Cyclodextrin-Based Systems of Cetraria islandica Extracts: A Novel Approach to Improve Solubility and Biological Activity of Lichen-Derived Natural Products
Abstract
1. Introduction
2. Results and Discussion
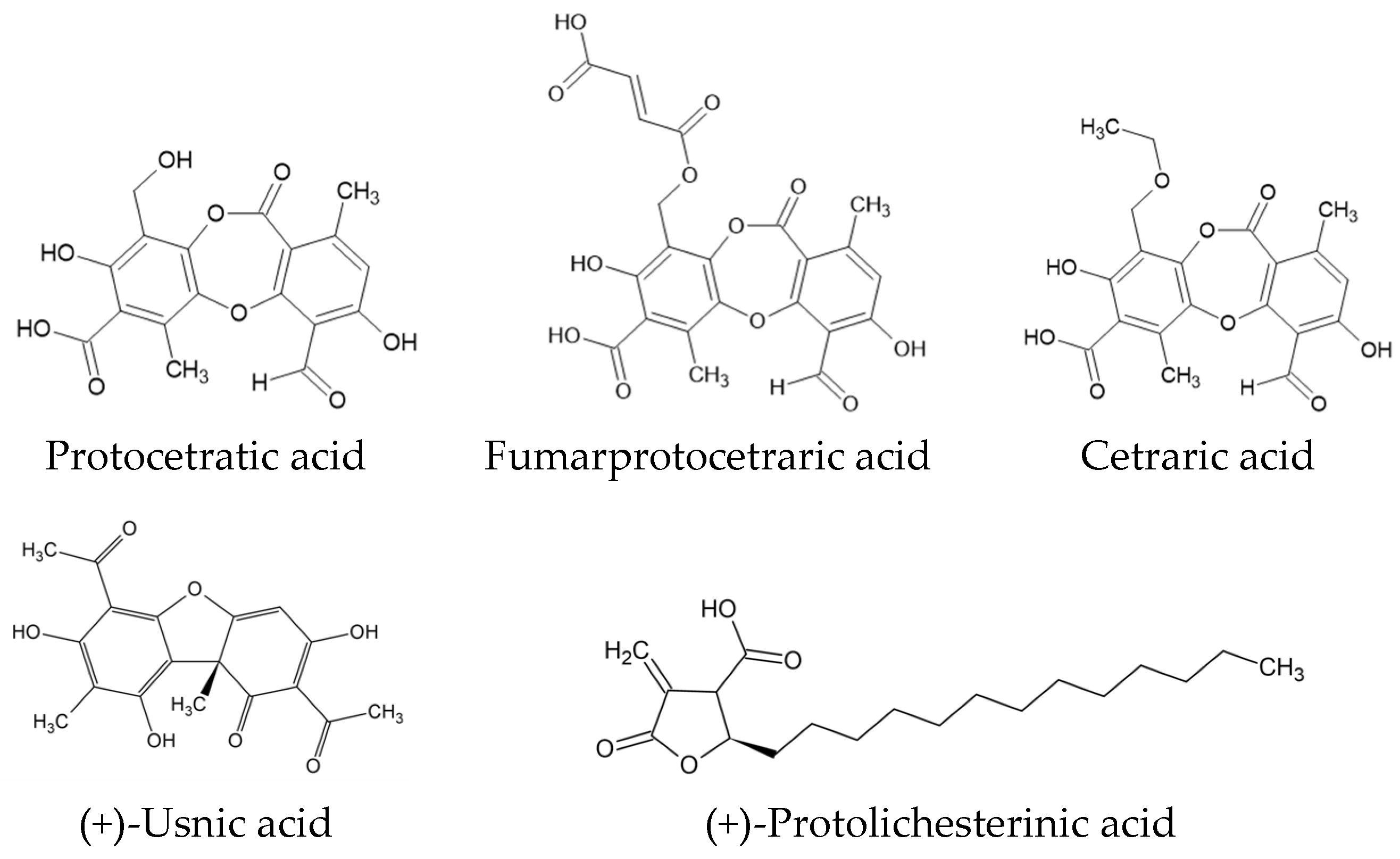
2.1. Phytochemical Analysis of C. islandica Extracts
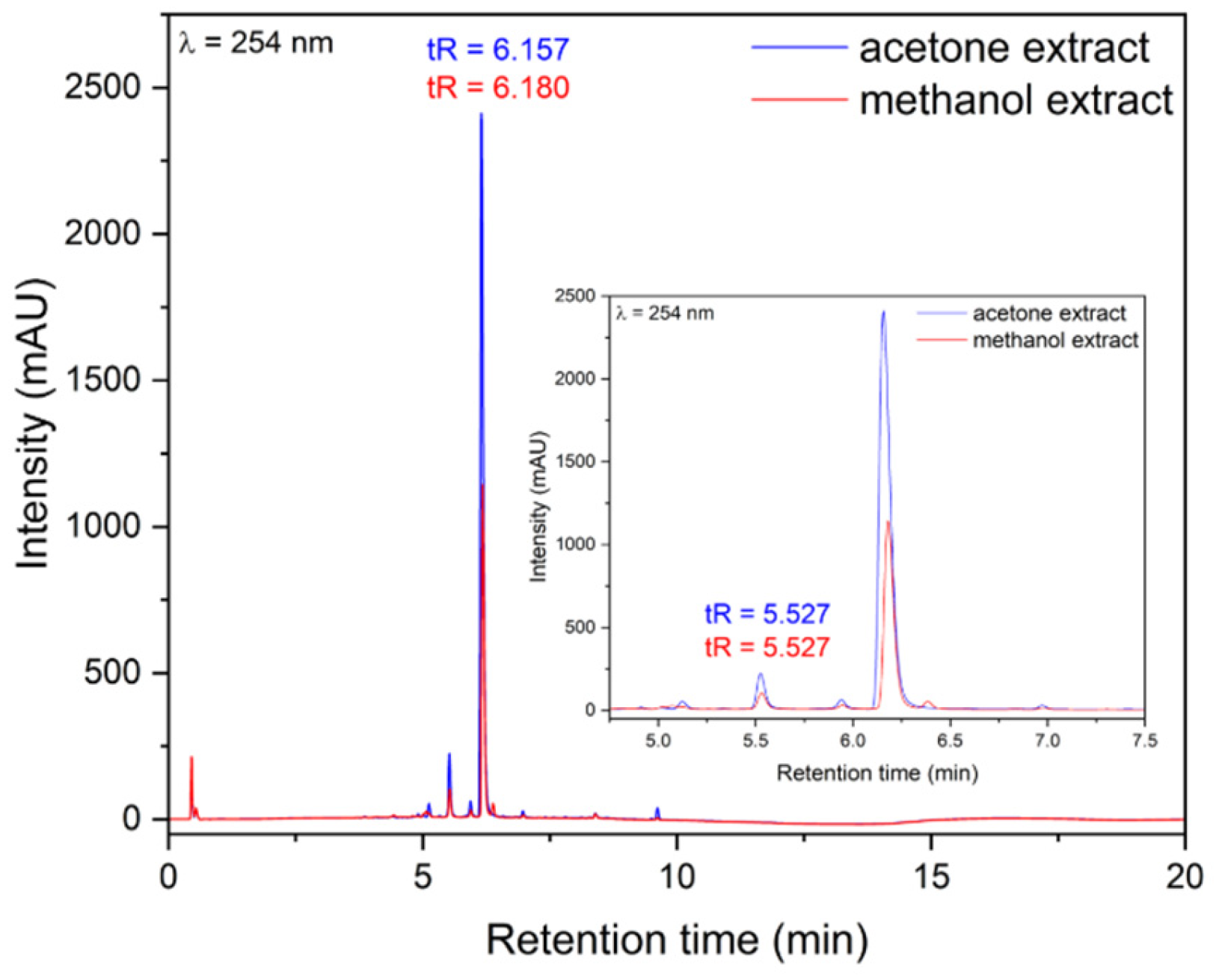
2.1.1. High-Performance Liquid Chromatography (HPLC) and Total Polyphenol Content (TPC) Analysis
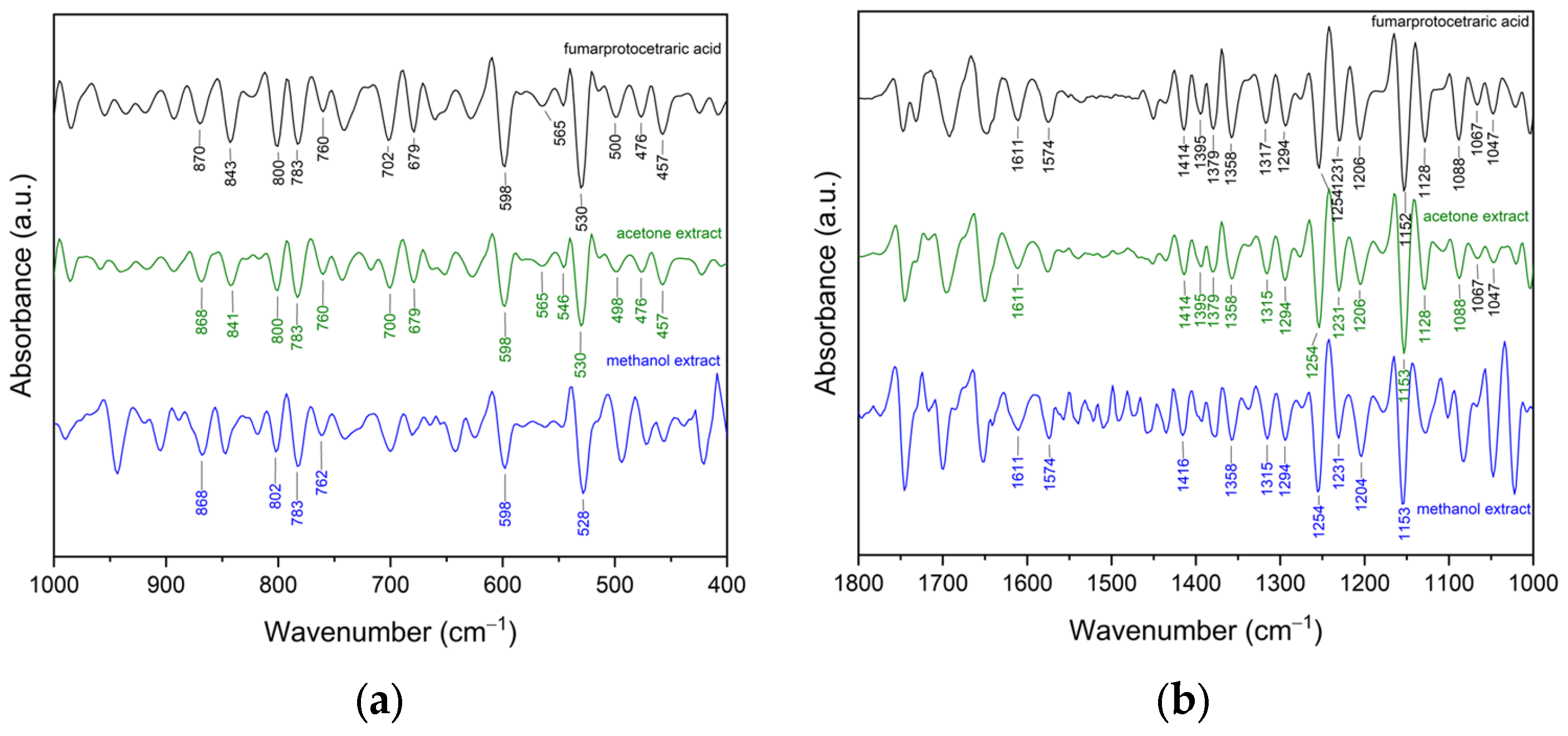
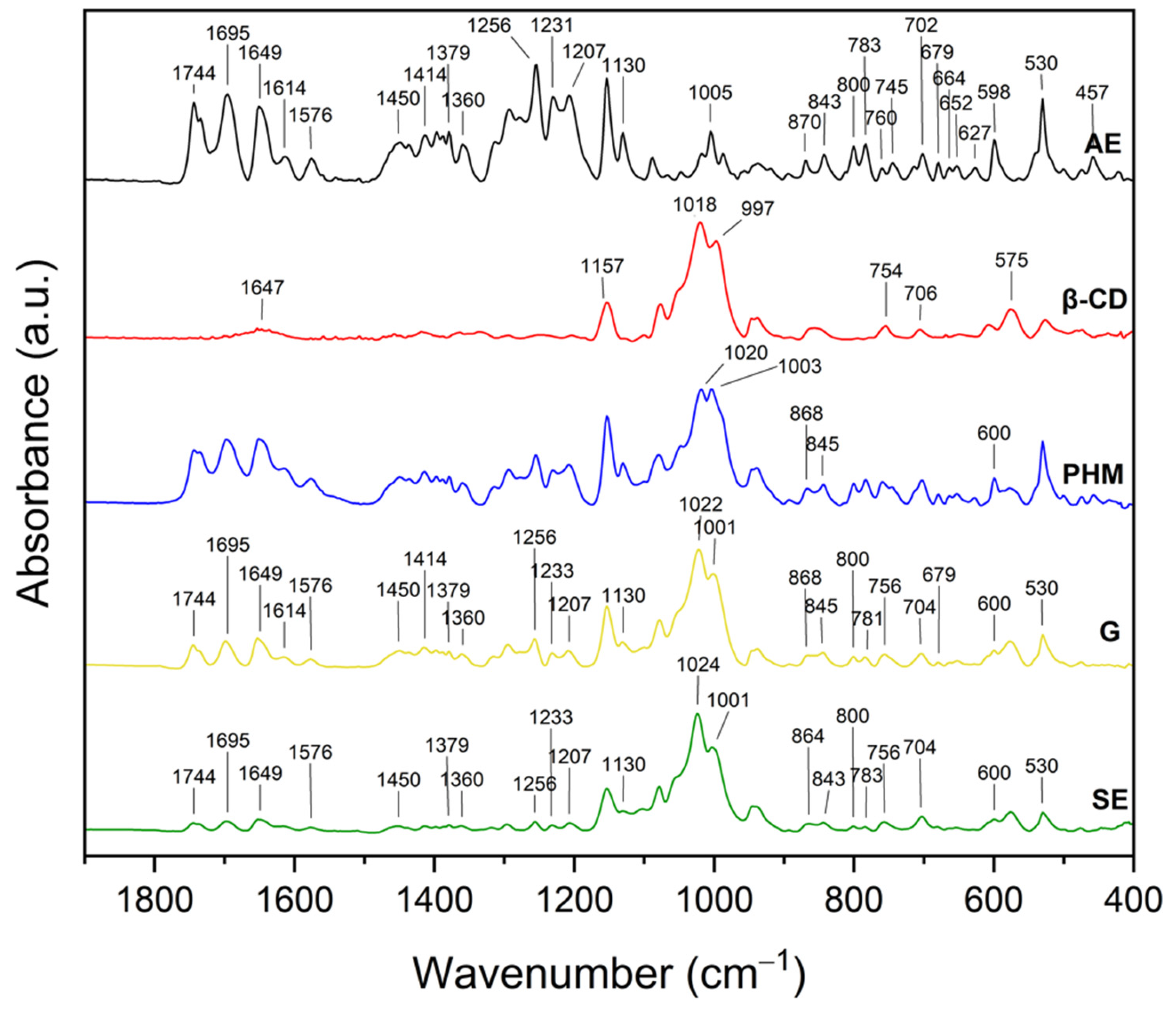
2.1.2. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
2.1.3. GC-MS Analysis of the C. islandica Extracts
2.2. Preparation and Identification of C. islandica Extract/CD Systems
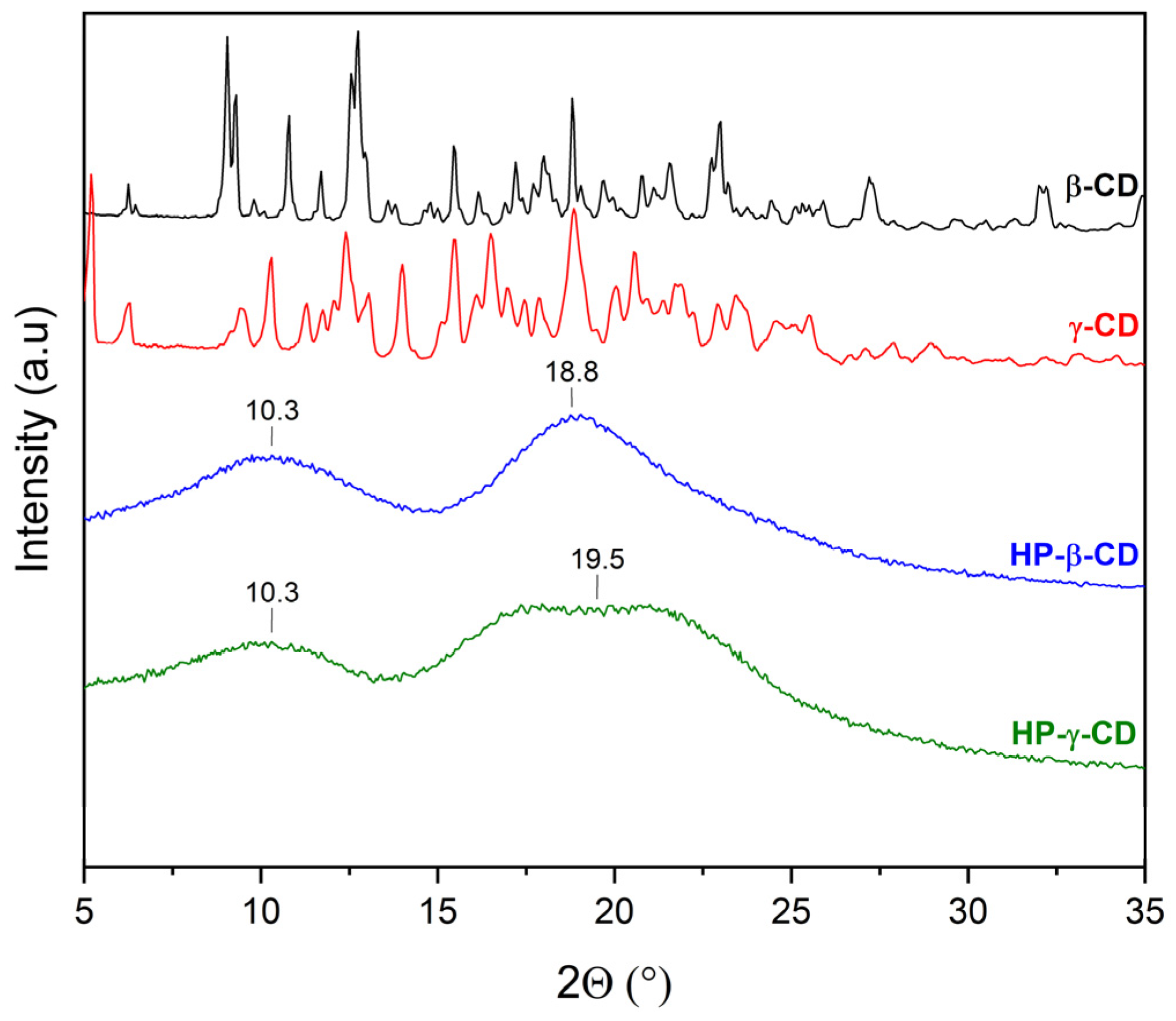
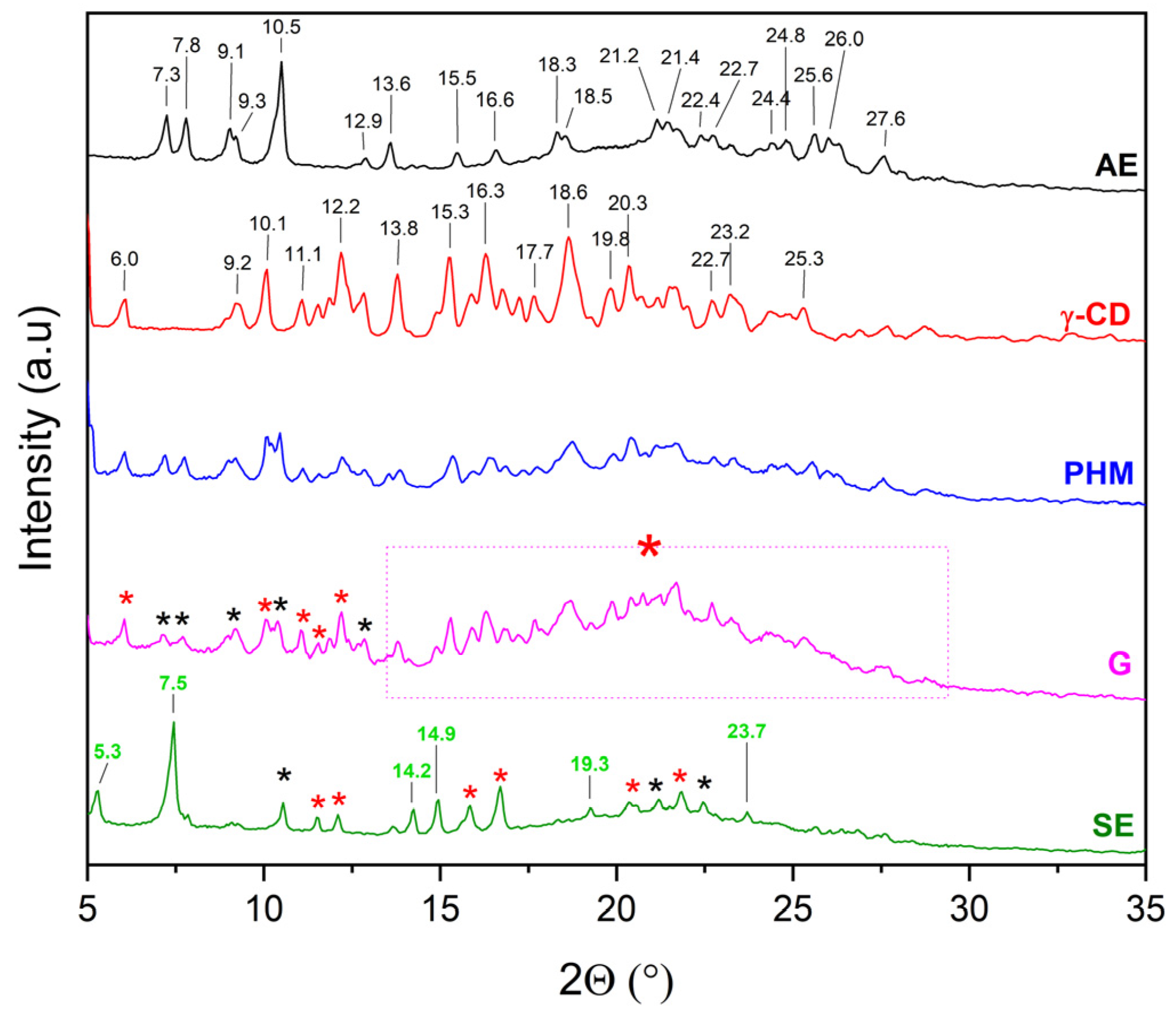
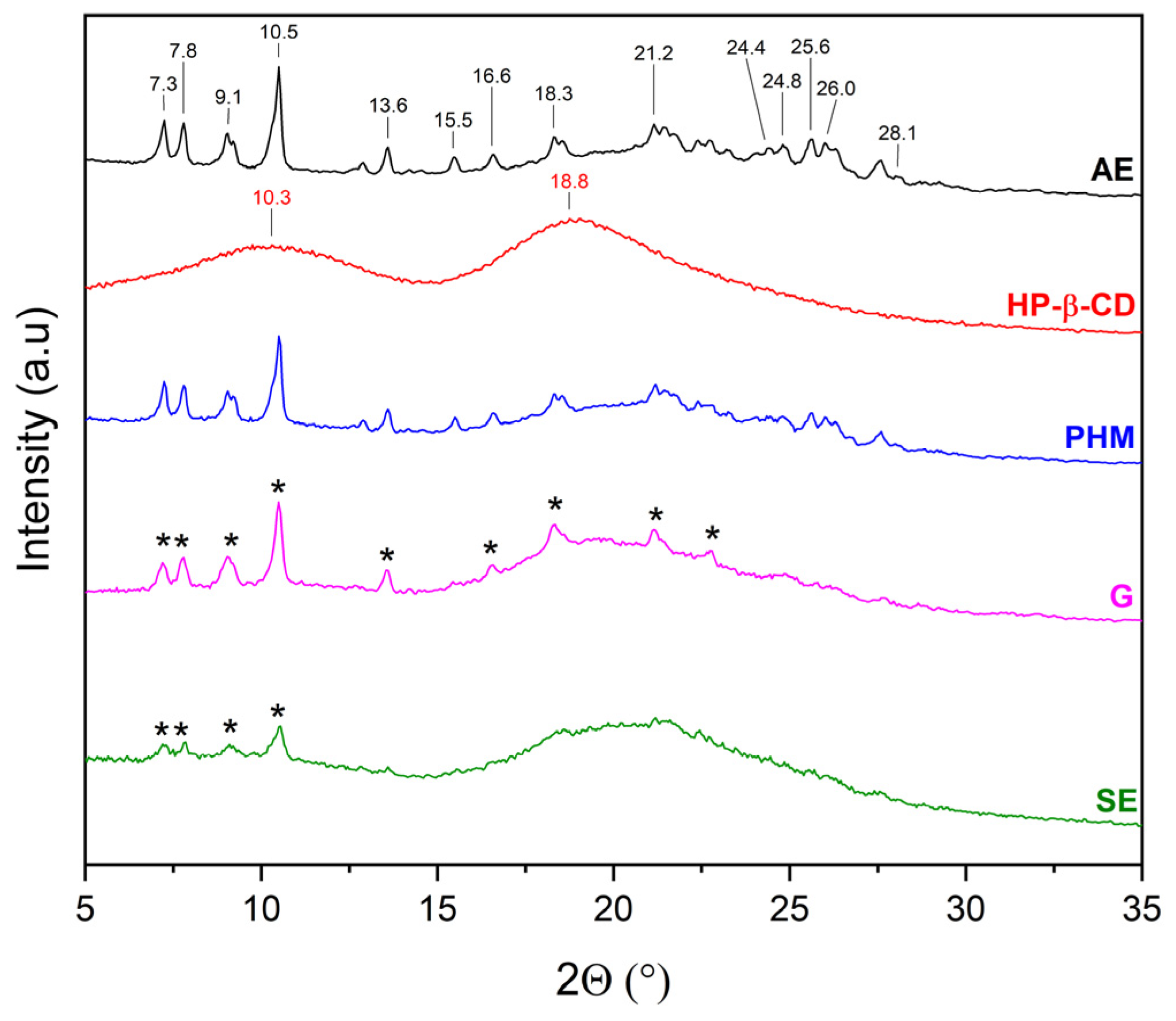
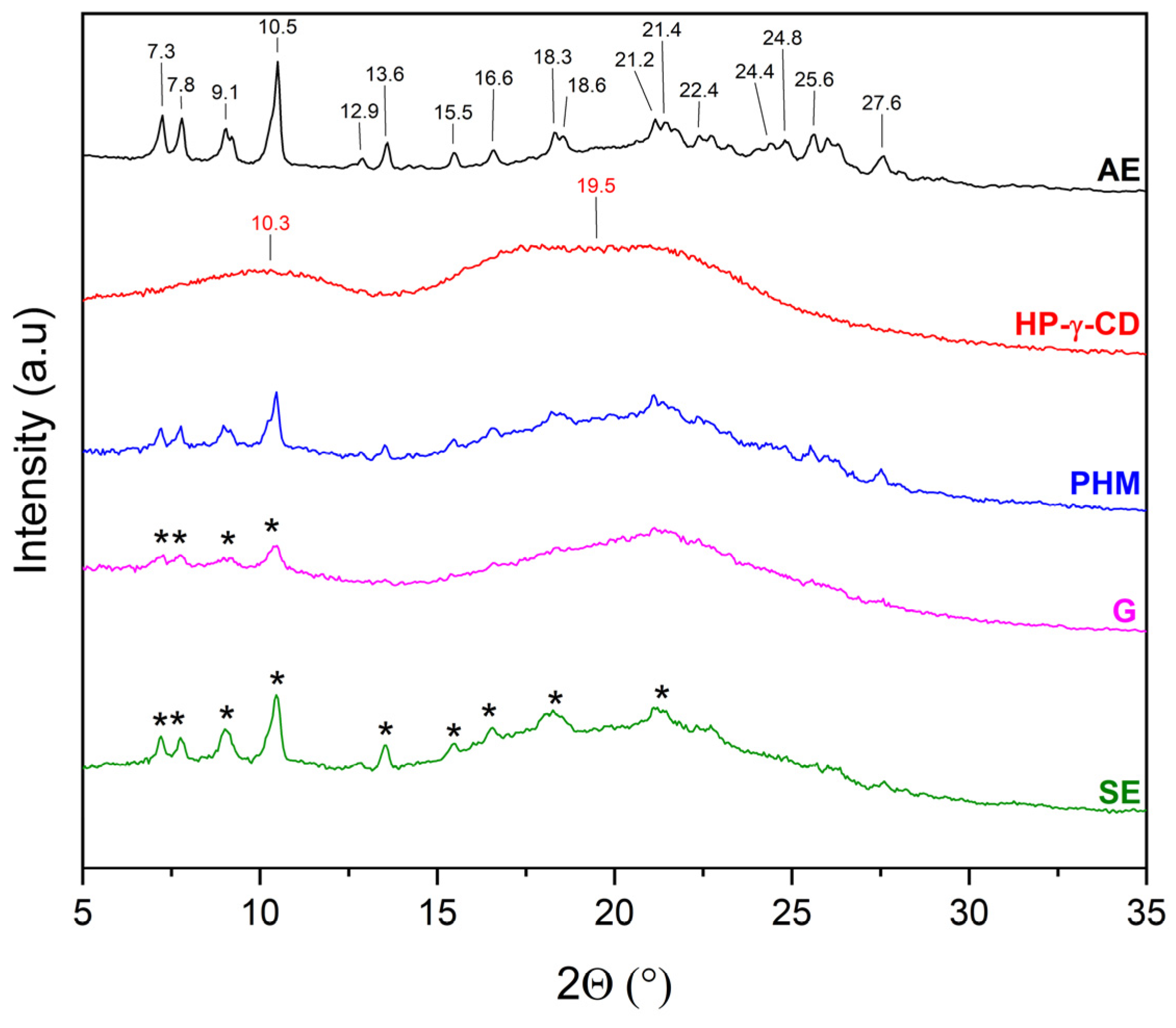
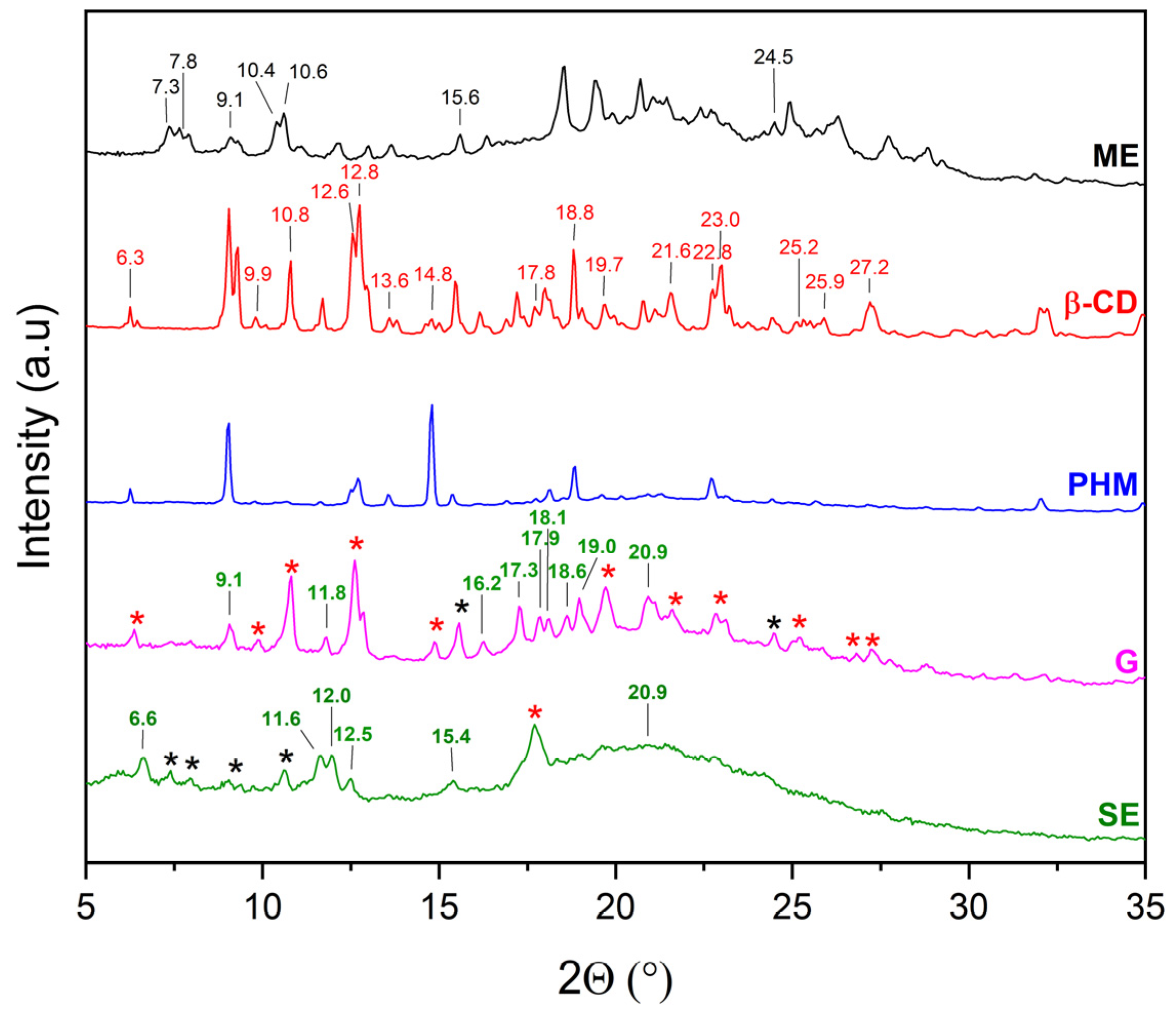
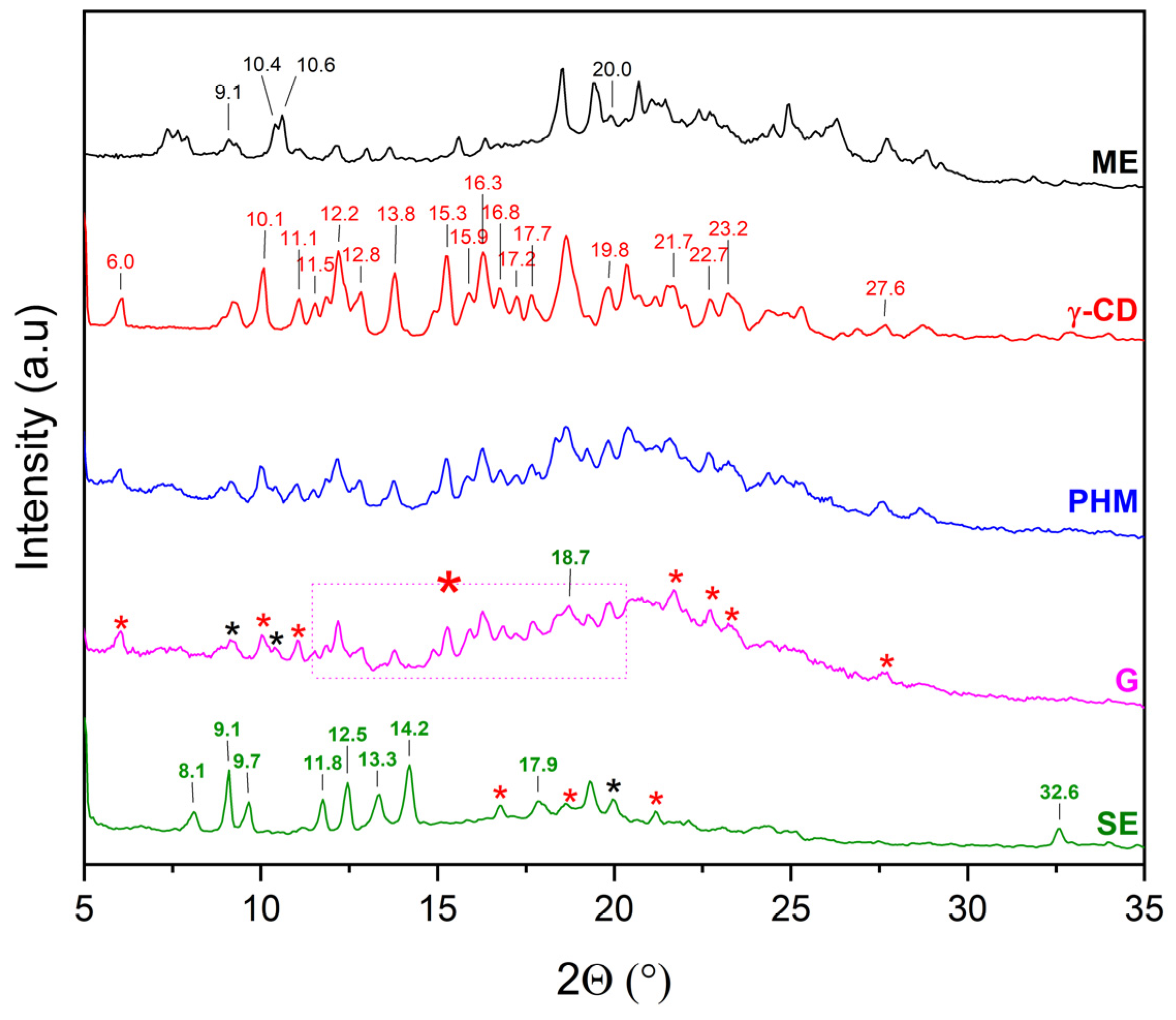
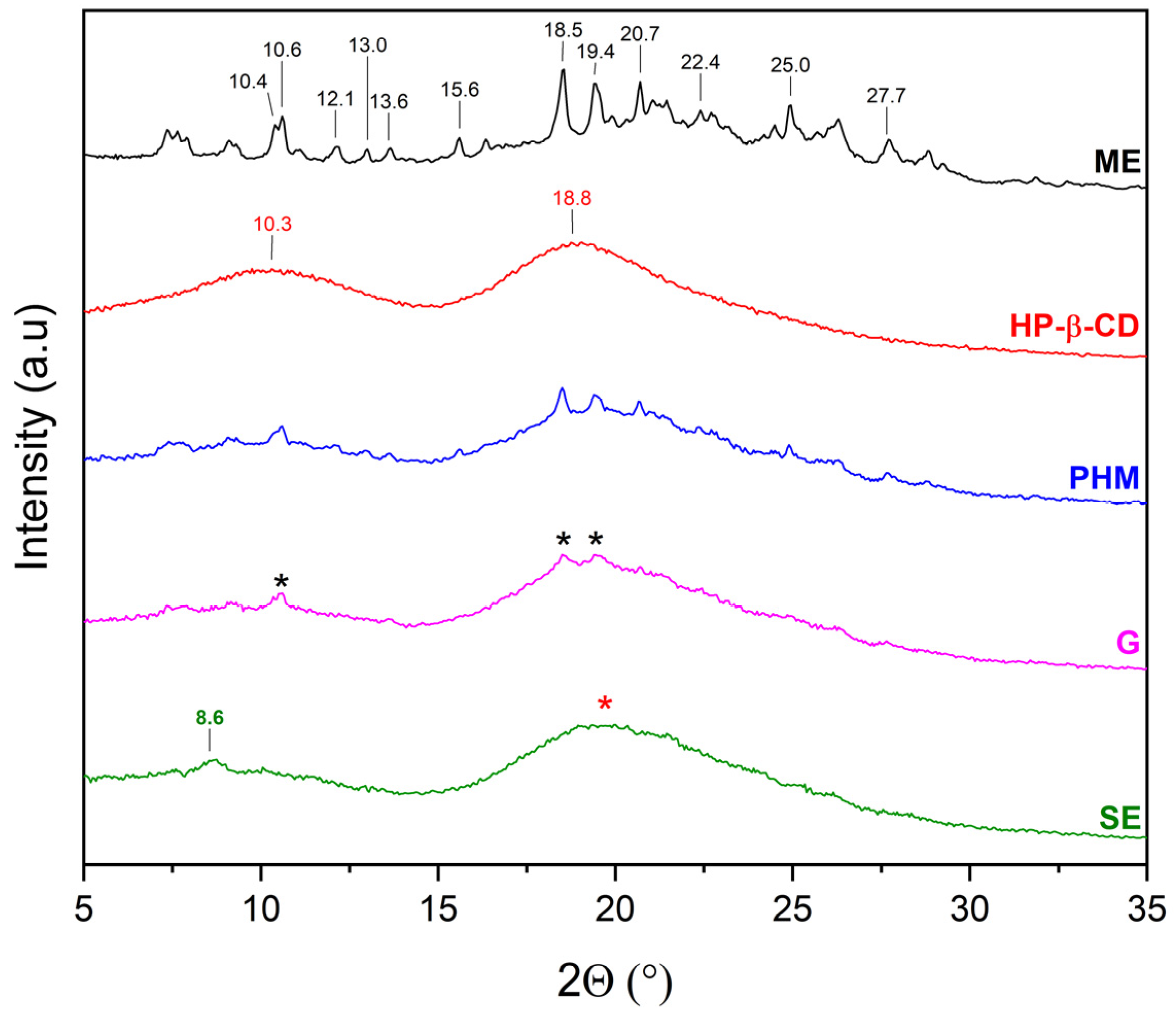
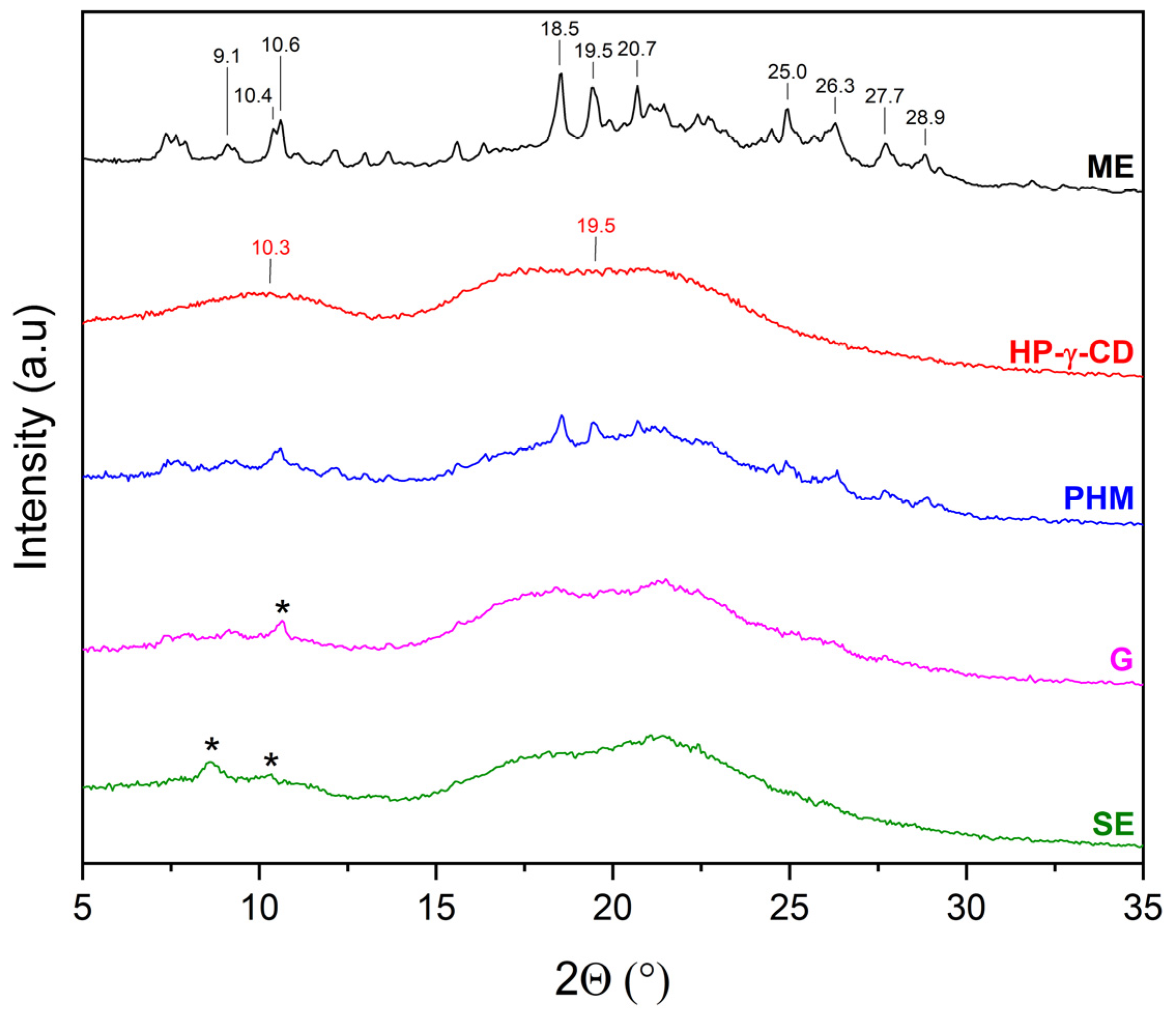
2.2.1. X-Ray Powder Diffraction (XRPD)
2.2.2. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
2.3. Characterization of Biological Potential of C. islandica Extract/CD Systems
2.3.1. Total Polyphenol Content in C. islandica Extract/CD Systems
2.3.2. Antioxidant Activity of C. islandica Extract/CD Systems
2.3.3. Inhibition of AChE and BChE Enzymes by C. islandica Extract/CD Systems
2.3.4. Inhibition of Tyrosinase by Lichen Extract/CD Systems
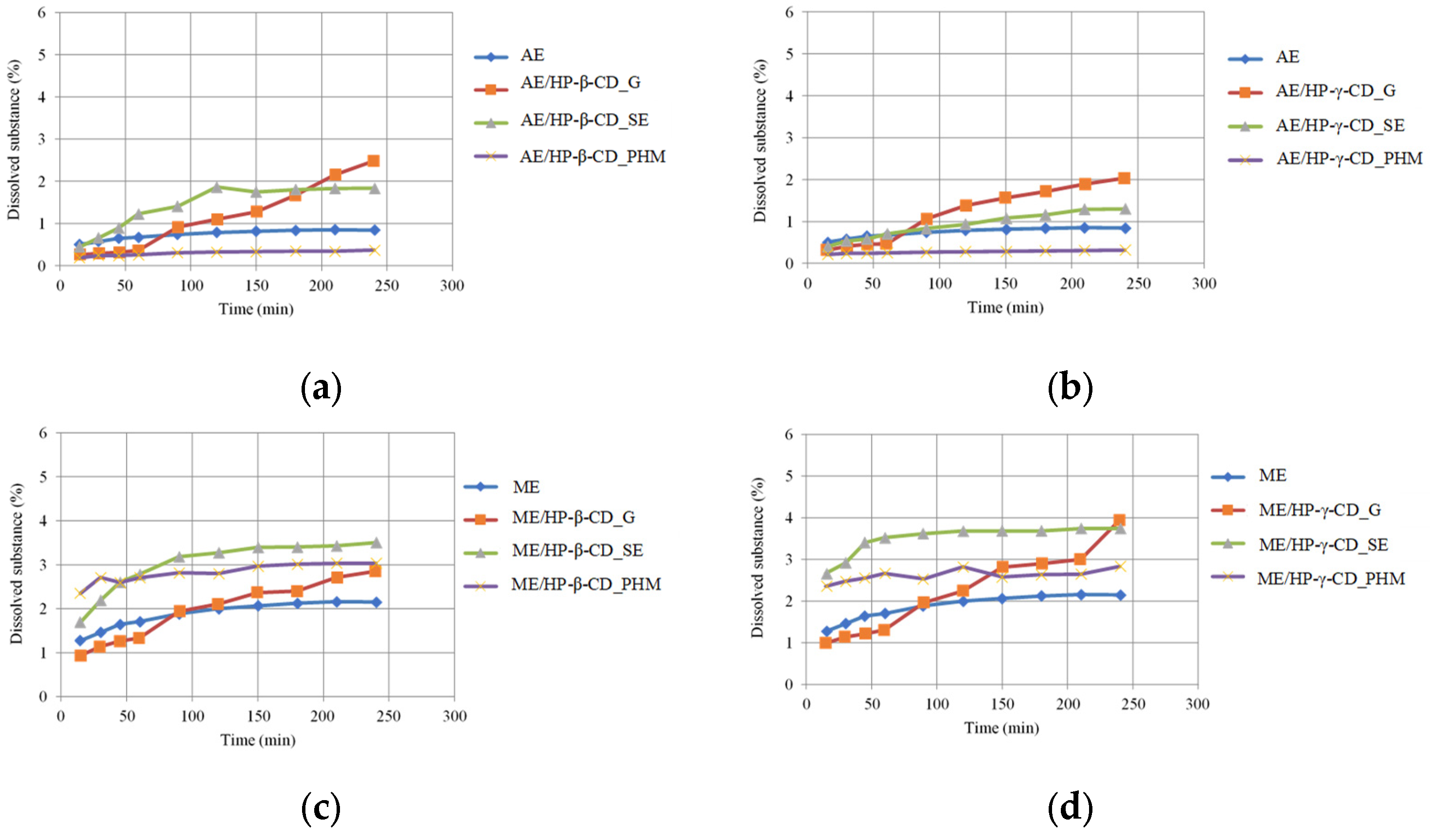
2.3.5. Dissolution Study of Lichen Extract/CD Systems
2.3.6. Limitations and Assumptions
3. Materials and Methods
3.1. Plant Material and Reagents
3.2. Preparation of Extract
3.3. Phytochemical Characteristic of C. islandica Extracts
3.3.1. High-Performance Liquid Chromatography (HPLC) Analyses
3.3.2. Total Polyphenol Content (TPC)
3.3.3. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
3.3.4. GC-MS Analysis of the C. islandica Extracts
3.4. System Preparation
3.5. Identification of C. islandica Extract/CD Systems
3.5.1. X-Ray Powder Diffraction Analysis (XRPD)
3.5.2. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
3.6. Biological Activity of C. islandica Extract/CD Systems
3.6.1. Total Phenolic Content (TPC) of Extract/CD Systems
3.6.2. Antioxidant Activity
3.6.3. Enzymatic Activity
Effect on Acetylcholinesterase (AChE) and Butyrylcholinesterase (BChE) Activity
Effect on Tyrosinase Activity
3.7. Dissolution Study
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hecker, M.; Völp, A. Tolerability of Icelandic Moss Lozenges in Upper Respiratory Tract Diseases-Multicentric Drug Monitoring Study with 3143 Children. Forsch. Komplementarmedizin Klass. Naturheilkunde Res. Complement. Nat. Class. Med. 2004, 11, 76–82. [Google Scholar]
