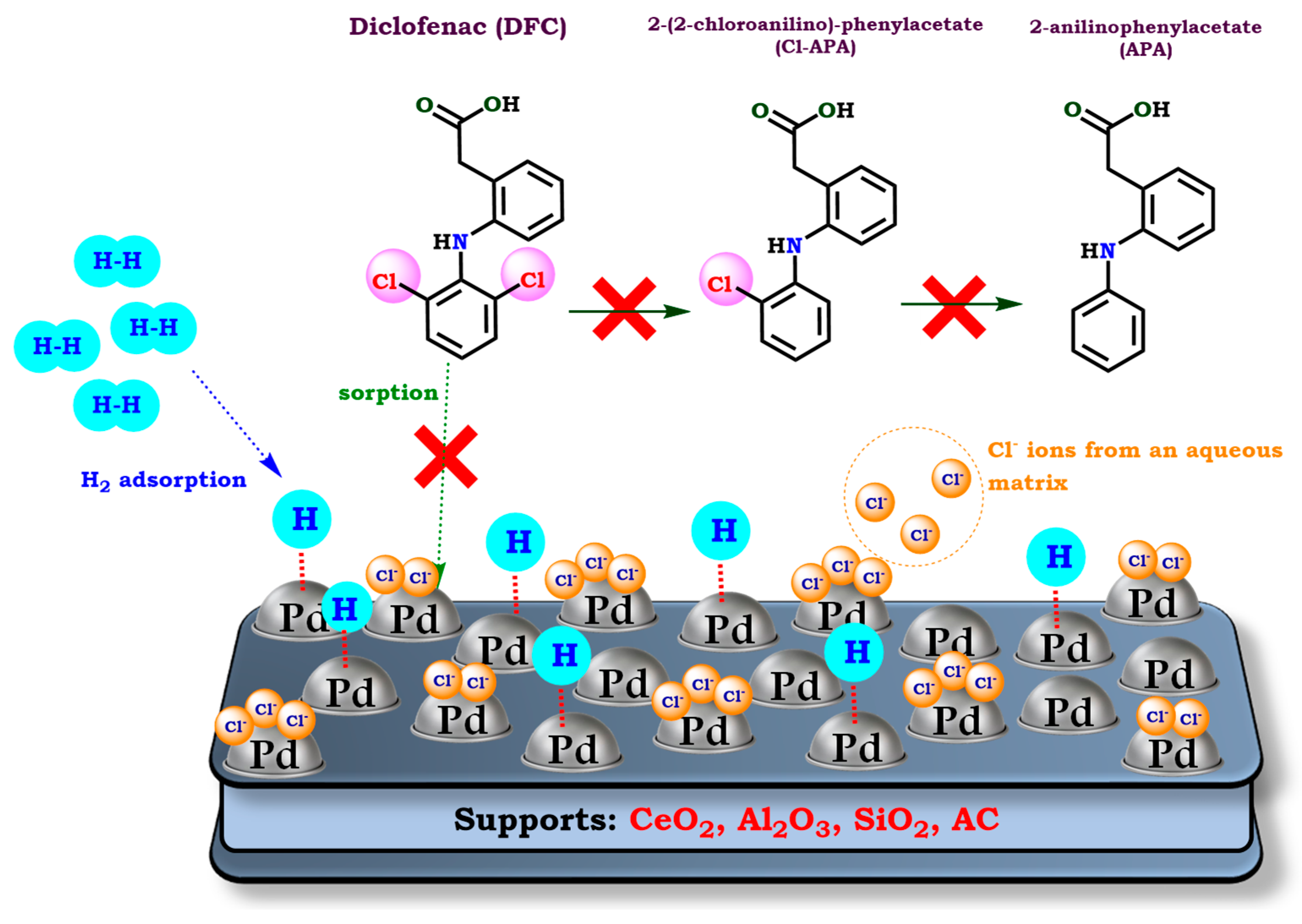
3. HDC of DFC: Kinetic Models and Mechanistic Insights
4. Toxicity Evaluation of Post-Treated DFC Solutions by HDC
5. HDC of DFC in Real Environmentally Relevant Water Matrices
6. Emergent Approaches for HDC: Biocatalytic and Electrocatalytic Treatments for DFC Removal
6.1. Biocatalytic Hydrodechlorination Processes
6.2. Electrocatalytic Hydrodechlorination (ECHDC) Processes
7. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Hydrodechlorination | HDC |
| Diclofenac | DFC |
| Active pharmaceutical ingredients | APIs |
| Non-steroidal anti-inflammatory drugs | NSAIDs |
| Wastewater treatment plants | WWTPs |
| Advanced oxidation processes | AOPS |
| Acetaminophen | ACE |
| Ciprofloxacin | CPFX |
| Nanoparticles | NPs |
| Extracellular polymeric substance | EPS |
| Membrane biofilm reactors | MBfRs |
| Electrocatalytic hydrodechlorination | ECHDC |
| Chloramphenicol | CAP |
| Triclosan | TCL |
| Sertraline | SRT |
| Ecological structure–activity relationships | ECOSARs |
| Density functional theory X-ray diffraction | DFT XRD |
| Transmission electron microscopy | TEM |
| X-ray photoelectron spectroscopy | XPS |
| Scanning electron microscopy | SEM |
| High-resolution TEM | HRTEM |
| Total reflection X-ray fluorescence | TXRF |
| 2-anilinophenylacetate | APA |
| 2-cyclohexylaminophenylacetate | CPA |
| Drinking water treatment plants | DWTPs |
| Temperature-programmed hydride decomposition | TPHD |
| Hydrodehalogenation | HDH |
| Catalytic membrane reactor | CMR |
| Inductively coupled plasma mass spectrometry | ICP-MS |
| Thermogravimetric analysis | TGA |
| Turnover frequency | TOF |
| (2-(2-chloroanilino)-phenylacetate) | Cl-APA |
| Toxicity units | TUs |
| Total organic carbon | TOC |
| Conventional activated sludge | CAS |
| Synchrotron X-ray diffraction | SXRD |
| X-ray absorption spectroscopy | XAS |
| Anaerobic granular sludge | AGS |
| Reduced graphene oxide | rGO |
| Polyaniline-reduced graphene oxide/nickel foam | PANI-rGO/NF |
| Ultraviolet photoelectron spectroscopy | UPS |
| Cyclic voltammetry | CV |
| Carbon microspheres | CMs |
References
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and Personal Care Products in the Environment: Agents of Subtle Change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Bhandari, A.; Das, K.; Pillar, G. Occurrence and Fate of Pharmaceuticals and Personal Care Products (PPCPs) in Biosolids. J. Environ. Qual. 2005, 34, 91–104. [Google Scholar] [CrossRef]
- Alessandretti, I.; Rigueto, C.V.T.; Nazari, M.T.; Rosseto, M.; Dettmer, A. Removal of Diclofenac from Wastewater: A Comprehensive Review of Detection, Characteristics and Tertiary Treatment Techniques. J. Environ. Chem. Eng. 2021, 9, 106743. [Google Scholar] [CrossRef]
- Ternes, T.; Bonerz, M.; Schmidt, T. Determination of Neutral Pharmaceuticals in Wastewater and Rivers by Liquid Chromatography-Electrospray Tandem Mass Spectrometry. J. Chromatogr. A 2001, 938, 175–185. [Google Scholar] [CrossRef]
- Nunes, B.; Daniel, D.; Canelas, G.G.; Barros, J.; Correia, A.T. Toxic Effects of Environmentally Realistic Concentrations of Diclofenac in Organisms from Two Distinct Trophic Levels, Hediste Diversicolor and Solea Senegalensis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 231, 108722. [Google Scholar] [CrossRef]
- Parolini, M. Toxicity of the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Acetylsalicylic Acid, Paracetamol, Diclofenac, Ibuprofen and Naproxen towards Freshwater Invertebrates: A Review. Sci. Total Environ. 2020, 740, 140043. [Google Scholar] [CrossRef]
- aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the Environment-Global Occurrences and Perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Kuvarega, A.T.; Onwudiwe, D.C. Recent Strategies for Environmental Remediation of Organochlorine Pesticides. Appl. Sci. 2020, 10, 6286. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The Removal of Pharmaceuticals, Personal Care Products, Endocrine Disruptors and Illicit Drugs during Wastewater Treatment and Its Impact on the Quality of Receiving Waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef]
