Bioactive Compounds and Antioxidant Activity of Boletus edulis, Imleria badia, Leccinum scabrum in the Context of Environmental Conditions and Heavy Metals Bioaccumulation
Abstract
1. Introduction
2. Results and Discussion
2.1. Soil Properties
2.2. Bioactive Compounds in Boletus edulis, Imleria badia, and Leccinum scabrum
2.2.1. Polysaccharides, Carotenes, and Ascorbic Acid
2.2.2. Organic Acids in Mushroom
2.3. Antioxidant Activity of Boletus edulis, Imleria badia, and Leccinum scabrum
2.4. Heavy Metals in Mushrooms
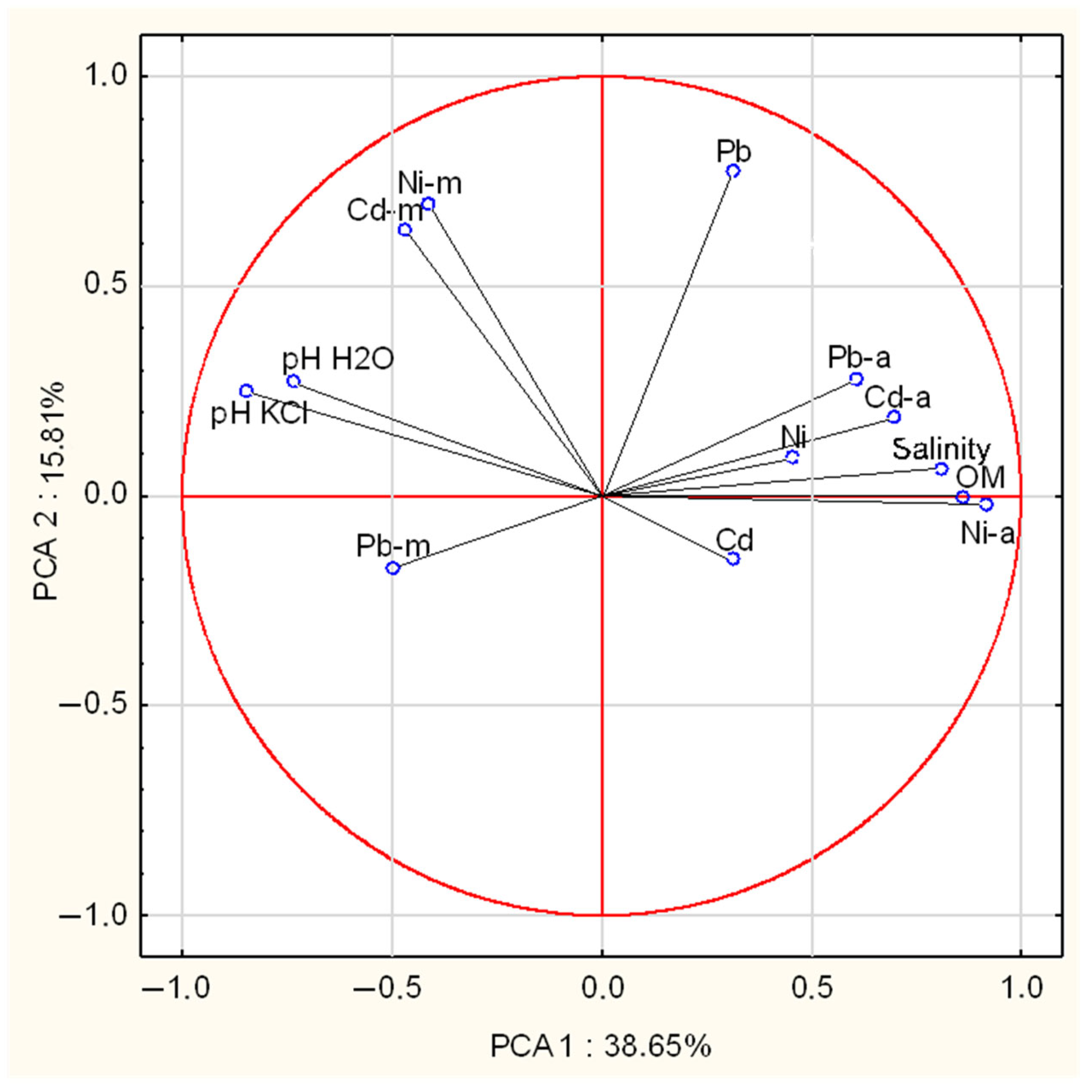
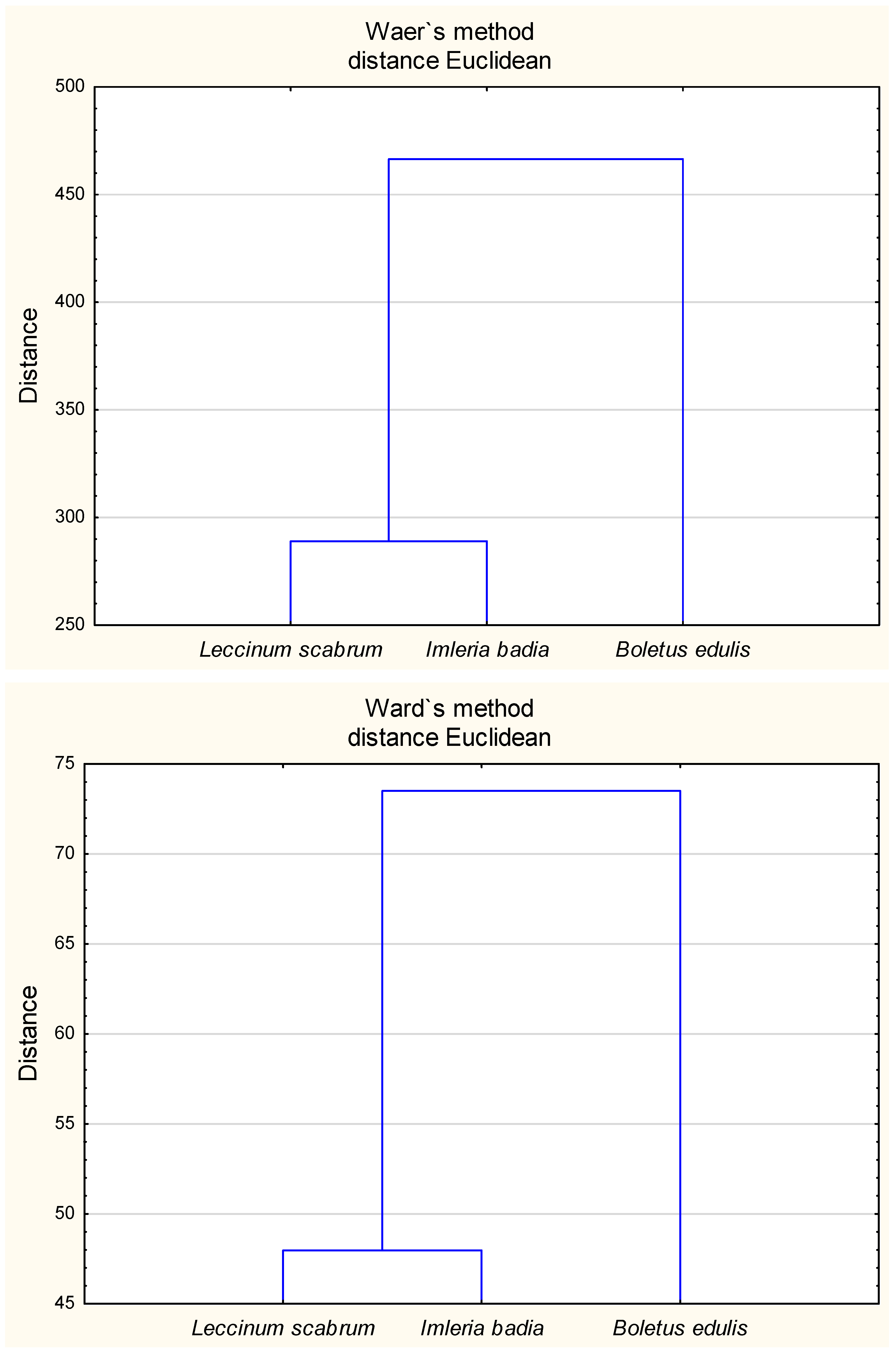
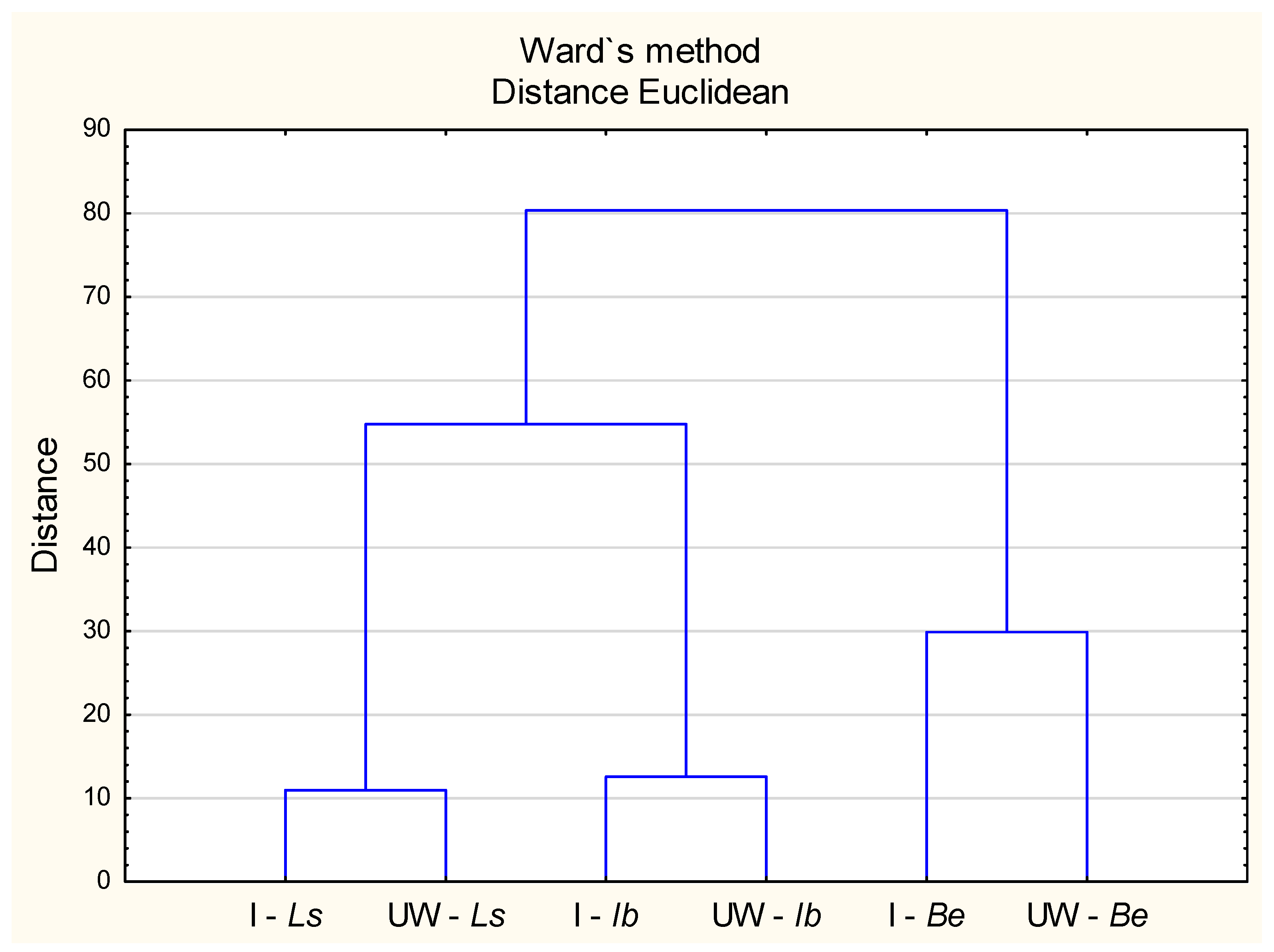
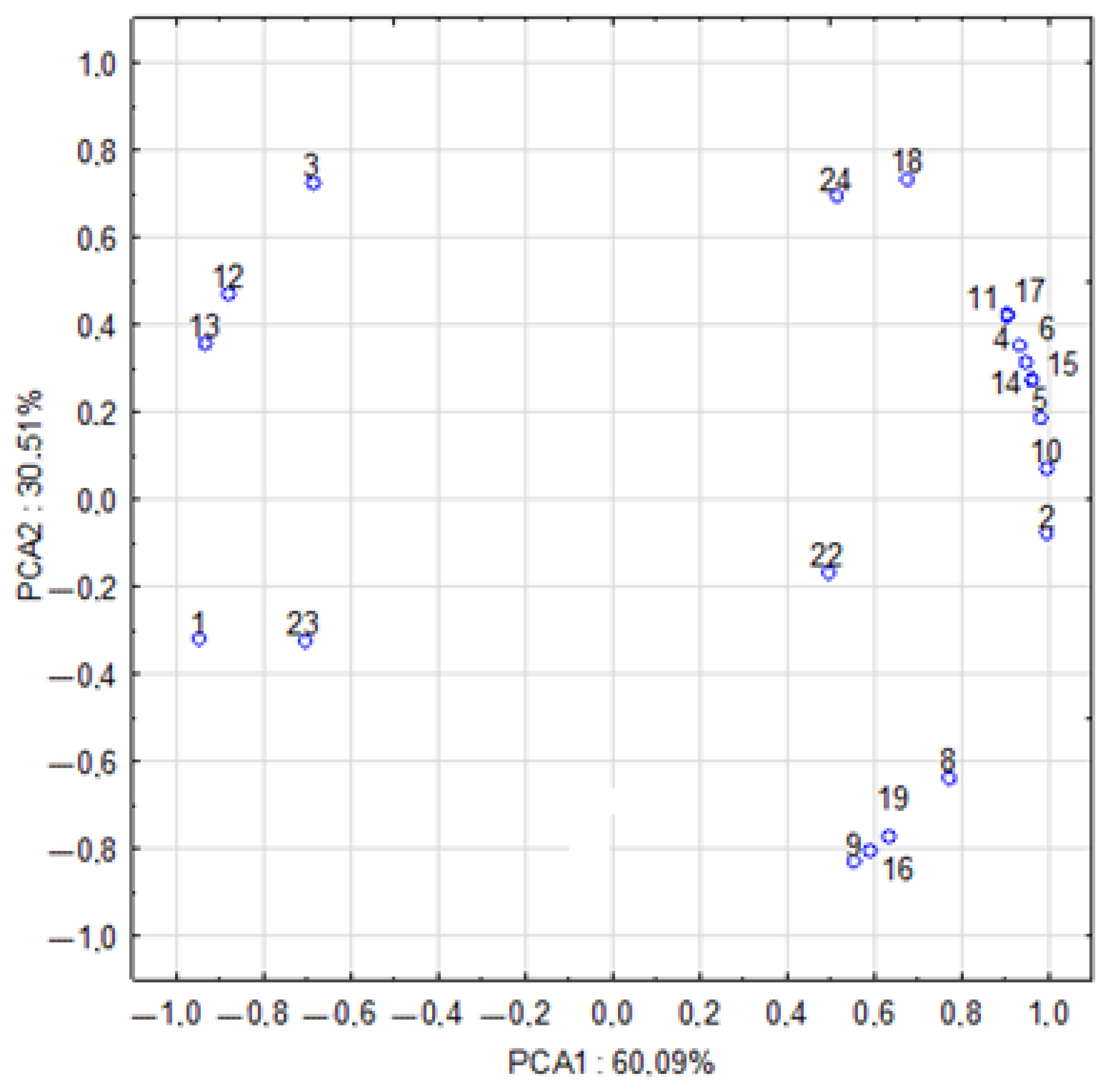
2.5. Relationships Between the Chemical Composition of Mushroom Fruiting Bodies, Antioxidant Activity, Phenolic Compounds, and Heavy Metal Content
3. Materials and Methods
3.1. Study Area and Sampling
3.2. Elemental Analysis
3.2.1. Soil
3.2.2. Mushrooms
3.3. Bioactive Compounds and Health-Promoting Properties
3.3.1. Determination of Bioactive Compounds
3.3.2. Antioxidant Assays
3.3.3. Organic Acids
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, S.T.; Wasser, S.P. The role of culinary-medicinal mushrooms on human welfare with a pyramid model for human health. Int. J. Med. Mushrooms 2012, 14, 95–134. [Google Scholar] [CrossRef] [PubMed]
- Turtiainen, M.; Nuutinen, T. Evaluation of information on wild berry and mushroom markets in European countries. Small-Scale For. 2012, 11, 131–145. [Google Scholar] [CrossRef]
- Fusté-Forné, F. Seasonality in food tourism: Wild foods in peripheral areas. Tour. Geogr. 2019, 24, 578–598. [Google Scholar] [CrossRef]
- Agronomist.pl. Zbiory Grzybów Leśnych w Polsce w 2023 roku. 2024. Available online: https://agronomist.pl/articles/poland-approximately-100-thousand-tons-of-mushrooms-gathered-in-poland-every-year?utm_source=chatgpt.com (accessed on 14 December 2024).
- Zhang, D.; Frankowska, A.; Jarzyńska, G.; Kojta, A.K.; Drewnowska, M.; Wydmańska, D.; Bielawski, L.; Wang, J.; Falandysz, J. Metals of King Bolete (Boletus edulis) Bull.: Fr. collected at the same site over two years. Afr. J. Agric. Res. 2010, 5, 3050–3055. [Google Scholar]
- Zeb, M.; Lee, C.H. Medicinal properties and bioactive compounds from wild mushrooms native to North America. Molecules 2021, 26, 251. [Google Scholar] [CrossRef]
- Robinson, B.; Winans, K.; Kendall, A.; Dlott, J.; Dlott, F. A life cycle assessment of Agaricus bisporus mushroom production in the USA. Int. J. Life Cycle Assess. 2019, 24, 456–467. [Google Scholar] [CrossRef]