- Bradley British Herbal Compendium Pharmacopoeia. A Handbook of Scientific Information on Widely Used Plant Drugs; British Herbal Medicine Association: Exeter, UK, 2006. [Google Scholar]
- Gudjónsdóttir, G.A.; Ingólfsdóttir, K. Quantitative Determination of Protolichesterinic-and Fumarprotocetraric Acids in Cetraria islandica by High-Performance Liquid Chromatography. J. Chromatogr. A 1997, 757, 303–306. [Google Scholar] [CrossRef]
- Patriche, S.; Ghinea, I.O.; Adam, G.; Gurau, G.; Furdui, B.; Dinica, R.M.; Rebegea, L.F.; Lupoae, M. Characterization of Bioactive Compounds from Romanian Cetraria islandica (L.) Ach. Rev. Chim. 2019, 70, 2186–2191. [Google Scholar] [CrossRef]
- Stepanenko, L.S.; Krivoshchekova, O.E.; Dmitrenok, P.S.; Maximov, O.B. Quinones of Cetraria islandica. Phytochemistry 1997, 46, 565–568. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report on Cetraria islandica (L.) Acharius s.l., Thallus; European Medicines Agency: London, UK, 2014. [Google Scholar]
- Sánchez, M.; Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. The Genus Cetraria s. Str.—A Review of Its Botany, Phytochemistry, Traditional Uses and Pharmacology. Molecules 2022, 27, 4990. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Neuroprotective Activity and Cytotoxic Potential of Two Parmeliaceae lichens: Identification of Active Compounds. Phytomedicine 2015, 22, 847–855. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. In Vitro Neuroprotective Potential of Lichen Metabolite Fumarprotocetraric Acid via Intracellular Redox Modulation. Toxicol. Appl. Pharmacol. 2017, 316, 83–94. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Żarowski, M.; Plech, T.; Cielecka-Piontek, J. Permeability of Hypogymnia Physodes Extract Component—Physodic Acid through the Blood–Brain Barrier as an Important Argument for Its Anticancer and Neuroprotective Activity within the Central Nervous System. Cancers 2021, 13, 1717. [Google Scholar] [CrossRef]
- Munro, I.C.; Newberne, P.M.; Young, V.R.; Bär, A. Safety Assessment of γ-Cyclodextrin. Regul. Toxicol. Pharmacol. 2004, 39, 3–13. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, P.; Wang, J.; Wu, Y.; Han, Y.; Zhou, J. Combining Various Wall Materials for Encapsulation of Blueberry Anthocyanin Extracts: Optimization by Artificial Neural Network and Genetic Algorithm and a Comprehensive Analysis of Anthocyanin Powder Properties. Powder Technol. 2017, 311, 77–87. [Google Scholar] [CrossRef]
- Jaski, J.M.; Barão, C.E.; Morais Lião, L.; Silva Pinto, V.; Zanoelo, E.F.; Cardozo-Filho, L. β-Cyclodextrin Complexation of Extracts of Olive Leaves Obtained by Pressurized Liquid Extraction. Ind. Crops Prod. 2019, 129, 662–672. