Protective Effects of Sodium Copper Chlorophyllin and/or Ascorbic Acid Against Barium Chloride-Induced Oxidative Stress in Mouse Brain and Liver
Abstract
1. Introduction
2. Results
2.1. Safety Profile and Behavioral Effects of SCC and ASC
2.1.1. SCC and ASC Toxicity
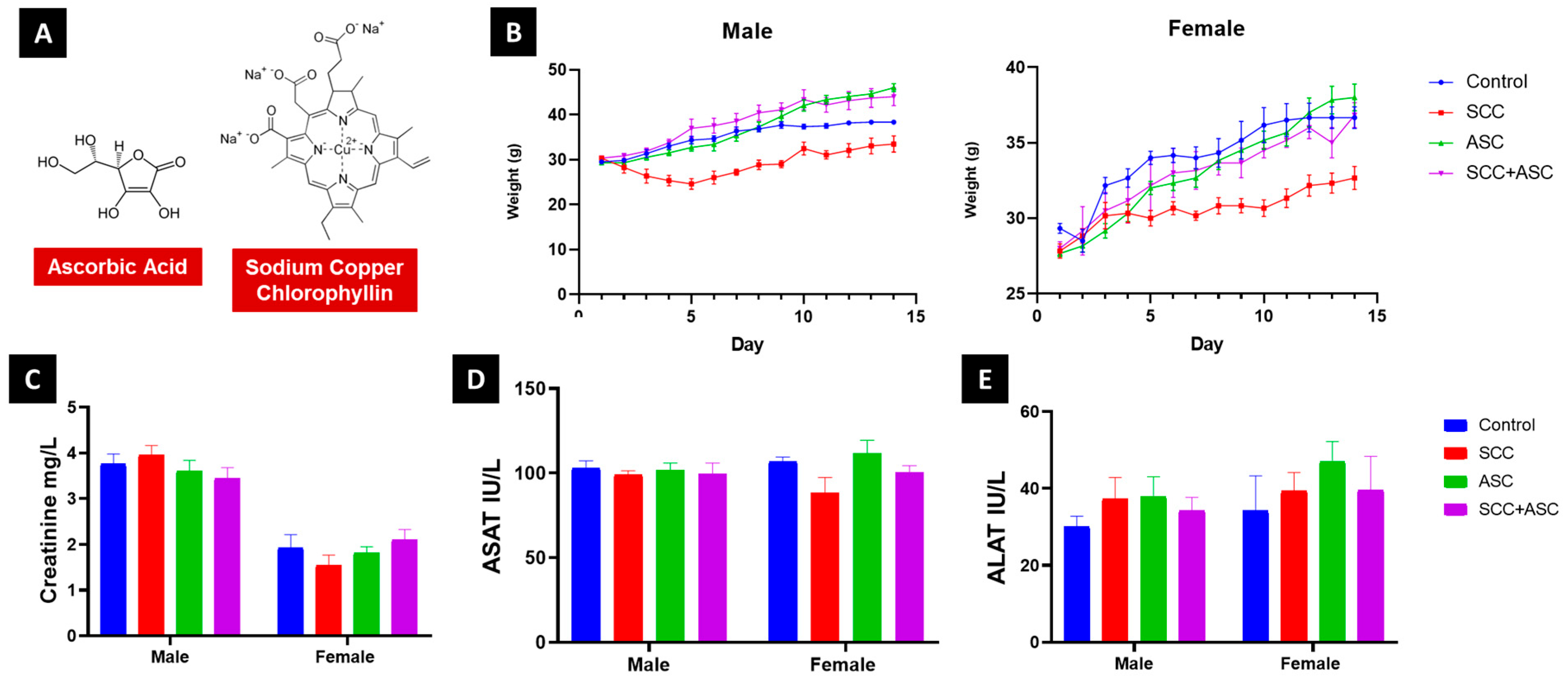
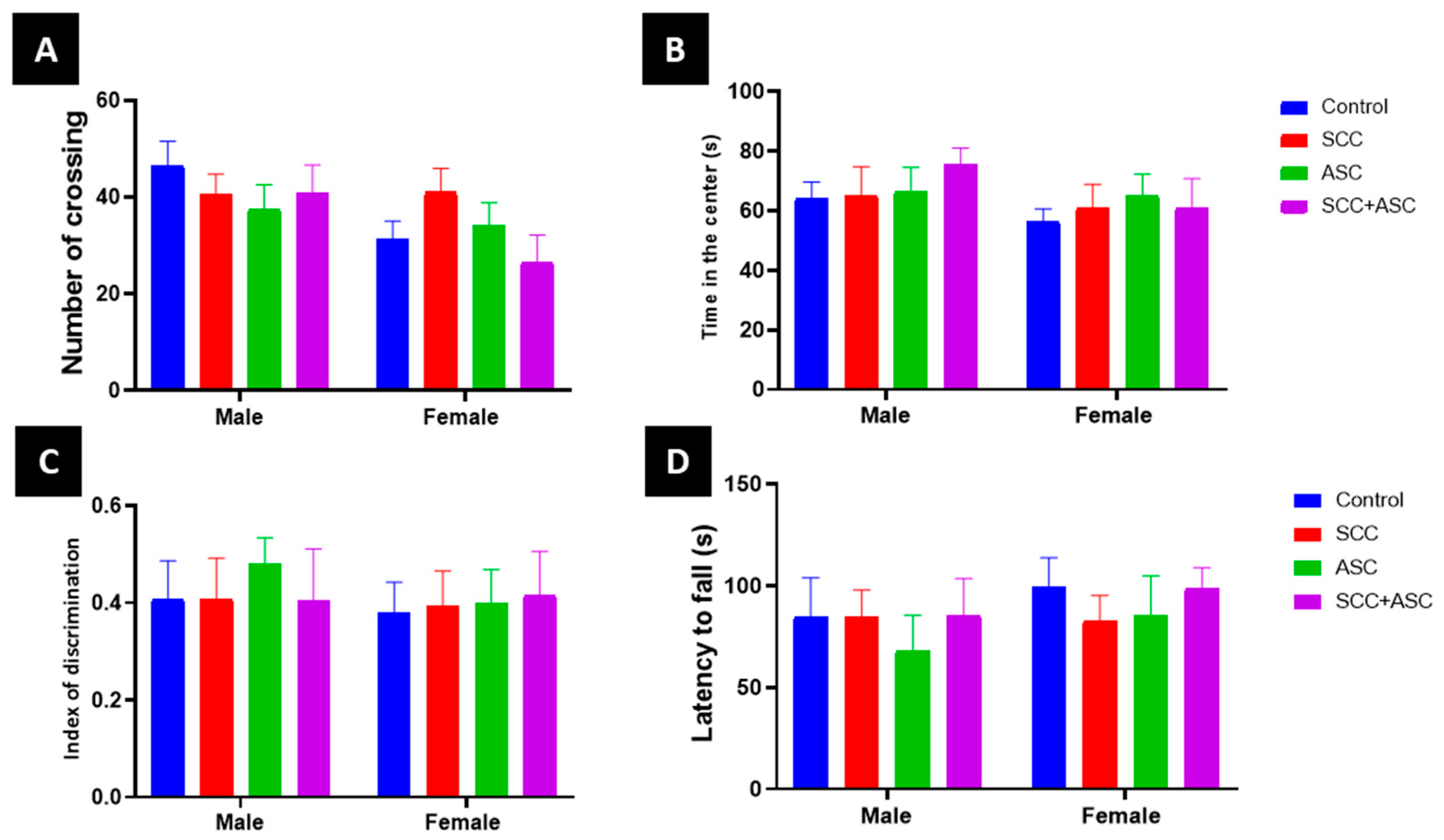
2.1.2. SCC and ASC Effect on Behavior
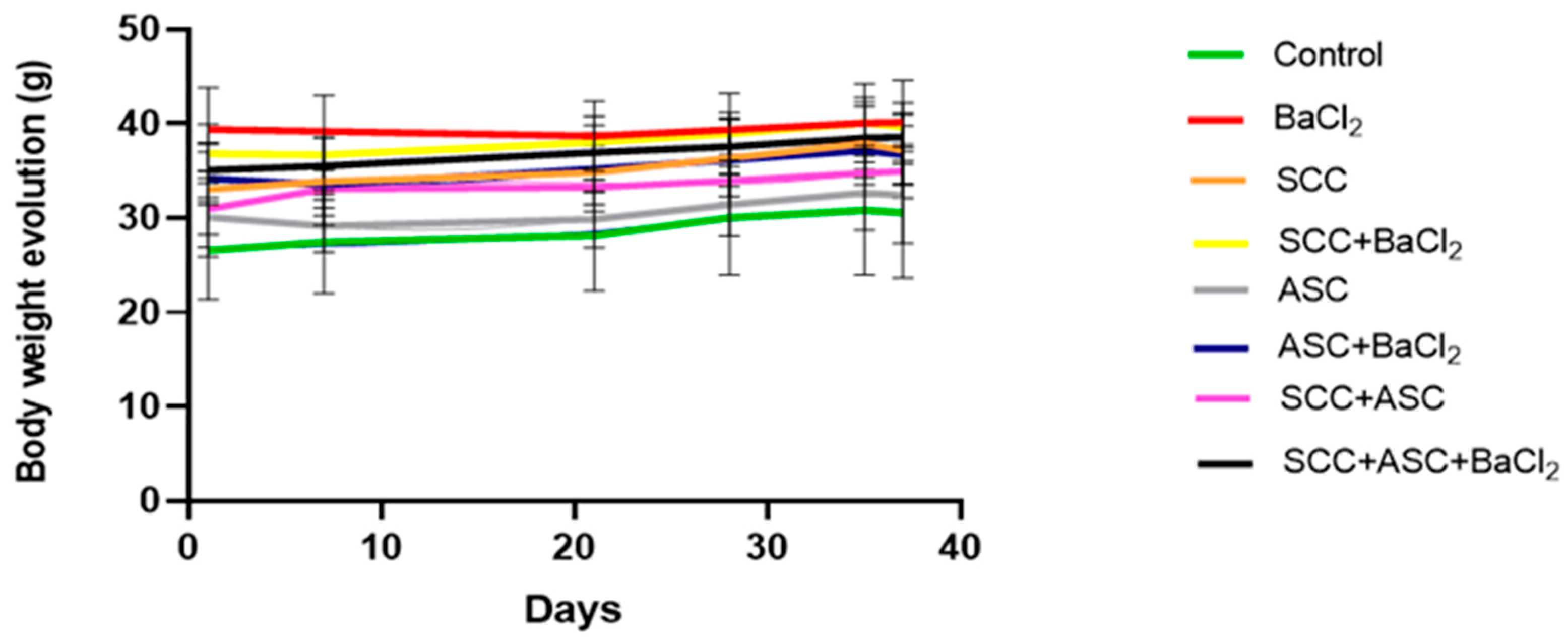
2.2. Effects on Body Weight and Relative Weight
2.3. Serum Biochemical Markers
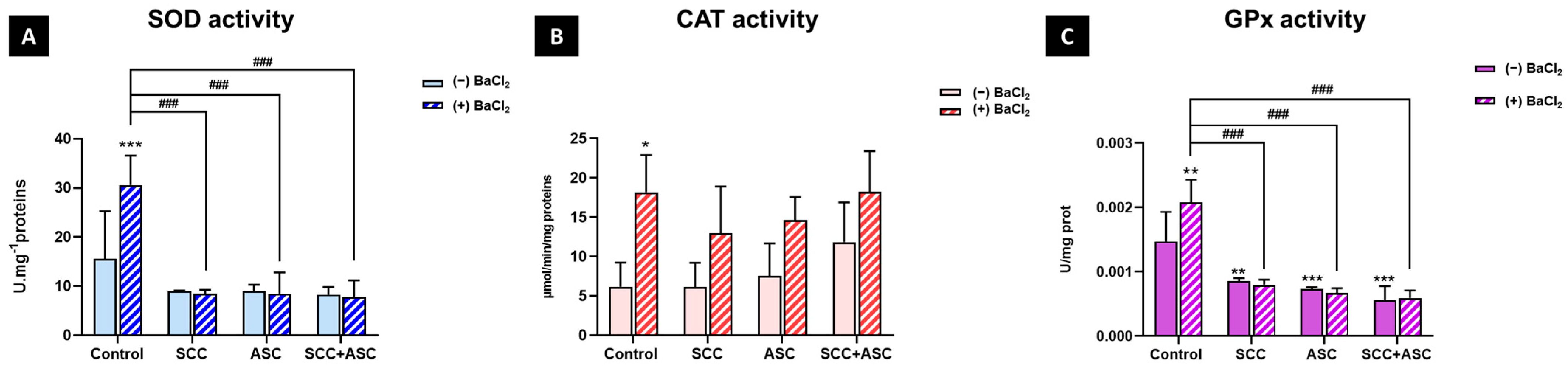
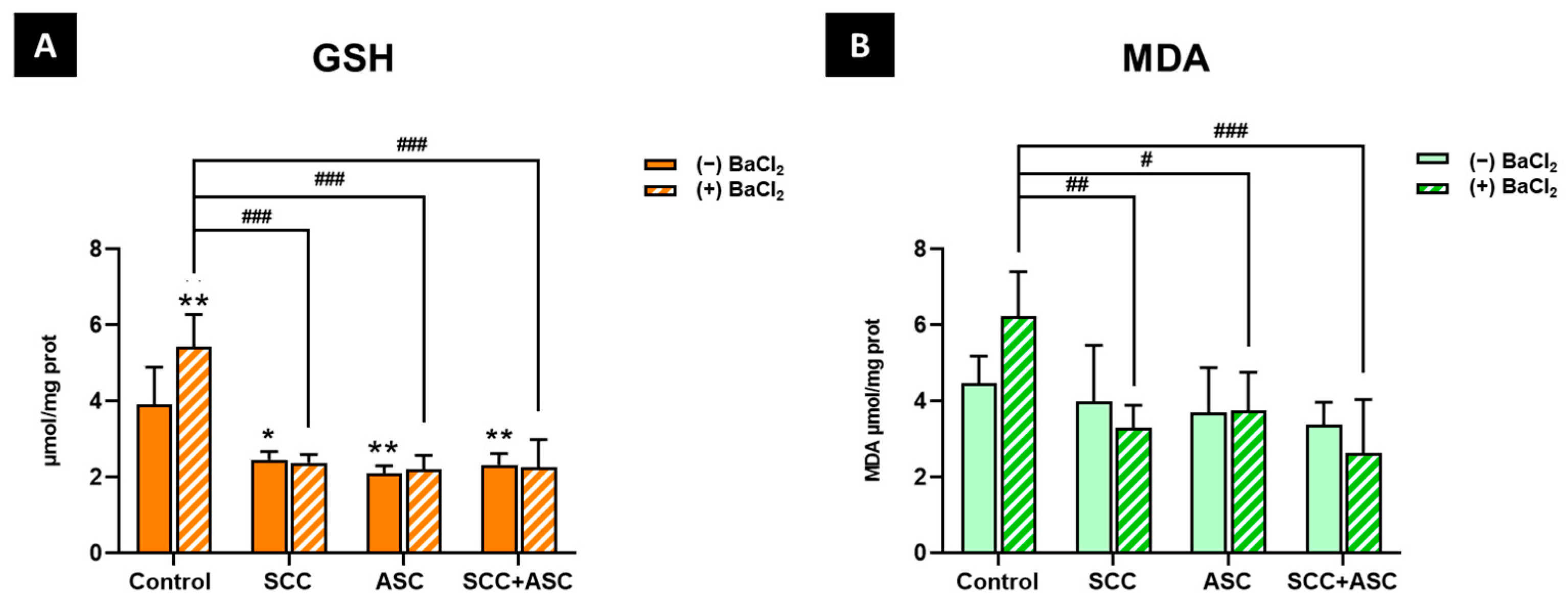
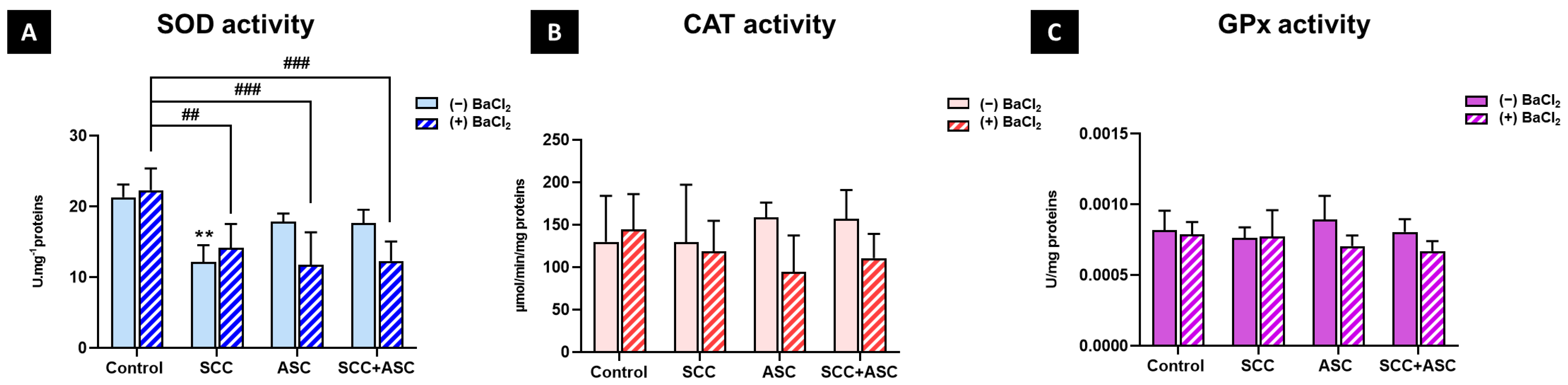
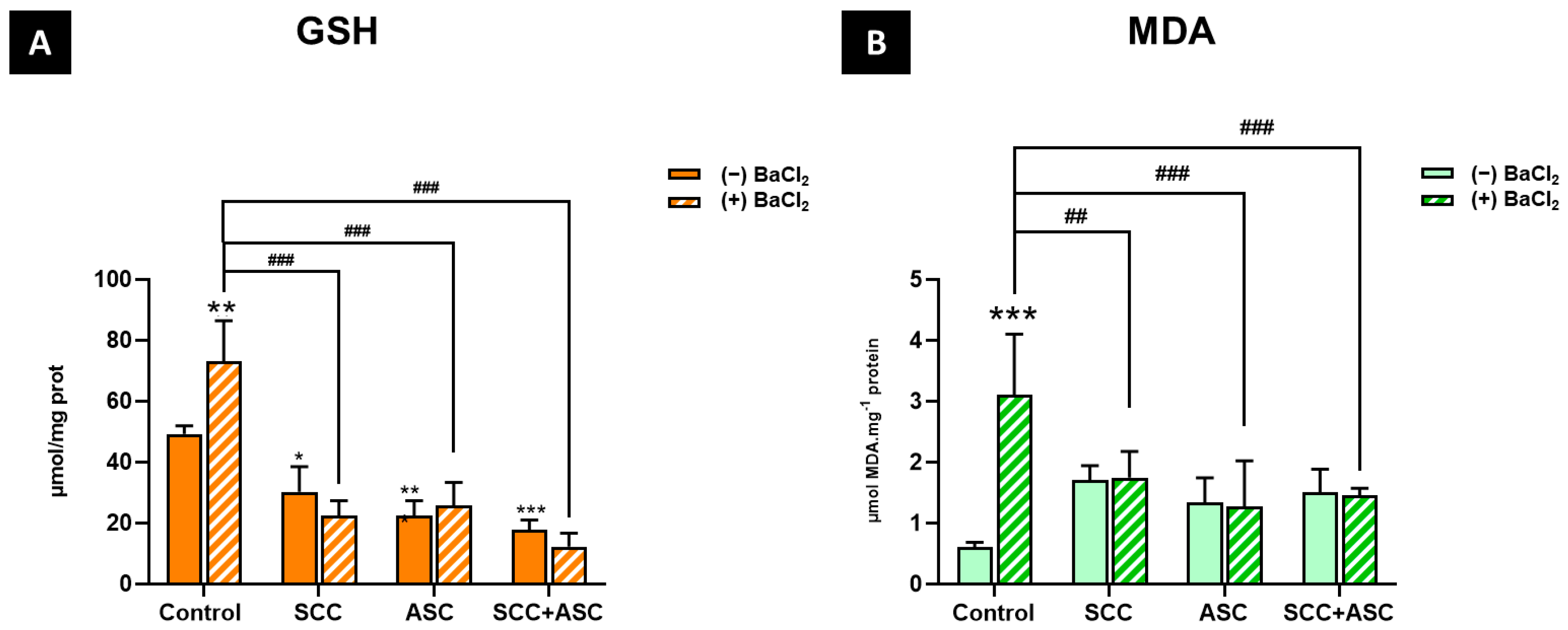
2.4. Effect on Cerebral Antioxidant Markers
2.5. Effects on Hepatic Antioxidants Markers
2.6. Histopathological Examination
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Toxicological Study Design
4.3.1. Behavioral and Neurotoxicity Assessments
4.3.2. Cognitive and Motor Function Evaluation
4.4. Animals and Experimental Design
4.5. Serum Biochemical and Ionic Parameters
4.6. Homogenate Preparation
4.7. Protein Content
4.8. Superoxide Dismutase
4.9. Catalase
4.10. Lipid Peroxidation
4.11. Glutathione
4.12. Glutathione Peroxidase
4.13. Histopathological Analysis
4.14. Statistical Analysis
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BaCl2 | Barium chloride |
| SCC | Sodium copper chlorophyllin |
| ASC | Ascorbic acid (Vitamin C) |
| ROS | Reactive oxygen species |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| ETC | Electron transport chain |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NOX | NADPH oxidase |
| H2O2 | Hydrogen peroxide |
| O2•− | Superoxide anion |
| DTNB | 5,5′-dithiobis (2-nitrobenzoic acid) |
| TNB− | 5-thio-2-nitrobenzoate |