- FAO. World Mushroom Production Report 2021; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023. [Google Scholar]
- Yadav, D.; Negi, P.S. Bioactive components of mushrooms: Processing effects and health benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta glucan: Supplement or drug? From laboratory to clinical trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef]
- Xue, X.; Zhou, H.; Gao, J.; Li, X.; Wang, J.; Bai, W.; Bai, Y.; Fan, L.; Chang, H.; Shi, S. The impact of traditional Chinese medicine and dietary compounds on modulating gut microbiota in hepatic fibrosis: A review. Heliyon 2024, 10, e38339. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Barros, L.; Abreu, R.M. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, G.M.; Petraglia, T.; Latronico, T.; Crescenzi, A.; Rossano, R. Antioxidant compounds from edible mushrooms as potential candidates for treating age-related neurodegenerative diseases. Nutrients 2023, 15, 1913. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, L.; Feng, J.; Zhang, J.; Geng, J.; Wang, J.; Yang, Y.; Wu, D.; Liu, Y.; Guo, Q. Preparation, structural characterization, and in vitro modulation of the gut microbiota activity of a novel α-glucan in Hericium erinaceus. J. Agric. Food Chem. 2025, 73, 8338–8351. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Xiao, J.; Xu, B. A critical review on health promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.K.; Pal, R.S.; Arunkumar, R. Antioxidant activities and bioactive compound determination from caps and stipes of specialty medicinal mushrooms Calocybe indica and Pleurotus sajor-caju (higher Basidiomycetes) from India. Int. J. Med. Mushrooms 2014, 16, 555–567. [Google Scholar] [CrossRef]
- Abdulhadi, S.Y.; Gergees, R.N.; Hasan, G.Q. Molecular identification, antioxidant efficacy of phenolic compounds, and antimicrobial activity of beta-carotene isolated from fruiting bodies of Suillus sp. Karbala Int. J. Mod. Sci. 2020, 6, 365–374. [Google Scholar] [CrossRef]
- Heleno, S.A.; Ferreira, R.C.; Antonio, A.L.; Queiroz, M.J.R.; Barros, L.; Ferreira, I.C. Nutritional value, bioactive compounds and antioxidant properties of three edible mushrooms from Poland. Food Biosci. 2015, 11, 48–55. [Google Scholar] [CrossRef]