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Simal-Gándara, J.; Torrado-Agrasar, A. Antioxidant and Antimicrobial Properties of Encapsulated Guava Leaf Oil in Hydroxypropyl-Beta-Cyclodextrin. Ind. Crops Prod. 2018, 111, 219–225. [Google Scholar] [CrossRef]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Kovačević, D.B.; Putnik, P.; Donsì, F.; Barba, F.J.; Jambrak, A.R. Innovative Technologies for Encapsulation of Mediterranean Plants Extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Dos Santos, P.H.; Mesquita, T.; Miguel-dos-Santos, R.; de Almeida, G.K.M.; de Sá, L.A.; dos Passos Menezes, P.; de Souza Araujo, A.A.; Lauton-Santos, S. Inclusion Complex with β-Cyclodextrin Is a Key Determining Factor for the Cardioprotection Induced by Usnic Acid. Chem. Biol. Interact. 2020, 332, 109297. [Google Scholar] [CrossRef]
- Kristmundsdóttir, T.; Jónsdóttir, E.; Ögmundsdóttir, H.M.; Ingólfsdóttir, K. Solubilization of Poorly Soluble Lichen Metabolites for Biological Testing on Cell Lines. Eur. J. Pharm. Sci. 2005, 24, 539–543. [Google Scholar] [CrossRef]
- Manassov, N.; Samy, M.N.; Datkhayev, U.; Avula, B.; Adams, S.J.; Katragunta, K.; Raman, V.; Khan, I.A.; Ross, S.A. Ultrastructural, Energy-Dispersive X-Ray Spectroscopy, Chemical Study and LC-DAD-QToF Chemical Characterization of Cetraria islandica (L.) Ach. Molecules 2023, 28, 4493. [Google Scholar] [CrossRef] [PubMed]
- Tas, I.; Yildirim, A.B.; Ozyigitoglu, G.C.; Yavuz, M.Z.; Turker, A.U. Determination of Biological Activities (Antibacterial, Antioxidant and Antiproliferative) and Metabolite Analysis of Some Lichen Species from Turkey. Eur. J. Biomed. 2017, 4, 13–20. [Google Scholar]
- Zarabska-Bożejewicz, D.; Studzińska-Sroka, E.; FaŁtynowicz, W. Transplantation of Lichen Thalli: A Case Study on Cetraria islandica for Conservation and Pharmaceutical Purposes. Fungal Ecol. 2015, 16, 34–43. [Google Scholar] [CrossRef]
- Chanaj-Kaczmarek, J.; Rosiak, N.; Szymanowska, D.; Rajewski, M.; Wender-Ozegowska, E.; Cielecka-Piontek, J. The Chitosan-Based System with Scutellariae baicalensis radix Extract for the Local Treatment of Vaginal Infections. Pharmaceutics 2022, 14, 740. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Bańdurska, M.; Rosiak, N.; Szwajgier, D.; Baranowska-Wójcik, E.; Szymański, M.; Gruszka, W.; Cielecka-Piontek, J. Is Caperatic Acid the Only Compound Responsible for Activity of Lichen Platismatia glauca within the Nervous System? Antioxidants 2022, 11, 2069. [Google Scholar] [CrossRef]
- Jung, E.P.; Alves, R.C.; Rocha, W.F.d.C.; Monteiro, S.d.S.; Ribeiro, L.d.O.; Moreira, R.F.A. Chemical Profile of the Volatile Fraction of Bauhinia forficata Leaves: An Evaluation of Commercial and in Natura Samples. Food Sci. Technol. 2022, 42, e34122. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, Q.; Liang, Z.; Zhao, M.; Yu, X.; Yang, D.; Xu, X. The GC/MS Analysis of Volatile Components Extracted by Different Methods from Exocarpium Citri Grandis. J. Anal. Methods Chem. 2013, 2013, 918406. [Google Scholar] [CrossRef]
