From Nutrition to Innovation: Biomedical Applications of Egg Components
Abstract
1. Introduction
| Bioactive Compound | Functional Properties |
|---|---|
| Lysozyme | Antibacterial [11,12], anti-cancer [13], antiviral [14], immunomodulation [15], antihypertensive [16] |
| Ovalbumin | Antibacterial [17,18], antioxidant [19], immunomodulation [20] |
| Ovotransferrin | Antibacterial [21], anti-inflammatory [22], antioxidant [23], antiviral [24], immunomodulation [25] |
| Ovomucin | Antibacterial [26], anti-cancer [27,28], anti-adhesive [29], antioxidant [30], antiviral [28], immunomodulation [31] |
| Avidin | Antibacterial [32] |
| Phosvitin | Antibacterial [33], anti-inflammatory [34], antioxidant [35] |
| IgY | Antibacterial [36], anti-cancer [37], antiviral [38], immunomodulation [39] |
| Phospholipids | Anti-inflammatory [40], antioxidant [41] |
| Lutein/Zeaxanthin | Anti-inflammatory, antioxidant [42] |
| High-density Lipoproteins | Anti-inflammatory, antioxidant [43] |
| Cystatin | Protease inhibition [44] |
| Ovoinhibitor | Antibacterial [45], protease inhibition [46] |
| Egg Yolk Hydrolysates | Antihypertensive [47] |
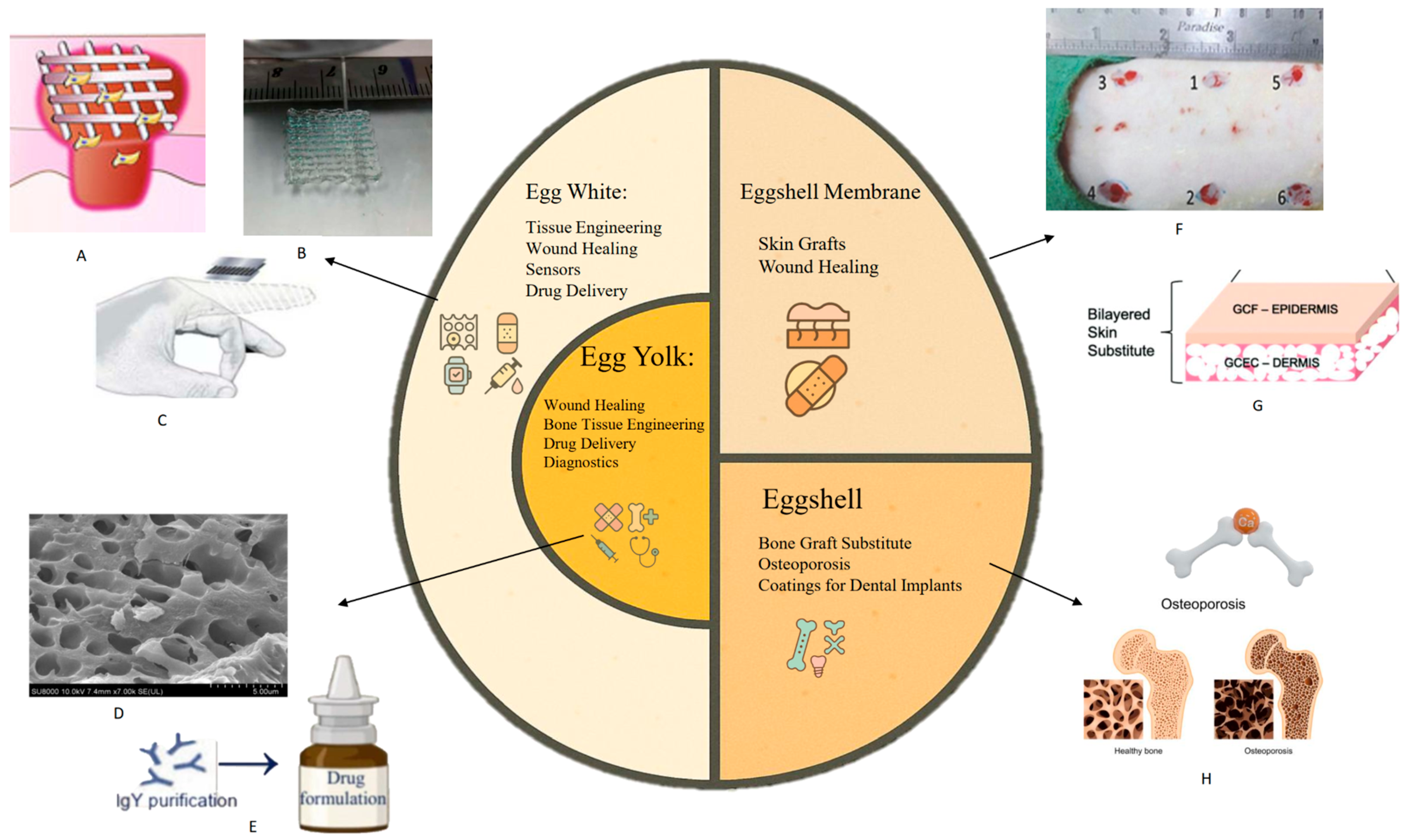
2. Applications of Eggs in Biomedical Engineering
2.1. Eggshell
2.2. Eggshell Membrane
2.3. Egg White
2.4. Egg Yolk
2.5. Other Applications
| Egg Component | Application | Key Components | Role of the Egg Components | Performance |
|---|---|---|---|---|
| Eggshell | Bone repair | PCL/ES powder/CNT [71] | Provides a source of hydroxyapatite, a key component of bone | Abundant, inexpensive, biocompatible, osteoconductive, brittle, potential immune response |
| Used as a filler for improving strength | ES-derived Calcium Oxide/CNT [110] | Source of calcium, environmentally friendly | Improved tensile strength, impact strength, and hardness, reduced flammability | |
| ES powder in Polyamide composites [66] | Low-cost biocompatible filler material enhances flexural and tensile modulus. | Improved adsorption due to the porous structure and increased surface area | ||
| ES powder and date palm fiber in Bio-epoxy composites [67] | Improves the mechanical properties of the composite | Improve tensile strength/modulus, flexural strength/modulus, and stiffness | ||
| Bone scaffold | Sodium alginate/EW and ES powder/PCL [73] | ES improves mechanical stability; EW promotes cell adhesion | ES enhances bioactivity and compressive strength; EW improves biocompatibility | |
| Treatment of osteoporosis | ES powder [55] | Providing a natural source of calcium and other elements for bone health | Similar or better bioavailability than food grade purified calcium carbonate | |
| Eggshell membrane | Skin grafts Wound healing | ES membrane and Polycaprolactone (PCL) [77] | Provides a scaffold for cell attachment and growth | Enhanced biocompatibility, antibacterial activity, mechanical strength, appropriate water uptake and degradation properties |
| Egg white | Tissue engineering | Raw EW/Gelatin [96] | Improves the scaffold’s bioactivity by providing bioactive proteins that promote cell growth and adhesion | Hydrocolloids with tunable viscosity, mechanical properties and high biocompatibility, supporting cell growth |
| Raw EW/Gelatin/Carboxymethylcellulose [94] | Acts as a reinforcing biopolymer that enhances hydrogel stability | Self-adhesive, self-healing, and sensing capabilities with great biocompatibility | ||
| Proteins derived from raw EW/PCL [111] | Improves hydrophilicity, enhances bioactivity, promotes cell proliferation/differentiation | Nanofibrous mats with enhanced water uptake, tensile strength, and cell interactions, suitable for tissue engineering scaffolds | ||
| Raw EW/Alginate [112] | Enhances the gelation process and promotes cell attachment and growth | More affordable option with a slower degradation rate compared to Matrigel® | ||
| EW/PVA film [113] | The EW provides bioactivity and improves cell adhesion by providing a rougher texture | Suitable mechanical strength, degradation rate, high swelling ratio, and optimal water vapor transmission | ||
