The Effect of Bee Venom and Melittin on Glioblastoma Cells in Zebrafish Model
Abstract
1. Introduction
2. Results
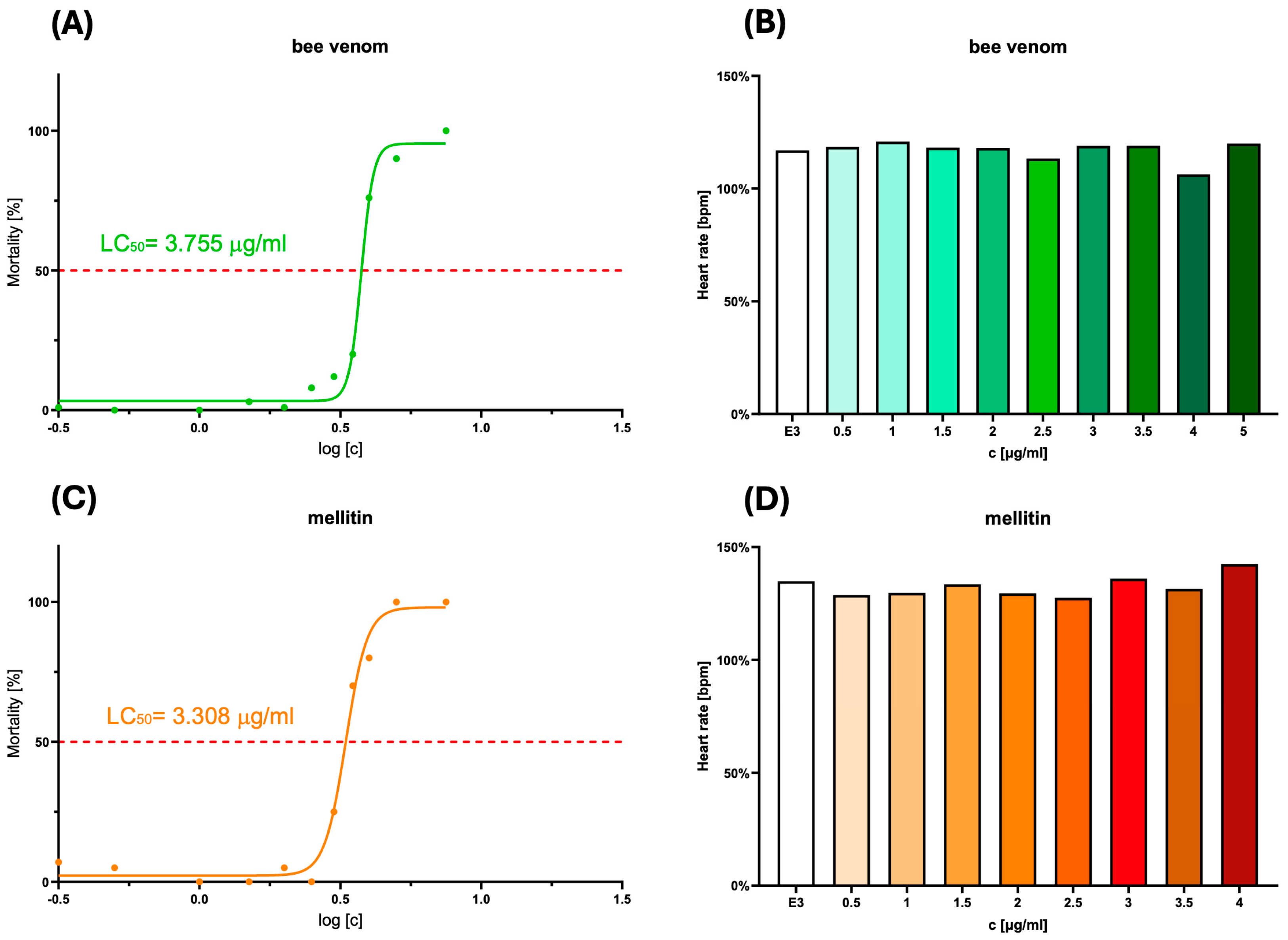
2.1. Estimation of LC50 and Assessment of the Potential Cardiotoxicity of the Substances
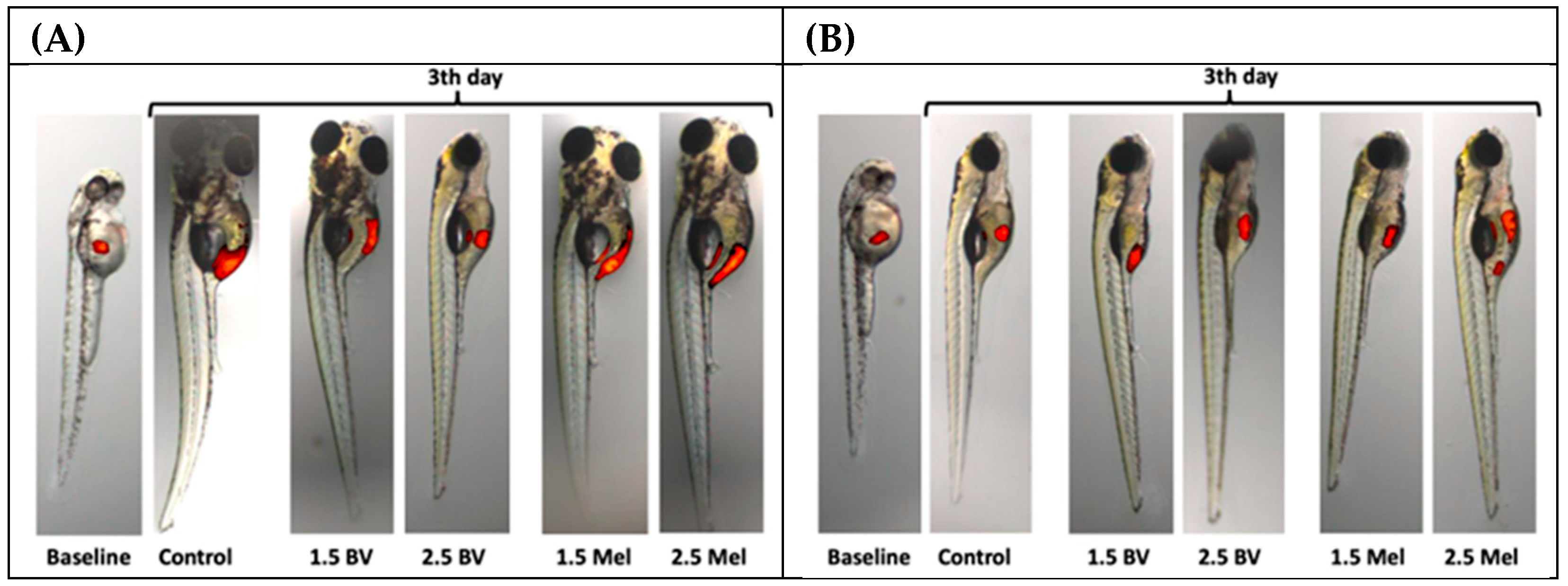
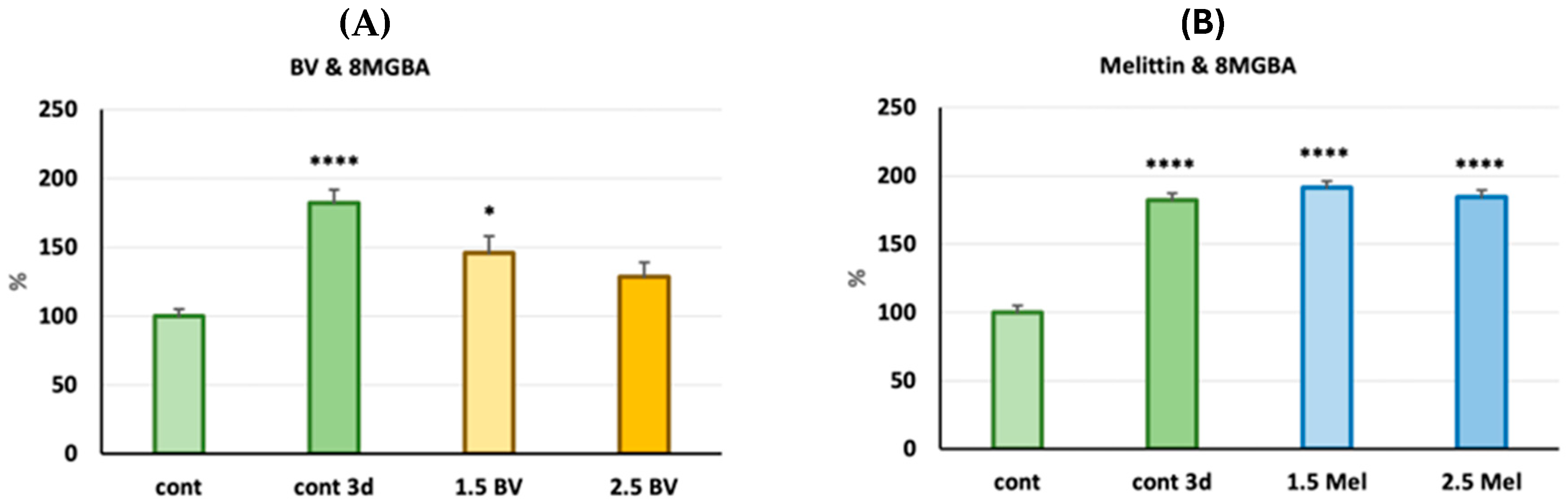
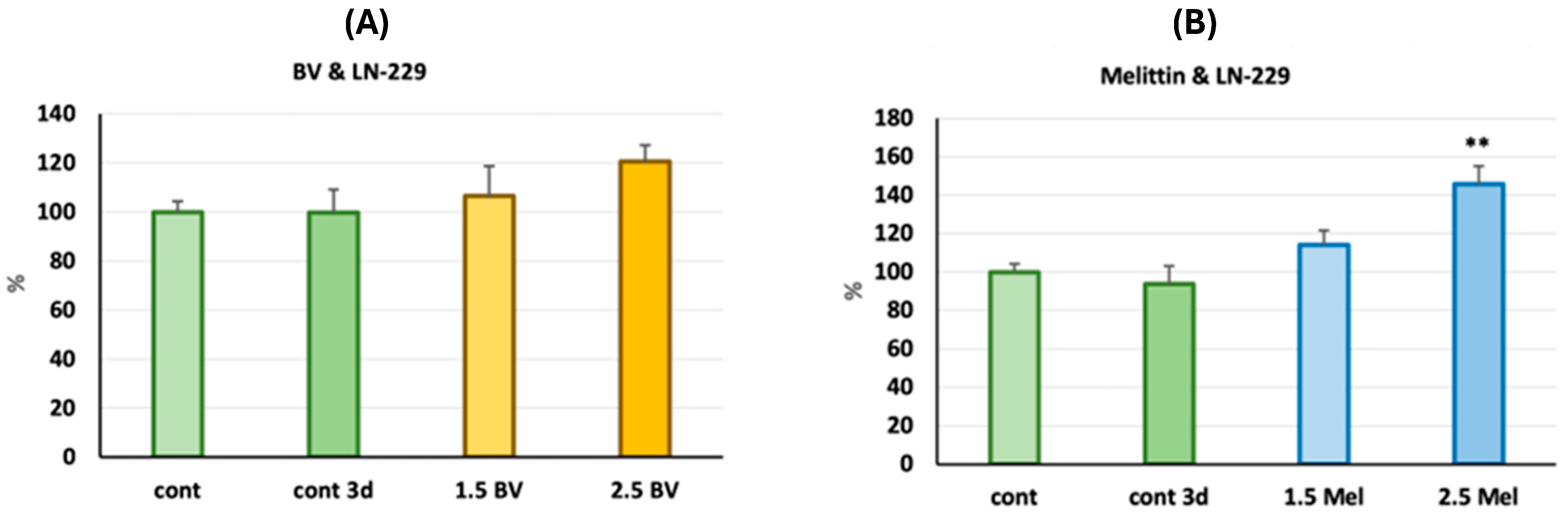
2.2. Assessment of the Antiglioblastoma Activity of Bee Venom vs. Melittin
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Zebrafish Husbandry
4.3. Drug Toxicity
4.4. Zebrafish Xenotransplantation
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BV | Bee venom |
| JAK2 | Janus Kinase 2 |
| MCD | Mast Cell Degranulating |
| NF-κB | Nuclear Factor-κB |
| PLA2 | Phospholipase A2 |
| PLB | Phospholipase B |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
References
- Chang, C.; Chavarro, V.S.; Gerstl, J.V.E.; Blitz, S.E.; Spanehl, L.; Dubinski, D.; Valdes, P.A.; Tran, L.N.; Gupta, S.; Esposito, L.; et al. Recurrent Glioblastoma-Molecular Underpinnings and Evolving Treatment Paradigms. Int. J. Mol. Sci. 2024, 25, 6733. [Google Scholar] [CrossRef]
- Roda, D.; Veiga, P.; Melo, J.B.; Carreira, I.M.; Ribeiro, I.P. Principles in the Management of Glioblastoma. Genes 2024, 15, 501. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Sosnovtseva, A.O.; Valikhov, M.P.; Chernysheva, A.A.; Abramova, O.V.; Pavlov, K.A.; Chekhonin, V.P. Systemic and local immunosuppression in glioblastoma and its prognostic significance. Front. Immunol. 2024, 15, 1326753. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Guz-Montgomery, K.; Lowe, D.B.; Saha, D. Pathogenetic Features and Current Management of Glioblastoma. Cancers 2021, 13, 856. [Google Scholar] [CrossRef]