- Gąsecka, M.; Siwulski, M.; Mleczek, M. Evaluation of bio-active compounds content and antioxidant properties of soil-growing and wood-growing edible mushrooms. J. Food Process. Preserv. 2018, 42, e13386. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of temperatures on polyphenols during extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Narra, F.; Piragine, E.; Benedetti, G.; Ceccanti, C.; Florio, M.; Spezzini, J.; Troisi, F.; Giovannoni, R.; Martelli, A.; Guidi, Ł. Impact of thermal processing on polyphenols, carotenoids, glucosinolates, and ascorbic acid in fruit and vegetables and their cardiovascular benefits. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13426. [Google Scholar] [CrossRef]
- Pérez-Bassart, Z.; Méndez, D.A.; Martínez-Abad, A.; López-Rubio, A.; Fabra, M.J. Emulsifying properties of β-glucan-rich extracts obtained from Pleurotus ostreatus whole mushroom and stipes. Food Hydrocoll. 2024, 153, 109961. [Google Scholar] [CrossRef]
- Espejel-Sánchez, K.I.; Espinosa-Solares, T.; Reyes-Trejo, B.; Hernández-Rodríguez, G.; Cunill-Flores, J.M.; Guerra-Ramírez, D. Nutritional value and thermal degradation of bioactive compounds in wild edible mushrooms. Rev. Chapingo Ser. Cienc. For. Ambient. 2021, 27, 337–354. [Google Scholar] [CrossRef]
- Izham, I.; Avin, F.; Raseetha, S. Systematic review: Heat treatments on phenolic content, antioxidant activity, and sensory quality of Malaysian mushroom: Oyster (Pleurotus spp.) and Black Jelly (Auricularia spp.). Front. Sustain. Food Syst. 2022, 6, 882939. [Google Scholar] [CrossRef]
- Cocchi, L.; Vescovi, L.; Petrini, L.E.; Petrini, O. Heavy metals in edible mushrooms in Italy. Food Chem. 2006, 98, 277–284. [Google Scholar] [CrossRef]
- Pelkonen, R.; Alfthan, G.; Järvinen, O. Cadmium, Lead, Arsenic, and Nickel in Wild Edible Mushrooms; Environment Institute: Helsinki, Finland, 2006. [Google Scholar]
- Falandysz, J.; Borovička, J. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2013, 97, 477–501. [Google Scholar] [CrossRef]
- Balta, I.; Mireșan, V.; Răducu, C.; Odagiu, A.; Tripon, I.; Longodor, A.L.; Butucel, E.; Marchis, Z.; Andronie, L.; Coroian, A. The physico-chemical composition and the level of metals from Boletus edulis and Cantharellus cibarius from the Vatra Dornei area. ProEnviron. Promediu 2018, 11, 241–248. [Google Scholar]
- Kalač, P. Edible Mushrooms: Chemical Composition and Nutritional Value; Elsevier, Academic Press: London, UK, 2016. [Google Scholar]
- Brzezicha-Cirocka, J.; Mędyk, M.; Falandysz, J.; Szefer, P. Bio- and toxic elements in edible wild mushrooms from two regions of potentially different environmental conditions in eastern Poland. Environ. Sci. Pollut. Res. 2016, 23, 21517–21522. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Cadmium in Food: A Public Health Perspective. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cadmium-in-food (accessed on 5 August 2022).
