Toxic Effects of the Most Common Components of Energetic Co-Crystals
Abstract
1. Introduction
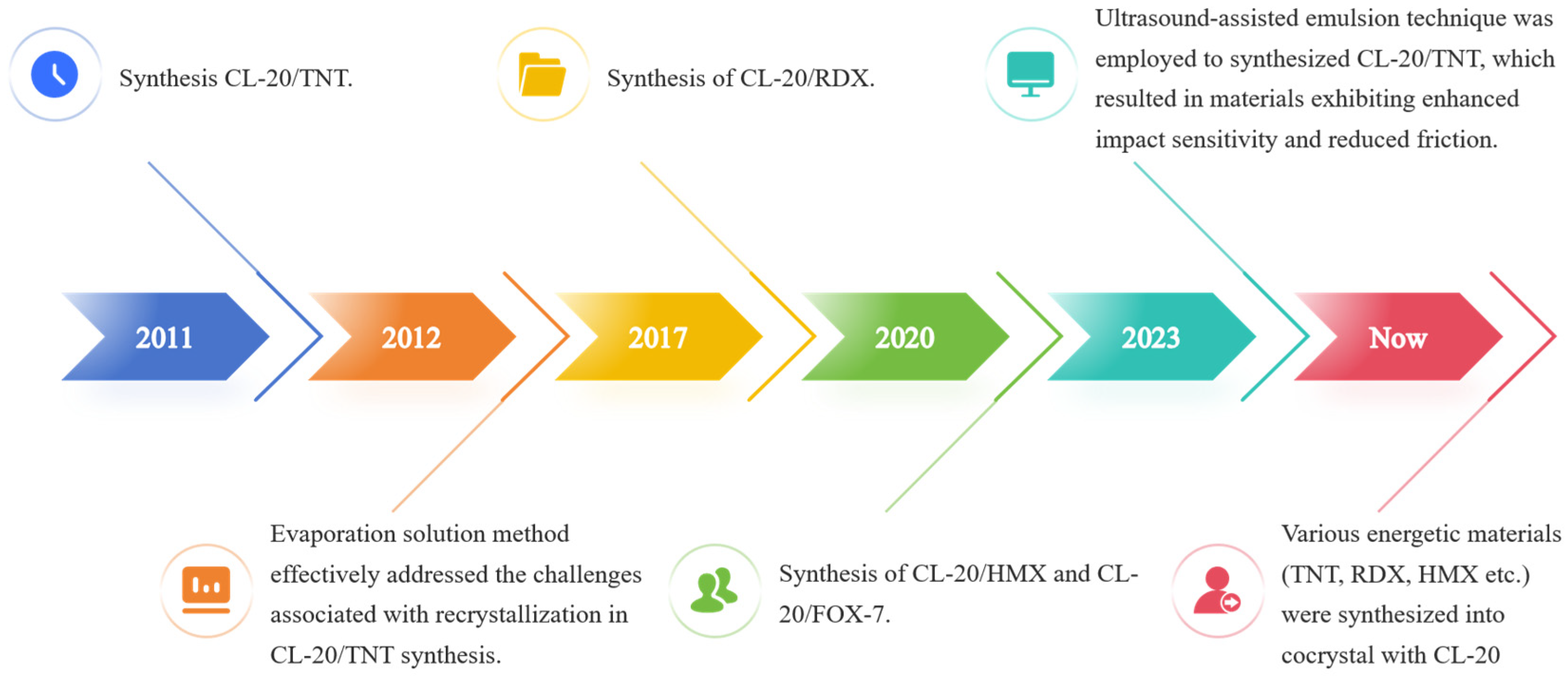
2. Advances in Cocrystal Materials Research
3. Toxic Effects of Common Components in CL-20 Cocrystal Materials
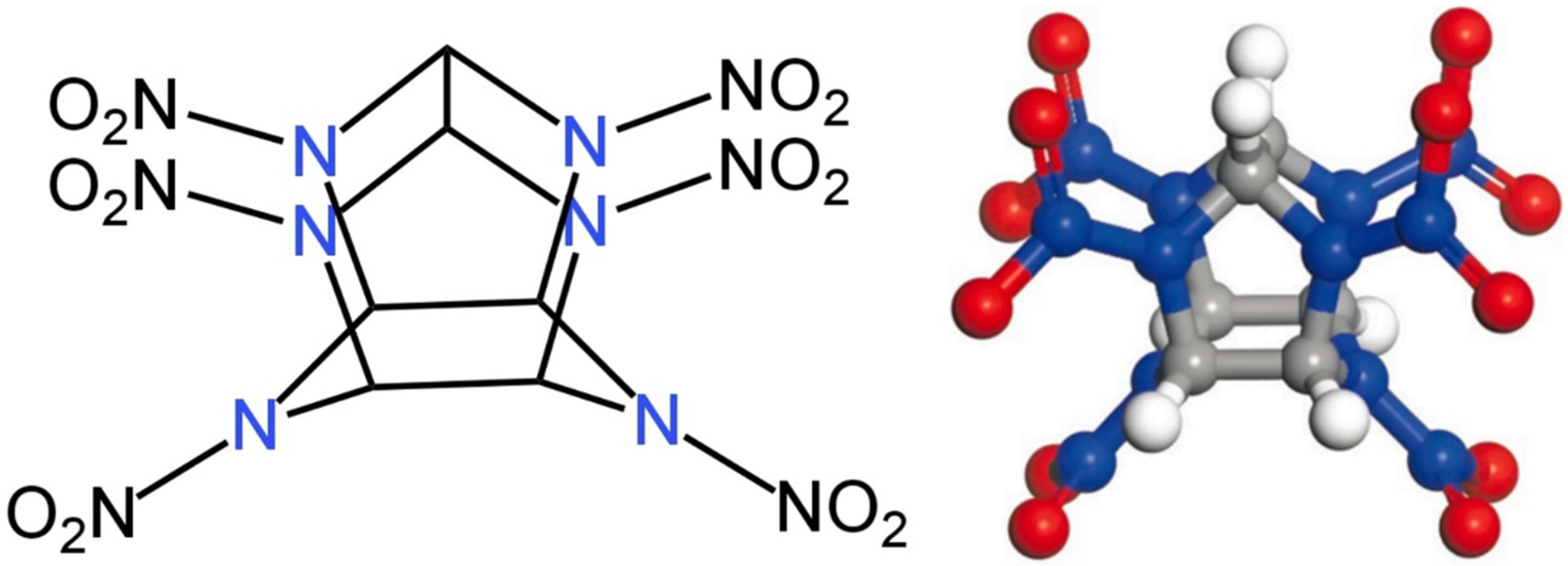
3.1. CL-20
3.2. TNT
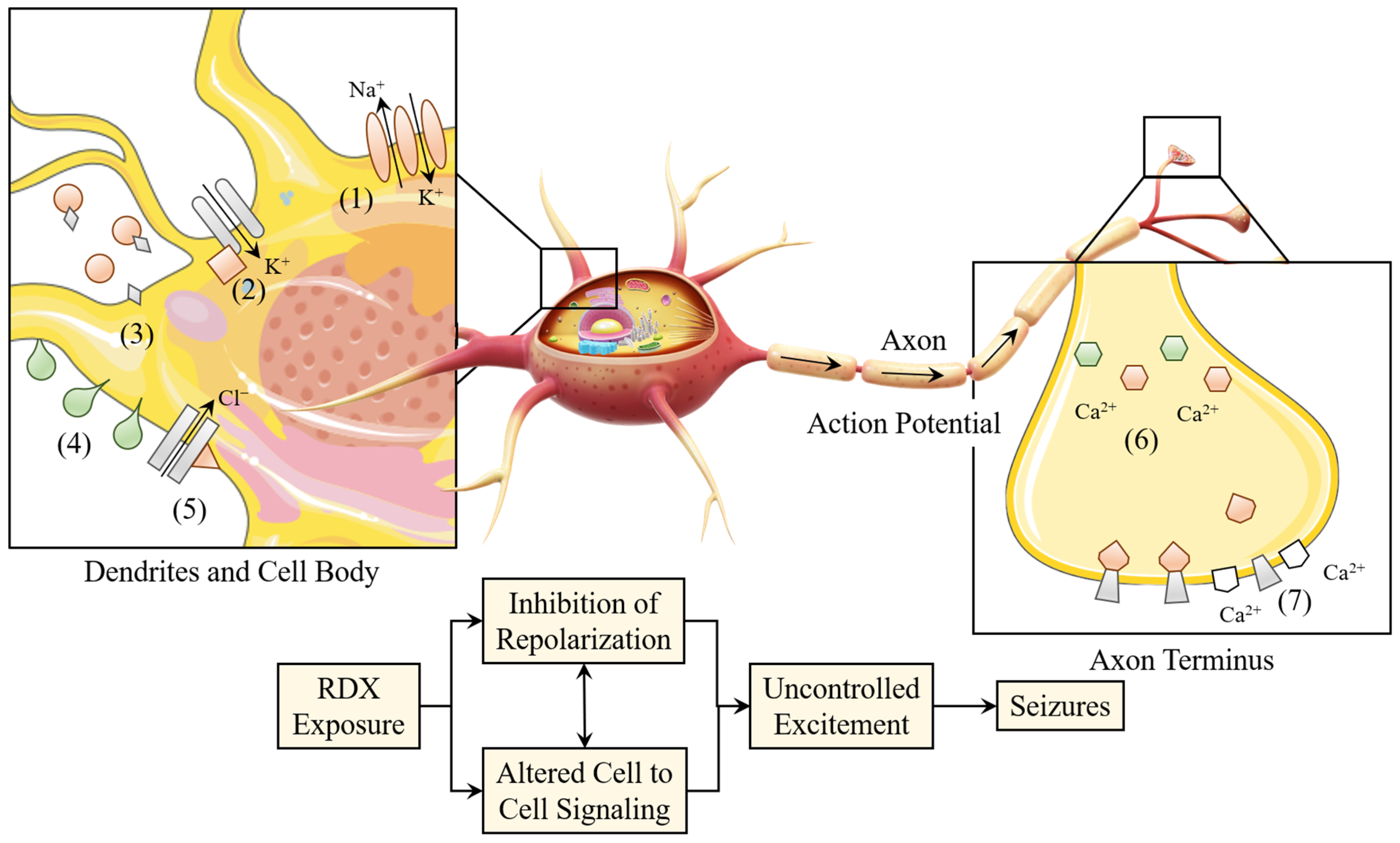
3.3. RDX
3.4. HMX
4. Toxicity Mechanism of Common Components in CL-20 Cocrystal Materials
4.1. The Toxic Mechanism of Action of CL-20
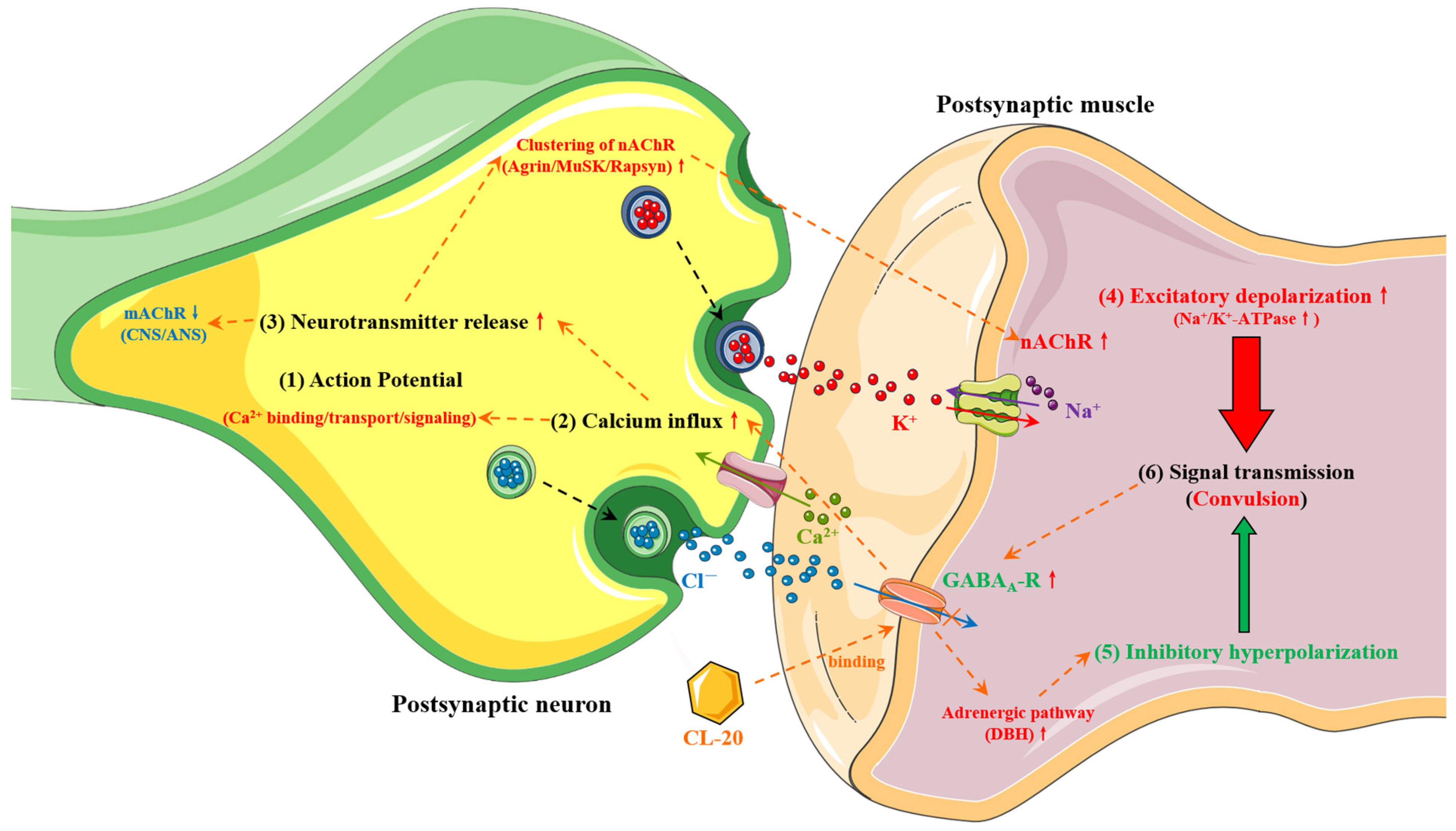
4.1.1. Neurotoxicity
4.1.2. Genotoxicity
4.2. The Mechanism of Toxic Action of TNT
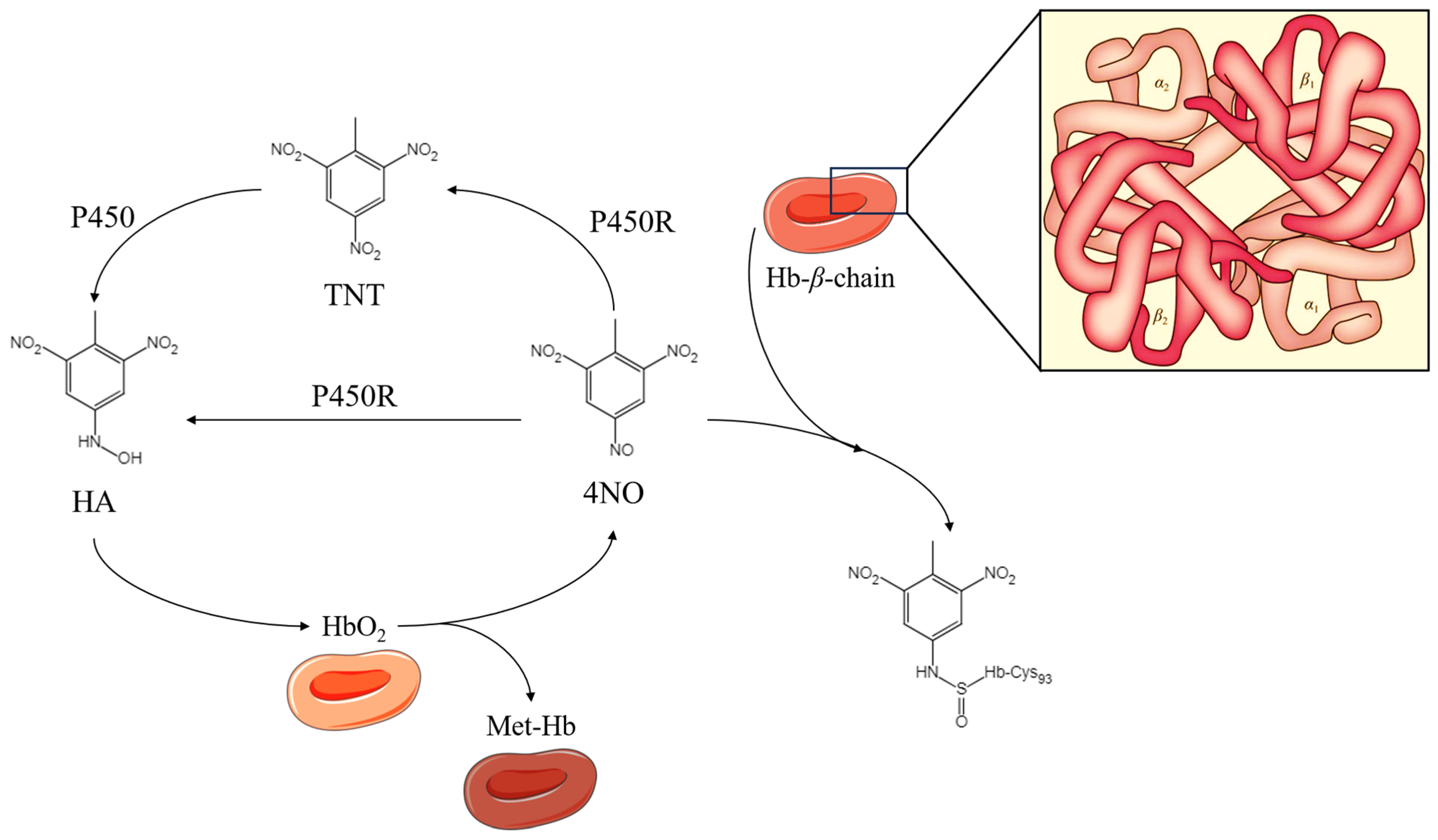
4.2.1. Oxidative Stress and Covalent Addition Reactions
4.2.2. Mitochondrial Dysfunction
4.2.3. The Transmembrane Transport Mechanism of TNT
4.2.4. Endoplasmic Reticulum Stress and Cellular Apoptosis Pathways
4.3. The Toxic Mechanism of Action of RDX
4.3.1. Neurotoxicity
4.3.2. Reproductive Toxicity
4.4. The Mechanism of Toxic Action of HMX
5. Speculation of the Toxicities of CL-20 Cocrystal Materials and Their Influencing Factors
6. Conclusions and Future Directions
- Acute toxicity, long-term toxicity, and ecological toxicity of cocrystal materials on different organisms, and their toxic action mechanism. Researchers should conduct comprehensive studies on acute, chronic, and ecological toxicity of cocrystal materials across different organisms. A deeper investigation into the metabolic pathways of eutectic materials in various species is essential to understanding their physiological impacts and potential disruptions to ecosystem balance.
- The interaction of eutectic material components and its influence on the overall toxic effect of materials. The interactions between different energetic material components can significantly influence overall toxicity. Understanding these interactions is crucial for designing safer eutectic materials with minimized adverse effects.
- Utilizing computational chemistry and molecular simulation techniques, researchers can develop QSAR models. These models provide a scientific basis for designing safer eutectic materials by predicting potential toxicity risks at the early stages of material development.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.; Tang, J.; Lei, C.J.; Xue, C.; Yang, H.; Xiao, C.; Cheng, G. A novel synthesis method for nitrogen-rich energetic frameworks containing bistetrazoles: Assembling an advanced high-energy density material with high nitrogen content and good oxygen balance. J. Mater. Chem. A 2024, 12, 29638–29644. [Google Scholar] [CrossRef]
- Wang, H.; Liu, G.; Su, G.; Wei, H.; Dang, L. Solubility measurement, correlation, and thermodynamic analysis of ε-CL-20 in four binary solvents (ethyl acetate plus halogenated hydrocarbon) from 288.15 to 328.15 K. J. Chem. Thermodyn. 2025, 201, 107414. [Google Scholar] [CrossRef]
- Huang, Y.F.; Wu, H.; Wang, X.M.; Du, L.; Zhang, Y.J.; Sun, B.C. Computational study on the thermal decomposition mechanism and mechanical sensitivity of CL-20/4,5-MDNI. Chem. Eng. Sci. 2025, 317, 122085. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Liu, X.; Wang, N. Characterization of CL-20/HMX cocrystallization and its effects on GAP-based propellants during thermal aging process. Polym. Test 2024, 140, 108623. [Google Scholar] [CrossRef]