- Ou, A.; Yung, W.K.A.; Majd, N. Molecular Mechanisms of Treatment Resistance in Glioblastoma. Int. J. Mol. Sci. 2020, 22, 351. [Google Scholar] [CrossRef]
- Lin, S.; Li, K.; Qi, L. Cancer stem cells in brain tumors: From origin to clinical implications. MedComm 2023, 4, e341. [Google Scholar] [CrossRef] [PubMed]
- Baumann, F.; Bjeljac, M.; Kollias, S.S.; Baumert, B.G.; Brandner, S.; Rousson, V.; Yonekawa, Y.; Bernays, R.L. Combined thalidomide and temozolomide treatment in patients with glioblastoma multiforme. J. Neurooncol. 2004, 67, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Riva, M.; Imbesi, F.; Beghi, E.; Galli, C.; Citterio, A.; Trapani, P.; Sterzi, R.; Collice, M. Temozolomide and thalidomide in the treatment of glioblastoma multiforme. Anticancer. Res. 2007, 27, 1067–1071. [Google Scholar]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Nižnanský, Ľ.; Osinová, D.; Kuruc, R.; Hengerics Szabó, A.; Szórádová, A.; Masár, M.; Nižnanská, Ž. Natural Taxanes: From Plant Composition to Human Pharmacology and Toxicity. Int. J. Mol. Sci. 2022, 23, 15619. [Google Scholar] [CrossRef]
- Małek, A.; Strzemski, M.; Kurzepa, J.; Kurzepa, J. Can Bee Venom Be Used as Anticancer Agent in Modern Medicine? Cancers 2023, 15, 3714. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Leonova, E.; Sjakste, N. Bee Venom: Composition and Anticancer Properties. Toxins 2024, 16, 117. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef]