- Shamsudin, M.S.; Azha, S.F.; Ismail, S. A Review of Diclofenac Occurrences, Toxicology, and Potential Adsorption of Clay-Based Materials with Surfactant Modifier. J. Environ. Chem. Eng. 2022, 10, 107541. [Google Scholar] [CrossRef]
- Pizzichetti, R.; Martín-Gamboa, M.; Pablos, C.; Reynolds, K.; Stanley, S.; Dufour, J.; Marugán, J. Environmental Life Cycle Assessment of UV-C LEDs vs. Mercury Lamps and Oxidant Selection for Diclofenac Degradation. Sustain. Mater. Technol. 2024, 41, e01002. [Google Scholar] [CrossRef]
- Parra-Enciso, C.; Avila, B.S.; Rubio-Clemente, A.; Peñuela, G.A. Degradation of Diclofenac through Ultrasonic-Based Advanced Oxidation Processes at Low Frequency. J. Environ. Chem. Eng. 2022, 10, 108296. [Google Scholar] [CrossRef]
- Ebal, J.C.C.; Marchadesch, A.N.R.; Oro, R.A.G.; Ortega, R.M.P.; Polinar, P.J.B.; Bolaños, I.F.Y.; Halabaso, E.; Rubi, R.V.C.; Roque, E.C.; Lopez, E.C.R. Fenton-like Degradation of Diclofenac in Water Using Iron-Impregnated Mussel Shell Biochar (Fe@MSBC). Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Jiang, L.; Xie, S.; Chen, H.; Yang, J.; Wang, X.; Li, W.; Peng, X.; Wu, Z.; Wang, H.; Wang, J.; et al. Visible-Light-Promoted Peroxymonosulfate Activation for ACE Degradation: Overlooked Role of Photogenerated Hole. Appl. Catal. B Environ. 2025, 365, 124881. [Google Scholar] [CrossRef]
- Xie, S.; Jiang, L.; Liu, W.; Lu, Q.; Zeng, G.; Wang, H.; Wang, J.; Yuan, X.; Chen, H.; Jiang, H. Network Capture Effect-Driven Enhanced Activation of Peroxymonosulfate by Iron-Doped Carbon Quantum Dots Derived from Ferrous Gluconate for Efficient Ciprofloxacin Degradation: DFT Calculations and Mechanism Analysis. J. Mater. Chem. A 2025, 13, 20868–20883. [Google Scholar] [CrossRef]
- Farré, M.; Ferrer, I.; Ginebreda, A.; Figueras, M.; Olivella, L.; Tirapu, L.; Vilanova, M.; Barceló, D. Determination of Drugs in Surface Water and Wastewater Samples by Liquid Chromatography-Mass Spectrometry: Methods and Preliminary Results Including Toxicity Studies with Vibrio Fischeri. J. Chromatogr. A 2001, 938, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Stülten, D.; Zühlke, S.; Lamshöft, M.; Spiteller, M. Occurrence of Diclofenac and Selected Metabolites in Sewage Effluents. Sci. Total Environ. 2008, 405, 310–316. [Google Scholar] [CrossRef]
- Naddeo, V.; Belgiorno, V.; Ricco, D.; Kassinos, D. Degradation of Diclofenac during Sonolysis, Ozonation and Their Simultaneous Application. Ultrason. Sonochem. 2009, 16, 790–794. [Google Scholar] [CrossRef]
- Pizzichetti, R.; Reynolds, K.; Pablos, C.; Casado, C.; Moore, E.; Stanley, S.; Marugán, J. Removal of Diclofenac by UV-B and UV-C Light-Emitting Diodes (LEDs) Driven Advanced Oxidation Processes (AOPs): Wavelength Dependence, Kinetic Modelling and Energy Consumption. Chem. Eng. J. 2023, 471, 144520. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Rani, N.; Kumar, V.; Mythili, R.; Jain, D. A Review on Simultaneous Heavy Metal Removal and Organo-Contaminants Degradation by Potential Microbes: Current Findings and Future Outlook. Microbiol. Res. 2023, 273, 127419. [Google Scholar] [CrossRef]
- Nisar, J.; Sayed, M.; Khan, F.U.; Khan, H.M.; Iqbal, M.; Khan, R.A.; Anas, M. Gamma-Irradiation Induced Degradation of Diclofenac in Aqueous Solution: Kinetics, Role of Reactive Species and Influence of Natural Water Parameters. J. Environ. Chem. Eng. 2016, 4, 2573–2584. [Google Scholar] [CrossRef]
- Barrimi, M.; Aalouane, R.; Aarab, C.; Hafidi, H.; Baybay, H.; Soughi, M.; Tachfouti, N.; Nejjari, C.; Mernissi, F.Z.; Rammouz, I.; et al. Implications of the Use of Organochlorine in the Environment, and Public Health. Abanico Vet. 2013, 53, 59–65. [Google Scholar]