- Hang, G.Y.; Wang, J.T.; Wang, T.; Shen, H.M.; Yu, W.L.; Shen, R.Q. Theoretical investigations on stability, sensitivity, energetic performance, and mechanical properties of CL-20/TNAD cocrystal explosive by molecular dynamics method. J. Mol. Model. 2022, 28, 58. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, S.; Zhao, H.; Wu, W.; Wang, N.; Wang, T.; Huang, X.; Zhou, L.; Bao, Y.; Hao, H. Polymorphism of CL-20 and the Modification and Inhibition Strategies for Its Crystal Transformation. Cryst. Growth Des. 2024, 24, 9266–9296. [Google Scholar] [CrossRef]
- Patil, V.; Zeman, S. Novel approach for preparation of the energy-safety balanced cocrystals of attractive nitramines via coagglomeration. FirePhysChem 2024, 4, 283–294. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Liu, Y.; Huang, H. Research Progress and Prospect of Energetic Cocrystal Materials. Mater. China 2022, 41, 81–91. [Google Scholar]
- Yanwei, T. Study on preparation, characterization and properties of CL-20/nitroimidazole cocrystal. Ph.D. Thesis, North University of China, Taiyuan, China, 2020. [Google Scholar]
- Du, J.; Wang, B.G.; Chen, Y.; Li, X.; Wang, C. Theoretical investigation of potential energetic material CL-20/TNBP co-crystal explosive based on molecular dynamics method. J. Mol. Model. 2024, 30, 348. [Google Scholar] [CrossRef]
- Bolton, O.; Matzger, A.J. Improved Stability and Smart-Material Functionality Realized in an Energetic Cocrystal. Angew. Chem.-Int. Edit. 2011, 50, 8960–8963. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Li, H.; Zhou, X.; Nie, F.; Li, J.; Huang, H. Preparation, Structure and Properties of CL-20/TNT Cocrystal. Energ. Mater. 2012, 20, 674–679. [Google Scholar]
- Tariq, Q.-U.; Tariq, M.-U.; Dong, W.-S.; Manzoor, S.; Arshad, F.; Zhang, J.-G. Comparative Studies of Synthesis, Performance, and Applications of Recently Developed CL-20 Based Co-crystals. Cryst. Growth Des. 2023, 23, 6974–6987. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; An, C.; Guo, W. Preparation and Characterization of Ultrafine CL-20/TNT Cocrystal Explosive by Spray Drying Method. Chin. J. Energ. Mater. 2015, 23, 1103–1106. [Google Scholar]
- Jia, Q.; Zhang, J.Q.; Zhang, S.J.; Shi, Q.; Lei, D.; Xu, Y.L.; Kou, K.C. Low-temperature heat capacities, standard molar enthalpies of formation and detonation performance of two CL-20 cocrystal energetic materials. Fluid Phase Equilib. 2020, 518, 112638. [Google Scholar] [CrossRef]
- Ghosh, M.; Sikder, A.; Banerjee, S.; Talawar, M.; Sikder, N. Preparation of reduced sensitivity co-crystals of cyclic nitramines using spray flash evaporation. Def. Technol. 2020, 16, 188–200. [Google Scholar] [CrossRef]
- Gao, H.; Du, P.; Ke, X.; Liu, J.; Hao, G.Z.; Chen, T.; Jiang, W. A Novel Method to Prepare Nano-sized CL-20/NQ Co-crystal: Vacuum Freeze Drying. Propellants Explos. Pyrotech. 2017, 42, 889–895. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.; Ma, Y.; Huang, Q.; Zhang, J.; Nie, F.; Zhang, J.; Li, H. Isomeric Cocrystals of CL-20: A Promising Strategy for Development of High-Performance Explosives. Cryst. Growth Des. 2018, 18, 6399–6403. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, Z.; Wang, H.; Li, H.; Nie, F.; Liu, Y.; Yu, Y. High Energy Explosive with Low Sensitivity: A New Energetic Cocrystal Based on CL-20 and 1,4-DNI. Cryst. Growth Des. 2019, 19, 4476–4482. [Google Scholar] [CrossRef]
