Stimulation of Transient Receptor Potential Channels TRPM3 and TRPM8 Increases Human Prostaglandin Endoperoxide Synthase-2 Promoter Activity
Abstract
1. Introduction
2. Results
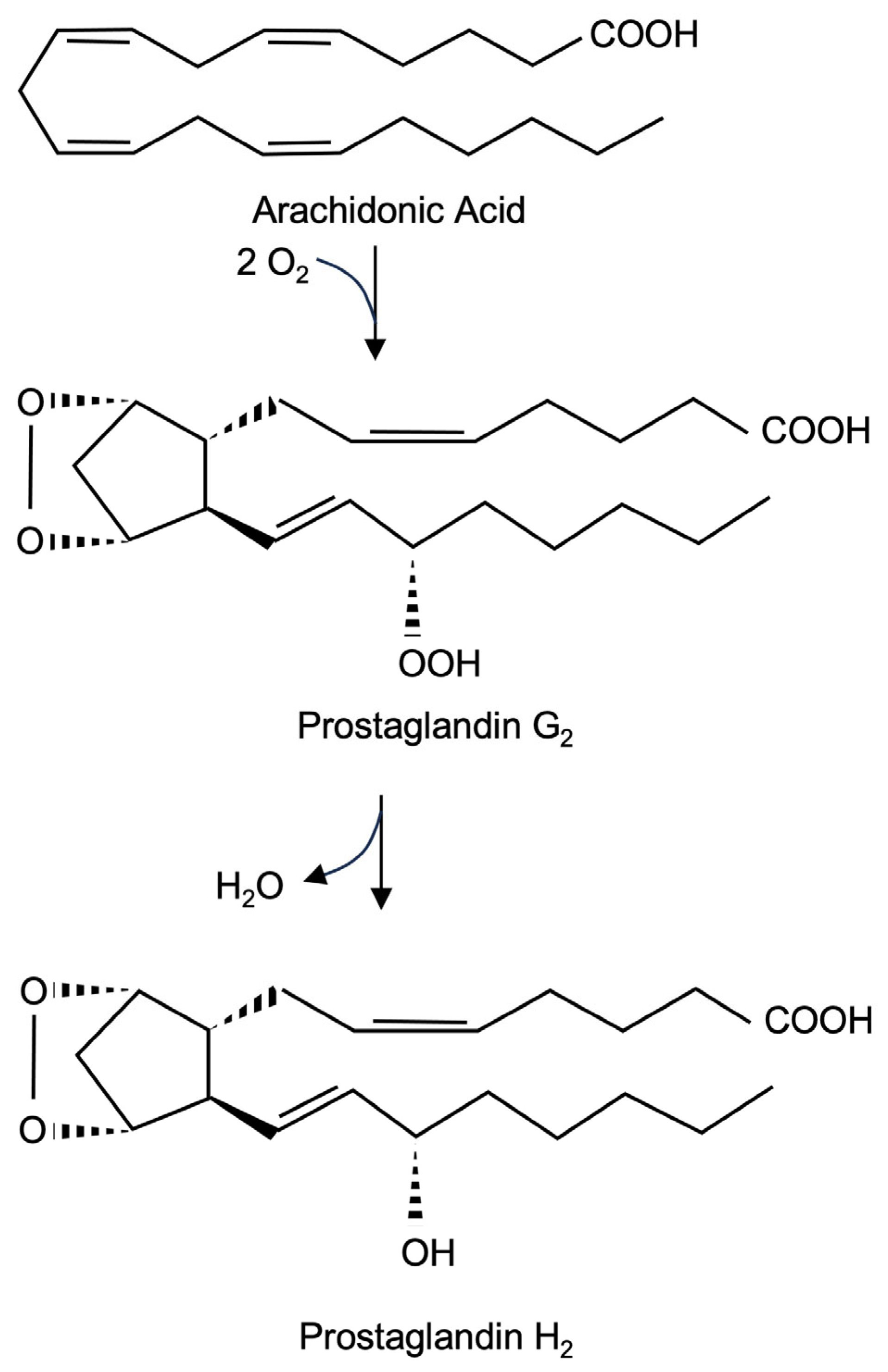
2.1. Prostaglandin Endoperoxide Synthase-2 Catalyzes the Biosynthesis of Prostaglandin H2 (PGH2)
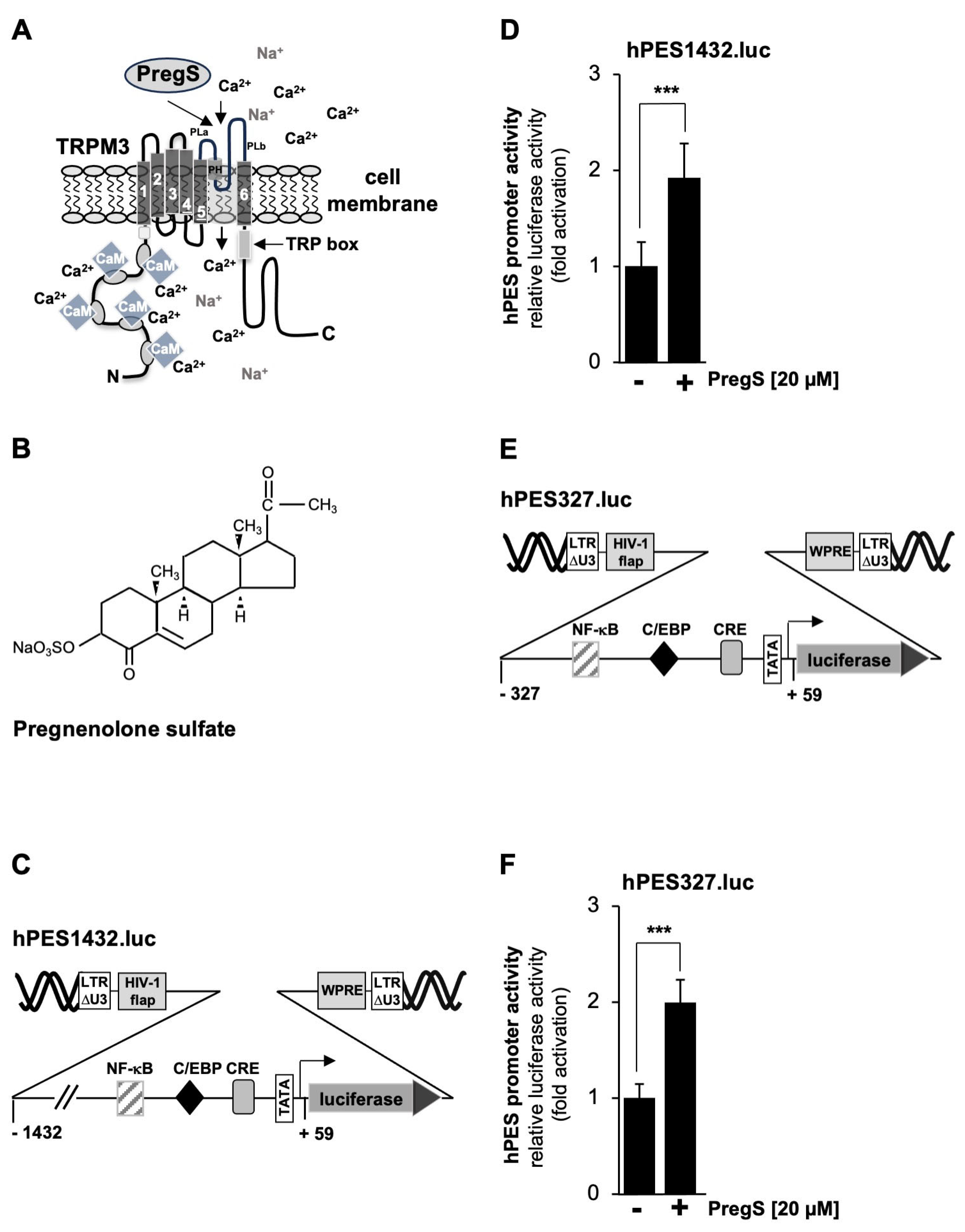
2.2. TRPM3 Channel Stimulation Activates the Prostaglandin Endoperoxide Synthase-2 Promoter
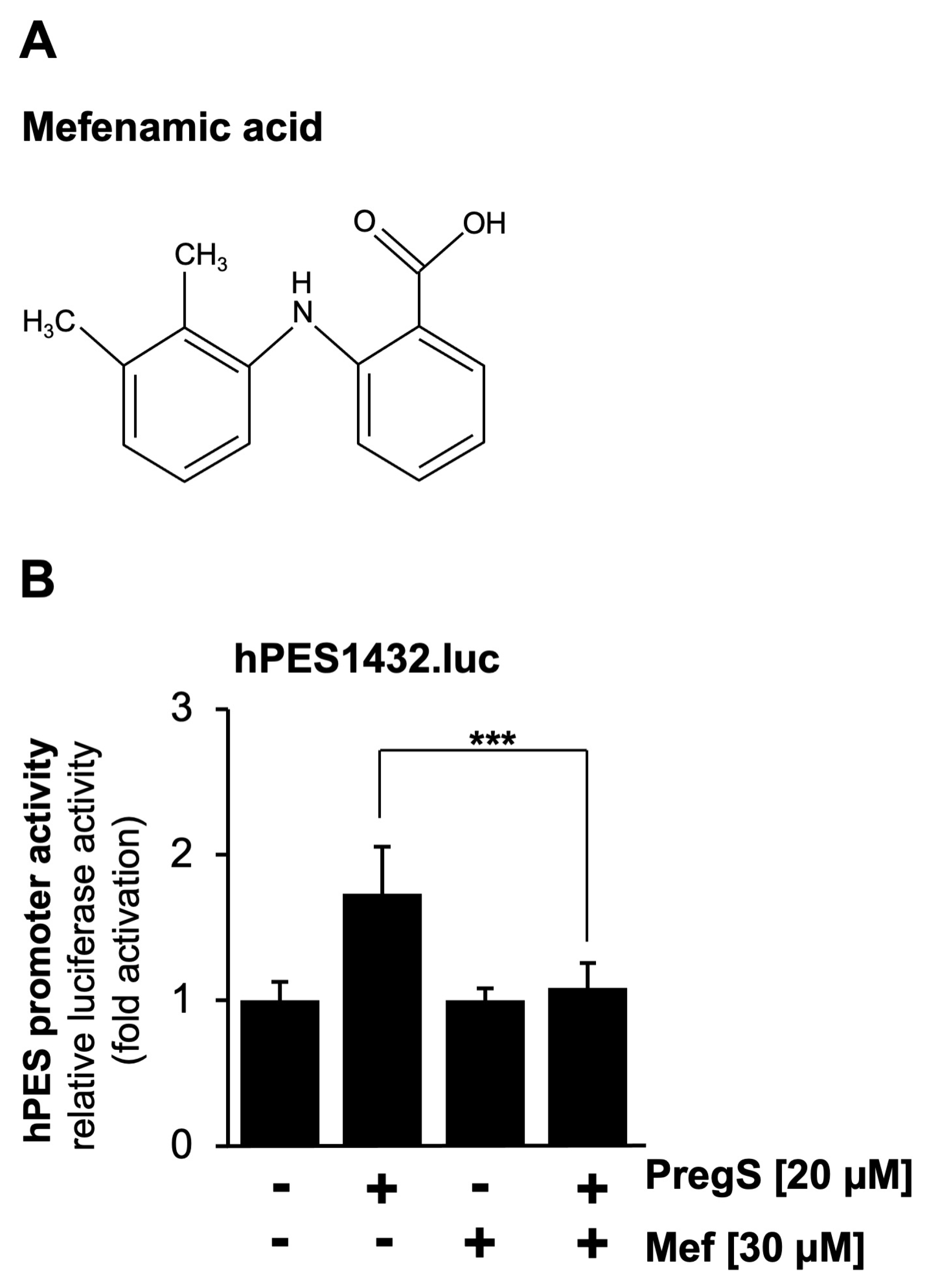
2.3. Pharmacological Inhibition of TRPM3 Channels Impairs Pregnenolone Sulfate-Induced Upregulation of Prostaglandin Endoperoxide Synthase-2 Promoter Activity
2.4. Mutational Analysis of the Human Prostaglandin Endoperoxide Synthase-2 Promoter Identified the CRE as the Pregnenolone Sulfate-Responsive Element
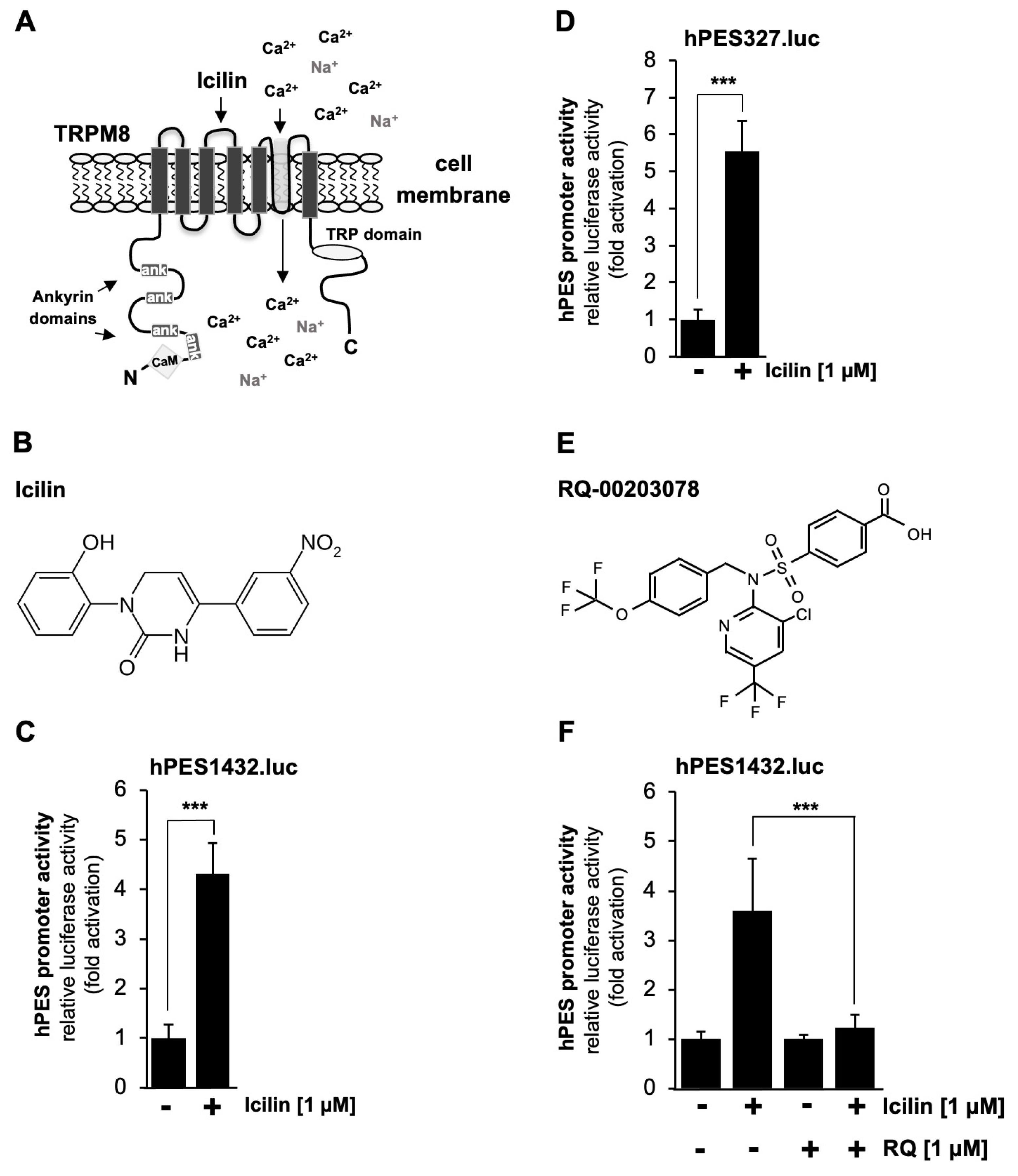
2.5. TRPM8 Channel Stimulation Activates the Prostaglandin Endoperoxide Synthase-2 Promoter
2.6. Pharmacological Inhibition of TRPM8 Channels Impairs Icilin-Induced Upregulation of the Prostaglandin Endoperoxide Synthase-2 Promoter
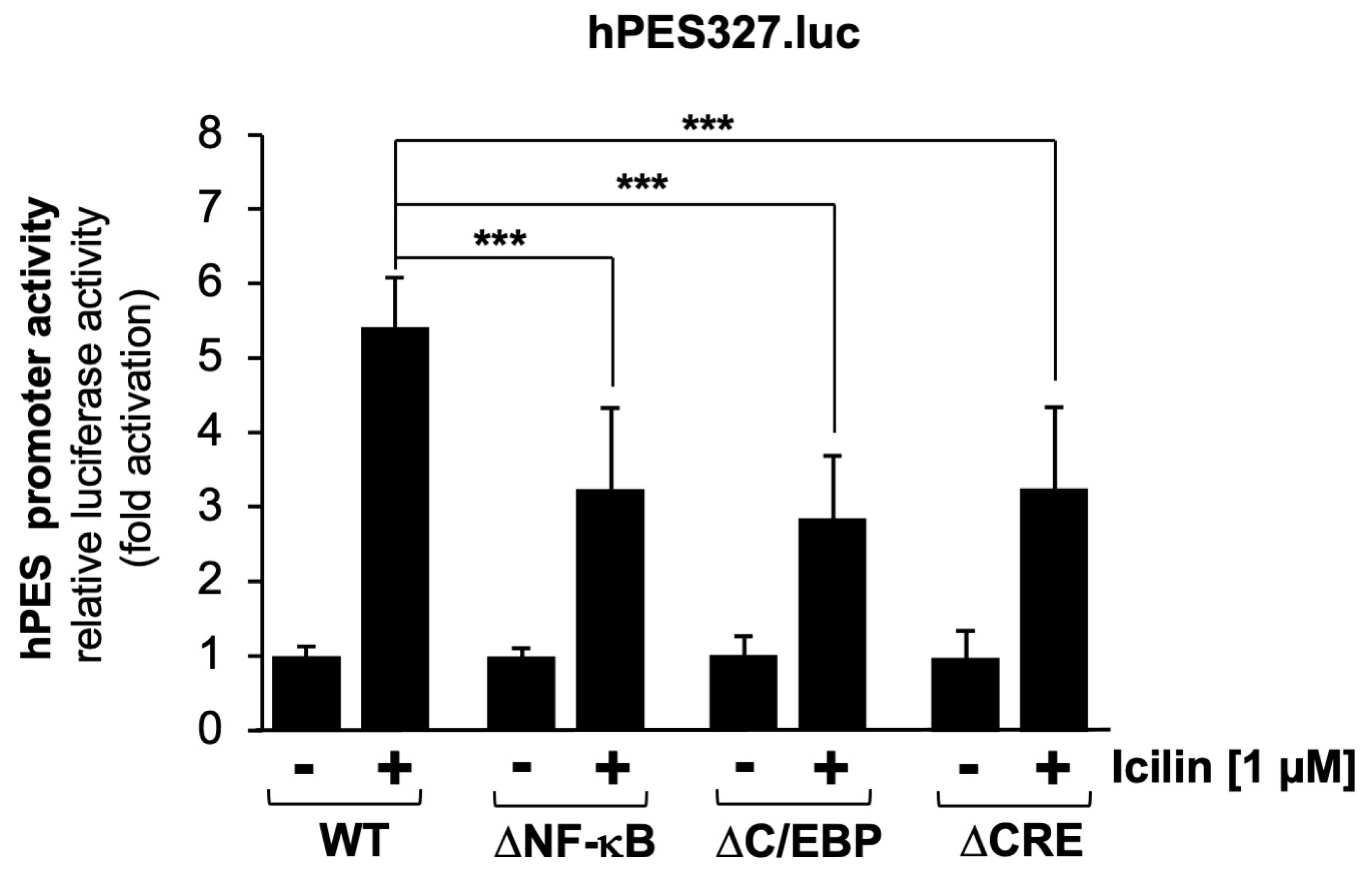
2.7. Mutational Analysis of the Human Prostaglandin Endoperoxide Synthase-2 Promoter Identified Multiple Genetic Elements as Icilin-Responsive Elements
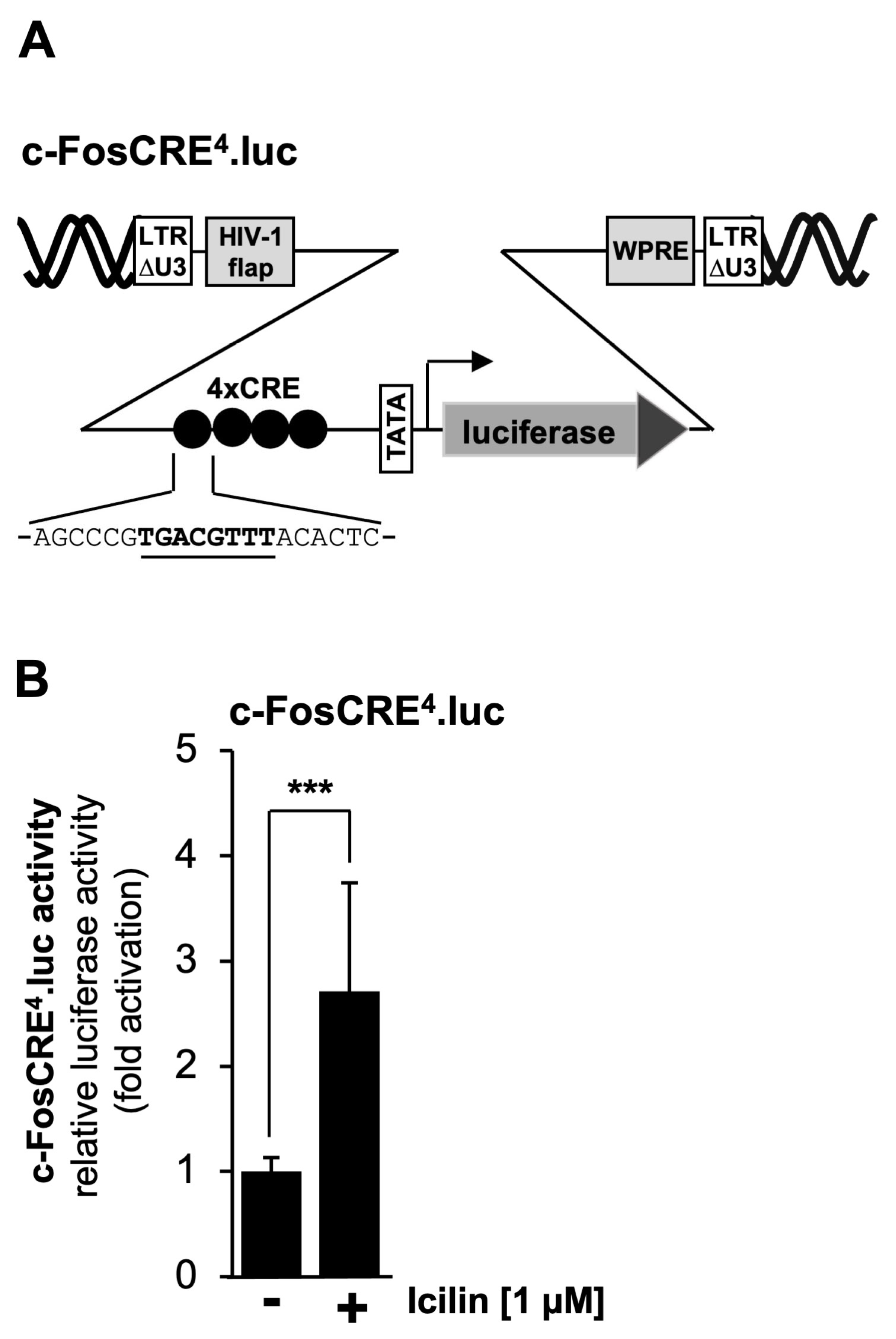
2.8. TRPM8 Channel Stimulation Activates a CRE-Containing Reporter Gene
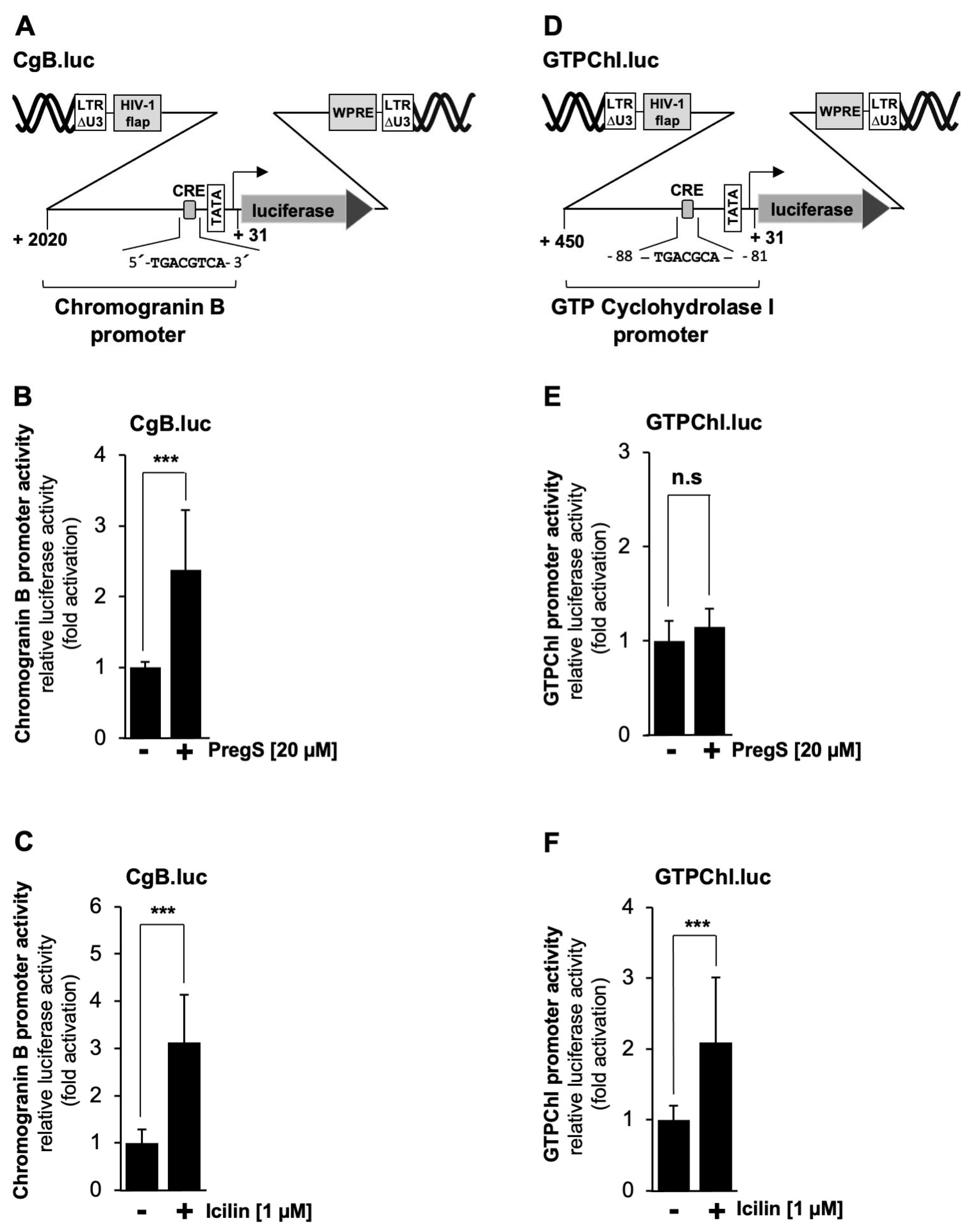
2.9. Activation of CRE-Containing Genes Following Stimulation of TRPM3 or TRPM8 Channels
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Lentiviral Infection
4.3. Reporter Assays
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRE | cyclic AMP response element |
| CREB | cyclic AMP response element-binding protein |
| ERK | extracellular signal-regulated protein kinase |
| PKC | protein kinase C |
| PregS | pregnenolone sulfate |
| TRP | transient receptor potential |
References
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient receptor potential (TRP) channels. Subcell. Biochem. 2018, 87, 141–165. [Google Scholar]
- Nilius, B.; Szallasi, A. Transient receptor potential channels as drug targets: From the science of basic research to the art of medicine. Pharmacol. Rev. 2014, 66, 676–814. [Google Scholar] [CrossRef]
- Thiel, G.; Rubil, S.; Lesch, A.; Guethlein, L.A.; Rössler, O.G. Transient receptor potential TRPM3 channels: Pharmacology, signaling, and biological functions. Pharmacol. Res. 2017, 124, 92–99. [Google Scholar] [CrossRef]
- Held, K.; Tóth, B.I. TRPM3 in brain (patho)physiology. Front. Cell Dev. Biol. 2021, 9, 635659. [Google Scholar] [CrossRef]