| TCA | Trichloroacetic acid |
| BSA | Bovine serum albumin |
| H&E | Hematoxylin and eosin |
| RITA | Registry of Industrial Toxicology Animal-data |
| NACAD | National Advisory Committee for Acute Exposure Guidelines |
| ANOVA | Analysis of variance |
| LD50 | Lethal dose, 50% |
References
- Jacobs, I.A.; Taddeo, J.; Kelly, K.; Valenziano, C. Poisoning as a result of barium styphnate explosion. Am. J. Ind. Med. 2002, 41, 285–288. [Google Scholar] [CrossRef]
- Elwej, A.; Grojja, Y.; Ghorbel, I.; Boudawara, O.; Jarraya, R.; Boudawara, T.; Zeghal, N. Barium chloride induces redox status unbalance, upregulates cytokine genes expression and confers hepatotoxicity in rats-alleviation by pomegranate peel. Environ. Sci. Pollut. Res. Int. 2016, 23, 7559–7571. [Google Scholar] [CrossRef] [PubMed]
- Copeland, C.S.; Rock, K.L.; Pinhal, A.; Chapman, R.C.; Chilcott, R.P. A Fatal Case Report of Barium Chloride Toxicity. J. Anal. Toxicol. 2023, 47, 33–41. [Google Scholar] [CrossRef]
- McCauley, P.T.; Washington, I.S. Barium bioavailability as the chloride, sulfate, or carbonate salt in the rat. Drug Chem. Toxicol. 1983, 6, 209–217. [Google Scholar] [CrossRef]
- Diwan, J.J. Ba2+ uptake and the inhibition by Ba2+ of K+ flux into rat liver mitochondria. J. Membr. Biol. 1985, 84, 165–171. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Wafaa Taha, O.A.; Houssam, A.; Chaimaa, S.; Asmae, T.; El Mariame, M.; Faiza, B.; Fatima, C. Unraveling the Triad: A Bioinformatics Analysis of the Interplay between Prenatal Depression, Inflammation, and the Gut Microbiota. CNS Neurol. Disord.-Drug Targets 2025, 24, 846–864. [Google Scholar] [CrossRef]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Trofin, D.-M.; Sardaru, D.-P.; Trofin, D.; Onu, I.; Tutu, A.; Onu, A.; Onită, C.; Galaction, A.I.; Matei, D.V. Oxidative Stress in Brain Function. Antioxidants 2025, 14, 297. [Google Scholar] [CrossRef]