- Alharbi, O.M.L.; Basheer, A.A.; Khattab, R.A.; Ali, I. Health and Environmental Effects of Persistent Organic Pollutants. J. Mol. Liq. 2018, 263, 442–453. [Google Scholar] [CrossRef]
- Quan, X.; Wang, X.; Sun, Y.; Li, W.; Chen, L.; Zhao, J. Degradation of Diclofenac Using Palladized Anaerobic Granular Sludge: Effects of Electron Donor, Reaction Medium and Deactivation Factors. J. Hazard. Mater. 2019, 365, 155–163. [Google Scholar] [CrossRef]
- Hennebel, T.; Van Nevel, S.; Verschuere, S.; De Corte, S.; De Gusseme, B.; Cuvelier, C.; Fitts, J.P.; Van Der Lelie, D.; Boon, N.; Verstraete, W. Palladium Nanoparticles Produced by Fermentatively Cultivated Bacteria as Catalyst for Diatrizoate Removal with Biogenic Hydrogen. Appl. Microbiol. Biotechnol. 2011, 91, 1435–1445. [Google Scholar] [CrossRef]
- De Corte, S.; Sabbe, T.; Hennebel, T.; Vanhaecke, L.; De Gusseme, B.; Verstraete, W.; Boon, N. Doping of Biogenic Pd Catalysts with Au Enables Dechlorination of Diclofenac at Environmental Conditions. Water Res. 2012, 46, 2718–2726. [Google Scholar] [CrossRef]
- Liu, Y.; Xi, Y.; Xie, T.; Liu, H.; Su, Z.; Huang, Y.; Xu, W.; Wang, D.; Zhang, C.; Li, X. Enhanced Removal of Diclofenac via Coupling Pd Catalytic and Microbial Processes in a H2-Based Membrane Biofilm Reactor: Performance, Mechanism and Biofilm Microbial Ecology. Chemosphere 2022, 307, 135597. [Google Scholar] [CrossRef]
- Li, J.; Peng, Y.; Zhang, W.; Shi, X.; Chen, M.; Wang, P.; Zhang, X.; Fu, H.; Lv, X.; Dong, F.; et al. Hierarchical Pd/MnO2 Nanosheet Array Supported on Ni Foam: An Advanced Electrode for Electrocatalytic Hydrodechlorination Reaction. Appl. Surf. Sci. 2020, 509, 145369. [Google Scholar] [CrossRef]
- Ma, X.; Sun, Z.; Hu, X. Electrocatalytic Dechlorination of Chlorophenols on Palladium/Graphene-Nafion/Titanium Mesh Electrode. J. Water Process Eng. 2018, 26, 72–82. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, X.; Wang, S.; Cui, C.; Xin, Y.; Zhang, G.; Zhou, C. In Situ-Synthesized Amorphous Pd/N-C Microspheres Derived from Shrimp Shells as a Three-Dimensional Electrocatalyst for Hydrodechlorination of Diclofenac. Chem. Eng. J. 2022, 428, 131231. [Google Scholar] [CrossRef]
- Li, J.; Kong, K.; Chong, Y.; Ding, J.; Wang, L.; Ba, Z.; Zhang, J. Unveiling the Mechanism and Performance of Electrocatalytic Hydrodechlorination of Chlorinated PPCPs by Electron-Rich Palladium Electrode Modulated through PANI-RGO Interlayer. Sep. Purif. Technol. 2023, 323, 124452. [Google Scholar] [CrossRef]
- Wang, S.; Yang, M.; Cui, C.; Zheng, Q.; Zhou, C.Z.; Xin, Y. Duckweed Derived N, P-Doped Carbon Microspheres Supported Ru as Highly Active Catalyst for Electrocatalytic Hydrodechlorination Diclofenac. Electrochim. Acta 2023, 462, 142707. [Google Scholar] [CrossRef]
- Wu, Y.; Gan, L.; Zhang, S.; Song, H.; Lu, C.; Li, W.; Wang, Z.; Jiang, B.; Li, A. Carbon-Nanotube-Doped Pd-Ni Bimetallic Three-Dimensional Electrode for Electrocatalytic Hydrodechlorination of 4-Chlorophenol: Enhanced Activity and Stability. J. Hazard. Mater. 2018, 356, 17–25. [Google Scholar] [CrossRef]
- Radjenovic, J.; Sedlak, D.L. Challenges and Opportunities for Electrochemical Processes as Next-Generation Technologies for the Treatment of Contaminated Water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, C.; Zhang, G.; Xin, Y.; Wang, S. Electrocatalytic Hydrodechlorination of Pentachlorophenol on Pd-Supported Magnetic Biochar Particle Electrodes. Sep. Purif. Technol. 2021, 258, 118017. [Google Scholar] [CrossRef]
- Wu, K.; Qian, X.; Chen, L.; Xu, Z.; Zheng, S.; Zhu, D. Effective Liquid Phase Hydrodechlorination of Diclofenac Catalysed by Pd/CeO2. RSC Adv. 2015, 5, 18702–18709. [Google Scholar] [CrossRef]