- Vriens, J.; Owsianik, G.; Hofmann, T.; Philipp, S.E.; Stab, J.; Chen, X.; Benoit, M.; Xue, F.; Janssens, A.; Kerselaers, S.; et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011, 70, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Vandewauw, I.; De Clercq, K.; Mulier, M.; Held, K.; Pinto, S.; Van Ranst, N.; Segal, A.; Voet, T.; Vennekens, R.; Zimmermann, K.; et al. A TRP channel trio mediates acute noxious heat sensing. Nature 2018, 555, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Cabanas, H.; Muraki, K.; Balinas, C.; Eaton-Fitch, N.; Staines, D.; Marshall-Gradisnik, S. Validation of impaired transient receptor potential melastatin 3 ion channel activity in natural killer cells from chronic fatigue syndrome/myalgic encephalomyelitis patients. Mol. Med. 2019, 25, 14. [Google Scholar] [CrossRef] [PubMed]
- Roelens, R.; Peigneur, A.N.F.; Voets, T.; Vriens, J. Neurodevelopmental disorders caused by variants in TRPM3. Biochim. Biophys. Acta—Mol. Cell Res. 2024, 1871, 119709. [Google Scholar] [CrossRef]
- Zhou, Y.; Bennett, T.M.; Shiels, A. Mutation of the TRPM3 cation channel underlies progressive cataract development and lens calcification associated with pro-fibrotic and immune cell responses. FASEB J. 2021, 35, e21288. [Google Scholar] [CrossRef]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role of TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stuck, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Colburn, R.W.; Lubin, M.L.; Stone, D.J., Jr.; Wang, Y.; Lawrence, D.; D’Andrea, M.R.; Brandt, M.R.; Liu, Y.; Flores, C.M.; Qin, N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007, 54, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 is required for cold sensation in mice. Neuron 2007, 54, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Caceres, A.I.; Liu, B.; Jabba, S.V.; Achanta, S.; Morris, J.B.; Jordt, S.-E. Transient receptor potential cation channel subfamily M member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br. J. Pharmacol. 2017, 174, 867–879. [Google Scholar] [CrossRef]
- Hantute-Ghesquier, A.; Haustrate, A.; Prevarskaya, N.; Lehen’kyi, V. TRPM family channels in cancer. Pharmaceuticals 2018, 11, 58. [Google Scholar] [CrossRef]
- Thiel, G.; Rössler, O.G. Stimulus-transcription coupling of TRPM3 channels: A signaling pathway from the plasma membrane to the nucleus. Biomolecules 2025, 15, 521. [Google Scholar] [CrossRef]
- Ulrich, M.; Wissenbach, U.; Thiel, G. The super-cooling compound icilin stimulates c-Fos and Egr-1 expression and activity involving TRPM8 channel activation, Ca2+ ion influx and activation of the ternary complex factor Elk-1. Biochem. Pharmacol. 2020, 177, 113936. [Google Scholar] [CrossRef]