- Benayad, S.; Wahnou, H.; El Kebbaj, R.; Liagre, B.; Sol, V.; Oudghiri, M.; Saad, E.M.; Duval, R.E.; Limami, Y. The Promise of Piperine in Cancer Chemoprevention. Cancers 2023, 15, 5488. [Google Scholar] [CrossRef]
- Es-Sai, B.; Wahnou, H.; Benayad, S.; Rabbaa, S.; Laaziouez, Y.; El Kebbaj, R.; Limami, Y.; Duval, R.E. Gamma-Tocopherol: A Comprehensive Review of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties. Molecules 2025, 30, 653. [Google Scholar] [CrossRef]
- Tahri-Joutey, M.; Saih, F.-E.; El Kebbaj, R.; Gondcaille, C.; Vamecq, J.; Latruffe, N.; Lizard, G.; Savary, S.; Nasser, B.; Cherkaoui-Malki, M.; et al. Protective Effect of Nopal Cactus (Opuntia ficus-indica) Seed Oil against Short-Term Lipopolysaccharides-Induced Inflammation and Peroxisomal Functions Dysregulation in Mouse Brain and Liver. Int. J. Mol. Sci. 2022, 23, 11849. [Google Scholar] [CrossRef] [PubMed]
- Bouchab, H.; Abbas, I.; Riad, E.L.K.; Boubker, N.; Saretzki, G. Protective effect of argan oil on DNA damage in vivo and in vitro. Biomarkers 2021, 26, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Rabbaa, S.; Bouchab, H.; Laaziouez, Y.; Limami, Y.; Nasser, B.; Andreoletti, P.; Cherkaoui-Malki, M.; El Kebbaj, R. Argan Oil: A Natural Bioactive Lipid Modulating Oxidative Stress and Inflammation. Antioxidants 2025, 14, 515. [Google Scholar] [CrossRef]
- Hba, S.; Ghaddar, S.; Wahnou, H.; Pinon, A.; El Kebbaj, R.; Pouget, C.; Sol, V.; Liagre, B.; Oudghiri, M.; Limami, Y. Natural Chalcones and Derivatives in Colon Cancer: Pre-Clinical Challenges and the Promise of Chalcone-Based Nanoparticles. Pharmaceutics 2023, 15, 2718. [Google Scholar] [CrossRef] [PubMed]