| Drug delivery | Raw EW/Chitosan hydrogel [99] | Enhances biodegradability; improves cellular interaction and adhesion | Biocompatible, antioxidant, anti-inflammatory, and antibacterial, accelerating healing of burn wounds | |
| Homogenized raw EW/Silk fibroin [114] | Enhances mechanical properties and flexibility; promotes cellular attachment and proliferation | A composite bioink with improved mechanical properties, controlled degradation, and enhanced cell compatibility | ||
| Biosensors | Raw EW/Carbon Dots [101] | Provides a biodegradable and biocompatible matrix for integrating carbon dots | Compatible strength with skin; porous structure promoting cell infiltration; degradation aligned with hair follicles regeneration | |
| Raw EW hydrogel/CNT [6] | Provides excellent biocompatibility, shear thinning property for 3D printing | Highly stretchable and self-healing; 3D printable at room temperature, suitable for electronic sensors and humidity-responsive actuators | ||
| Egg yolk | Wound healing and Bone tissue engineering | Egg yolk derivatives such as IgY or phospholipids [105] | Promote wound healing and bone tissue engineering; provide antibodies for diagnostics | Contains various bioactive components, including lipids, proteins, vitamins, and minerals |
3. Summary and Future Prospectives
Funding
Conflicts of Interest
References
- Zhang, Y.; Pham, H.M.; Tran, S.D. The Chicken Egg: An Advanced Material for Tissue Engineering. Biomolecules 2024, 14, 439. [Google Scholar] [CrossRef]
- Zhang, X.; Chelliappan, B.; Rajeswari, S.; Antonysamy, M. Recent Advances in Applications of Bioactive Egg Compounds in Nonfood Sectors. Front. Bioeng. Biotechnol. 2021, 9, 738993. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Huang, X.; He, Y.; Su, T.; Niu, X.; Gao, J.; Lu, F.; Chang, Q. Radial Egg White Hydrogel Releasing Extracellular Vesicles for Cell Fate Guidance and Accelerated Diabetic Skin Regeneration. Adv. Healthc. Mater. 2024, 13, e2400016. [Google Scholar] [CrossRef] [PubMed]
- Mankad, A.; Hobman, E.; Carter, L.; Tizard, M. Ethical Eggs: Can Synthetic Biology Disrupt the Global Egg Production Industry? Front. Sustain. Food Syst. 2022, 6, 915454. [Google Scholar] [CrossRef]
- Wang, X.H.; Ma, Y.; Niu, X.T.; Su, T.; Huang, X.Q.; Lu, F.; Chang, Q. Direct three-dimensional printed egg white hydrogel wound dressing promotes wound healing with hitching adipose stem cells. Front. Bioeng. Biotechnol. 2022, 10, 930551. [Google Scholar] [CrossRef]
- Chang, Q.; Darabi, M.A.; Liu, Y.; He, Y.; Zhong, W.; Mequanin, K.; Li, B.; Lu, F.; Xing, M.M.Q. Hydrogels from natural egg white with extraordinary stretchability, direct-writing 3D printability and self-healing for fabrication of electronic sensors and actuators. J. Mater. Chem. A 2019, 7, 24626–24640. [Google Scholar] [CrossRef]
- Tian, Y.; Lin, S.; Bao, Z. Characterization and Mechanism of Gel Deterioration of Egg Yolk Powder during Storage. Foods 2023, 12, 2477. [Google Scholar] [CrossRef]
- Sharifi, A.; Rasooli, I.; Jahangiri, A. Egg-yolk antibodies raised to VacJ passively immunize mice against Acinetobacter baumannii pneumonia in a neutropenic murine model. Process Biochem. 2023, 131, 13–18. [Google Scholar] [CrossRef]
- Vinayak, M.N.; Jana, S.; Datta, P.; Das, H.; Chakraborty, B.; Mukherjee, P.; Mondal, S.; Kundu, B.; Nandi, S.K. Accelerating full-thickness skin wound healing using Zinc and Cobalt doped-bioactive glass-coated eggshell membrane. J. Drug Deliv. Sci. Technol. 2023, 81, 104273. [Google Scholar] [CrossRef]
- Saha, R.; Patkar, S.; Maniar, D.; Pillai, M.M.; Tayalia, P. A bilayered skin substitute developed using an eggshell membrane crosslinked gelatin–chitosan cryogel. Biomater. Sci. 2021, 9, 7921–7933. [Google Scholar] [CrossRef]
- Biswas, T.T.; Yu, J.C.; Nierstrasz, V.A. Sequential Inkjet Printing of Lysozyme and Tyrosinase on Polyamide Fabric: Sustainable Enzyme Binding on Textile Surface. Adv. Mater. Interfaces 2022, 9, 2200723. [Google Scholar] [CrossRef]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of Lysozyme, an Innate Immune Defense Factor, as an Alternative Antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.H.; Madden, L.A.; Paunov, V.N. Enhanced anticancer effect of lysozyme-functionalized metformin-loaded shellac nanoparticles on a 3D cell model: Role of the nanoparticle and payload concentrations. Biomater. Sci. 2024, 12, 4735–4746. [Google Scholar] [CrossRef]