- Rubil, S.; Lesch, A.; Mukaida, N.; Thiel, G. Stimulation of transient receptor potential M3 (TRPM3) increases interleukin-8 gene promoter activity involving AP-1 and extracellular signal-regulated protein kinase. Cytokine 2018, 103, 133–141. [Google Scholar] [CrossRef]
- Held, K.; Kichko, T.; De Clercq, K.; Klaassen, H.; Van Bree, R.; Vanherck, J.-C.; Marchand, A.; Reeh, P.W.; Chaltin, P.; Voets, T.; et al. Activation of TRPM3 by a potent synthetic ligand reveals a role in peptide release. Proc. Natl. Acad. Sci. USA 2015, 112, E1363–E1372. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Mbonye, U.R.; DeLong, C.J.; Wada, M.; Smith, W.L. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog. Lipid Res. 2007, 46, 108–125. [Google Scholar] [CrossRef]
- Yin, Y.; Park, C.-G.; Feng, S.; Guan, Z.; Lee, H.-J.; Zhang, F.; Sharma, K.; Borgnia, M.J.; Im, W.; Lee, S.-Y. Molecular basis of neurosteroid and anticonvulsant regulation of TRPM3. Nat. Struct. Mol. Biol. 2025, 32, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Peri, K.G.; Almazan, G.; Varma, D.R.; Chemtob, S. A role for protein kinase C alpha in stimulation of prostaglandin G/H synthase-2 transcription by 14,15-epoxyeicosatrienoic acid. Biochem. Biophys. Res. Comm. 1998, 244, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.; Sakaue, M.; Subbaramaiah, K.; Kamitani, H.; Eling, T.E.; Dannenberg, A.J.; Tanabe, T.; Inoue, H.; Arata, J.; Jetten, A.M. Regulation of cyclooxygenase-2 by interferon g and transforming growth factor a in normal human epidermal keratinocytes and squamous carcinoma cells. Role of mitogen-activated protein kinases. J. Biol. Chem. 1999, 274, 29138–29148. [Google Scholar] [CrossRef] [PubMed]
- McGinty, A.; Foschi, M.; Chang, Y.W.; Han, J.; Dunn, M.J.; Sorokin, A. Induction of prostaglandin endoperoxide synthase-2 by mitogen-activated protein kinase cascades. Biochem. J. 2000, 352, 419–424. [Google Scholar] [CrossRef]
- Guo, Y.-S.; Hellmich, M.R.; Wen, X.D.; Townsend, C.M., Jr. Activator protein-1 transcription factor mediates bombesin-stimulated cyclooxygenase-2 expression in intestinal epithelial cells. J. Biol. Chem. 2001, 276, 22941–22947. [Google Scholar] [CrossRef]
- Ogata, S.; Kubota, Y.; Satoh, S.; Ito, S.; Takeuchi, H.; Ashizuka, M.; Shirasuna, K. Ca2+ stimulates COX-2 expression through calcium-sensing receptor in fibroblasts. Biochem. Biophys. Res. Comm. 2006, 351, 808–814. [Google Scholar] [CrossRef]