- Dorofte, A.L.; Dima, C.; Bleoanca, I.; Aprodu, I.; Alexe, P.; Kharazmi, M.S.; Jafari, S.M.; Dima, Ș.; Borda, D. Mechanism of β–Cyclodextrin—Thyme Nanocomplex Formation and Release: In Silico Behavior, Structural and Functional Properties. Carbohydr. Polym. Technol. Appl. 2024, 7, 100422. [Google Scholar] [CrossRef]
- Lima Nascimento, J.; Coelho, A.G.; Oliveira Barros, Y.S.; Sousa Oliveira, I.; Vieira da Silva, F.; Custódio Viana, A.F.; Araújo, B.Q.; dos Santos Rocha, M.; das Chagas Pereira de Andrade, F.; de Oliveira Barbosa, C.; et al. Production and Characterization of a β-Cyclodextrin Inclusion Complex with Platonia Insignis Seed Extract as a Proposal for a Gastroprotective System. Appl. Sci. 2023, 13, 58. [Google Scholar] [CrossRef]
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of Beta-Cyclodextrin Inclusion Complexes Containing an Essential Oil Component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Stasiłowicz, A.; Rosiak, N.; Tykarska, E.; Kozak, M.; Jenczyk, J.; Szulc, P.; Kobus-Cisowska, J.; Lewandowska, K.; Płazińska, A.; Płaziński, W. Combinations of Piperine with Hydroxypropyl-β-Cyclodextrin as a Multifunctional System. Int. J. Mol. Sci. 2021, 22, 4195. [Google Scholar] [CrossRef]
- Stasiłowicz-Krzemień, A.; Rosiak, N.; Płazińska, A.; Płaziński, W.; Miklaszewski, A.; Tykarska, E.; Cielecka-Piontek, J. Cyclodextrin Derivatives as Promising Solubilizers to Enhance the Biological Activity of Rosmarinic Acid. Pharmaceutics 2022, 14, 2098. [Google Scholar] [CrossRef]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Żarowski, M.; Płazińska, A.; Płaziński, W.; Cielecka-Piontek, J. Amorphous Inclusion Complexes: Molecular Interactions of Hesperidin and Hesperetin with HP-Β-CD and Their Biological Effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Costa, B.S.B.; Circuncisão, A.R.; Silva, A.M.S.; Cardoso, S.M.; Braga, S.S. γ-Cyclodextrin Inclusion of Phloroglucinol: Solid State Studies and Antioxidant Activity throughout the Digestive Tract. Appl. Sci. 2022, 12, 2340. [Google Scholar] [CrossRef]
- Braga, S.S.; Lysenko, K.; El-Saleh, F.; Almeida Paz, F.A. Cyclodextrin-Efavirenz Complexes Investigated by Solid State and Solubility Studies. Proceedings 2021, 78, 15. [Google Scholar]
- Shrestha, M.; Ho, T.M.; Bhandari, B.R. Encapsulation of Tea Tree Oil by Amorphous Beta-Cyclodextrin Powder. Food Chem. 2017, 221, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Guo, X.; Yang, G.; Yao, Y.; Zhao, L.; Gao, S.; Ye, F.; Fu, Y. Direct Electrospinning for Producing Multiple Activity Nanofibers Consisting of Aggregated Luteolin/Hydroxypropyl-Gamma-Cyclodextrin Inclusion Complex. Int. J. Biol. Macromol. 2024, 270, 132344. [Google Scholar] [CrossRef] [PubMed]
- Manojlović, N.T.; Rančić, A.B.; Décor, R.; Vasiljević, P.; Tomović, J. Determination of Chemical Composition and Antimicrobial, Antioxidant and Cytotoxic Activities of Lichens Parmelia conspersa and Parmelia perlata. J. Food Meas. Charact. 2021, 15, 686–696. [Google Scholar] [CrossRef]