- Tan, Y.W.; Liu, Y.C.; Wang, H.J.; Li, H.Z.; Nie, F.D.; Yang, Z.W. Different Stoichiometric Ratios Realized in Energetic-Energetic Cocrystals Based on CL-20 and 4,5-MDNI: A Smart Strategy to Tune Performance. Cryst. Growth Des. 2020, 20, 3826–3833. [Google Scholar] [CrossRef]
- Gao, H.; Jiang, W.; Liu, J.; Hao, G.; Xiao, L.; Ke, X.; Chen, T. Synthesis and Characterization of a New Co-Crystal Explosive with High Energy and Good Sensitivity. J. Energ. Mater. 2017, 35, 490–498. [Google Scholar] [CrossRef]
- Guo, C.; Zhu, Y.; Hu, Y.; Zhang, X.; Wang, D.; Gao, B. Supramolecular self-assembly of micro spherical CL-20/TNT energetic Co-crystal. Mater. Chem. Phys. 2023, 309, 128366. [Google Scholar] [CrossRef]
- Li, H.J.; Li, L.; Sun, J.; Han, J.M.; Yang, L.; Ren, X.T.; Tong, W.C. Construction and Preparation of the Novel ADN/CL-20 Cocrystal via Directional Hydrogen Bonding Design for Turning Hygroscopicity. Inorg. Chem. 2024, 63, 16713–16725. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, Z.; Han, T.; Li, J.; Han, P.; Wang, J. Preparation and Molecular Dynamic Simulation of Superfine CL-20/TNT Cocrystal Based on the Opposite Spray Method. Int. J. Mol. Sci. 2024, 25, 9501. [Google Scholar] [CrossRef]
- Du, J.; Wang, B.; Chen, Y.; Li, X.; Wang, C. Theoretical study of potential energetic material CL-20/DNAN eutectic explosive based on molecular dynamics method. J. Mol. Model. 2024, 30, 311. [Google Scholar] [CrossRef]
- Zhang, M.; Tan, Y.; Zhao, X.; Zhang, J.; Huang, S.; Zhai, Z.; Liu, Y.; Yang, Z. Seeking a novel energetic co-crystal strategy through the interfacial self-assembly of CL-20 and HMX nanocrystals. Crystengcomm 2020, 22, 61–67. [Google Scholar] [CrossRef]
- Shu, Y.; Wu, Z.; Liu, N.; Ding, X.; Wu, M.; Wang, K.; Lu, Y. Crystal Control and Cocrystal Formation:Important Route of Modification Research of Energetic Materials. Chin. J. Explos. Propellants 2015, 38, 1–9. [Google Scholar] [CrossRef]
- Liu, Q.C.; Yang, F.; Zhang, Q.; Duan, Y.W.; Deng, H.; Shang, L.P. Research on vibrational features of CL-20/MTNP cocrystal by terahertz spectroscopy. Acta Phys. Sin. 2024, 73, 193201. [Google Scholar] [CrossRef]
- Lv, X.; Liu, Z.; Li, C.; Deng, H.; Gao, J. Research Progress of Toxicology of Typical Energetic Compounds. Chin. J. Energ. Mater. 2023, 31, 729–740. [Google Scholar]
- Xia, J.; Gao, J.; Liu, Z.; Wang, H.; Yue, H.; Gao, Y.; Sun, C.; Song, J. Study on Teratogenicity of Hexa-benzyl-hexa-aza-iso-wurtzitane in Rats. J. Environ. Hyg. 2016, 6, 259–262. [Google Scholar]
- Liu, Z.; Gao, J.; Li, J.; Yue, H.; Wang, H.; Lu, Q.; Sun, C.; Song, J. Study on skin sensitization of hexabellzylhexaazaisowurtzita in guinea pigs. Occup. Health 2014, 30, 1468–1470. [Google Scholar]
- Xing, Y.; Chang, Z.; Du, W.; Wang, Y.; Liu, Y. Two new types of high energy density materials on rabbit skin irritation. J. Environ. Health 2014, 31, 226. [Google Scholar]
- Liu, Z.; Gao, J.; Wang, H.; Yue, H.; Gao, Y.; Lu, Q.; Sun, C.; Song, J. Study of sub-chronic oral toxicity of hexabenzylhexaazaisowurtzitane. Ind. Health Occup. Dis. 2018, 44, 255–259. [Google Scholar]