- Alique, M.; Herrero, J.F.; Lucio-Cazana, J. All-trans retinoic acid induce COX-2 and prostaglandin E2 synthesis in SH-SY5Y human neuroblastoma cells: Involvement of retinoid acid receptor and extracellular-regulated protein kinase 1/2. J. Neuroinflamm. 2007, 4, 1. [Google Scholar] [CrossRef]
- Naidich, M.; Shterntal, B.; Furman, R.; Pawson, A.J.; Jabbour, H.N.; Morgan, K.; Millar, R.P.; Jia, J.; Tomic, M.; Stojikovic, S.; et al. Elucidation of mechanisms of the reciprocal cross talk between gonadotropin-releasing hormone and prostaglandin receptors. Endocrinology 2010, 151, 2700–2712. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Chen, B.-K.; Wang, Y.-S.; Tsai, Y.-T.; Chen, W.-C.; Chang, W.-C.; Hou, M.-F.; Wu, Y.-C.; Chang, W.-C. Involvement of store-operated calcium signaling in EGF-mediated COX-2 gene activation in cancer cells. Cell. Signal. 2012, 24, 162–169. [Google Scholar] [CrossRef]
- Wouters, E.; Hudson, C.A.; McArdle, C.A.; Bernal, A.L. Central role for protein kinase C in oxytocin and epidermal growth factor stimulated cyclooxygenase 2 expression in human myometrial cells. BMC Res. Notes 2014, 7, 357. [Google Scholar] [CrossRef]
- Wong, J.-H.; Ho, K.-H.; Nam, S.; Hsu, W.-L.; Lin, C.-H.; Chang, C.-M.; Wang, J.-Y.; Chang, W.-C. Store-operated Ca2+ entry facilitates the lipopolysaccharide-induced cyclooxygenase-2 expression in gastric cancer cells. Sci. Rep. 2017, 7, 12813. [Google Scholar] [CrossRef]
- Mayer, S.I.; Müller, I.; Mannebach, S.; Endo, T.; Thiel, G. Signal transduction of pregnenolone sulfate in insulinoma cells. Activation of Egr-1 expression involving TRPM3, voltage-gated calcium channels, ERK, and ternary complex factors. J. Biol. Chem. 2011, 286, 10084–10096. [Google Scholar] [CrossRef]
- Lesch, A.; Hui, X.; Lipp, P.; Thiel, G. Transient receptor potential melastatin-3 (TRPM3)-induced activation of AP-1 requires Ca2+ ions and the transcription factors c-Jun, ATF2, and ternary complex factor. Mol. Pharmacol. 2015, 87, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Lesch, A.; Rössler, O.G.; Thiel, G. Extracellular signal-regulated protein kinase, c-Jun N-terminal protein kinase, and calcineurin regulate transient receptor potential M3 (TRPM3) induced activation of AP-1. J. Cell. Biochem. 2017, 118, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Stein, A. Micrococcal nuclease digestion of nuclei reveals extended nucleosome ladders having anomalous DNA lengths for chromatin assembled on non-replicating plasmids in transfected cells. Nucleic Acids Res. 1994, 22, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Hager, G.L. Transcriptional regulation of mammalian genes in vivo. J. Biol. Chem. 1997, 272, 27493–27496. [Google Scholar] [CrossRef]