- Paczkowska, M.; Mizera, M.; Piotrowska, H.; Szymanowska-Powałowska, D.; Lewandowska, K.; Goscianska, J.; Pietrzak, R.; Bednarski, W.; Majka, Z.; Cielecka-Piontek, J. Complex of Rutin with β-Cyclodextrin as Potential Delivery System. PLoS ONE 2015, 10, e0120858. [Google Scholar] [CrossRef]
- Rachmawati, H.; Edityaningrum, C.A.; Mauludin, R. Molecular Inclusion Complex of Curcumin–β-Cyclodextrin Nanoparticle to Enhance Curcumin Skin Permeability from Hydrophilic Matrix Gel. AAPS PharmSciTech 2013, 14, 1303–1312. [Google Scholar] [CrossRef]
- Negi, J.S.; Singh, S. Spectroscopic Investigation on the Inclusion Complex Formation between Amisulpride and γ-Cyclodextrin. Carbohydr. Polym. 2013, 92, 1835–1843. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, A.; Binou, P.; Igoumenidis, P.E.; Chiou, A.; Yannakopoulou, K.; Karathanos, V.Τ. Host–Guest Inclusion Complexes of Hydroxytyrosol with Cyclodextrins: Development of a Potential Functional Ingredient for Food Application. J. Food Sci. 2022, 87, 2678–2691. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Wang, W.; Chen, C.; Jiao, L.; Wu, W. Preparation, Characterization, and Bioavailability of Host-Guest Inclusion Complex of Ginsenoside Re with Gamma-Cyclodextrin. Molecules 2021, 26, 7227. [Google Scholar] [CrossRef]
- Gallo, G.; Zannini, D.; Immirzi, B.; De Bruno, A.; Fiorentino, G.; Dal Poggetto, G. Host–Guest Complexes HP-β-CD/Citrus Antioxidants: Exploratory Evaluations of Enhanced Properties in Biodegradable Film Packaging. Antioxidants 2023, 12, 763. [Google Scholar] [CrossRef]
- Wang, C.; Xia, N.; Yu, M.; Zhu, S. Physicochemical Properties and Mechanism of Solubilised Neohesperidin System Based on Inclusion Complex of Hydroxypropyl-β-Cyclodextrin. Int. J. Food Sci. Technol. 2023, 58, 107–115. [Google Scholar] [CrossRef]
- Misiuk, W. Investigation of Inclusion Complex of HP-γ-Cyclodextrin with Ceftazidime. J. Mol. Liq. 2016, 224, 387–392. [Google Scholar] [CrossRef]
- Misiuk, W.; Jasiuk, E. Study of the Inclusion Interaction of HP-γ-Cyclodextrin with Bupropion and Its Analytical Application. J. Mol. Struct. 2014, 1060, 272–279. [Google Scholar] [CrossRef]
- Liu, P.; Gan, N.; Li, Q.; Zeng, Z.; Zhong, J.; Wang, X.; Sun, Y.; Wu, D. Investigating the Differences in β-Cyclodextrin Derivatives/Hyperoside Inclusion Complexes: Dissolution Properties, Thermal Stability, and Antioxidant Activity. Food Chem. 2025, 481, 144044. [Google Scholar] [CrossRef] [PubMed]
- Kung, H.-C.; Lin, K.-J.; Kung, C.-T.; Lin, T.-K. Oxidative Stress, Mitochondrial Dysfunction, and Neuroprotection of Polyphenols with Respect to Resveratrol in Parkinson’s Disease. Biomedicines 2021, 9, 918. [Google Scholar] [CrossRef]