- Ndayambaje, M.; Hicham, W.; Marieme, S.; Oumaima, C.; Thierry, H.; Mehdi, K.; Youness, L.; Abdallah, N.; Oudghiri, M. Exploring the multifaceted effects of Ammi visnaga: Subchronic toxicity, antioxidant capacity, immunomodulatory, and anti-inflammatory activities. J. Toxicol. Environ. Health Part A 2024, 87, 150–165. [Google Scholar] [CrossRef]
- Kamat, J.P.; Boloor, K.K.; Devasagayam, T.P. Chlorophyllin as an effective antioxidant against membrane damage in vitro and ex vivo. Biochim. Biophys. Acta 2000, 1487, 113–127. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Ascorbic acid as antioxidant. Vitam. Horm. 2023, 121, 247–270. [Google Scholar] [CrossRef]
- Monacelli, F.; Acquarone, E.; Giannotti, C.; Borghi, R.; Nencioni, A. Vitamin C, Aging and Alzheimer’s Disease. Nutrients 2017, 9, 670. [Google Scholar] [CrossRef]
- Dashwood, R.H. The importance of using pure chemicals in (anti) mutagenicity studies: Chlorophyllin as a case in point. Mutat. Res. 1997, 381, 283–286. [Google Scholar] [CrossRef]
- Ramani, N.; Patwardhan, R.S.; Checker, R.; Singh, B.; Morjaria, S.; Kumar, B.K.; Gurjar, M.; Gota, V.; Sharma, D. Preclinical evaluation of sodium copper chlorophyllin: Safety, pharmacokinetics, and therapeutic potential in breast cancer chemotherapy and cyclophosphamide-induced bladder toxicity. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 1, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Mohammed, A.T.; Ismail, H.T.H. Hematological, biochemical, and histopathological impacts of barium chloride and barium carbonate accumulation in soft tissues of male Sprague-Dawley rats. Environ. Sci. Pollut. Res. Int. 2017, 24, 26634–26645. [Google Scholar] [CrossRef]