- Akira, S.; Isshiki, H.; Sugita, T.; Tanabe, O.; Kinoshita, S.; Nishio, Y.; Nakajima, T.; Hirano, T.; Kishimoto, T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990, 9, 1897–1906. [Google Scholar] [CrossRef]
- Klose, C.; Straub, I.; Riehle, M.; Ranta, F.; Krautwurst, D.; Ullrich, S.; Mayerhof, W.; Harteneck, C. Fenamates as TRP channel blocker: Mefenamic acid selectively blocks TRPM3. Br. J. Pharmacol. 2011, 162, 1757–1769. [Google Scholar] [CrossRef]
- Straub, I.; Krügel, U.; Mohr, F.; Teichert, J.; Rizun, O.; Konrad, M.; Oberwinkler, J.; Schaefer, M. Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo. Mol. Pharmacol. 2013, 84, 736–750. [Google Scholar] [CrossRef]
- Lesch, A.; Rubil, S.; Thiel, G. Activation and inhibition of transient receptor potential TRPM3-induced gene transcription. Br. J. Pharmacol. 2014, 171, 2645–2658. [Google Scholar] [CrossRef]
- Souza Bomfim, G.H.; Niemeyer, B.A.; Lacruz, R.S.; Lis, A. On the connections between TRPM channels and SOCE. Cells 2022, 11, 1190. [Google Scholar] [CrossRef]
- Thiel, G.; Backes, T.M.; Welck, J.; Steinhausen, S.; Fischer, A.-L.; Langfermann, D.S.; Ulrich, M.; Wissenbach, U.; Rössler, O.G. Pharmacological inhibition of TRPM8-induced gene transcription. Biochem. Pharmacol. 2019, 170, 113678. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Ohkubo, T.; Ikebe, T.; Yamazaki, J. Blockade of TRPM8 activity reduces the invasion potential of oral squamous carcinoma cell lines. Int. J. Oncol. 2012, 40, 1231–1440. [Google Scholar] [CrossRef] [PubMed]
- Ohmi, M.; Shishido, Y.; Inoue, T.; Ando, K.; Fujiuchi, A.; Yamada, A.; Watanabe, S.; Kawamura, K. Identification of a novel 2-pyridyl-benzensulfonamide derivative, RQ-0020378, as a selective and orally active TRPM8 antagonist. Bioorg. Med. Chem. Lett. 2014, 24, 5364–5368. [Google Scholar] [CrossRef] [PubMed]
- Müller, I.; Rössler, O.G.; Thiel, G. Pregnenolone sulfate activates basic region leucine zipper transcription factors in insulinoma cells: Role of voltage-gated Ca2+ channels and transient receptor potential melastatin 3 channels. Mol. Pharmacol. 2011, 80, 1179–1189. [Google Scholar] [CrossRef]
- Rubil, S.; Rössler, O.G.; Thiel, G. CREB, AP-1, ternary complex factors and MAP kinases connect transient receptor potential melastatin-3 (TRPM3) channel stimulation with increased c-Fos expression. Br. J. Pharmacol. 2016, 173, 305–318. [Google Scholar] [CrossRef]
- Thiel, G.; Rössler, O.G. TRPM3-induced gene transcription is under epigenetic control. Pharmaceuticals 2022, 15, 846. [Google Scholar] [CrossRef]