- Bergamo, A.; Sava, G. Lysozyme: A Natural Product with Multiple and Useful Antiviral Properties. Molecules 2024, 29, 652. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Shi, Y.D.; Hu, H.K.; Dai, Z.Y.; Liu, Z.C.; Li, X.N. A phase-transited lysozyme coating doped with strontium on titanium surface for bone repairing via enhanced osteogenesis and immunomodulatory. Front. Cell Dev. Biol. 2025, 12, 1506671. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Wang, L.; Guo, J.; Liu, W.; Meng, G.; Zhang, L.; Li, M.; Cong, L.; Sun, M. Recent Insights Into the Prognostic and Therapeutic Applications of Lysozymes. Front. Pharmacol. 2021, 12, 767642. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Giri, S.; Sadhukhan, R.; Ghosh, A.; Santra, S.; Goswami, D.K. A Metal-Insulator-Metal Humidity Sensor Using Albumin-WO3 Composites for Enhanced Responses. IEEE Sens. Lett. 2025, 9, 3498605. [Google Scholar] [CrossRef]
- Shojaee, M.; Navaee, F.; Jalili-Firoozinezhad, S.; Faturechi, R.; Majidi, M.; Bonakdar, S. Fabrication and characterization of ovalbumin films for wound dressing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 158–164. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Huang, X.; Ahn, D.U. Antioxidant, angiotensin-converting enzyme inhibitory activity and other functional properties of egg white proteins and their derived peptides—A review. Poult. Sci. 2018, 97, 1462–1468. [Google Scholar] [CrossRef]
- Zeng, Y.; Lyu, S.; Yang, Q.; Du, Z.; Liu, X.; Shang, X.; Xu, M.; Liu, J.; Zhang, T. Preparation, physicochemical characterization, and immunomodulatory activity of ovalbumin peptide-selenium nanoparticles. Food Chem. 2025, 472, 142852. [Google Scholar] [CrossRef]
- Zhuo, Z.H.; Yin, C.F.; Zhang, Z.Q.; Han, Y.M.; Teng, H.Y.; Xu, Q.; Li, C.M. Nano-Reactors Based on Ovotransferrin Organic Skeleton through a Ferroptosis-like Strategy Efficiently Enhance Antibacterial Activity. J. Funct. Biomater. 2024, 15, 205. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Yao, Y.; Xu, M.S.; Du, H.Y.; Zhang, M.Y.; Tu, Y.G. Anti-inflammatory activity of di-peptides derived from ovotransferrin by CrossMark simulated peptide-cut in TNF-α-induced Caco-2 cells. J. Funct. Foods 2017, 37, 424–432. [Google Scholar] [CrossRef]
- Moon, S.H.; Lee, J.H.; Ahn, D.U.; Paik, H.D. In vitro antioxidant and mineral-chelating properties of natural and autocleaved ovotransferrin. J. Sci. Food Agric. 2015, 95, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, F.; Massucci, M.T.; Giardi, M.F.; Nozza, F.; Pulsinelli, E.; Nicolini, C.; Botti, D.; Antonini, G. Antiviral activity of ovotransferrin derived peptides. Biochem. Biophys. Res. Commun. 2005, 331, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, F.; Leboffe, L.; Pitari, G.; Ippoliti, R.; Antonini, G. Physiological roles of ovotransferrin. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2012, 1820, 218–225. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, J.E.; Kim, K.T.; Ahn, D.U.; Paik, H.D. Anti-biofilm effect of enzymatic hydrolysates of ovomucin in Listeria monocytogenes and Staphylococcus aureus. Microb. Pathog. 2024, 193, 106771. [Google Scholar] [CrossRef]
- Oguro, T.; Ohaki, Y.; Asano, G.; Ebina, T.; Watanabe, K. Ultrastructural and immunohistochemical characterization on the effect of ovomucin in tumor angiogenesis. Jpn. J. Clin. Electron Microsc. 2001, 33, 89–99. [Google Scholar]
- Wang, Z.H.; Tu, A.B.; Tang, D.Y.; Shan, Y.Y. Effectively preparing soluble ovomucin with high antiviral activity from egg white. Int. J. Biol. Macromol. 2018, 118, 504–510. [Google Scholar] [CrossRef]
- Sun, X.; Gänzle, M.; Wu, J. Glycopeptides from egg white ovomucin inhibit K88ac enterotoxigenic Escherichia coli adhesion to porcine small intestinal epithelial cell-line. J. Funct. Foods 2019, 54, 320–328. [Google Scholar] [CrossRef]
- Chang, O.K.; Ha, G.E.; Han, G.S.; Seol, K.H.; Kim, H.W.; Jeong, S.G.; Oh, M.H.; Park, B.Y.; Ham, J.S. Novel Antioxidant Peptide Derived from the Ultrafiltrate of Ovomucin Hydrolysate. J. Agric. Food Chem. 2013, 61, 7294–7300. [Google Scholar] [CrossRef]