- Du, W.; Liu, Y.; Wang, Y.; Yang, X.; Jia, Z.; Wang, S.; Xue, X.; Zhang, Y. Study on mutagenicity and teratogenicity of hexanitrohexaazaisowurtzitane. China J. Ind. Hyg. Occup. Dis. 2007, 25, 2. [Google Scholar]
- Li, C.; Deng, H.; Liu, Z.; Lv, X.; Gao, W.; Gao, Y.; Gao, J.; Hu, L. Salidroside protect Chinese hamster V79 cells from genotoxicity and oxidative stress induced by CL-20. Toxicol. Res. 2023, 12, 133–142. [Google Scholar] [CrossRef]
- Bardai, G.K.; Halasz, A.; Sunahara, G.I.; Dodard, S.; Spear, P.A.; Grosse, S.; Hoang, J.; Hawari, J. In vitro degradation of hexanitrohexaazaisowurtzitane (CL-20) by cytosolic enzymes of Japanese quail and the rabbit. Environ. Toxicol. Chem. 2006, 25, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Kuperman, R.G.; Checkai, R.T.; Simini, M.; Sunahara, G.I.; Hawari, J. Energetic contaminants inhibit plant litter decomposition in soil. Ecotox. Environ. Saf. 2018, 153, 32–39. [Google Scholar] [CrossRef]
- Gong, P.; Basu, N.; Scheuhammer, A.M.; Perkins, E.J. Neurochemical and electrophysiological diagnosis of reversible neurotoxicity in earthworms exposed to sublethal concentrations of CL-20. Environ. Sci. Pollut. Res. 2010, 17, 181–186. [Google Scholar] [CrossRef]
- Gong, P.; Guan, X.; Pirooznia, M.; Liang, C.; Perkins, E.J. Gene Expression Analysis of CL-20-Induced Reversible Neurotoxicity Reveals GABA(A) Receptors as Potential Targets in the Earthworm Eisenia fetida. Environ. Sci. Technol. 2012, 46, 1223–1232. [Google Scholar] [CrossRef]
- Gong, P.; Escalon, B.L.; Hayes, C.A.; Perkins, E.J. Uptake of hexanitrohexaazaisowurtzitane (CL-20) by the earthworm Eisenia fetida through dermal contact. Sci. Total Environ. 2008, 390, 295–299. [Google Scholar] [CrossRef]
- Rocheleau, S.; Lachance, B.; Kuperman, R.G.; Hawari, J.; Thiboutot, S.; Ampleman, G.; Sunahara, G.I. Toxicity and uptake of cyclic nitrarnine explosives in ryegrass Lolium perenne. Environ. Pollut. 2008, 156, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Sunahara, G.I.; Rocheleau, S.; Dodard, S.G.; Robidoux, P.Y.; Hawari, J. Preliminary ecotoxicological characterization of a new energetic substance, CL-20. Chemosphere 2004, 56, 653–658. [Google Scholar] [CrossRef]
- Bradley, M.D. 2,4,6-Trinitrotoluene (TNT) air concentrations, hemoglobin changes, and anemia cases in respirator protected TNT munitions demilitarization workers. Int. Arch. Occup. Environ. Health 2011, 84, 239–250. [Google Scholar] [CrossRef]
- Wei, T.; Zhou, Y.; Yang, Z.; Yang, H. Progress of Toxicity Effects and Mechanisms of Typical Explosives. Chin. J. Energ. Mater. 2019, 27, 558–568. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Wang, C.Y.; Nie, Y.G.; Wu, L.J.; Xu, A. 2,4,6-trinitrotoluene causes mitochondrial toxicity in Caenorhabditis elegans by affecting electron transport. Environ. Res. 2024, 252, 118820. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, Y.; Xia, L.; Song, Y.; Li, W.; Kong, L.; Liu, Y.; Shen, C.; Huang, C.; Chu, Y. A study on the determination of the metabolites of 2,4,6-Trinitrotoluene using a dual-drift tube ion mobility spectrometer. Talanta 2025, 281, 126857. [Google Scholar] [CrossRef]