- Lou, Z.; Yu, C.; Wen, X.; Xu, Y.; Yu, J.; Xu, X. Construction of Pd Nanoparticles/Two-Dimensional Co-MOF Nanosheets Heterojunction for Enhanced Electrocatalytic Hydrodechlorination. Appl. Catal. B Environ. 2022, 317, 121730. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhao, B.; Ding, J.; Zhang, J.; Yin, M.; Zhang, Z.; Ma, S.; Liu, Y.; Tan, Z.; et al. Unraveling Kinetics and Mechanism of Electrocatalytic Hydrodechlorination of Chlorinated PPCPs by Nickel-Cobalt Metal Organic Framework Supported Palladium Composite Electrode. Appl. Catal. B Environ. 2023, 332, 122754. [Google Scholar] [CrossRef]
- Yang, L.; Pang, S.; Zhou, J.; Li, X.; Yao, M.; Xia, S. Biological Reduction and Hydrodechlorination of Chlorinated Nitroaromatic Antibiotic Chloramphenicol under H2-Transfer Membrane Biofilm Reactor. Bioresour. Technol. 2023, 376, 128881. [Google Scholar] [CrossRef]
- Li, L.X.; Zhang, G.C.; Sun, W.J.; Zhang, H.Y.; Wang, S.X.; Wei, J.L.; He, J.H.; Song, K.; Lu, J.M. Construction of Ultra-Small Pt Nanoparticles @Ti3C2Tx MXene Electrocatalyst for Efficient and Stable Electrochemical Hydrodechlorination of Chloramphenicol. Chem. Eng. J. 2022, 433, 134415. [Google Scholar] [CrossRef]
- Li, J.; Ma, S.; Qi, Z.; Ding, J.; Yin, M.; Zhao, B.; Zhang, Z.; Wang, Y.; Zhang, H.; Wang, L.; et al. Insights into the Removal of Chloramphenicol by Electrochemical Reduction on Pd/NiFe-MOF/Foam-Ni Electrode: Performance and Mechanism. Appl. Catal. B Environ. 2023, 322, 122076. [Google Scholar] [CrossRef]
- Zheng, Q.; Xu, H.; Yao, Y.; Dai, J.; Wang, J.; Hou, W.; Zhao, L.; Zou, X.; Zhan, G.; Wang, R.; et al. Cobalt Single-Atom Reverse Hydrogen Spillover for Efficient Electrochemical Water Dissociation and Dechlorination. Angew. Chem. Int. Ed. 2024, 63, e202401386. [Google Scholar] [CrossRef]
- Song, G.; Wu, H.; Wang, X.; Li, S.; Liang, R.; Zhou, M. Regulating the Electronic Structure by P-Doping Cobalt-Based Catalyst for Atomic Hydrogen Mediated Electrocatalytic Dechlorination. J. Hazard. Mater. 2024, 474, 134750. [Google Scholar] [CrossRef]
- Huang, J.; Lin, M.; Cai, Y.; Luo, Y.H.; Zhou, D.; Rittmann, B.E. Complete Dehalogenation of Chloramphenicol by Bimetallic Alloy Pd-Au Nanoparticles in a H2-Based Membrane Catalyst-Film Reactor. Chem. Eng. J. 2024, 497, 154758. [Google Scholar] [CrossRef]
- Han, B.; Liu, W.; Li, J.; Wang, J.; Zhao, D.; Xu, R.; Lin, Z. Catalytic Hydrodechlorination of Triclosan Using a New Class of Anion-Exchange-Resin Supported Palladium Catalysts. Water Res. 2017, 120, 199–210. [Google Scholar] [CrossRef]
- Pérko, J.; Kamenická, B.; Weidlich, T. Degradation of the Antibacterial Agents Triclosan and Chlorophene Using Hydrodechlorination by Al-Based Alloys. Monatshefte Chem. 2018, 149, 1777–1786. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Luan, C.; Wang, H.; Cheng, X.; Fang, L.; Wang, L.; Zhao, B.; Ma, C.; Zhang, H.; et al. Preparation and Characterization of Palladium/Polypyrrole-Reduced Graphene Oxide/Foamed Nickel Composite Electrode and Its Electrochemical Dechlorination of Triclosan. Arab. J. Chem. 2020, 13, 3963–3973. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Jia, J.; Yan, B. Triclosan Detoxification through Dechlorination and Oxidation via Microbial Pd-NPs under Aerobic Conditions. Chemosphere 2022, 286, 131836. [Google Scholar] [CrossRef]
- Mao, R.; Huang, C.; Zhao, X.; Ma, M.; Qu, J. Dechlorination of Triclosan by Enhanced Atomic Hydrogen-Mediated Electrochemical Reduction: Kinetics, Mechanism, and Toxicity Assessment. Appl. Catal. B Environ. 2019, 241, 120–129. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.R.; Fu, M.L.; Yuan, B.; Cui, H.J.; Wang, Y.F. Fabrication and Evaluation of Au-Pd Core-Shell Nanocomposites for Dechlorination of Diclofenac in Water. Environ. Technol. (U. K.) 2015, 36, 1510–1518. [Google Scholar] [CrossRef]
- Nieto-Sandoval, J.; Munoz, M.; de Pedro, Z.M.; Casas, J.A. Fast Degradation of Diclofenac by Catalytic Hydrodechlorination. Chemosphere 2018, 213, 141–148. [Google Scholar] [CrossRef]