- Gomes, B.B.; Barros, S.B.M.; Andrade-Wartha, E.R.S.; Silva, A.M.O.; Silva, V.V.; Lanfer-Marquez, U.M. Bioavailability of dietary sodium copper chlorophyllin and its effect on antioxidant defence parameters of Wistar rats. J. Sci. Food Agric. 2009, 89, 2003–2010. [Google Scholar] [CrossRef]
- Ballaz, S.J.; Rebec, G.V. Neurobiology of vitamin C: Expanding the focus from antioxidant to endogenous neuromodulator. Pharmacol. Res. 2019, 146, 104321. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef] [PubMed]
- El Riad, K.; Habiba, B.; Mounia, T.-J.; Boubker, N.; Melford, C.E.; Paolo, T.; Mustapha, C.-M.; Pierre, A.; Soufiane, R.; Youness, L.; et al. The Potential Role of Major Argan Oil Compounds as Nrf2 Regulators and Their Antioxidant Effects. Antioxidants 2024, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Ogboo, B.C.; Grabovyy, U.V.; Maini, A.; Scouten, S.; van der Vliet, A.; Mattevi, A.; Heppner, D.E. Architecture of the NADPH oxidase family of enzymes. Redox Biol. 2022, 52, 102298. [Google Scholar] [CrossRef]
- Bayir, H.; Kagan, V.E. Bench-to-bedside review: Mitochondrial injury, oxidative stress and apoptosis--there is nothing more practical than a good theory. Crit. Care (Lond. Engl.) 2008, 12, 206. [Google Scholar] [CrossRef]
- Lewandowski, Ł.; Kepinska, M.; Milnerowicz, H. Inhibition of copper-zinc superoxide dismutase activity by selected environmental xenobiotics. Environ. Toxicol. Pharmacol. 2018, 58, 105–113. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Sen, S.; Sharma, A.; Talukder, G. Effect of chlorophyllin on mercuric chloride-induced clastogenicity in mice. Food Chem. Toxicol. 1991, 29, 777–779. [Google Scholar] [CrossRef]