- Lin, D.; Chen, Y.Q.; Liang, L.S.; Huang, Z.X.; Guo, Y.W.; Cai, P.; Wang, W.X. Effects of exposure to the explosive and environmental pollutant 2,4,6-trinitrotoluene on ovarian follicle development in rats. Environ. Sci. Pollut. Res. 2023, 30, 96412–96423. [Google Scholar] [CrossRef]
- Kober, S.L.; Hollert, H.; Frohme, M. Quantification of nitroaromatic explosives in contaminated soil using MALDI-TOF mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 5993–6003. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Arif, M.; Seher, S.; Haleem, A.; Farhat, S.M.; Hassan, S.F.; Ahmed, S. Evaluation of Treatment Methods for Nitro-Aromatic Compounds from TNT Contaminated Soil in Pebbles Packed Reed-Beds Mesocosm. Water Air Soil Pollut. 2024, 235, 577. [Google Scholar] [CrossRef]
- Oates, N.C.; Nay, E.R.; Cary, T.J.; Rylott, E.L.; Bruce, N.C. New weapons explosive exhibits persistent toxicity in plants. Nat. Plants 2025, 11, 16–22. [Google Scholar] [CrossRef]
- Ni, S.Y.; Zhang, H.J.; Zhao, Y.N.; Pei, C.C.; Nie, Y.G.; Liu, X.D.; Wu, L.J.; Xu, A. Transgenerational reproductive toxicity of 2,4,6-trinitrotoluene (TNT) and its metabolite 4-ADNT in Caenorhabditis elegans. Environ. Toxicol. Pharmacol. 2022, 92, 103865. [Google Scholar] [CrossRef]
- Li, Y.; Luo, J.P.; Liao, X.Y.; Cao, H.Y.; Pan, J.T.; James, A.; Li, H.N. Multiomics insights into the TNT degradation mechanism by Pantoea sp. BJ2 isolated from an ammunition destruction site. Chem. Eng. J. 2024, 497, 154957. [Google Scholar] [CrossRef]
- Sharamok, T.S.; Khromykh, N.O.; Yesipova, N.B.; Marenkov, O.M.; Koptieva, S.D.; Korzhenevska, P.O.; Holub, I. Study on tnt toxic effects on the functional state of hydrobionts in the model contaminated water pond. J. Chem. Technol. 2024, 32, 518–527. [Google Scholar]
- Maser, E.; Bünning, T.H.; Strehse, J.S. Environmental and human toxicology studies on explosive chemicals leaking from submerged munitions. Propellants Explos. Pyrotech. 2024, 49, e202300181. [Google Scholar] [CrossRef]
- Gust, K.A.; Indest, K.J.; Lotufo, G.; Everman, S.J.; Jung, C.M.; Ballentine, M.L.; Hoke, A.V.; Sowe, B.; Gautam, A.; Hammamieh, R.; et al. Genomic investigations of acute munitions exposures on the health and skin microbiome composition of leopard frog (Rana pipiens) tadpoles. Environ. Res. 2021, 192, 110245. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Bahkali, A.H.; Elgorban, A.M.; Al-Otaibi, M.S.; Ghfar, A.A.; Gabr, S.A.; Wabaidur, S.M.; Habila, M.A.; Ahmed, A. Bioremediation of Explosive TNT by Trichoderma viride. Molecules 2020, 25, 1393. [Google Scholar] [CrossRef]
- Davies, J.O.J.; Roberts, D.M.; Hittarage, A.; Buckley, N.A. Oral C-4 plastic explosive in humans—A case series. Clin. Toxicol. 2007, 45, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Kucukardali, Y.; Acar, H.V.; Ozkan, S.; Nalbant, S.; Yazgan, Y.; Atasoyu, E.M.; Keskin, O.; Naz, A.; Akyatan, N.; Gokben, M.; et al. Accidental oral poisoning caused by RDX (cyclonite): A report of 5 cases. J. Intensive Care Med. 2003, 18, 42–46. [Google Scholar] [CrossRef]
- Gao, T.; Liu, Z.; Gao, Y.; Li, C.; Zhao, B.; Li, H.; Fan, P.; Gao, J. Research progress on neurotoxicity of hexogen. Chin. J. Ind. Med. 2022, 35, 42–44. [Google Scholar]
- Abadin, H.; Smith, C.; Ingerman, L.; Llados, F.T.; Barber, L.E.; Plewak, D.; Diamond, G.L. Toxicological Profile for RDX; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2012; p. 243.