- Gąsecka, M.; Rzymski, P.; Mleczek, M.; Siwulski, M.; Budzyńska, S.; Magdziak, Z.; Niedzielski, P.; Sobieralski, K. The relationship between metal composition, phenolic acid and flavonoid content in Imleria badia from non-polluted and polluted areas. J. Environ. Sci. Health B 2017, 52, 171–177. [Google Scholar] [CrossRef]
- Thabede, P.M.; Shooto, N.D.; Xaba, T.; Naidoo, E.B. Adsorption studies of toxic cadmium (II) and chromium (VI) ions from aqueous solution by activated black cumin (Nigella sativa) seeds. J. Environ. Chem. Eng. 2020, 8, 104045. [Google Scholar] [CrossRef]
- Ghosh, S. Fungi-mediated detoxification of heavy metals. In Recent Advancements in Bioremediation of Metal Contaminants; IGI Global: Hershey, PA, USA, 2021. [Google Scholar] [CrossRef]
- Werkneh, A.A.; Gebru, S.B.; Redae, G.H.; Tsige, A.G. Removal of endocrine disruptors from the contaminated environments: Public health concerns, treatment strategies, and future perspectives—A review. Heliyon 2022, 8, e09206. [Google Scholar] [CrossRef]
- Kavčič, A.; Mikuš, K.; Debeljak, M.; van Elteren, J.T.; Arčon, I.; Kodre, A.; Kump, P.; Karydas, A.G.; Migliori, A.; Czyzycki, M.; et al. Localization, ligand environment, bioavailability and toxicity of mercury in Boletus spp. and Scutiger pes-caprae mushrooms. Ecotoxicol. Environ. Saf. 2019, 184, 109623. [Google Scholar] [CrossRef]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens metropolitan area (Greece). Sci. Total Environ. 2019, 685, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Đurđić, S.; Stanković, V.; Ražić, S.; Mutić, J. Lead isotope ratios as tool for elucidation of chemical environment in a system of Macrolepiota procera (Scop.) Singer-soil. Environ. Sci. Pollut. Res. 2021, 28, 59003–59014. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yan, H.; Chen, J.; Zhang, X. Bioactive proteins from mushrooms. Biotechnol. Adv. 2011, 29, 667–674. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Santoyo, S.; Ramírez-Anguiano, A.C.; Aldars-García, L.; Reglero, G.; Soler-Rivas, C. Antiviral activities of Boletus edulis, Pleurotus ostreatus and Lentinus edodes extracts and polysaccharide fractions against Herpes simplex virus type 1. J. Food Nutr. Res. 2012, 51, 225–235. [Google Scholar]
- Valentão, P.; Lopes, G.; Valente, M.; Barbosa, P.; Andrade, P.B.; Silva, B.M.; Baptista, P.; Seabra, R.M. Quantitation of nine organic acids in wild mushrooms. J. Agric. Food Chem. 2005, 53, 3626–3630. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M. Evaluation of Polish wild mushrooms as beta-glucan sources. Int. J. Environ. Res. Public Health 2020, 17, 7299. [Google Scholar] [CrossRef]
- Ćirić, M.Z.; Dabetić, N.; Todorović, V.; Đuriš, J.; Vidović, B. Beta-glucan content and antioxidant activities of mushroom-derived food supplements. J. Serb. Chem. Soc. 2020, 85, 439–451. [Google Scholar] [CrossRef]
- Wunjuntuk, K.; Ahmad, M.; Techakriengkrai, T.; Chunhom, R.; Jaraspermsuk, E.; Chaisri, A.; Kiwwongngam, R.; Wuttimongkolkul, S.; Charoenkiatkul, S. Proximate composition, dietary fibre, beta-glucan content, and inhibition of key enzymes linked to diabetes and obesity in cultivated and wild mushrooms. J. Food Compos. Anal. 2022, 105, 104226. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Wijesekara, T.; Xu, B. New insights into sources, bioavailability, health-promoting effects, and applications of chitin and chitosan. J. Agric. Food Chem. 2024, 72, 17138–17152. [Google Scholar] [CrossRef]
- Kalač, P. Chemical composition and nutritional value of European species of wild-growing mushrooms: A review. Food Chem. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Jaworska, G.; Pogoń, K.; Skrzypczak, A.; Bernaś, E. Composition and antioxidant properties of wild mushrooms Boletus edulis and Xerocomus badius prepared for consumption. J. Food Sci. Technol. 2015, 52, 7944–7953. [Google Scholar] [CrossRef]
- Valentão, P.; Andrade, P.B.; Rangel, J.; Ribeiro, B.; Silva, B.M.; Baptista, P.; Seabra, R.M. Effect of the conservation procedure on the contents of phenolic compounds and organic acids in chanterelle (Cantharellus cibarius) mushroom. J. Agric. Food Chem. 2005, 53, 4925–4931. [Google Scholar] [CrossRef] [PubMed]
- Dubost, N.J.; Ou, B.; Beelman, R.B. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem. 2007, 105, 727–735. [Google Scholar] [CrossRef]
- Ribeiro, B.; Valentão, P.; Baptista, P.; Seabra, R.M.; Andrade, P.B. Phenolic compounds, organic acids profiles and antioxidative properties of beefsteak fungus (Fistulina hepatica). Food Chem. Toxicol. 2007, 45, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.; Jovin, E.; Malbaša, R.; Matavuly, M.; Popović, M. Medicinal and edible lignicolous fungi as natural sources of antioxidative and antibacterial agents. Phytother. Res. 2010, 24, 1473–1481. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms: Technology and Applications; Springer: Cham, Switzerland, 2017; pp. 5–13. [Google Scholar] [CrossRef]
- Noonan, S.C.; Savage, G.P. Oxalate content of foods and its effect on humans. Asia Pac. J. Clin. Nutr. 1999, 8, 64–74. [Google Scholar] [CrossRef]
- Salgado, N.; Silva, M.A.; Figueira, M.E.; Costa, H.S.; Albuquerque, T.G. Oxalate in foods: Extraction conditions, analytical methods, occurrence, and health implications. Foods 2023, 12, 3201. [Google Scholar] [CrossRef]