- Jüngling, S.; Cibelli, G.; Czaeybon, M.; Gerdes, H.H.; Thiel, G. Differential regulation of chromogranin B and synapsin I gene promoter activity by cAMP and cAMP-dependent protein kinase. Eur. J. Biochem. 1994, 226, 925–935. [Google Scholar] [CrossRef]
- Al Sarraj, J.; Vinson, C.; Han, J.; Thiel, G. Regulation of GTP cyclohydrolase I gene transcription by basic region leucine zipper transcription factors. J. Cell. Biochem. 2005, 96, 1003–1030. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef]
- Mayer, S.I.; Rössler, O.G.; Endo, T.; Charnay, P.; Thiel, G. Epidermal growth factor-induced proliferation of astrocytes requires Egr transcription factors. J. Cell Sci. 2009, 122, 3340–3350. [Google Scholar] [CrossRef]
- Müller, I.; Rössler, O.G.; Wittig, C.; Menger, M.D.; Thiel, G. Critical role of Egr transcription factors in regulating insulin biosynthesis, blood glucose homeostasis and islet size. Endocrinology 2012, 153, 3040–3053. [Google Scholar] [CrossRef] [PubMed]
- Thiel, G.; Backes, T.M.; Guethlein, L.A.; Rössler, O.G. Critical protein—Protein interactions determine the biological activity of Elk-1, a master regulator of stimulus-induced gene transcription. Molecules 2021, 26, 6125. [Google Scholar] [CrossRef] [PubMed]
- Loviscach, L.; Backes, T.M.; Langfermann, D.S.; Ulrich, M.; Thiel, G. Zn2+ ions inhibit gene transcription following stimulation of the Ca2+ channels Cav1.2 and TRPM3. Metallomics 2020, 12, 1735–1747. [Google Scholar] [CrossRef] [PubMed]
- Tsagareli, M.G.; Nozadze, I.; Tsiklauri, N.; Gurtskaia, G. Non-steroidal anti-inflammatory drugs attenuate agonist-evoked activation of transcient receptor potential channels. Biomed. Pharmacother. 2018, 97, 745–751. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, W.; Gonzales, M.S.; Finch, J.; Inoue, H.; Bowden, G.T. Role of cyclic AMP responsive element in the UVB induction of cyclooxygenase-2 transcription in human keratinocytes. Oncogene 2001, 20, 5164–5172. [Google Scholar] [CrossRef]
- Xie, W.; Herschman, H.R. Transcriptional regulation of prostaglandin synthase-2 gene expression by platelet-derived growth factor and serum. J. Biol. Chem. 1996, 271, 31742–31748. [Google Scholar] [CrossRef]
- Park, S.-W.; Sung, M.-W.; Heo, D.-S.; Inoue, H.; Shim, S.-H.; Kim, K.-H. Nitric oxide upregulates the cyclooxygenase-2 expression though the cAMP-response element in its promoter in several cancer cell lines. Oncogene 2005, 24, 6689–6698. [Google Scholar] [CrossRef][Green Version]
- Kapatos, G.; Stegenga, S.L.; Hirayama, K. Identification and characterization of basal and cyclic AMP response elements in the promoter of the rat GTP cyclohydrolase I gene. J. Biol. Chem. 2000, 275, 5947–5957. [Google Scholar] [CrossRef]