- Nordberg, G.; Fowler, B.; Nordberg, M. Handbook on the Toxicology of Metals, 4th ed.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Muser, J.; Bienvenu, J.; Blanckaert, N.; Brandslund, I.; Delattre, J.; Soffiati, G.; Swaminathan, R.; Maggini, S.; Mastall, H. Inter-laboratory evaluation of the COBAS INTEGRA 400 analytical system. Clin. Chem. Lab. Med. 2001, 39, 539–559. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hafeman, D.G.; Sunde, R.A.; Hoekstra, W.G. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J. Nutr. 1974, 104, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Morawietz, G.; Ruehl-Fehlert, C.; Kittel, B.; Bube, A.; Keane, K.; Halm, S.; Heuser, A.; Hellmann, J. Revised guides for organ sampling and trimming in rats and mice--Part 3. A joint publication of the RITA and NACAD groups. Exp. Toxicol. Pathol. 2004, 55, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Ruehl-Fehlert, C.; Kittel, B.; Morawietz, G.; Deslex, P.; Keenan, C.; Mahrt, C.R.; Nolte, T.; Robinson, M.; Stuart, B.P.; Deschl, U. Revised guides for organ sampling and trimming in rats and mice—Part 1. Exp. Toxicol. Pathol. 2003, 55, 91–106. [Google Scholar] [CrossRef]



| Control | BaCl2 | SCC | SCC + BaCl2 | ASC | ASC + BaCl2 | SCC + ASC | SCC + ASC + BaCl2 | |
|---|---|---|---|---|---|---|---|---|
| Triglyceride (g/L) | 0.54 ± 0.06 | 0.54 ± 0.05 | 0.60 ± 0.07 | 0.57 ± 0.07 | 0.6 ± 0.04 | 0.6± 0.14 # | 0.55± 0.03 | 0.66 ± 0.06 |
| Urea (mg/L) | 0.15 ± 0.03 | 0.19 ± 0.03 | 0.16 ± 0.02 | 0.2 ± 0.03 | 0.19 ± 0.02 | 0.2 ± 0.02 | 0.18 ± 0.01 | 0.19 ± 0.02 |
| Creatinine (mg/L) | 3.68 ± 0.84 | 3.23 ± 0.68 | 2.49 ± 1.04 | 2.83 ± 0.63 | 3.54 ± 0.96 | 3.47 ± 0.51 | 2.94 ± 0.43 | 3.75 ± 0.72 |
| Uric acid (mg/L) | 23.43 ± 2.51 | 27.83 ± 5.23 | 30.75 ± 1.82 | 25.22 ± 2.64 | 27.67 ± 2.48 | 27.03 ± 2.82 | 25.86 ± 4.39 | 30.36 ± 2.1 |