- Omana, D.A.; Wang, J.; Wu, J. Ovomucin—A glycoprotein with promising potential. Trends Food Sci. Technol. 2010, 21, 455–463. [Google Scholar] [CrossRef]
- Duggleby, R.G.; Attwood, P.V.; Wallace, J.C.; Keech, D.B. Inhibition of Pyruvate-Carboxylase by the Antibacterial Protein, Avidin. Clin. Exp. Pharmacol. Physiol. 1982, 9, 547. [Google Scholar]
- Khan, M.A.S.; Nakamura, S.; Ogawa, M.; Akita, E.; Azakami, H.; Kato, A. Bactericidal action of egg yolk phosvitin against Escherichia coli under thermal stress. J. Agric. Food Chem. 2000, 48, 1503–1506. [Google Scholar] [CrossRef]
- Lee, J.E.; An, B.J.; Jo, C.; Min, B.; Paik, H.D.; Ahn, D.U. The elastase and melanogenesis inhibitory and anti-inflammatory activities of phosvitin phosphopeptides produced using high-temperature and mild-pressure (HTMP) pretreatment and enzyme hydrolysis combinations. Poult. Sci. 2023, 102, 102680. [Google Scholar] [CrossRef]
- Lee, S.K.; Han, J.H.; Decker, E.A. Antioxidant activity of phosvitin in phosphatidylcholine liposomes and meat model systems. J. Food Sci. 2002, 67, 37–41. [Google Scholar] [CrossRef]
- Zhang, M.J.; Liu, L.L.; Yang, X.L.; Guo, J.F.; Wang, H.Y. Multispectral Analysis of Interaction Between Catechins and Egg Yolk Immunoglobulin and the Change of Bacteriostasis. Spectrosc. Spectr. Anal. 2022, 42, 2297–2303. [Google Scholar] [CrossRef]
- Amirijavid, S.; Hashemi, M. Detection of Anticancer and Apoptotic Effect of the Produced IgYs against the Three Extracellular Domain of Human DR5 Protein. Iran. J. Cancer Prev. 2015, 8, 109–115. [Google Scholar] [PubMed]
- Gao, E.Y.; Wu, S.W.; Xu, Q.; Zeng, Y.L.; Tan, N.; He, S.Q.; Yang, Y.; Wei, J.C. Enterovirus type 71-immunized chicken egg yolk immunoglobulin has cross antiviral activity against coxsackievirus A16 in vitro. Exp. Ther. Med. 2019, 18, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.L.; Bao, L.R.; Shen, X.; Yan, C.X.; Zhang, C.; Wei, W.; Yang, Y.T.; Li, J.; Dong, J.J.; Xiao, L.Y.; et al. The preparation of N-IgY targeting SARS-CoV-2 and its immunomodulation to IFN-γ production in vitro. Int. Immunopharmacol. 2021, 96, 107797. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Decker, E. Phospholipids in Foods: Prooxidants or Antioxidants? J. Sci. Food Agric. 2015, 96, 18–31. [Google Scholar] [CrossRef]
- Stringham, N.T.; Green, M.; Roche, W.; Prado-Cabrero, A.; Mulcahy, R.; Nolan, J. Lutein, zeaxanthin, and meso-zeaxanthin supplementation attenuates inflammatory cytokines and markers of oxidative cardiovascular processes in humans. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, P.; Kothari, V.; Thomas, D.G.; Westerterp, M.; Molusky, M.M.; Altin, E.; Abramowicz, S.; Wang, N.; He, Y.; Heinecke, J.W.; et al. Anti-Inflammatory Effects of HDL (High-Density Lipoprotein) in Macrophages Predominate Over Proinflammatory Effects in Atherosclerotic Plaques. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e253–e272. [Google Scholar] [CrossRef] [PubMed]
- Zavasnik-Bergant, T. Cystatin protease inhibitors and immune functions. Front. Biosci. 2008, 13, 4625–4637. [Google Scholar] [CrossRef] [PubMed]
- Słowińska, M.; Liszewska, E.; Nynca, J.; Bukowska, J.; Hejmej, A.; Bilińska, B.; Szubstarski, J.; Kozłowski, K.; Jankowski, J.; Ciereszko, A. Isolation and Characterization of an Ovoinhibitor, a Multidomain Kazal-Like Inhibitor from Turkey (Meleagris gallopavo) Seminal Plasma1. Biol. Reprod. 2014, 91, 108. [Google Scholar] [CrossRef]
- Bourin, M.; Gautron, J.; Berges, M.; Attucci, S.; Le Blay, G.; Labas, V.; Nys, Y.; Rehault-Godbert, S. Antimicrobial potential of egg yolk ovoinhibitor, a multidomain Kazal-like inhibitor of chicken egg. J. Agric. Food Chem. 2011, 59, 12368–12374. [Google Scholar] [CrossRef]
- Miguel, M.; Aleixandre, A. Antihypertensive Peptides Derived from Egg Proteins1. J. Nutr. 2006, 136, 1457–1460. [Google Scholar] [CrossRef]
- Opris, H.; Dinu, C.; Baciut, M.; Baciut, G.; Mitre, I.; Crisan, B.; Armencea, G.; Prodan, D.A.; Bran, S. The Influence of Eggshell on Bone Regeneration in Preclinical In Vivo Studies. Biology 2020, 9, 476. [Google Scholar] [CrossRef]