- Ciupei, D.; Colişar, A.; Leopold, L.; Stănilă, A.; Diaconeasa, Z.M. Polyphenols: From Classification to Therapeutic Potential and Bioavailability. Foods 2024, 13, 4131. [Google Scholar] [CrossRef]
- Sarabia-Vallejo, Á.; Caja, M.D.; Olives, A.I.; Martín, M.A.; Menéndez, J.C. Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects. Pharmaceutics 2023, 15, 2345. [Google Scholar] [CrossRef]
- Christaki, S.; Spanidi, E.; Panagiotidou, E.; Athanasopoulou, S.; Kyriakoudi, A.; Mourtzinos, I.; Gardikis, K. Cyclodextrins for the Delivery of Bioactive Compounds from Natural Sources: Medicinal, Food and Cosmetics Applications. Pharmaceuticals 2023, 16, 1274. [Google Scholar] [CrossRef]
- Jullian, C.; Moyano, L.; Yanez, C.; Olea-Azar, C. Complexation of Quercetin with Three Kinds of Cyclodextrins: An Antioxidant Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 230–234. [Google Scholar] [CrossRef]
- Vhangani, L.N.; Favre, L.C.; Rolandelli, G.; Van Wyk, J.; del Pilar Buera, M. Optimising the Polyphenolic Content and Antioxidant Activity of Green Rooibos (Aspalathus linearis) Using Beta-Cyclodextrin Assisted Extraction. Molecules 2022, 27, 3556. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Cheng, B.; Hu, Y.; Zhang, Y.; Zou, G. Complexation of Resveratrol with Cyclodextrins: Solubility and Antioxidant Activity. Food Chem. 2009, 113, 17–20. [Google Scholar] [CrossRef]
- Iskineyeva, A.; Fazylov, S.; Bakirova, R.; Sarsenbekova, A.; Pustolaikina, I.; Seilkhanov, O.; Alsfouk, A.A.; Elkaeed, E.B.; Eissa, I.H.; Metwaly, A.M. Combined in Silico and Experimental Investigations of Resveratrol Encapsulation by Beta-Cyclodextrin. Plants 2022, 11, 1678. [Google Scholar] [CrossRef]
- Chen, J.; Qin, X.; Zhong, S.; Chen, S.; Su, W.; Liu, Y. Characterization of Curcumin/Cyclodextrin Polymer Inclusion Complex and Investigation on Its Antioxidant and Antiproliferative Activities. Molecules 2018, 23, 1179. [Google Scholar] [CrossRef]
- Ahmad, V.; Alotibi, I.; Alghamdi, A.A.; Ahmad, A.; Jamal, Q.M.; Srivastava, S. Computational Approaches to Evaluate the Acetylcholinesterase Binding Interaction with Taxifolin for the Management of Alzheimer’s Disease. Molecules 2024, 29, 674. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Picciotto, M.R. The Role of Acetylcholine in Negative Encoding Bias: Too Much of a Good Thing? Eur. J. Neurosci. 2021, 53, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Jaipea, S.; Saehlim, N.; Sutcharitruk, W.; Athipornchai, A.; Ingkaninan, K.; Saeeng, R. Synthesis of Piperine Analogues as AChE and BChE Inhibitors for the Treatment of Alzheimer’s Disease. Phytochem. Lett. 2023, 53, 216–221. [Google Scholar] [CrossRef]
- Furmanek, Ł.; Czarnota, P.; Seaward, M.R.D. A Review of the Potential of Lichen Substances as Antifungal Agents: The Effects of Extracts and Lichen Secondary Metabolites on Fusarium Fungi. Arch. Microbiol. 2022, 204, 523. [Google Scholar] [CrossRef]