- Hwang, J.; Peng, Z.K.; Najar, F.Z.; Xu, C.; Agnew, R.J.; Xu, X.; Yang, Z.B.; Ahsan, N. Urine proteome profile of firefighters with exposure to emergency fire-induced smoke: A pilot study to identify potential carcinogenic effects. Sci. Total Environ. 2024, 927, 172273. [Google Scholar] [CrossRef]
- Mukhi, S.; Pan, X.P.; Cobb, G.P.; Patiño, R. Toxicity of hexahydro-1,3,5-trinitro-1,3,5-triazine to larval zebrafish (Danio rerio). Chemosphere 2005, 61, 178–185. [Google Scholar] [CrossRef]
- Mukhi, S.; Patiño, R. Effects of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in zebrafish:: General and reproductive toxicity. Chemosphere 2008, 72, 726–732. [Google Scholar] [CrossRef]
- Talmage, S.S.; Opresko, D.M.; Maxwell, C.J.; Welsh, C.J.; Cretella, F.M.; Reno, P.H.; Daniel, F.B. Nitroaromatic munition compounds: Environmental effects and screening values. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 1999; Volume 161, pp. 1–156. [Google Scholar] [CrossRef]
- Johnson, M.S.; Paulus, H.I.; Salice, C.J.; Checkai, R.T.; Simini, M. Toxicologic and histopathologic response of the terrestrial salamander Plethodon cinereus to soil exposures of 1,3,5-trinitrohexahydro-1,3,5-triazine. Arch. Environ. Contam. Toxicol. 2004, 47, 496–501. [Google Scholar] [CrossRef]
- Gogal, R.M.; Johnson, M.S.; Larsen, C.T.; Prater, M.R.; Duncan, R.B.; Ward, D.L.; Lee, R.B.; Salice, C.J.; Jortner, B.; Holladay, S.D. Dietary oral exposure to 1,3,5-trinitro-1,3,5-triazine in the northern bobwhite (Colinus virginianus). Environ. Toxicol. Chem. 2003, 22, 381–387. [Google Scholar] [CrossRef]
- Sun, L.Y.; Zhou, Y.P.; Wang, C.Y.; Nie, Y.G.; Xu, A.; Wu, L.J. Multi-generation reproductive toxicity of RDX and the involved signal pathways in Caenorhabditis elegans. Ecotox. Environ. Saf. 2023, 260, 115074. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.S.; McFarland, C.A.; Bazar, M.A.; Quinn, M.J.; LaFiandra, E.M.; Talent, L.G. Toxicity of Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine (HMX) in Three Vertebrate Species. Arch. Environ. Contam. Toxicol. 2010, 58, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Bhanot, P.; Celin, S.M.; Sharma, P.; Sahai, S.K.; Kalsi, A. Comparative Evaluation of Low- and Medium-Pressure UV Lamps for Photo-degradation of RDX Wastewater. Water Air Soil Pollut. 2023, 234, 587. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, K.; Chai, G.; Xi, P.; Yang, H.; Xie, L.; Qin, L.; Lin, Y.; Li, X.; Yan, W.; et al. Performance Optimization and Toxicity Effects of the Electrochemical Oxidation of Octogen. Catalysts 2022, 12, 815. [Google Scholar] [CrossRef]
- Meda, A.; Sangwan, P.; Bala, K. Optimization of process parameters for degradation of HMX with Bacillus toyonensis using response surface methodology. Int. J. Environ. Sci. Technol. 2020, 17, 4601–4610. [Google Scholar] [CrossRef]
- Sharma, K.; Sangwan, P.; Sharma, P. Microbial Degradation of Explosive Manufacturing Facility Wastewater in a Bioreactor. Int. J. Environ. Res. 2023, 17, 49. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, C.; Xue, X.; Liu, Y.; Wang, S.; Zhang, Y.; Cheng, X.; Hao, Y.; Liu, M. The impact of octogen on the neurobehavioral functions of workers. Ind. Health Occup. Dis. 1998, 2, 94–96. [Google Scholar]
- Singh, B.; Kaur, J.; Singh, K. Microbial remediation of explosive waste. Crit. Rev. Microbiol. 2012, 38, 152–167. [Google Scholar] [CrossRef]
- Robidoux, P.Y.; Hawari, J.; Bardai, G.; Paquet, L.; Ampleman, G.; Thiboutot, S.; Sudahara, G.I. TNT, RDX, and HMX decrease earthworm (Eisenia andrei) life-cycle responses in a spiked natural forest soil. Arch. Environ. Contam. Toxicol. 2002, 43, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Panz, K.; Miksch, K. Phytoremediation of explosives (TNT, RDX, HMX) by wild-type and transgenic plants. J. Environ. Manag. 2012, 113, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Panz, K.; Miksch, K.; Sójka, T. Synergetic Toxic Effect of an Explosive Material Mixture in Soil. Bull. Environ. Contam. Toxicol. 2013, 91, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Grisold, W.; Carozzi, V.A. Toxicity in Peripheral Nerves: An Overview. Toxics 2021, 9, 218. [Google Scholar] [CrossRef]
- Volkov, E.M.; Nurullin, L.F.; Volkov, M.E.; Nikolsky, E.E.; Vyskocil, F. Mechanisms of carbacholine and GABA action on resting membrane potential and Na+/K+-ATPase of Lumbricus terrestris body wall muscles. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2011, 158, 520–524. [Google Scholar] [CrossRef]
- Atchison, W.D. Effects of neurotoxicants on synaptic transmission: Lessons learned from electrophysiological studies. Neurotoxicology Teratol. 1988, 10, 393–416. [Google Scholar] [CrossRef]
- Deng, H.; Liu, Z.; Li, C.; Lv, X.; Gao, T.; Li, H.; Gao, J. Research Progress on Toxicity and Biodegradation of CL-20. Guangzhou Chem. Ind. 2023, 51, 14–16. [Google Scholar] [CrossRef]
- Allemang, A.; Lester, C.; Roth, T.; Pfuhler, S.; Peuschel, H.; Kosemund, K.; Mahony, C.; Bergeland, T.; O’Keeffe, L. Assessing the genotoxicity and carcinogenicity of 2-chloroethanol through structure activity relationships and in vitro testing approaches. Food Chem. Toxicol. 2022, 168, 113290. [Google Scholar] [CrossRef]