- Baláz, M. Eggshell membrane biomaterial as a platform for applications in materials science. Acta Biomater. 2014, 10, 3827–3843. [Google Scholar] [CrossRef]
- Li, Z.; Huang, X.; Tang, Q.Y.; Ma, M.H.; Jin, Y.G.; Sheng, L. Functional Properties and Extraction Techniques of Chicken Egg White Proteins. Foods 2022, 11, 2434. [Google Scholar] [CrossRef]
- Patil, S.; Rao, B.; Matondkar, M.; Bhushette, P.; Sonawane, S.K. A REVIEW ON UNDERSTANDING OF EGG YOLK AS FUNCTIONAL INGREDIENTS. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e4627. [Google Scholar] [CrossRef]
- Mahdavi, S.; Amirsadeghi, A.; Jafari, A.; Niknezhad, S.V.; Bencherif, S.A. Avian Egg: A Multifaceted Biomaterial for Tissue Engineering. Ind. Eng. Chem. Res. 2021, 60, 17348–17364. [Google Scholar] [CrossRef] [PubMed]
- Zghair Chfat, A.H.; Yaacob, H.; Mohd Kamaruddin, N.H.; Al-Saffar, Z.H.; Putra Jaya, R. Effects of nano eggshell powder as a sustainable bio-filler on the physical, rheological, and microstructure properties of bitumen. Results Eng. 2024, 22, 102061. [Google Scholar] [CrossRef]
- Huang, X.; Dong, K.; Liu, L.; Luo, X.; Yang, R.; Song, H.; Li, S.; Huang, Q. Physicochemical and structural characteristics of nano eggshell calcium prepared by wet ball milling. LWT 2020, 131, 109721. [Google Scholar] [CrossRef]
- Rovensky, J.; Stancíková, M.; Masaryk, P.; Švík, K.; Istok, R. Eggshell calcium in the prevention and treatment of osteoporosis. Int. J. Clin. Pharmacol. Res. 2003, 23, 83–92. [Google Scholar]
- Bartter, J.; Diffey, H.; Yeung, Y.H.; O’Leary, F.; Häsler, B.; Maulaga, W.; Alders, R. Use of chicken eggshell to improve dietary calcium intake in rural sub-Saharan Africa. Matern. Child Nutr. 2018, 14 (Suppl. S3), e12649. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, A.; Pakan, I. Short-term effects of a chicken egg shell powder enriched dairy-based products on bone mineral density in persons with osteoporosis or osteopenia. Bratisl. Lek. Listy 1999, 100, 651–656. [Google Scholar] [PubMed]
- Bijarimi, M.; Alya Azlan, S.; Norazmi, M.; Normaya, E.; Mat Desa, M.S.Z. Sustainable green poly(lactic acid) (PLA)/eggshell powder (ESP) biocomposites. Mater. Today Proc. 2023, 85, 83–86. [Google Scholar] [CrossRef]
- Owuamanam, S.; Soleimani, M.; Cree, D.E. Fabrication and Characterization of Bio-Epoxy Eggshell Composites. Appl. Mech. 2021, 2, 694–713. [Google Scholar] [CrossRef]
- Hiremath, P.; Shettar, M.; Shankar, M.C.G.; Mohan, N.S. Investigation on Effect of Egg Shell Powder on Mechanical Properties of GFRP Composites. Mater. Today Proc. 2018, 5, 3014–3018. [Google Scholar] [CrossRef]
- Tanahashi, M. Development of Fabrication Methods of Filler/Polymer Nanocomposites: With Focus on Simple Melt-Compounding-Based Approach without Surface Modification of Nanofillers. Materials 2010, 3, 1593–1619. [Google Scholar] [CrossRef]
- Habte, L.; Shiferaw, N.; Mulatu, D.; Thenepalli, T.; Chilakala, R.; Ahn, J.W. Synthesis of Nano-Calcium Oxide from Waste Eggshell by Sol-Gel Method. Sustainability 2019, 11, 3196. [Google Scholar] [CrossRef]
- Abu Bakar, N.H.; Yusop, H.M.; Ismail, W.N.W.; Zulkifli, N.F. Sol-Gel Finishing for Protective Fabrics. Biointerface Res. Appl. Chem. 2023, 13, 283. [Google Scholar] [CrossRef]
- Toibah, A.R.; Misran, F.; Juoi, J.M.; Shaaban, A. Synthesis of hydroxyapatite from eggshells with different pH by hydrothermal method. In Proceedings of the 5th Mechanical Engineering Research Day (MERD), Univ Teknikal Malaysia, Melaka, Malaysia, 3 May 2018; pp. 241–242. [Google Scholar]
- Yoshimura, M.; Byrappa, K. Hydrothermal processing of materials: Past, present and future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Bin-Dahman, O.A.; Saleh, T.A. Synthesis of polyamide grafted on biosupport as polymeric adsorbents for the removal of dye and metal ions. Biomass Convers. Biorefin. 2024, 14, 2439–2452. [Google Scholar] [CrossRef]
- Sarmin, S.N.; Jawaid, M.; Zaki, S.A.; Ali, M.R.; Fouad, H.; Khiari, R.; Rahayu, S.; Salim, N. The Effect of Eggshell Fillers on the Physical, Mechanical, and Morphological Properties of Date palm Fibre Reinforced Bio-epoxy Composites. J. Polym. Environ. 2023, 31, 5015–5027. [Google Scholar] [CrossRef]