- Badawi, J.K. Bee Venom Components as Therapeutic Tools against Prostate Cancer. Toxins 2021, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Garaj-Vrhovac, V. Melittin: A lytic peptide with anticancer properties. Environ. Toxicol. Pharmacol. 2013, 36, 697–705. [Google Scholar] [CrossRef]
- Oršolić, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Bee Venom Phospholipase A2: Yesterday’s Enemy Becomes Today’s Friend. Toxins 2016, 8, 48. [Google Scholar] [CrossRef]
- Gu, H.; Han, S.M.; Park, K.K. Therapeutic Effects of Apamin as a Bee Venom Component for Non-Neoplastic Disease. Toxins 2020, 12, 195. [Google Scholar] [CrossRef]
- Alvarez-Fischer, D.; Noelker, C.; Vulinović, F.; Grünewald, A.; Chevarin, C.; Klein, C.; Oertel, W.H.; Hirsch, E.C.; Michel, P.P.; Hartmann, A. Bee venom and its component apamin as neuroprotective agents in a Parkinson disease mouse model. PLoS ONE 2013, 8, e61700. [Google Scholar] [CrossRef]
- Proulx, É.; Power, S.K.; Oliver, D.K.; Sargin, D.; McLaurin, J.; Lambe, E.K. Apamin Improves Prefrontal Nicotinic Impairment in Mouse Model of Alzheimer’s Disease. Cereb. Cortex 2020, 30, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hong, J.Y.; Lee, J.; Jeon, W.J.; Ha, I.H. Apamin Enhances Neurite Outgrowth and Regeneration after Laceration Injury in Cortical Neurons. Toxins 2021, 13, 603. [Google Scholar] [CrossRef]
- Lyu, C.; Fang, F.; Li, B. Anti-Tumor Effects of Melittin and Its Potential Applications in Clinic. Curr. Protein Pept. Sci. 2019, 20, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Rady, I.; Siddiqui, I.A.; Rady, M.; Mukhtar, H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017, 402, 16–31. [Google Scholar] [CrossRef]