- Nieto-Sandoval, J.; Munoz, M.; de Pedro, Z.M.; Casas, J.A. Catalytic Hydrodechlorination as Polishing Step in Drinking Water Treatment for the Removal of Chlorinated Micropollutants. Sep. Purif. Technol. 2019, 227, 115717. [Google Scholar] [CrossRef]
- Kowalewski, E.; Asztemborska, M.; Bonarowska, M.; Matus, K.; Śrębowata, A. Effect of Unimodality and Bimodality of Pd Nanoparticles on the Catalytic Activity of Pd/SiO2 in the Removal of Diclofenac from Water. Catal. Commun. 2020, 143, 106056. [Google Scholar] [CrossRef]
- Zawadzki, B.; Kowalewski, E.; Asztemborska, M.; Matus, K.; Casale, S.; Dzwigaj, S.; Śrębowata, A. Palladium Loaded BEA Zeolites as Efficient Catalysts for Aqueous-Phase Diclofenac Hydrodechlorination. Catal. Commun. 2020, 145, 3–7. [Google Scholar] [CrossRef]
- De Corte, S.; Hennebel, T.; Fitts, J.P.; Sabbe, T.; Bliznuk, V.; Verschuere, S.; Van Der Lelie, D.; Verstraete, W.; Boon, N. Biosupported Bimetallic Pd-Au Nanocatalysts for Dechlorination of Environmental Contaminants. Environ. Sci. Technol. 2011, 45, 8506–8513. [Google Scholar] [CrossRef] [PubMed]
- Bendová, H.; Kamenická, B.; Weidlich, T.; Beneš, L.; Vlček, M.; Lacina, P.; Švec, P. Application of Raney Al-Ni Alloy for Simple Hydrodehalogenation of Diclofenac and Other Halogenated Biocidal Contaminants in Alkaline Aqueous Solution under Ambient Conditions. Materials 2022, 15, 3939. [Google Scholar] [CrossRef]
- Nieto-Sandoval, J.; Gomez-Herrero, E.; Munoz, M.; de Pedro, Z.M.; Casas, J.A. Palladium-Based Catalytic Membrane Reactor for the Continuous Flow Hydrodechlorination of Chlorinated Micropollutants. Appl. Catal. B Environ. 2021, 293, 120235. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Qi, Z.; Ma, C.; Zhang, Z.; Zhao, B.; Wang, L.; Zhang, H.; Chong, Y.; Chen, X.; et al. Kinetics and Mechanisms of Electrocatalytic Hydrodechlorination of Diclofenac on Pd-Ni / PPy-RGO / Ni Electrodes. Appl. Catal. B Environ. 2020, 268, 118696. [Google Scholar] [CrossRef]
- Pintar, A.; Batista, J.; Levec, J.; Kajiuchi, T. Kinetics of the Catalytic Liquid-Phase Hydrogenation of Aqueous Nitrate Solutions. Appl. Catal. B Environ. 1996, 11, 81–98. [Google Scholar] [CrossRef]
- Baeza, J.A.; Calvo, L.; Gilarranz, M.A.; Mohedano, A.F.; Casas, J.A.; Rodriguez, J.J. Catalytic Behavior of Size-Controlled Palladium Nanoparticles in the Hydrodechlorination of 4-Chlorophenol in Aqueous Phase. J. Catal. 2012, 293, 85–93. [Google Scholar] [CrossRef]
- Díaz, E.; Mohedano, A.F.; Calvo, L.; Gilarranz, M.A.; Casas, J.A.; Rodríguez, J.J. Kinetics of the Hydrodechlorination of 4-Chlorophenol in Water Using Pd, Pt and Rh/Al 2 O 3 Catalysts, Ind. Eng. Chem. Res. 2008, 47, 3840–3846. [Google Scholar] [CrossRef]
- Chang, W.; Kim, H.; Lee, G.Y.; Ahn, B.J. Catalytic Hydrodechlorination Reaction of Chlorophenols by Pd Nanoparticles Supported on Graphene. Res. Chem. Intermed. 2016, 42, 71–82. [Google Scholar] [CrossRef]
- Dorathi, P.J.; Kandasamy, P. Dechlorination of Chlorophenols by Zero Valent Iron Impregnated Silica. J. Environ. Sci. 2012, 24, 765–773. [Google Scholar] [CrossRef]
- Ordóñez, S.; Vivas, B.P.; Díez, F.V. Minimization of the Deactivation of Palladium Catalysts in the Hydrodechlorination of Trichloroethylene in Wastewaters. Appl. Catal. B Environ. 2010, 95, 288–296. [Google Scholar] [CrossRef]
- Flid, M.R.; Kartashov, L.M.; Treger, Y.A. Theoretical and Applied Aspects of Hydrodechlorination Processes—Catalysts and Technologies. Catalysts 2020, 10, 216. [Google Scholar] [CrossRef]
- Kümmerer, K. The Presence of Pharmaceuticals in the Environment Due to Human Use-Present Knowledge and Future Challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geißen, S.U.; Gal, C. Carbamazepine and Diclofenac: Removal in Wastewater Treatment Plants and Occurrence in Water Bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Munoz, M.; Mora, F.J.; de Pedro, Z.M.; Alvarez-Torrellas, S.; Casas, J.A.; Rodriguez, J.J. Application of CWPO to the Treatment of Pharmaceutical Emerging Pollutants in Different Water Matrices with a Ferromagnetic Catalyst. J. Hazard. Mater. 2017, 331, 45–54. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Liu, G.; Xie, Y. Oxidation of Diclofenac by Aqueous Chlorine Dioxide: Identification of Major Disinfection Byproducts and Toxicity Evaluation. Sci. Total Environ. 2014, 473–474, 437–445. [Google Scholar] [CrossRef]