- Strehse, J.S.; Brenner, M.; Kisiela, M.; Maser, E. The explosive trinitrotoluene (TNT) induces gene expression of carbonyl reductase in the blue mussel (Mytilus spp.): A new promising biomarker for sea dumped war relicts? Arch. Toxicol. 2020, 94, 4043–4054. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.Y.; Kao, C.M.; Yao, C.L.; Chiu, P.W.; Yao, C.C.; Chen, S.C. 2,4,6-Trinitrotoluene Induces Apoptosis via ROS-Regulated Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in HepG2 and Hep3B Cells. Sci. Rep. 2017, 7, 8148. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Hassan, H.A.; Ahmed, H.S.; Hassan, D.F. Free radicals and oxidative stress: Mechanisms and therapeutic targets. Hum. Antibodies 2024, 32, 151–167. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Fan, X.; Wang, H.; Niu, H.; Gao, J. Effects of octogen and its intermediate product on apoptosis and oxidative stress of Chinese hamster lung cells. J. Environ. Occup. Med. 2020, 37, 808–811. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, M.; Li, H.R.; Liu, L.; Zhou, Y.; Long, X.P. Collective absorption of 2,4,6-trinitrotoluene into lipid membranes and its effects on bilayer properties. A computational study. RSC Adv. 2019, 9, 39046–39054. [Google Scholar] [CrossRef]
- Möller, C.; Davis, W.C.; Thompson, V.R.; Mari, F.; DeCaprio, A.P. Proteomic Analysis of Thiol Modifications and Assessment of Structural Changes in Hemoglobin Induced by the Aniline Metabolites N-Phenylhydroxylamine and Nitrosobenzene. Sci. Rep. 2017, 7, 14794. [Google Scholar] [CrossRef]
- Salvatici, M.; Caslini, C.; Alesci, S.; Arosio, G.; Meroni, G.; Ceriotti, F.; Ammirabile, M.; Drago, L. The Application of Clinical and Molecular Diagnostic Techniques to Identify a Rare Haemoglobin Variant. Int. J. Mol. Sci. 2024, 25, 6781. [Google Scholar] [CrossRef]
- Kiese, M.; Taeger, K. The fate of phenylhydroxylamine in human red cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1976, 292, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Borsche, M.; Pereira, S.L.; Klein, C.; Grünewald, A. Mitochondria and Parkinson’s Disease: Clinical, Molecular, and Translational Aspects. J. Park. Dis. 2021, 11, 45–60. [Google Scholar] [CrossRef]
- Esteve-Núñez, A.; Caballero, A.; Ramos, J.L. Biological degradation of 2,4,6-trinitrotoluene. Microbiol. Mol. Biol. Rev. 2001, 65, 335–352. [Google Scholar] [CrossRef]
- Rylott, E.L.; Bruce, N.C. Right on target: Using plants and microbes to remediate explosives. Int. J. Phytoremediat. 2019, 21, 1051–1064. [Google Scholar] [CrossRef]
- Loverde, S.M. Molecular Simulation of the Transport of Drugs across Model Membranes. J. Phys. Chem. Lett. 2014, 5, 1659–1665. [Google Scholar] [CrossRef]
- Yang, H.; Li, H.; Liu, L.; Zhou, Y.; Long, X. Molecular Simulation Studies on the Interactions of 2,4,6-Trinitrotoluene and Its Metabolites with Lipid Membranes. J. Phys. Chem. B 2019, 123, 6481–6491. [Google Scholar] [CrossRef]
- Li, G.Z.; Yang, H.M.; Zhang, P.Y.; Guo, Y.; Yuan, L.L.; Xu, S.J.; Yuan, Y.X.; Xiong, H.B.; Yin, H.Y. Insights into the molecular underlying mechanisms and therapeutic potential of endoplasmic reticulum stress in sensorineural hearing loss. Front. Molec. Neurosci. 2024, 17, 1443401. [Google Scholar] [CrossRef]
- Liu, Z.H.; Liu, Q.; Zeng, A.Q.; Song, L.J. Regulatory function of endoplasmic reticulum stress in colorectal cancer: Mechanism, facts, and perspectives. Int. Immunopharmacol. 2025, 147, 114024. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.R.; Cui, W.; Liu, H.P. Research progress and advances in endoplasmic reticulum stress regulation of acute kidney injury. Ren. Fail. 2024, 46, 2433160. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xia, G.; Li, X.; Gong, M.; Lv, X. Endoplasmic reticulum stress in liver fibrosis: Mechanisms and therapeutic potential. Biochim. Biophys. acta. Mol. Basis Dis. 2025, 1871, 167695. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.C.; Zhou, L.; Jin, Y.J.; Wu, D.L.; Chen, N.; Zhang, C.C.; Liu, H.P.; Li, C.L.; Ning, R.; Yang, X.C.; et al. Calcium bridges built by mitochondria-associated endoplasmic reticulum membranes: Potential targets for neural repair in neurological diseases. Neural Regen. Res. 2025, 20, 3349–3369. [Google Scholar] [CrossRef]
- Song, L.; Wang, Y.; Wang, J.; Yang, F.; Li, X.J.; Wu, Y.H. Trinitrotoluene Induces Endoplasmic Reticulum Stress and Apoptosis in HePG2 Cells. Med. Sci. Monit. 2015, 21, 3434–3441. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Reyero, N.; Habib, T.; Pirooznia, M.; Gust, K.A.; Gong, P.; Warner, C.; Wilbanks, M.; Perkins, E. Conserved toxic responses across divergent phylogenetic lineages: A meta-analysis of the neurotoxic effects of RDX among multiple species using toxicogenomics. Ecotoxicology 2011, 20, 580–594. [Google Scholar] [CrossRef]
- Ehrich, M.; Wu, X.H.; Werre, S.R.; Major, M.A.; McCain, W.C.; Reddy, G. Calcium Signaling in Neuronal Cells Exposed to the Munitions Compound Cyclotrimethylenetrinitramine (RDX). Int. J. Toxicol. 2009, 28, 425–435. [Google Scholar] [CrossRef]
- Catoni, C.; Calì, T.; Brini, M. Calcium, Dopamine and Neuronal Calcium Sensor 1: Their Contribution to Parkinson’s Disease. Front. Molec. Neurosci. 2019, 12, 8. [Google Scholar] [CrossRef]
- Gust, K.A.; Pirooznia, M.; Quinn, M.J.; Johnson, M.S.; Escalon, L.; Indest, K.J.; Guan, X.; Clarke, J.; Deng, Y.P.; Gong, P.; et al. Neurotoxicogenomic Investigations to Assess Mechanisms of Action of the Munitions Constituents RDX and 2,6-DNT in Northern Bobwhite (Colinus virginianus). Toxicol. Sci. 2009, 110, 168–180. [Google Scholar] [CrossRef]
- Williams, L.R.; Aroniadou-Anderjaska, V.; Qashu, F.; Finne, H.; Pidoplichko, V.; Bannon, D.I.; Braga, M.F.M. RDX Binds to the GABAA Receptor-Convulsant Site and Blocks GABAA Receptor-Mediated Currents in the Amygdala: A Mechanism for RDX-Induced Seizures. Environ. Health Perspect. 2011, 119, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Mundy, P.C.; Werner, A.; Singh, L.; Singh, V.; Mendieta, R.; Patullo, C.E.; Wulff, H.; Lein, P.J. Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) causes seizure activity in larval zebrafish via antagonism of γ-aminobutyric acid type A receptor α1β2γ2. Arch. Toxicol. 2023, 97, 1355–1365. [Google Scholar] [CrossRef]