- Karr, T.; Guptha, L.S.; Bell, K.; Thenell, J. Oxalates: Dietary oxalates and kidney inflammation—A literature review. Integr. Med. Clin. J. 2024, 23, 36–44. [Google Scholar]
- Zayed, A.; Adly, G.M.; Farag, M.A. Management strategies for the anti-nutrient oxalic acid in foods: A comprehensive overview of its dietary sources, roles, metabolism, and processing. Food Bioprocess Technol. 2025, 18, 4280–4300. [Google Scholar] [CrossRef]
- Balik, M.; Sułkowska-Ziaja, K.J.; Ziaja, M.; Muszyńska, B. Phenolic acids—Occurrence and significance in the world of higher fungi. Med. Int. Rev. 2020, 29, 72–81. [Google Scholar]
- Shen, Y.; Song, X.; Li, L.; Sun, J.; Jaiswal, Y.; Huang, J.; Liu, C.; Yang, W.; Williams, L.; Zhang, H.; et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia—An in vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 111, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Lim, I.F.; Pearson, A.E.; Ralph, J.; Harris, P.J. Bacterial antimutagenesis by hydroxycinnamic acids from plant cell walls. Mutat. Res. Toxicol. Environ. Mutagen. 2003, 542, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.S.; Jeong, C.H.; Choi, J.S.; Kim, K.J.; Jeong, J.W. Antiangiogenic effects of p-coumaric acid in human endothelial cells. Phytother. Res. 2013, 27, 317–323. [Google Scholar] [CrossRef]
- Singha, K.; Banerjee, A.; Jana, A.; Bandyopadhyay, P.; Maiti, S.; Pati, B.R.; Mohapatra, P.K.D. Molecular exposition of broad-spectrum antibacterial efficacy by p-coumaric acid from an edible mushroom Termitomyces heimii: In vitro and in silico approach. Syst. Microbiol. Biomanuf. 2023, 3, 750–764. [Google Scholar] [CrossRef]
- Kim, M.Y.; Seguin, P.; Ahn, J.K.; Kim, J.J.; Chun, S.C.; Kim, E.H.; Seo, S.H.; Kang, E.Y.; Kim, S.L.; Park, Y.J.; et al. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef]
- Palmer, I.A.; Chen, H.; Chen, J.; Chang, M.; Li, M.; Liu, F.; Fu, Z.Q. Novel salicylic acid analogs induce a potent defense response in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 3356. [Google Scholar] [CrossRef]
- Cappellari, L.D.R.; Santoro, M.V.; Schmidt, A.; Gershenzon, J.; Banchio, E. Improving phenolic total content and monoterpene in Mentha × piperita by using salicylic acid or methyl jasmonate combined with Rhizobacteria inoculation. Int. J. Mol. Sci. 2020, 21, 50. [Google Scholar] [CrossRef]
- Çayan, F.; Deveci, E.; Tel-Çayan, G.; Duru, M.E. Identification and quantification of phenolic acid compounds of twenty-six mushrooms by HPLC–DAD. J. Food Meas. Charact. 2020, 14, 1690–1698. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.Y.; Kim, Y.J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Mleczek, M.; Magdziak, Z.; Gąsecka, M.; Niedzielski, P.; Kalač, P.; Siwulski, M.; Rzymski, P.; Zalicka, S.; Sobieralski, K. Content of selected elements and low-molecular-weight organic acids in fruiting bodies of edible mushroom Boletus badius (Fr.) Fr. from unpolluted and polluted areas. Environ. Sci. Pollut. Res. 2016, 23, 20609–20618. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Schmitt-Schillig, S.; Müller, W.E.; Eckert, G.P. Antioxidant properties of Mediterranean food plant extracts: Geographical differences. J. Physiol. Pharmacol. Suppl. 2005, 56, 115–124. [Google Scholar]
- Sotek, Z.; Białecka, B.; Pilarczyk, B.; Kruzhel, B.; Drozd, R.; Pilarczyk, R.; Tomza-Marciniak, A.; Lysak, H.; Bąkowska, M.; Vovk, S. The content of selenium, polyphenols and antioxidative activity in selected medicinal plants from Poland and Western Ukraine. Acta Pol. Pharm. Drug Res. 2018, 75, 1107–1116. [Google Scholar] [CrossRef]
- Piwowarczyk, R.; Ochmian, I.; Lachowicz, S.; Kapusta, I.; Malinowska, K.; Ruraż, K. Correlational nutritional relationships and interactions between expansive holoparasite Orobanche laxissima and woody hosts on metal-rich soils. Phytochemistry 2021, 190, 112844. [Google Scholar] [CrossRef]
- Fogarasi, M.; Socaciu, M.I.; Sălăgean, C.D.; Ranga, F.; Fărcaș, A.C.; Socaci, S.A.; Socaciu, C.; Țibulcă, D.; Fogarasi, S.; Semeniuc, C.A. Comparison of different extraction solvents for characterization of antioxidant potential and polyphenolic composition in Boletus edulis and Cantharellus cibarius mushrooms from Romania. Molecules 2021, 26, 7508. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.C.; Chang, J.S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Phillips, J.M.; Ooi, S.L.; Pak, S.C. Health-promoting properties of medicinal mushrooms and their bioactive compounds for the COVID-19 era—An appraisal: Do the pro-health claims measure up? Molecules 2022, 27, 2302. [Google Scholar] [CrossRef]
- Vetter, J. The mushroom glucans: Molecules of high biological and medicinal importance. Foods 2023, 12, 1009. [Google Scholar] [CrossRef]
- Bernaś, E.; Jaworska, G.; Kmiecik, W. Storage and processing of edible mushrooms. Acta Sci. Pol. Technol. Aliment. 2006, 5, 5–23. [Google Scholar]
- Benchamas, G.; Huang, S.; Huang, G. The influence of traditional and new processing technologies on the structure and function of food polysaccharide. Food Funct. 2020, 11, 5718–5725. [Google Scholar] [CrossRef]
- Shah, P.; Modi, H.A. Comparative study of DPPH, ABTS and FRAP assays for determination of antioxidant activity. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 636–641. [Google Scholar]