- Lee, B.; Dziema, H.; Lee, K.H.; Choi, Y.-S.; Obrietan, K. CRE-mediated transcription and COX-2 expression in the pilocarpine model of status epilepticus. Neurobiol. Dis. 2007, 25, 80–91. [Google Scholar] [CrossRef][Green Version]
- Chen, L.-C.; Chen, B.-K.; Chang, W.-C. Activating protein 1-mediated cyclooxygenase-2 expression is independent of N-terminal phosphorylation of c-Jun. Mol. Pharmacol. 2005, 67, 2057–2069. [Google Scholar] [CrossRef]
- Thiel, G.; Rössler, O.G. Signal transduction of transient receptor potential TRPM8 channels: Role of PIP5K, Gq-Proteins, and c-Jun. Molecules 2024, 29, 2602. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Yokoyama, S.; Hara, S.; Tone, Y.; Tanabe, T. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharides and phorbol ester in vascular endothelial cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element. J. Biol. Chem. 1995, 270, 24965–24971. [Google Scholar] [CrossRef]
- Inoue, H.; Umesono, K.; Nishimori, T.; Hirata, Y.; Tanabe, T. Glucocorticoid-mediated suppression of the promoter activity of the cyclooxygenase-2 gene is modulated by expression of its receptor in vascular endothelial cells. Biochem. Biophys. Res. Comm. 1999, 254, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Font-Nieves, M.; Sans-Fons, M.G.; Gorina, R.; Bonfill-Teixidor, E.; Slas-Pérdomo, A.; Márquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Induction of COX-2 enzyme and down-regulation of COX-1 expression by lipopolysaccharide (LPS) control prostaglandin E2 production in astrocytes. J. Biol. Chem. 2012, 287, 6454–6468. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Pang, L.; Inoue, H.; Knox, A.J. Transcriptional regulation of cyclooxygenase 2 by bradykinin and interleukin-1b in human airway smooth muscle cells: Involvement of different promoter elements, transcription factors, and histone H4 acetylation. Mol. Cell. Biol. 2003, 23, 9233–9244. [Google Scholar] [CrossRef] [PubMed]
- Naylor, J.; Milligan, C.J.; Zeng, F.; Jones, C.; Beech, D.J. Production of a specific extracellular inhibitor of TRPM3 channels. Br. J. Pharmacol. 2008, 55, 567–573. [Google Scholar] [CrossRef]
- Bödding, M.; Wissenbach, U.; Flockerzi, V. Characterization of TRPM8 as a pharmacophore receptor. Cell Calcium 2007, 42, 618–628. [Google Scholar] [CrossRef]
- Rössler, O.G.; Thiel, G. Regulation of gene transcription following stimulation of Gαq-coupled designer receptors. In Designer Receptors Exclusively Activated by Designer Drugs; Thiel, G., Ed.; Springer: New York, NY, USA; Humana Press, Neuromethods: Totowa, NJ, USA, 2015; Volume 108, pp. 49–60. [Google Scholar]