- Taoufik, N.; Boumya, W.; Achak, M.; Sillanpää, M.; Barka, N. Comparative Overview of Advanced Oxidation Processes and Biological Approaches for the Removal Pharmaceuticals. J. Environ. Manag. 2021, 288. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Chamanehpour, E.; Fahoul, N. Recent Advances and Future Outlook for Treatment of Pharmaceutical from Water: An Overview. Int. J. Environ. Sci. Technol. 2023, 20, 3437–3454. [Google Scholar] [CrossRef]
- Ortúzar, M.; Esterhuizen, M.; Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Pharmaceutical Pollution in Aquatic Environments: A Concise Review of Environmental Impacts and Bioremediation Systems. Front. Microbiol. 2022, 13, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Conde, J.; de Pedro, Z.M.; Casas, J.A. Antibiotics Abatement in Synthetic and Real Aqueous Matrices by H2O2/Natural Magnetite. Catal. Today 2018, 313, 142–147. [Google Scholar] [CrossRef]
- Hoeger, B.; Köllner, B.; Dietrich, D.R.; Hitzfeld, B. Water-Borne Diclofenac Affects Kidney and Gill Integrity and Selected Immune Parameters in Brown Trout (Salmo Trutta f. Fario). Aquat. Toxicol. 2005, 75, 53–64. [Google Scholar] [CrossRef]
- de Pedro, Z.M.; Diaz, E.; Mohedano, A.F.; Casas, J.A.; Rodriguez, J.J. Compared Activity and Stability of Pd/Al2O3 and Pd/AC Catalysts in 4-Chlorophenol Hydrodechlorination in Different PH Media. Appl. Catal. B Environ. 2011, 103, 128–135. [Google Scholar] [CrossRef]
- Mackenzie, K.; Frenzel, H.; Kopinke, F.D. Hydrodehalogenation of Halogenated Hydrocarbons in Water with Pd Catalysts: Reaction Rates and Surface Competition. Appl. Catal. B Environ. 2006, 63, 161–167. [Google Scholar] [CrossRef]
- Angeles-Wedler, D.; Mackenzie, K.; Kopinke, F.D. Sulphide-Induced Deactivation of Pd/Al2O3 as Hydrodechlorination Catalyst and Its Oxidative Regeneration with Permanganate. Appl. Catal. B Environ. 2009, 90, 613–617. [Google Scholar] [CrossRef]
- Hennebel, T.; Simoen, H.; De Windt, W.; Verloo, M.; Boon, N.; Verstraete, W. Biocatalytic Dechlorination of Trichloroethylene with Bio-Palladium in a Pilot-Scale Membrane Reactor. Biotechnol. Bioeng. 2009, 102, 995–1002. [Google Scholar] [CrossRef]
- Ding, S.; Parlett, C.M.A.; Fan, X. Recent Developments in Multifunctional Catalysts for Fatty Acid Hydrodeoxygenation as a Route towards Biofuels. Mol. Catal. 2022, 523, 111492. [Google Scholar] [CrossRef]
- Bennett, J.A.; Mikheenko, I.P.; Deplanche, K.; Shannon, I.J.; Wood, J.; Macaskie, L.E. Nanoparticles of Palladium Supported on Bacterial Biomass: New Re-Usable Heterogeneous Catalyst with Comparable Activity to Homogeneous Colloidal Pd in the Heck Reaction. Appl. Catal. B Environ. 2013, 140–141, 700–707. [Google Scholar] [CrossRef]
- Creamer, N.J.; Mikheenko, I.P.; Yong, P.; Deplanche, K.; Sanyahumbi, D.; Wood, J.; Pollmann, K.; Merroun, M.; Selenska-Pobell, S.; Macaskie, L.E. Novel Supported Pd Hydrogenation Bionanocatalyst for Hybrid Homogeneous/Heterogeneous Catalysis. Catal. Today 2007, 128, 80–87. [Google Scholar] [CrossRef]
- Song, X.; Shi, Q.; Wang, H.; Liu, S.; Tai, C.; Bian, Z. Preparation of Pd-Fe/Graphene Catalysts by Photocatalytic Reduction with Enhanced Electrochemical Oxidation-Reduction Properties for Chlorophenols. Appl. Catal. B Environ. 2017, 203, 442–451. [Google Scholar] [CrossRef]
- Sun, Z.; Wei, X.; Zhang, H.; Hu, X. Dechlorination of Pentachlorophenol (PCP) in Aqueous Solution on Novel Pd-Loaded Electrode Modified with PPy–SDBS Composite Film. Environ. Sci. Pollut. Res. 2015, 22, 3828–3837. [Google Scholar] [CrossRef]