- Nelumdeniya, N.R.M.; Ranatunga, R.J.K.U. Complex Forming Behaviour of α, β and γ-Cyclodextrins with Varying Size Probe Particles in Silico. Ceylon J. Sci. 2021, 50, 329. [Google Scholar] [CrossRef]
- Lai, W.-F.; Rogach, A.L.; Wong, W.-T. Chemistry and Engineering of Cyclodextrins for Molecular Imaging. Chem. Soc. Rev. 2017, 46, 6379–6419. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.M.M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Omidian, H.; Akhzarmehr, A.; Gill, E.J. Cyclodextrin–Hydrogel Hybrids in Advanced Drug Delivery. Gels 2025, 11, 177. [Google Scholar] [CrossRef]
- Sussman, J.L.; Harel, M.; Frolow, F.; Oefner, C.; Goldman, A.; Toker, L.; Silman, I. Atomic Structure of Acetylcholinesterase from Torpedo Californica: A Prototypic Acetylcholine-Binding Protein. Science 1991, 253, 872–879. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal Structure of Human Butyrylcholinesterase and of Its Complexes with Substrate and Products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-S. An Updated Review of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Kavetsou, E.; Pitterou, I.; Katopodi, A.; Petridou, G.; Adjali, A.; Grigorakis, S.; Detsi, A. Preparation, Characterization, and Acetylcholinesterase Inhibitory Ability of the Inclusion Complex of β-Cyclodextrin–Cedar (Juniperus phoenicea) Essential Oil. Micro 2021, 1, 250–266. [Google Scholar] [CrossRef]
- Zengin, G.; Nilofar; Yildiztugay, E.; Bouyahya, A.; Cavusoglu, H.; Gevrenova, R.; Zheleva-Dimitrova, D. A Comparative Study on UHPLC-HRMS Profiles and Biological Activities of Inula Sarana Different Extracts and Its Beta-Cyclodextrin Complex: Effective Insights for Novel Applications. Antioxidants 2023, 12, 1842. [Google Scholar] [CrossRef] [PubMed]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and Synthetic Flavonoid Derivatives as New Potential Tyrosinase Inhibitors: A Systematic Review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef] [PubMed]
- Jakupović, L.; Bačić, I.; Jablan, J.; Marguí, E.; Marijan, M.; Inić, S.; Nižić Nodilo, L.; Hafner, A.; Zovko Končić, M. Hydroxypropyl-β-Cyclodextrin-Based Helichrysum Italicum Extracts: Antioxidant and Cosmeceutical Activity and Biocompatibility. Antioxidants 2023, 12, 855. [Google Scholar] [CrossRef]
- Pan, T.; Li, X.; Jankovic, J. The Association between Parkinson’s Disease and Melanoma. Int. J. Cancer 2011, 128, 2251–2260. [Google Scholar] [CrossRef]
- Li, J.; Feng, L.; Liu, L.; Wang, F.; Ouyang, L.; Zhang, L.; Hu, X.; Wang, G. Recent Advances in the Design and Discovery of Synthetic Tyrosinase Inhibitors. Eur. J. Med. Chem. 2021, 224, 113744. [Google Scholar] [CrossRef]