- Astell, K.R.; Sieger, D. Zebrafish In Vivo Models of Cancer and Metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a037077. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.H.; Chua, H.L.; Gong, Z.; Lam, T.J.; Sin, Y.M. Development and maturation of the immune system in zebrafish, Danio rerio: A gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, A.N. The Importance of Honey Consumption in Human Evolution. Food Foodways 2011, 19, 257–273. [Google Scholar] [CrossRef]
- Yoo, J.; Lee, G. Adverse Events Associated with the Clinical Use of Bee Venom: A Review. Toxins 2022, 14, 562. [Google Scholar] [CrossRef]
- Lebel, A.A.; Kisembo, M.V.; Soucy, M.N.; Hébert, M.P.A.; Morin, P.J.; Boudreau, L.H. Molecular characterization of the anticancer properties associated with bee venom and its components in glioblastoma multiforme. Chem. Biol. Interact. 2021, 347, 109622. [Google Scholar] [CrossRef]
- Ertilav, K.; Nazıroğlu, M. Honey bee venom melittin increases the oxidant activity of cisplatin and kills human glioblastoma cells by stimulating the TRPM2 channel. Toxicon 2023, 222, 106993. [Google Scholar] [CrossRef]
- Małek, A.; Strzemski, M.; Kapka-Skrzypczak, L.; Kurzepa, J. Anticancer Activity of Melittin-Containing Bee Venom Fraction Against Glioblastoma Cells In Vitro. Int. J. Mol. Sci. 2025, 26, 2376. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.; Currie, P. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, T.; Ji, N.; Lu, T.; Yuan, R.; Wu, L.; Zhang, J.; Li, M.; Cao, P.; Zhao, J.; et al. Multi-omics and pharmacological characterization of patient-derived glioma cell lines. Nat. Commun. 