- Sivakumar, A.; Srividhya, S.; Prakash, R.; Padma, S. Properties of biocomposites from waste eggshell as fillers. Glob. Nest J. 2023, 25, 109–115. [Google Scholar] [CrossRef]
- Opris, H.; Baciut, M.; Bran, S.; Dinu, C.; Opris, D.; Armencea, G.; Onisor, F.; Bumbu, B.; Baciut, G. Biocompatibility and histological responses of eggshell membrane for dental implant-guided bone regeneration. J. Med. Life 2023, 16, 1007–1012. [Google Scholar] [CrossRef]
- Panchal, M.; Raghavendra, G.; Reddy, A.R.; Omprakash, M.; Ojha, S. Experimental investigation of mechanical and erosion behavior of eggshell nanoparticulate epoxy biocomposite. Polym. Polym. Compos. 2021, 29, 897–908. [Google Scholar] [CrossRef]
- Zhang, P.S.; Xin, Y.; Ai, F.R.; Cao, C.L. Preparation and properties of multi-walled carbon nanotubes and eggshell dual-modified polycaprolactone composite scaffold. J. Polym. Eng. 2019, 39, 343–350. [Google Scholar] [CrossRef]
- Shafiei, S.; Omidi, M.; Nasehi, F.; Golzar, H.; Mohammadrezaei, D.; Rad, M.R.; Khojasteh, A. Egg shell-derived calcium phosphate/carbon dot nanofibrous scaffolds for bone tissue engineering: Fabrication and characterization. Mater. Sci. Eng. C—Mater. Biol. Appl. 2019, 100, 564–575. [Google Scholar] [CrossRef]
- Rezaei, H.; Shahrezaee, M.; Monfared, M.J.; Nikjou, M.; Shahrezaee, M.H.; Mohseni, M. Fabrication and characterization of three-dimensional polycaprolactone/sodium alginate and egg whites and eggshells hybrid scaffold in bone tissue engineering. J. Polym. Eng. 2023, 43, 47–52. [Google Scholar] [CrossRef]
- Yuan, Z.C.; Wu, S.Y.; Fu, L.W.; Shafiq, M.; Liang, Y.Q.; Li, P.; Wang, X.Y.; Feng, H.; Hashim, R.; Lou, S.Q.; et al. Composite scaffolds based on egg membrane and eggshell-derived inorganic particles promote soft and hard tissue repair. Compos. Part B—Eng. 2025, 292, 112071. [Google Scholar] [CrossRef]
- Fladerer, J.P.; Grollitsch, S. Eggshell membrane as promising supplement to maintain bone health: A systematic review. Bone Rep. 2024, 21, 101776. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Suso, H.-P.; Maqbool, A.; Hincke, M.T. Processed eggshell membrane powder: Bioinspiration for an innovative wound healing product. Mater. Sci. Eng. C 2019, 95, 192–203. [Google Scholar] [CrossRef]
- Bello, M.; Abdullah, F.; Mahmood, W.M.A.W.; Malek, N.A.N.N.; Jemon, K.; Siddiquee, S.; Chee, T.Y.; Sathishkumar, P. Electrospun poly (Ɛ-caprolactone)-eggshell membrane nanofibrous mat as a potential wound dressing material. Biochem. Eng. J. 2022, 187, 108563. [Google Scholar] [CrossRef]
- Kim, D.; Gwon, Y.; Park, S.; Kim, W.; Yun, K.; Kim, J. Eggshell membrane as a bioactive agent in polymeric nanotopographic scaffolds for enhanced bone regeneration. Biotechnol. Bioeng. 2021, 118, 1862–1875. [Google Scholar] [CrossRef]
- Mensah, R.A.; Cook, M.T.; Kirton, S.B.; Hutter, V.; Chau, D.Y.S. A drug-incorporated-microparticle-eggshell-membrane-scaffold (DIMES) dressing: A novel biomaterial for localised wound regeneration. Eur. J. Pharm. Biopharm. 2023, 190, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wang, W.; Xie, Z. Porous egg microparticles for controllable drug delivery and oncotherapy. Precis. Med. Sci. 2025, 14, 46–53. [Google Scholar] [CrossRef]
- Ruan, G.P.; Yao, X.; Wang, K.; He, J.; Zhu, X.Q.; Pang, R.Q.; Pan, X.H. The Effect of Different Chicken Ovalbumin Extracts on Cell Proliferation. Indian J. Anim. Res. 2024, 58, 1883–1886. [Google Scholar] [CrossRef]
- Mousseau, Y.; Mollard, S.; Qiu, H.; Richard, L.; Cazal, R.; Nizou, A.; Vedrenne, N.; Rémi, S.; Baaj, Y.; Fourcade, L.; et al. In vitro 3D angiogenesis assay in egg white matrix: Comparison to Matrigel, compatibility to various species, and suitability for drug testing. Lab. Investig. 2014, 94, 340–349. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Y.Q. An insight on egg white: From most common functional food to biomaterial application. J. Biomed. Mater. Res. Part B—Appl. Biomater. 2021, 109, 1045–1058. [Google Scholar] [CrossRef]
- Chang, Q.; He, Y.; Liu, Y.; Zhong, W.; Wang, Q.; Lu, F.; Xing, M. Protein Gel Phase Transition: Toward Superiorly Transparent and Hysteresis-Free Wearable Electronics. Adv. Funct. Mater. 2020, 30, 1910080. [Google Scholar] [CrossRef]