- Sun, Z.; Wei, X.; Shen, H.; Hu, X. Preparation of Palladium-Nickel Loaded Titanium Electrode with Surfactant Assistance and Its Application in Pentachlorophenol Reductive Dechlorination. Sep. Purif. Technol. 2014, 124, 224–230. [Google Scholar] [CrossRef]
- Zhou, J.; Lou, Z.; Yang, K.; Xu, J.; Li, Y.; Liu, Y.; Baig, S.A.; Xu, X. Electrocatalytic Dechlorination of 2,4-Dichlorobenzoic Acid Using Different Carbon-Supported Palladium Moveable Catalysts: Adsorption and Dechlorination Activity. Appl. Catal. B Environ. 2019, 244, 215–224. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Shan, J.; Zhang, J. Electrodeposition of Palladium and Reduced Graphene Oxide Nanocomposites on Foam-Nickel Electrode for Electrocatalytic Hydrodechlorination of 4-Chlorophenol. J. Hazard. Mater. 2015, 290, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Cao, L.; Li, B.; Huang, X.; Ye, K.; Zhu, K.; Cao, D.; Cheng, K.; Wang, G. Highly Efficient Palladium Nanoparticles Decorated Reduced Graphene Oxide Sheets Supported on Nickel Foam for Hydrogen Peroxide Electroreduction. Appl. Surf. Sci. 2017, 426, 1046–1054. [Google Scholar] [CrossRef]



| Catalysts | Reaction Conditions | DFC Conv. (%) | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Name | Preparation Method | Particle Size ** (nm) | Metal Dispersion ** (%) | SBET (m2·g−1) | |||
| dp-Pd(1.8)/SiO2 | dp: Deposition–precipitation | 9.6 | --- | --- | Catalyst amount: 20 mg [DFC]0: 0.06–0.24 mM pH of DFC solution: 9.0 Hydrogen donor: H2. H2 flow: 250 mL·min−1 Stirring: 1400 rpm Temperature: 25 °C | Negligible | [36] |
| dp-Pd(1.8)/AC | 9.2 | --- | --- | 29 | |||
| dp-Pd(1.9)/Al2O3 | 6.2 | --- | --- | 89 | |||
| dp-Pd(1.7)/CeO2 | 1.3 | 86 | 45 | 98 | |||
| im-Pd(1.8)/CeO2 | im: Impregnation | 5.2 | 21.6 | 66 | 76 | ||
| Au–Pd core–shell NPs | Precipitation between H2PdCl4 and Au NPs | 8.0 | --- | --- | Catalyst amount: 0.56 mg [DFC]0: 30 mg·L−1 pH of DFC solution: 7.0 Hydrogen donor: H2 H2 flow: --- Stirring: --- Temperature: 25 °C | ≈100 | [50] |
| Pd/Al2O3 * | --- | --- | --- | 270 | Catalyst load: 0.5 g·L−1 [DFC]0: 68 μM pH of DFC solution: 6.9 Hydrogen donor: H2 H2 flow: 50 N·mL·min−1 Stirring: 900 rpm Temperature: 25 °C | 100 | [51] |
| Catalyst load: 0.25 g.L−1 [DFC]0: 3 mg·L−1 pH of DFC solution: 7.0 H2 flow: 50 N·mL·min−1 Hydrogen donor: H2 Stirring: 900 rpm Temperature: 17–35 °C | 100 | [52] | |||||
| Pd/SiO2(s) | (s): Ion exchange between hydroxyl group of SiO2 and [Pd(NH3)4](NO3)2 | 1.6 ± 0.1 | 86 | 240 ± 5 | Catalyst amount: 0.1 g [DFC]0: 240 μM pH of DFC solution: --- H2 flow: 1 mL·min−1 Hydrogen donor: H2 Stirring: --- Temperature: 25 °C | 40 | [53] |
| Pd/SiO2(bg) Pd/SiO2(bim) | Incipient wetness impregnation by using two different precursors: (bg): (CH3COO)2Pd and (bim): PdCl2 | 3.4 ± 0.2 2.9 ± 0.15 | 11 28 | 240 ± 5 240 ± 5 | 80 100 | ||
| PdSiBEA | Two-step post-synthesis | 8.0 | --- | 393 ± 39 | Catalyst load: --- [DFC]0: 240 μM. pH of DFC solution: --- Hydrogen donor: H2 H2 flow: --- Stirring: --- Temperature: 30 °C | 99 | [54] |
| PdHAlBEA | Wet impregnation | 9.0 | --- | 377 ± 37 | 88 | ||
| Pd/SiO2 | Ion exchange between hydroxyl group of SiO2 and [Pd(NH3)4](NO3)2 | 2.0 | --- | 240 ± 24 | 65 | ||
| Pd/Al2O3 | Incipient wetness impregnation | 6.0 | --- | 213 ± 21 | 33 | ||
| Bio-Pd/Au | Hosted in Shewanella oneidensis | 11 ± 13.65 | --- | --- | Catalyst load: 50 mg·L−1 [DFC]0: 20 mg·L−1 pH of DFC solution: 7.0 Hydrogen donor: H2 H2 pressure: 1 bar Stirring: 100 rpm Temperature: 25 °C | 78 | [55] |
| Bio-Pd/Au | Hosted in Shewanella oneidensis | 11 ± 13.65 | --- | --- | Catalyst load: 50 mg·L−1 [DFC]0: 20 mg·L−1 pH of DFC solution: 5.0–8.0 Hydrogen donor: H2 H2 pressure: 1 bar Stirring: 100 rpm Temperature: 25 °C | 43.8 | [26] |
| Pd-AGS | Hosted in AGS | 85% of particles within the size range of 0–10 | --- | --- | Catalyst load: 80 mg·L−1 [DFC]0: 20 mg·L−1 pH of DFC solution: 7.0 Hydrogen donor: H2 H2 pressure: --- Stirring: 180 rpm Temperature: 35 °C | 96.5 | [24] |