- Rosa, G.B.; Sganzerla, W.G.; Ferreira, A.L.A.; Xavier, L.O.; Veloso, N.C.; da Silva, J.; de Oliveira, G.P.; Amaral, N.C.; de Lima Veeck, A.P.; Ferrareze, J.P. Investigation of nutritional composition, antioxidant compounds, and antimicrobial activity of wild culinary-medicinal mushrooms Boletus edulis and Lactarius deliciosus from Brazil. Int. J. Med. Mushrooms 2020, 22, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, E.; Szefer, P.; Falandysz, J. Metals bioaccumulation by bay bolete, Xerocomus badius, from selected sites in Poland. Food Chem. 2004, 84, 405–416. [Google Scholar] [CrossRef]
- Alonso, J.; García, M.A.; Pérez-López, M.; Melgar, M.J. The concentrations and bioconcentration factors of copper and zinc in edible mushrooms. Arch. Environ. Contam. Toxicol. 2003, 44, 180–188. [Google Scholar] [CrossRef]
- Isildak, Ö.; Turkekul, I.; Elmastaş, M.; Tuzen, M. Analysis of heavy metals in some wild-grown edible mushrooms from the Middle Black Sea region, Turkey. Food Chem. 2004, 86, 547–552. [Google Scholar] [CrossRef]
- Rudawska, M.; Leski, T. Macro- and microelement contents in fruiting bodies of wild mushrooms from the Notecka forest in west-central Poland. Food Chem. 2005, 92, 499–506. [Google Scholar] [CrossRef]
- Falandysz, J.; Kunito, T.; Kubota, R.; Bielawski, L.; Mazur, A.; Falandysz, J.J.; Tanabe, S. Selected elements in brown birch scaber stalk, Leccinum scabrum. J. Environ. Sci. Health Part A 2007, 42, 2081–2088. [Google Scholar] [CrossRef]
- Wojciechowska-Mazurek, M.; Mania, M.; Starska, K.; Rebeniak, M.; Karłowski, K. Noxious elements in edible mushrooms in Poland. Bromatol. Chem. Toksykol. 2011, 44, 143–149. [Google Scholar]
- Mleczek, M.; Siwulski, M.; Kaczmarek, Z.; Rissmann, I.; Goliński, P.; Sobieralski, K.; Magdziak, Z. Nutritional elements and aluminum accumulation in Xerocomus badius mushrooms. Acta Sci. Pol. Technol. Aliment. 2013, 12, 411–420. [Google Scholar]
- Kalač, P.; Svoboda, L. A review of trace element concentrations in edible mushrooms. Food Chem. 2000, 69, 273–281. [Google Scholar] [CrossRef]
- García, M.Á.; Alonso, J.; Melgar, M.J. Lead in edible mushrooms: Levels and bioaccumulation factors. J. Hazard. Mater. 2009, 167, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Drewnowska, M.; Falandysz, J. Investigation on mineral composition and accumulation by popular edible mushroom common chanterelle (Cantharellus cibarius). Ecotoxicol. Environ. Saf. 2015, 113, 9–17. [Google Scholar] [CrossRef]
- Sun, L.; Chang, W.; Bao, C.; Zhuang, Y. Metal contents, bioaccumulation, and health risk assessment in wild edible Boletaceae mushrooms. J. Food Sci. 2017, 82, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Gast, C.H.; Jansen, E.; Bierling, J.; Haanstra, L. Heavy metals in mushrooms and their relationship with soil characteristics. Chemosphere 1988, 17, 789–799. [Google Scholar] [CrossRef]
- Michelot, D.; Siobud, E.; Doré, J.C.; Viel, C.; Poirier, F. Update on metal content profiles in mushrooms—Toxicological implications and tentative approach to the mechanisms of bioaccumulation. Toxicon 1998, 36, 1997–2012. [Google Scholar] [CrossRef]
- Frankowska, A.; Ziółkowska, J.; Bielawski, L.; Falandysz, J. Profile and bioconcentration of minerals by King Bolete (Boletus edulis) from the Płocka Dale in Poland. Food Addit. Contam. Part B 2010, 3, 1–6. [Google Scholar] [CrossRef]
- Širić, I.; Kasap, A.; Kos, I.; Markota, T.; Tomić, D.; Poljak, M. Heavy metal contents and bioaccumulation potential of some wild edible mushrooms. Šumarski List 2016, 140, 29–37. [Google Scholar]
- Vassiliou, A. COMMISSION REGULATION (EC) No 629/2008 of 2 July 2008 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2008, L173, 6–9. [Google Scholar]
- EFSA. Opinion of the scientific panel on contaminants in the food chain on a request from the European Parliament related to the safety assessment of wild and farmed fish. EFSA J. 2005, 236, 1–118. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 3rd ed.; Incorporating First and Second Addenda; World Health Organization: Geneva, Switzerland, 2008; Volume 1, Available online: www.who.int/water_sanitation_health/dwq/gdwq3rev/en/ (accessed on 1 May 2025).
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; Available online: www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/ (accessed on 1 May 2025).
- Chiocchetti, G.M.; Latorre, T.; Clemente, M.J.; Jadan-Piedra, C.; Devesa, V.; Vélez, D. Toxic trace elements in dried mushrooms: Effects of cooking and gastrointestinal digestion on food safety. Food Chem. 2020, 306, 125478. [Google Scholar] [CrossRef]
- Sun, L.; Liu, G.; Yang, M.; Zhuang, Y. Bioaccessibility of cadmium in fresh and cooked Agaricus blazei Murill assessed by in vitro biomimetic digestion system. Food Chem. Toxicol. 2012, 50, 1729–1733. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Bikiaris, D.N.; Kyzas, G.Z. Chitin adsorbents for toxic metals: A review. Int. J. Mol. Sci. 2017, 18, 114. [Google Scholar] [CrossRef]
- Yousefi, N.; Jones, M.; Bismarck, A.; Mautner, A. Fungal chitin-glucan nanopapers with heavy metal adsorption properties for ultrafiltration of organic solvents and water. Carbohydr. Polym. 2021, 253, 117273. [Google Scholar] [CrossRef]
- Büyükyörük, S. Chitosan for using food protection. In Chitin and Chitosan-Physicochemical Properties and Industrial Applications; Berrada, M., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V. Advances in porous chitosan-based composite hydrogels: Synthesis and applications. React. Funct. Polym. 2020, 146, 104372. [Google Scholar] [CrossRef]