- Kumbár, V.; Strnková, J.; Nedomová, Š.; Buchar, J. Fluid dynamics of liquid egg products. J. Biol. Phys. 2015, 41, 303–311. [Google Scholar] [CrossRef]
- Loebel, C.; Rodell, C.B.; Chen, M.H.; Burdick, J.A. Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nat. Protoc. 2017, 12, 1521–1541. [Google Scholar] [CrossRef] [PubMed]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2020, 137, 48668. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, Z.Y.; Hu, G.; Yang, Y.X.; Sun, H.Y.; Cai, Z.X. Hydrogel foam with designed structural properties of egg white protein through building aggregates as potential dysphagia food. Food Hydrocoll. 2025, 158, 110584. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Ma, J.; Long, P.Y.; Zheng, Y.J.; Zhang, Y.C. Improving gel properties of egg white protein using coconut endosperm dietary fibers modified by ultrasound and dual enzymolysis combined with carboxymethylation or phosphate crosslinking. Curr. Res. Food Sci. 2024, 9, 100941. [Google Scholar] [CrossRef]
- Mahdipour, E.; Mequanint, K. Films, Gels and Electrospun Fibers from Serum Albumin Globular Protein for Medical Device Coating, Biomolecule Delivery and Regenerative Engineering. Pharmaceutics 2022, 14, 2306. [Google Scholar] [CrossRef]
- Prasopdee, T.; Sinthuvanich, C.; Chollakup, R.; Uttayarat, P.; Smitthipong, W. The albumin/starch scaffold and its biocompatibility with living cells. Mater. Today Commun. 2021, 27, 102164. [Google Scholar] [CrossRef]
- Maier, F.I.; Klinger, D.; Grieshober, M.; Noschka, R.; Rodriguez, A.; Wiese, S.; Forssmann, W.G.; Ständker, L.; Stenger, S. Lysozyme: An endogenous antimicrobial protein with potent activity against extracellular, but not intracellular Mycobacterium tuberculosis. Med. Microbiol. Immunol. 2024, 213, 9. [Google Scholar] [CrossRef] [PubMed]
- Rasitanon, N.; Rattanapan, P.; Kaewpradub, K.; Buranachai, C.; Jeerapan, I. Glucose Oxidase/Egg White Protein Microparticles with a Redox Mediator for Glucose Biosensors on a Screen-Printed Electrode and a Decomposable Electrode. Biosensors 2023, 13, 772. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xu, Y.Z.; Maimaitiyiming, X. Egg white/gelatin/carboxymethylcellulose superbly bonded and biocompatible flexible self-adhesive multifunctional sensor. Cellulose 2024, 31, 6779–6795. [Google Scholar] [CrossRef]
- Babaei, J.; Mohammadian, M.; Madadlou, A. Gelatin as texture modifier and porogen in egg white hydrogel. Food Chem. 2019, 270, 189–195. [Google Scholar] [CrossRef]
- Pele, K.G.; Amaveda, H.; Mora, M.; Marcuello, C.; Lostao, A.; Alamán-Díez, P.; Pérez-Huertas, S.; Pérez, M.A.; García-Aznar, J.M.; García-Gareta, E. Hydrocolloids of Egg White and Gelatin as a Platform for Hydrogel-Based Tissue Engineering. Gels 2023, 9, 505. [Google Scholar] [CrossRef]
- Afrashi, M.; Semnani, D.; Hashemibeni, B.; Shokrgozar, M.A. Degradable and biocompatible nanofibrous scaffold incorporating a natural cell culture medium for skin tissue engineering. Phys. Scr. 2024, 99, 035029. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Pham, H.M.; Munguia-Lopez, J.G.; Kinsella, J.M.; Tran, S.D. The Optimization of a Novel Hydrogel-Egg White-Alginate for 2.5D Tissue Engineering of Salivary Spheroid-Like Structure. Molecules 2020, 25, 5751. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, Y.L.; Chu, Y.G.; Chang, Q. Facile synthesis of hydroxypropyl chitosan-egg white hydrogel dressing with antibacterial and antioxidative activities for accelerating the healing of burn wounds. J. Mater. Chem. B 2023, 11, 4330–4345. [Google Scholar] [CrossRef]
- You, R.C.; Zhang, J.; Gu, S.J.; Zhou, Y.S.; Li, X.F.; Ye, D.Z.; Xu, W.L. Regenerated egg white/silk fibroin composite films for biomedical applications. Mater. Sci. Eng. C—Mater. Biol. Appl. 2017, 79, 430–435. [Google Scholar] [CrossRef]
- Wu, J.; Lei, J.H.; Li, M.X.; Zhang, A.P.; Li, Y.; Liang, X.; de Souza, S.C.; Yuan, Z.; Wang, C.M.; Chen, G.K.; et al. Carbon Dots Crosslinked Egg White Hydrogel for Tissue Engineering. Adv. Sci. 2024, 11, e2404702. [Google Scholar] [CrossRef] [PubMed]