| Total protein | 64.89 ± 0.92 | 57.78 ± 3.03 | 64.84 ± 2.85 | 61.6 ± 5.91 | 62.38 ± 3.67 | 63.3 ± 3.43 | 61.67 ± 2.27 | 56.03 ± 5.17 |
| Cholesterol (g/L) | 0.84 ± 0.1 | 0.83 ± 0.04 | 0.84 ± 0.05 | 0.87 ± 0.04 | 0.76 ± 0.08 | 0.83 ± 0.07 | 0.85 ± 0.03 | 0.91 ± 0.09 |
| ASAT (IU/L) | 163.44 ± 3.91 | 173.41 ± 4.75 | 189.52 ± 15.83 | 175.33 ± 13.31 | 195.82 ± 48.98 | 250.93 ± 77.49 *,# | 178.58 ± 4.66 | 175.27 ± 10.6 |
| ALAT (IU/L) | 32.86 ± 11.85 | 27.92 ± 5.47 | 35.91 ± 10.33 | 32.8 ± 10.72 | 35.49 ± 7.85 | 39.71 ± 8.83 | 32.62 ± 3.42 | 30.24 ± 1.97 |
| Total bilirubin (mg/L) | 0.49 ± 0.18 | 0.38 ± 0.07 | 0.47 ± 0.18 | 0.37 ± 0.09 | 0.49 ± 0.1 | 0.4 ± 0.13 | 0.4 ± 0.04 | 0.33 ± 0.06 |
| Albumin (g/L) | 48.44 ± 3.71 | 63.43 ± 5.04 | 64 ± 2.39 | 61.16 ± 10.76 | 58.21 ± 10.44 | 62.39 ± 8.95 | 58.01 ± 9.34 | 69.83 ± 0.91 |
| Na+ (mmol/L) | 134.42 ± 1.92 | 134.58 ± 1.16 | 136.39 ± 1.98 | 134.31 ± 1.45 | 134.47 ± 2.56 | 133.23 ± 1.67 | 134.49 ± 0.85 | 133.26 ± 2.28 |
| K+ (mmol/L) | 5.4 ± 0.14 | 5.2 ± 0.32 | 5.23 ± 0.13 | 5.15 ± 0.49 | 5.10 ± 0.57 | 4.88 ± 0.3 | 5.25 ± 0.19 | 4.92 ± 0.47 |
| Ca2+ (mg/L) | 103.34 ± 3.01 | 98.62 ± 3.14 | 100.99 ± 2.31 | 102.73 ± 3.23 | 100.70 ± 1.15 | 100.51 ± 4.44 | 101.03 ± 2.15 | 99.19 ± 3.81 |
| Cl─ (mmol/L) | 94.44 ± 2.85 | 95.22 ± 2.04 | 96.6 ± 2.24 | 98.02 ± 2.56 | 97.07 ± 3.88 | 97.37 ± 2.05 | 96.81 ± 1.43 | 98.79 ± 1.73 |
| Group | Diet | SCC and ASC Supplementation | BaCl2 Supplementation |
|---|---|---|---|
| Control | Standard diet | Plain water | No |
| BaCl2 | 150 mg/L | ||
| SCC | In drinking water (40 mg/L) By gavage (40 mg/Kg) | No | |
| SCC + BaCl2 | 150 mg/L | ||
| Asc | In drinking water (3.3 g/L) By gavage (160 mg/Kg) | No | |
| Asc + BaCl2 | 150 mg/L | ||