- Papazov, P.; Denev, P.; Lozanov, V.; Sugareva, P. Profile of antioxidant properties in wild edible mushrooms, Bulgaria. Oxid. Commun. 2021, 44, 523–533. [Google Scholar]
- Li, N.; Shi, J.; Wang, K. Profile and antioxidant activity of phenolic extracts from 10 crabapples (Malus wild species). J. Agric. Food Chem. 2014, 62, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Słaba, M.; Długoński, J. Wiązanie metali ciężkich przez grzyby mikroskopowe. Biotechnologia 2003, 4, 101–109. [Google Scholar]
- Xiong, C. Adsorption of Cadmium (II) by chitin. J. Chem. Soc. Pak. 2010, 32, 429–435. [Google Scholar]
- Hartl, L.; Zach, S.; Seidl-Seiboth, V. Fungal chitinases: Diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol. 2012, 93, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Borówka, R.K. Geographic environment. In The Natural World of Western Pomerania; Borówka, R., Friedrich, S., Heese, T., Jasnowska, J., Kochanowska, R., Opęchowski, M., Stanecka, E., Zyska, W., Janicka, D., Gamrat, R., et al., Eds.; Oficyna in Plus: Szczecin, Poland, 2007. [Google Scholar]
- Koźmiński, C.; Michalska, B.; Czarnecka, M. Klimat Województwa Zachodniopomorskiego; AR Szczecin, Uniwersytet Szczeciński: Szczecin, Poland, 2007. [Google Scholar]
- Malinowski, R.; Sotek, Z.; Stasińska, M.; Malinowska, K.; Radke, P.; Malinowska, A. Bioaccumulation of macronutrients in edible mushrooms in various habitat conditions of NW Poland—Role in the human diet. Int. J. Environ. Res. Public Health 2021, 18, 8881. [Google Scholar] [CrossRef]
- Sotek, Z.; Stasińska, M.; Malinowski, R.; Pilarczyk, B.; Pilarczyk, R.; Bąkowska, M.; Malinowska, K.; Radke, P.; Kubus, M.; Malinowska, A.; et al. The role in the human diet of bioaccumulation of selenium, copper, zinc, manganese, and iron in edible mushrooms in various habitat conditions of NW Poland—A case study. Sustainability 2023, 15, 13334. [Google Scholar] [CrossRef]
- Knudsen, H.; Vesterholt, J. Funga Nordica: Agaricoid, Boletoid, Cyphelloid, and Gastroid Genera; Nordsvamp: Copenhagen, Denmark, 2012. [Google Scholar]
- Barros, L.; Cruz, T.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem. Toxicol. 2008, 46, 2742–2747. [Google Scholar] [CrossRef]
- Ride, J.P.; Drysdale, R.B. A rapid method for the chemical estimation of filamentous fungi in plant tissue. Physiol. Plant Pathol. 1972, 2, 7–15. [Google Scholar] [CrossRef]
- Mijowska, K.; Ochmian, I.; Oszmiański, J. Impact of cluster zone leaf removal on grapes cv. Regent polyphenol content by the UPLC-PDA/MS method. Molecules 2016, 21, 1688. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
| Species of Mushroom | ||||
|---|---|---|---|---|
| Location (Physical-Geographical Region) | Boletus edulis | Imleria badia | Leccinum scabrum | |
| OM [%] | Uznam–-Wolin | 5.06 a * | 31.76 ab | 64.83 b |
| Ińsko Lake District | 9.19 a | 23.78 a | 19.39 a | |
| mean | 7.12 A | 27.77 AB | 42.11 B | |
| pH in KCl | Uznam–Wolin | 3.39 b | 2.64 a | 2.58 a |
| Ińsko Lake District | 3.17 b | 2.98 ab | 3.20 b | |
| mean | 3.28 B | 2.81 A | 2.89 A | |
| pH in H2O | Uznam–Wolin | 3.72 ab | 3.43 ab | 3.32 a |
| Ińsko Lake District | 3.80 b | 3.67 ab | 3.53 ab | |
| mean | 3.76 B | 3.55 AB | 3.42 A | |
| Salinity [µS/cm] | Uznam–Wolin | 91.94 a | 271.35 c | 214.83 bc |
| Ińsko Lake District | 132.80 ab | 121.22 ab | 129.05 ab | |
| mean | 112.37 A | 196.28 B | 171.28 AB | |
| Ni [mg/kg] | Uznam–Wolin | 0.45 a | 2.34 c | 2.23 c |
| Ińsko Lake District | 1.10 b | 1.40 b | 1.37 b | |
| mean | 0.77 A | 1.87 B | 1.80 B | |
| Cd [mg/kg] | Uznam–Wolin | 0.23 c | 0.24 c | 0.28 c |
| Ińsko Lake District | 0.01 a | 0.06 ab | 0.17 b | |
| mean | 0.12 A | 0.15 A | 0.22 A | |
| Pb [mg/kg] | Uznam–Wolin | 10.08 ab | 23.00 b | 18.76 ab |
| Ińsko Lake District | 11.47 ab | 11.69 ab | 5.84 a | |
| mean | 10.77 A | 17.34 A | 12.30 A | |
| Species of Mushroom | ||||
|---|---|---|---|---|
| Location (Physical-Geographical Region) | Boletus edulis | Imleria badia | Leccinum scabrum | |
| Chitin (g/100 g) | Uznam–Wolin | 22.9 bc * | 20.9 ab | 27.9 e |
| Ińsko Lake District | 24.7 d | 23.6 cd | 19.1 a | |
| mean | 23.8 A | 22.2 A | 23.5 A | |
| β-glucan (g/100 g) | Uznam–Wolin | 48.6 f | 30.3 c | 23.7 a |
| Ińsko Lake District | 44.6 e | 37.7 d | 26.7 b | |
| mean | 46.6 C | 34.0 B | 25.2 A | |
| α-glucan (g/100 g) | Uznam–Wolin | 3.72 bc | 3.05 a | 3.06 a |
| Ińsko Lake District | 4.13 c | 3.56 b | 4.11 c | |
| mean | 3.93 B | 3.31 A | 3.59 AB | |
| L-ascorbic acid (mg/100 g) | Uznam–Wolin | 25.6 b | 48.9 d | 33.7 c |
| Ińsko Lake District | 14.8 a | 37.8 c | 24.5 b | |
| mean | 20.2 A | 43.35 C | 29.1 B | |
| β-carotene (mg/100 g) | Uznam–Wolin | 2.78 cd | 2.26 b | 2.33 b |
| Ińsko Lake District | 1.98 a | 2.82 d | 2.65 c | |
| mean | 2.38 A | 2.54 A | 2.49 A | |
| Lycopene (mg/100 g) | Uznam–Wolin | 1.43 d | 1.02 c | 0.74 bb |
| Ińsko Lake District | 1.28 d | 0.76 | 0.55 a | |
| mean | 1.36 C | 0.89 B | 0.65 A | |
| Species of Mushroom | ||||
|---|---|---|---|---|
| (mg/100 g d.w.) | Location (Physical-Geographical Region) | Boletus edulis | Imleria badia | Leccinum scabrum |
| Quinic acid | Uznam–Wolin | n.d. | n.d. | 1.04 b * |
| Ińsko Lake District | n.d. | n.d. | 0.55 a | |
| mean | 1.67 | |||
| Oxalic acid | Uznam–Wolin | 52.94 e | 24.67 b | 1.13 a |
| Ińsko Lake District | 37.06 c | 42.78 d | 0.78 a | |