2024, 15, 6740. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, T.Y.; Goo, B.; Park, Y. Bee Venom Stimulates Growth Factor Release from Adipose-Derived Stem Cells to Promote Hair Growth. Toxins 2024, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, W.; Jiang, H.; Ma, C.; Huang, M.; Wei, X.; Wang, W.; Jing, L. Zebrafish Xenograft Model for Studying Pancreatic Cancer-Instructed Innate Immune Microenvironment. Int. J. Mol. Sci. 2022, 23, 6442. [Google Scholar] [CrossRef]
- Cornet, C.; Dyballa, S.; Terriente, J.; Di Giacomo, V. ZeOncoTest: Refining and Automating the Zebrafish Xenograft Model for Drug Discovery in Cancer. Pharmaceuticals 2019, 13, 1. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małek, A.; Strzemski, M.; Kurzepa, J. The Effect of Bee Venom and Melittin on Glioblastoma Cells in Zebrafish Model. Molecules 2025, 30, 3306. https://doi.org/10.3390/molecules30153306
Małek A, Strzemski M, Kurzepa J. The Effect of Bee Venom and Melittin on Glioblastoma Cells in Zebrafish Model. Molecules. 2025; 30(15):3306. https://doi.org/10.3390/molecules30153306
Chicago/Turabian StyleMałek, Agata, Maciej Strzemski, and Jacek Kurzepa. 2025. "The Effect of Bee Venom and Melittin on Glioblastoma Cells in Zebrafish Model" Molecules 30, no. 15: 3306. https://doi.org/10.3390/molecules30153306
APA StyleMałek, A., Strzemski, M., & Kurzepa, J. (2025). The Effect of Bee Venom and Melittin on Glioblastoma Cells in Zebrafish Model. Molecules, 30(15), 3306. https://doi.org/10.3390/molecules30153306