| SCC + Asc | SCC: In drinking water (40 mg/L) By gavage (40 mg/Kg) ASC: In drinking water (3.3 g/L) By gavage (160 mg/Kg) | No | |
| SCC + Asc + BaCl2 | 150 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benayad, S.; Es-Sai, B.; Laaziouez, Y.; Rabbaa, S.; Wahnou, H.; Bouchab, H.; El Attar, H.; Benabdelkhalek, B.; Amahdar, L.; Abboussi, O.; et al. Protective Effects of Sodium Copper Chlorophyllin and/or Ascorbic Acid Against Barium Chloride-Induced Oxidative Stress in Mouse Brain and Liver. Molecules 2025, 30, 3231. https://doi.org/10.3390/molecules30153231
Benayad S, Es-Sai B, Laaziouez Y, Rabbaa S, Wahnou H, Bouchab H, El Attar H, Benabdelkhalek B, Amahdar L, Abboussi O, et al. Protective Effects of Sodium Copper Chlorophyllin and/or Ascorbic Acid Against Barium Chloride-Induced Oxidative Stress in Mouse Brain and Liver. Molecules. 2025; 30(15):3231. https://doi.org/10.3390/molecules30153231
Chicago/Turabian StyleBenayad, Salma, Basma Es-Sai, Yassir Laaziouez, Soufiane Rabbaa, Hicham Wahnou, Habiba Bouchab, Hicham El Attar, Bouchra Benabdelkhalek, Loubna Amahdar, Oualid Abboussi, and et al. 2025. "Protective Effects of Sodium Copper Chlorophyllin and/or Ascorbic Acid Against Barium Chloride-Induced Oxidative Stress in Mouse Brain and Liver" Molecules 30, no. 15: 3231. https://doi.org/10.3390/molecules30153231
APA StyleBenayad, S., Es-Sai, B., Laaziouez, Y., Rabbaa, S., Wahnou, H., Bouchab, H., El Attar, H., Benabdelkhalek, B., Amahdar, L., Abboussi, O., Duval, R. E., El Kebbaj, R., & Limami, Y. (2025). Protective Effects of Sodium Copper Chlorophyllin and/or Ascorbic Acid Against Barium Chloride-Induced Oxidative Stress in Mouse Brain and Liver. Molecules, 30(15), 3231. https://doi.org/10.3390/molecules30153231