| mean | 45.00 C | 33.73 B | 0.96 A | |
| Fumaric acid | Uznam–Wolin | 4.04 b | 21.56 d | 0.34 a |
| Ińsko Lake District | 5.11 bc | 5.78 c | 0.12 a | |
| mean | 4.58 B | 13.67 C | 0.23 A | |
| Citric acid | Uznam–Wolin | n.d. | 112.26 c | 11.51 b |
| Ińsko Lake District | 2.04 a | 128.34 d | 10.96 b | |
| mean | 2.04 A | 120.30 C | 11.24 B | |
| Succinic acid | Uznam–Wolin | 3.19 c | n.d. | 0.72 a |
| Ińsko Lake District | 1.17 b | n.d. | 0.68 a | |
| mean | 2.18 B | n.d. | 0.70 A | |
| Cinnamic acid | Uznam–Wolin | 0.23 b | 1.10 c | n.d. |
| Ińsko Lake District | 0.09 a | 2.58 d | n.d. | |
| mean | 0.16 A | 1.84 B | ||
| Caffeic acid | Uznam–Wolin | 0.18 a | n.d. | 0.35 a |
| Ińsko Lake District | 1.33 c | n.d. | 0.77 b | |
| mean | 0.76 A | n.d. | 0.56 A | |
| Chlorogenic acid | Uznam–Wolin | 12.55 f | 1.25 d | 0.17 a |
| Ińsko Lake District | 9.34 e | 1.06 c | 0.78 b | |
| mean | 10.95 C | 1.16 B | 0.48 A | |
| Ferulic acid | Uznam–Wolin | 1.04 c | n.d. | 0.21 a |
| Ińsko Lake District | 0.78 b | n.d. | 0.17 a | |
| mean | 0.91 B | n.d. | 0.19 A | |
| p-Coumaric acid | Uznam–Wolin | 78.36 b | n.d. | N.D. |
| Ińsko Lake District | 30.11 a | n.d. | N.D. | |
| mean | 54.24 | n.d. | ||
| Galic acid | Uznam–Wolin | 8.21 c | 0.98 b | 17.83 e |
| Ińsko Lake District | 1.09 b | 0.20 a | 11.28 d | |
| mean | 4.65 | 0.59 | 14.56 | |
| Salicylic acid | Uznam–Wolin | N.D. | 0.12 a | 1.79 c |
| Ińsko Lake District | N.D. | 0.09 a | 1.33 b | |
| mean | 0.11 A | 1.56 B | ||
| Vanillic acid | Uznam–Wolin | 18.33 c | n.d. | 3.05 b |
| Ińsko Lake District | 20.58 d | n.d. | 1.17 a | |
| mean | 19.46 B | n.d. | 2.11 A | |
| Quercetin | Uznam–Wolin | 2.38 c | 1.45 ab | 6.77 d |
| Ińsko Lake District | 1.85 b | 1.11 a | 9.12 e | |
| mean | 2.12 B | 1.28 A | 7.95 C | |
| Catechin | Uznam–Wolin | 4.90 a | 12.59 b | 25.61 d |
| Ińsko Lake District | 12.31 b | 17.33 c | 30.54 e | |
| mean | 8.61 A | 14.96 B | 28.08 C | |
| Species of Mushroom | ||||
|---|---|---|---|---|
| Location (Physical-Geographical Region) | Boletus edulis | Imleria badia | Leccinum scabrum | |
| DPPH· (mmol TE/100 g) | Uznam–Wolin | 28.9 c * | 40.1 d | 10.6 a |
| Ińsko Lake District | 26.9 c | 54.5 e | 17.8 b | |
| mean | 27.9 B | 47.3 C | 14.2 A | |
| ABTS·+ (mmol TE/100 g) | Uznam–Wolin | 12.4 ab | 29.8 c | 35.7 e |
| Ińsko Lake District | 10.9 a | 14.6 b | 32.1 d | |
| mean | 11.7 A | 22.2 B | 33.9 C | |
| FRAP (mmol Fe2+/100 g) | Uznam–Wolin | 22.9 b | 43.1 d | 58.3 f |
| Ińsko Lake District | 16.4 a | 27.9 c | 49.0 e | |
| mean | 19.7 A | 35.5 B | 53.7 C | |
| Species of Mushroom | ||||
|---|---|---|---|---|
| Location (Physical-Geographical Region) | Boletus edulis | Imleria badia | Leccinum scabrum | |
| Cd soil | Uznam–Wolin | 1.46 a * | 1.62 a | 1.71 a |
| Ińsko Lake District | 1.14 a | 1.75 a | 1.26 a | |
| mean | 1.30 A | 1.68 A | 1.49 A | |
| mushrooms | Uznam–Wolin | 2.83 d | 0.74 b | 0.31 ab |
| Ińsko Lake District | 2.64 d | 0.11 a | 1.93 c | |
| mean | 2.74 C | 0.43 A | 1.12 B | |
| Pb soil | Uznam–Wolin | 12.59 a | 25.65 a | 27.58 a |
| Ińsko Lake District | 30.96 a | 19.52 a | 17.53 a | |
| mean | 21.77 A | 22.58 A | 22.56 A | |
| mushrooms | Uznam–Wolin | 1.58 b | 0.61 a | 0.87 a |
| Ińsko Lake District | 1.64 b | 2.67 d | 2.22 c | |
| mean | 1.61 A | 1.64 A | 1.55 A | |
| Ni soil | Uznam–Wolin | 4.34 a | 7.84 b | 6.92 b |
| Ińsko Lake District | 7.75 b | 7.22 b | 6.48 ab | |
| mean | 6.04 A | 7.53 A | 7.70 A | |
| mushrooms | Uznam–Wolin | 3.92 b | 3.07 a | 3.09 a |
| Ińsko Lake District | 5.89 c | 3.34 a | 3.26 a | |
| mean | 4.91 B | 3.21 A | 3.18 A | |
| Species of Mushroom | ||||
|---|---|---|---|---|
| Location (Physical-Geographical Region) | Boletus edulis | Imleria badia | Leccinum scabrum | |
| Ni | Uznam–Wolin | 0.9 | 0.4 | 0.4 |
| Ińsko Lake District | 0.8 | 0.5 | 0.5 | |
| mean | 0.8 | 0.4 | 0.4 | |
| Cd | Uznam–Wolin | 1.9 | 0.5 | 0.2 |
| Ińsko Lake District | 2.3 | 0.1 | 1.5 | |
| mean | 2.1 | 0.3 | 0.8 | |
| Pb | Uznam–Wolin | 0.1 | 0.02 | 0.03 |
| Ińsko Lake District | 0.1 | 0.1 | 0.1 | |
| mean | 0.1 | 0.1 | 0.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotek, Z.; Malinowska, K.; Stasińska, M.; Ochmian, I. Bioactive Compounds and Antioxidant Activity of Boletus edulis, Imleria badia, Leccinum scabrum in the Context of Environmental Conditions and Heavy Metals Bioaccumulation. Molecules 2025, 30, 3277. https://doi.org/10.3390/molecules30153277
Sotek Z, Malinowska K, Stasińska M, Ochmian I. Bioactive Compounds and Antioxidant Activity of Boletus edulis, Imleria badia, Leccinum scabrum in the Context of Environmental Conditions and Heavy Metals Bioaccumulation. Molecules. 2025; 30(15):3277. https://doi.org/10.3390/molecules30153277
Chicago/Turabian StyleSotek, Zofia, Katarzyna Malinowska, Małgorzata Stasińska, and Ireneusz Ochmian. 2025. "Bioactive Compounds and Antioxidant Activity of Boletus edulis, Imleria badia, Leccinum scabrum in the Context of Environmental Conditions and Heavy Metals Bioaccumulation" Molecules 30, no. 15: 3277. https://doi.org/10.3390/molecules30153277
APA StyleSotek, Z., Malinowska, K., Stasińska, M., & Ochmian, I. (2025). Bioactive Compounds and Antioxidant Activity of Boletus edulis, Imleria badia, Leccinum scabrum in the Context of Environmental Conditions and Heavy Metals Bioaccumulation. Molecules, 30(15), 3277. https://doi.org/10.3390/molecules30153277