| Raney Al–Ni * | --- | --- | --- | --- | Catalyst load: 1.65 g.L−1 [DFC]0: 0.59 g.L−1 [KOH]0: 9.7 g.L−1 Stirring: 750 rpm Temperature: 25 °C | 100 | [56] |
| Pd/CMR | Ion adsorption | 6 ± 1.6 | --- | 3 | QEffluent: 0.1 and 0.2 mL·min−1 [DFC]0: 100–500 µg·L−1 pH of effluent: 6.9 H2 flow: 50 N·mL·min−1 Temperature: 25 °C | 58–64 | [57] |
| Pd/PANI-rGO/NF | Electrodeposition | 2.2 | --- | --- | [DFC]0: 20 mg·L−1 Anolyte: 50 mL Na2SO4 0.05 mol.L−1 Catholyte: 50 mL DFC mixed with Na2SO4 0.05 mol.L−1 Current density: 1.75 mA.cm−2 Temperature: 40 °C | 99.3 | [31] |
| Ru/CM-650 | Hydrothermal carbonization | 150 | --- | 193 | Anolyte: 0.2 mol.L−1 H2SO4 solution Catholyte: 80 mL of 25 mg·L−1 DFC solution containing 0.05 mol.L−1 Na2SO4 electrolyte with Ru/CM particle electrode [DFC]0: 20 mg·L−1 Cathode potential: −1.2 V | >90 | [32] |
| Catalyst | T (°C) | (min−1) | (min−1) | (min−1) | Kinetic Model | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| dp-Pd(1.9)/Al2O3 | 25 | 0.009 | 0.027 | 0.030 | 3.0 | 0.996 | Langmuir–Hinshelwood | [36] |
| dp-Pd(0.45)/CeO2 | 0.010 | 0.020 | 0.030 | 2.0 | 0.998 | |||
| dp-Pd(0.9)/CeO2 | 0.017 | 0.045 | 0.034 | 2.7 | 0.998 | |||
| dp-Pd(1.7)/CeO2 | 0.025 | 0.108 | 0.049 | 4.3 | 0.997 | |||
| dp-Pd(2.6)/CeO2 | 0.028 | 0.132 | 0.053 | 4.7 | 0.995 | |||
| Au-Pd core–shell NPs | 25 | 0.027 | --- | --- | --- | 0.994 | First order | [50] |
| 0.014 | 0.038 | --- | 2.7 | 0.997 | Second order | |||
| Pd/Al2O3 * | 17 | 0.107 | 0.069 | 0.286 | 0.6 | 0.949 | Pseudo-first order | [51] |
| 25 | 0.175 | 0.145 | 0.394 | 0.8 | 0.957 | |||
| 35 | 0.301 | 0.240 | 0.734 | 0.8 | 0.944 | |||
| Pd/SiBEA | 30 | 0.190 | --- | --- | --- | 0.993 | First order | [54] |
| Pd/HAlBEA | 0.110 | --- | --- | --- | 0.986 | |||
| Pd/SiO2 | 0.020 | --- | --- | --- | 0.948 | |||
| Pd/Al2O3 | 0.090 | --- | --- | --- | 0.997 |
| Matrices | pH | TOC (mg·L−1) | IC (mg·L−1) | Conductivity (μS.cm−1) | Cl− (mg·L−1) | (SO4)2- (mg·L−1) | Ref. |
|---|---|---|---|---|---|---|---|
| WWTP effluent | 7.1 | 2.6 | 19.7 | 462 | 75.0 | 39.5 | [51] |
| Surface water | 7.0 | 2.7 | 14.9 | 200 | 14.1 | 11.2 | |
| Hospital wastewater | 8.6 | 110 | 63 | 1185 | 216.8 | 33.8 | |
| Mineral water | 7.1 | 0.3 | 3.5 | 41 | 0.35 | --- | [52,57] |
| Tap water | 7.2 | 2.5 | 2.9 | 67 | 8.7 | --- | |
| Hospital WWTP | 7.4 | --- | --- | --- | --- | 30.5 | [26] |
| Technology | Catalyst Type | Reaction Medium | Advantages | Limitations |
|---|---|---|---|---|
| Conventional HDC | Pd/SiO2, Pd/AC, Pd/Al2O3, Pd/CeO2, Pd/zeolite | H2 in aqueous phase | High removal efficiency, well-studied | Requires external H2, catalyst deactivation |
| Biocatalytic HDC | Bio-Pd/AGS, Bio-Pd/MBfR, Bio-Pd/Au NPs | Anaerobic/neutral media | Low-energy, self-regenerating systems | Lower rates, biomass variability |
| ECHDC | Pd/N-CMs, Pd/PANI-rGO | Electrochemical cell | No H2 needed, high control of conditions | Requires a power source, electrode degradation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, C.; Mora, J.A.; Brijaldo, M.H. Advances in Hydrodechlorination Technologies for Diclofenac Removal from Aqueous Systems. Molecules 2025, 30, 3332. https://doi.org/10.3390/molecules30163332
Castillo C, Mora JA, Brijaldo MH. Advances in Hydrodechlorination Technologies for Diclofenac Removal from Aqueous Systems. Molecules. 2025; 30(16):3332. https://doi.org/10.3390/molecules30163332
Chicago/Turabian StyleCastillo, Cristian, Jorge A. Mora, and Maria H. Brijaldo. 2025. "Advances in Hydrodechlorination Technologies for Diclofenac Removal from Aqueous Systems" Molecules 30, no. 16: 3332. https://doi.org/10.3390/molecules30163332
APA StyleCastillo, C., Mora, J. A., & Brijaldo, M. H. (2025). Advances in Hydrodechlorination Technologies for Diclofenac Removal from Aqueous Systems. Molecules, 30(16), 3332. https://doi.org/10.3390/molecules30163332