- Pressly, B.; Lee, R.D.; Singh, V.; Pessah, I.N.; Wulff, H. The seizure-inducing plastic explosive RDX inhibits the α1β2y2 GABAA receptor. Ann. Clin. Transl. Neurol. 2022, 9, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Gust, K.A.; Brasfield, S.M.; Stanley, J.K.; Wilbanks, M.S.; Chappell, P.; Perkins, E.J.; Lotufo, G.R.; Lance, R.F. Genomic investigation of year-long and multigenerational exposures of fathead minnow to the munitions compound RDX. Environ. Toxicol. Chem. 2011, 30, 1852–1864. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Deb, U.; Datta, S.; Walther, C.; Gupta, D.K. Common explosives (TNT, RDX, HMX) and their fate in the environment: Emphasizing bioremediation. Chemosphere 2017, 184, 438–451. [Google Scholar] [CrossRef]
- Laschet, J.J.; Minier, F.; Kurcewicz, I.; Bureau, M.H.; Trottier, S.; Jeanneteau, F.; Griffon, N.; Samyn, B.; Van Beeumen, J.; Louvel, J.; et al. Glyceraldehyde-3-phosphate dehydrogenase is a GABAA receptor kinase linking glycolysis to neuronal inhibition. J. Neurosci. 2004, 24, 7614–7622. [Google Scholar] [CrossRef]
- Laschet, J.J.; Kurcewicz, I.; Minier, F.; Trottier, S.; Khallou-Laschet, J.; Louvel, J.; Gigout, S.; Turak, B.; Biraben, A.; Scarabin, J.M.; et al. Dysfunction of GABAA receptor glycolysis-dependent modulation in human partial epilepsy. Proc. Natl. Acad. Sci. USA 2007, 104, 3472–3477. [Google Scholar] [CrossRef]
- Bannon, D.I.; Johnson, M.; Williams, L.; Adams, V.; Perkins, E.; Gust, K.; Gong, P. RDX and miRNA Expression in B6C3F1 Mice Response. Environ. Health Perspect. 2009, 117, A98. [Google Scholar] [CrossRef]
- Deng, H.; Lv, X.; Li, C.; Gao, T.; Li, H.; Gao, J. Oxidative stress damage of octogen to DNA in V79 cells. J. Environ. Health 2024, 41, 202–205. [Google Scholar][Green Version]
- Song, J.; Wang, T.; Hong, J.-S.; Wang, Y.; Feng, J. TFEB-dependent autophagy-lysosomal pathway is required for NRF2-driven antioxidative action in obstructive sleep apnea-induced neuronal injury. Cell. Signal. 2025, 128, 111630. [Google Scholar] [CrossRef]
- Mingardi, J.; Meanti, R.; Paoli, C.; Cifani, C.; Torsello, A.; Popoli, M.; Musazzi, L. Ghrelin, Neuroinflammation, Oxidative Stress, and Mood Disorders: What Are the Connections? Curr. Neuropharmacol. 2025, 23, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Oliyaei, N.; Zekri, S.; Iraji, A.; Oliyaei, A.; Tanideh, R.; Mussin, N.M.; Tamadon, A.; Tanideh, N. Health benefits of algae and marine-derived bioactive metabolites for modulating ulcerative colitis symptoms. J. Funct. Food. 2025, 125, 106690. [Google Scholar] [CrossRef]
- Justi, L.H.Z.; Silva, J.F.; Santana, M.S.; Laureano, H.A.; Pereira, M.E.; Oliveira, C.S.; Guiloski, I.C. Non-steroidal anti-inflammatory drugs and oxidative stress biomarkers in fish: A meta-analytic review. Toxicol. Rep. 2025, 14, 101910. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.X.; Yang, D.Z.; Wang, W.W.; Hu, K.; Yan, M.; Zhang, L.; Gao, L.; Lu, Y. Recent advances in pharmaceutical cocrystals of theophylline. Nat. Product. Bioprospecting 2024, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.L.; Duan, C.X.; Qin, H.M.; Huang, C.A.; Hou, J.X.; Chen, Y.M.; Zhu, J.; Xu, C.C.; Jin, J.; Zhuang, T. Synthesis and structural characterization of a novel palbociclib-kaempferol cocrystal with improved tabletability and synergistic antitumor activity. J. Mol. Struct. 2023, 1281, 135101. [Google Scholar] [CrossRef]
- Li, D.; Li, J.; Deng, Z.; Zhang, H. The discovery of new cocrystals of 5-fluorocytosine using amine-carboxylate supramolecular synthon. J. Drug Deliv. Sci. Technol. 2022, 78, 103934. [Google Scholar] [CrossRef]
- Rathi, R.; Kushwaha, R.; Goyal, A.; Singh, I. Oxaliplatin-flavone pharmaceutical co-crystal-CN111205332A: Patent spotlight. Pharm. Pat. Anal. 2022, 11, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, H.; Zhou, M.; Wei, T.; Zhou, Y. Synergistic Effects of IMX-104 Components in Membrane Absorption: A Computational Study. ACS Omega 2022, 7, 40892–40899. [Google Scholar] [CrossRef] [PubMed]
- Binder, F.I.; Schuster, R.M.; Bünning, L.T.H.; Strehse, J.S.; Brockmann, M.; Herges, R.; Maser, E.; Brenner, M. Depuration kinetics of trinitrotoluene (TNT) and its metabolites in exposed blue mussels (Mytilus edulis L.). Front. Mar. Sci. 2025, 12, 15. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.L.; Sun, C.; Luo, Y.T.; Shi, Q.; Liang, R.P.; Qiu, J.D. Metallization-mediated charge transfer in cocrystals for efficient photocatalytic decontamination of mustard-gas simulant. J. Environ. Chem. Eng. 2025, 13, 115963. [Google Scholar] [CrossRef]
- Song, W.D.; Zhu, H.; Xiao, L.J.; Chen, J. Impact response and chemical reaction mechanisms of CL-20/TNT co-crystals with elliptical voids defects: A study of molecular dynamics. Mater. Today Commun. 2025, 47, 113253. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Li, C.; Li, H.; Deng, H.; Lv, X.; Gao, T.; Shen, J.; Zhao, B.; Liu, Z.; Gao, J. Toxic Effects of the Most Common Components of Energetic Co-Crystals. Molecules 2025, 30, 3234. https://doi.org/10.3390/molecules30153234
Peng X, Li C, Li H, Deng H, Lv X, Gao T, Shen J, Zhao B, Liu Z, Gao J. Toxic Effects of the Most Common Components of Energetic Co-Crystals. Molecules. 2025; 30(15):3234. https://doi.org/10.3390/molecules30153234
Chicago/Turabian StylePeng, Xinying, Cunzhi Li, Huan Li, Hui Deng, Xiaoqiang Lv, Ting Gao, Jiachen Shen, Bin Zhao, Zhiyong Liu, and Junhong Gao. 2025. "Toxic Effects of the Most Common Components of Energetic Co-Crystals" Molecules 30, no. 15: 3234. https://doi.org/10.3390/molecules30153234
APA StylePeng, X., Li, C., Li, H., Deng, H., Lv, X., Gao, T., Shen, J., Zhao, B., Liu, Z., & Gao, J. (2025). Toxic Effects of the Most Common Components of Energetic Co-Crystals. Molecules, 30(15), 3234. https://doi.org/10.3390/molecules30153234