- Smits, N.G.E.; De Dominicis, E.; Koops, A.J.; Kraan, R.; Saner, S.; Van Der Fels-Klerx, H.J.; Hoek-van den Hil, E. Comparison of commercial allergen ELISA kits for egg detection in food matrices. Heliyon 2023, 9, e19687. [Google Scholar] [CrossRef]
- Isah, M.B.; Yuzhang, H.; Dang, M.; Zhang, X. A novel and quick egg yolk immunoglobulin y antibody extraction method leveraging the protein liquid-liquid phase separation principle. Poult. Sci. 2025, 104, 104804. [Google Scholar] [CrossRef]
- Pereira, E.P.V.; van Tilburg, M.F.; Florean, E.; Guedes, M.I.F. Egg yolk antibodies (IgY) and their applications in human and veterinary health: A review. Int. Immunopharmacol. 2019, 73, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Ren, J.; Wu, J. Phosvitin Derived Phospho-Peptides Show Better Osteogenic Potential than Intact Phosvitin in MC3T3-E1 Osteoblastic Cells. Nutrients 2020, 12, 2998. [Google Scholar] [CrossRef] [PubMed]
- Herron, L.R.; Pridans, C.; Turnbull, M.L.; Smith, N.; Lillico, S.; Sherman, A.; Gilhooley, H.J.; Wear, M.; Kurian, D.; Papadakos, G.; et al. A chicken bioreactor for efficient production of functional cytokines. BMC Biotechnol. 2018, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Greenwood, H.E.; Tyrrell, W.E.; Edwards, R.S.; de Santis, V.; Baark, F.; Firth, G.; Tanc, M.; Terry, S.Y.A.; Herrmann, A.; et al. The chicken chorioallantoic membrane as a low-cost, high-throughput model for cancer imaging. Npj Imaging 2023, 1, 1. [Google Scholar] [CrossRef]
- Pomraenke, M.; Bolney, R.; Winkens, T.; Perkas, O.; Pretzel, D.; Theis, B.; Greiser, J.; Freesmeyer, M. A Novel Breast Cancer Xenograft Model Using the Ostrich Chorioallantoic Membrane-A Proof of Concept. Vet. Sci. 2023, 10, 349. [Google Scholar] [CrossRef]
- Chaudhari, B.C.; Khanderay, J.; Patil, C.; Patil, A.M.; Joshi, S.A.; Singh, V.; Palodkar, K.K.; Sesha Sainath, A.V.; Gite, V.V.; Jirimali, H.D. Waste Eggshells for the Decoration of Carbon Nanotubes and Graphene Nanosheets with Hydroxyapatite for Preparation of LLDPE Nanocomposites. J. Polym. Environ. 2019, 27, 2352–2359. [Google Scholar] [CrossRef]
- Renkler, N.Z.; Ergene, E.; Gokyer, S.; Tuzlakoglu Ozturk, M.; Yilgor Huri, P.; Tuzlakoglu, K. Facile modification of polycaprolactone nanofibers with egg white protein. J. Mater. Sci. Mater. Med. 2021, 32, 34. [Google Scholar] [CrossRef]
- Pham, H.M.; Zhang, Y.; Munguia-Lopez, J.G.; Tran, S.D. Egg White Alginate as a Novel Scaffold Biomaterial for 3D Salivary Cell Culturing. Biomimetics 2021, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Nour, S.; Imani, R.; Sharifi, A.M. Angiogenic Effect of a Nanoniosomal Deferoxamine-Loaded Poly(vinyl alcohol)–Egg White Film as a Promising Wound Dressing. ACS Biomater. Sci. Eng. 2022, 8, 3485–3497. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Zhao, S.-X.; Li, J.-X.; Zhang, Y.-Q. Silk Fibroin Improves the Biological Properties of Egg White-Based Bioink for the Bioprinting of Tissue Engineering Materials. ACS Omega 2023, 8, 46685–46696. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Vizely, K.; Li, C.Y.; Shen, K.; Shakeri, A.; Khosravi, R.; Smith, J.R.; Alteza, E.; Zhao, Y.; Radisic, M. Biomaterials for immunomodulation in wound healing. Regen. Biomater. 2024, 11, rbae032. [Google Scholar] [CrossRef]
- Meng, S.; Miao, A.; Wu, S.; Du, X.; Gao, F. Genetically modified chickens as bioreactors for protein-based drugs. Front. Genome Ed. 2024, 6, 1522837. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohseni Ghalehghazi, A.; Zhong, W. From Nutrition to Innovation: Biomedical Applications of Egg Components. Molecules 2025, 30, 3260. https://doi.org/10.3390/molecules30153260
Mohseni Ghalehghazi A, Zhong W. From Nutrition to Innovation: Biomedical Applications of Egg Components. Molecules. 2025; 30(15):3260. https://doi.org/10.3390/molecules30153260
Chicago/Turabian StyleMohseni Ghalehghazi, Amin, and Wen Zhong. 2025. "From Nutrition to Innovation: Biomedical Applications of Egg Components" Molecules 30, no. 15: 3260. https://doi.org/10.3390/molecules30153260
APA StyleMohseni Ghalehghazi, A., & Zhong, W. (2025). From Nutrition to Innovation: Biomedical Applications of Egg Components. Molecules, 30(15), 3260. https://doi.org/10.3390/molecules30153260