- Inoue, H.; Nanayama, T.; Hara, S.; Yokoyama, C.; Tanabe, T. The cyclic AMP response element plays an essential role in the expression of the human prostaglandin-endoperoxide synthase 2 gene in differentiated U937 monocytic cells. FEBS Lett. 1994, 350, 51–54. [Google Scholar] [CrossRef]
- Hirayama, K.; Shimoji, M.; Swick, L.; Meyer, A.; Kapatos, G. Characterization of GTP cyclohydrolase I gene expression in the human neuroblastoma SKN-BE(2)M17: Enhanced transcription in response to cAMP is conferred by the proximal promoter. J. Neurochem. 2001, 79, 576–587. [Google Scholar] [CrossRef]
- Mayer, S.I.; Dexheimer, V.; Nishida, E.; Kitajima, S.; Thiel, G. Expression of the transcriptional repressor ATF3 in gonadotrophs is regulated by Egr-1, CREB, and ATF2 after gonadotropin-releasing hormone receptor stimulation. Endocrinology 2008, 149, 6311–6325. [Google Scholar] [CrossRef]
- Sabnis, A.S.; Reilly, C.A.; Veranth, J.M.; Yost, G.S. Increased transcription of cytokine genes in human lung epithelial cells through activation of a TRPM8 variant by cold temperatures. Am. J. Physiol.—Lung Cell Mol. Physiol. 2008, 295, L194–L200. [Google Scholar] [CrossRef]
- Nakanishi, M.; Hata, K.; Nagayama, T.; Sakurai, T.; Nishisho, T.; Wakabayashi, H.; Hiraga, T.; Ebisu, S.; Yoneda, T. Acid activation of Trpv1 leads to an up-regulation of calcitonin gene-related peptide expression in dorsal root ganglion neurons via the CamK-CREB cascade: A potential mechanism of inflammatory pain. Mol. Biol. Cell 2010, 21, 2568–2577. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandmeier, N.; Rössler, O.G.; Thiel, G. Stimulation of Transient Receptor Potential Channels TRPM3 and TRPM8 Increases Human Prostaglandin Endoperoxide Synthase-2 Promoter Activity. Molecules 2025, 30, 3320. https://doi.org/10.3390/molecules30163320
Brandmeier N, Rössler OG, Thiel G. Stimulation of Transient Receptor Potential Channels TRPM3 and TRPM8 Increases Human Prostaglandin Endoperoxide Synthase-2 Promoter Activity. Molecules. 2025; 30(16):3320. https://doi.org/10.3390/molecules30163320
Chicago/Turabian StyleBrandmeier, Nikolas, Oliver G. Rössler, and Gerald Thiel. 2025. "Stimulation of Transient Receptor Potential Channels TRPM3 and TRPM8 Increases Human Prostaglandin Endoperoxide Synthase-2 Promoter Activity" Molecules 30, no. 16: 3320. https://doi.org/10.3390/molecules30163320
APA StyleBrandmeier, N., Rössler, O. G., & Thiel, G. (2025). Stimulation of Transient Receptor Potential Channels TRPM3 and TRPM8 Increases Human Prostaglandin Endoperoxide Synthase-2 Promoter Activity. Molecules, 30(16), 3320. https://doi.org/10.3390/molecules30163320