- Tan, X.; Song, Y.H.; Park, C.; Lee, K.-W.; Kim, J.Y.; Kim, D.W.; Kim, K.D.; Lee, K.W.; Curtis-Long, M.J.; Park, K.H. Highly Potent Tyrosinase Inhibitor, Neorauflavane from Campylotropis Hirtella and Inhibitory Mechanism with Molecular Docking. Bioorg. Med. Chem. 2016, 24, 153–159. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Bulicz, M.; Henkel, M.; Rosiak, N.; Paczkowska-Walendowska, M.; Szwajgier, D.; Baranowska-Wójcik, E.; Korybalska, K.; Cielecka-Piontek, J. Pleiotropic Potential of Evernia Prunastri Extracts and Their Main Compounds Evernic Acid and Atranorin: In Vitro and In Silico Studies. Molecules 2023, 29, 233. [Google Scholar] [CrossRef]
- Stasiłowicz-Krzemień, A.; Gołębiewski, M.; Płazińska, A.; Płaziński, W.; Miklaszewski, A.; Żarowski, M.; Adamska-Jernaś, Z.; Cielecka-Piontek, J. The Systems of Naringenin with Solubilizers Expand Its Capability to Prevent Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 755. [Google Scholar] [CrossRef]
- Nikolić, V.; Stanković, M.; Nikolić, L.; Nikolić, G.; Ilić-Stojanović, S.; Popsavin, M.; Zlatković, S.; Kundaković, T. Inclusion Complexes with Cyclodextrin and Usnic Acid. J. Incl. Phenom. Macrocycl. Chem. 2013, 76, 173–182. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Kaproń, B.; Plech, T.; Żarowski, M.; Cielecka-Piontek, J. Lichen-Derived Compounds and Extracts as Biologically Active Substances with Anticancer and Neuroprotective Properties. Pharmaceuticals 2021, 14, 1293. [Google Scholar] [CrossRef]
- Kulawik, A.; Rosiak, N.; Miklaszewski, A.; Cielecka-Piontek, J.; Zalewski, P. Investigation of Cyclodextrin as Potential Carrier for Lycopene. Arch. Pharm. 2024, 74, 178–205. [Google Scholar] [CrossRef]
- Medeleanu, M.A.; Hădărugă, D.I.; Muntean, C.V.; Popescu, G.; Rada, M.; Hegheş, A.; Zippenfening, S.E.; Lucan (Banciu), C.A.; Velciov, A.B.; Bandur, G.N.; et al. Structure-Property Relationships on Recrystallized β-Cyclodextrin Solvates: A Focus on X-Ray Diffractometry, FTIR and Thermal Analyses. Carbohydr. Polym. 2021, 265, 118079. [Google Scholar] [CrossRef] [PubMed]
- de Castro, D.P.; Garcia, R.H.L.; de Andrade, L.G. Characterization Using X-Ray Diffraction and Study of the Crystallinity of the Thermoplastic Starch/Poly (Butylene Adipate-Co-Terephthalate) Blends Irradiated by Gamma Rays/Caracterização Por DRX e Estudo de Cristalinidade de Blendas de Amido Termoplástic. Braz. J. Dev. 2020, 6, 9635–9643. [Google Scholar] [CrossRef][Green Version]
- Baranowska-Wójcik, E.; Szwajgier, D.; Winiarska-Mieczan, A. Regardless of the Brewing Conditions, Various Types of Tea Are a Source of Acetylcholinesterase Inhibitors. Nutrients 2020, 12, 709. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Paczkowska-Walendowska, M.; Erdem, C.; Paluszczak, J.; Kleszcz, R.; Hoszman-Kulisz, M.; Cielecka-Piontek, J. Anti-Aging Properties of Chitosan-Based Hydrogels Rich in Bilberry Fruit Extract. Antioxidants 2024, 13, 105. [Google Scholar] [CrossRef] [PubMed]


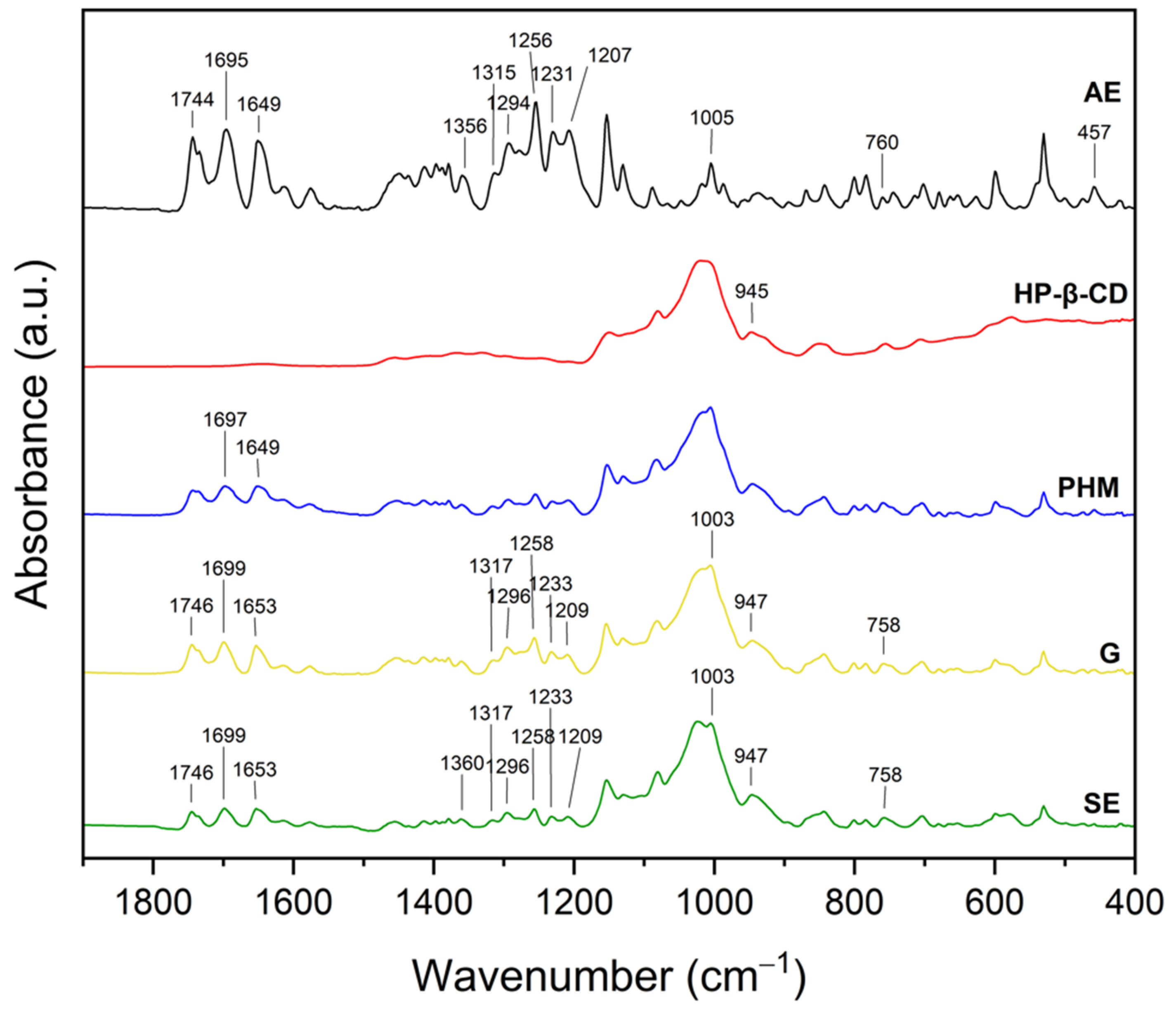
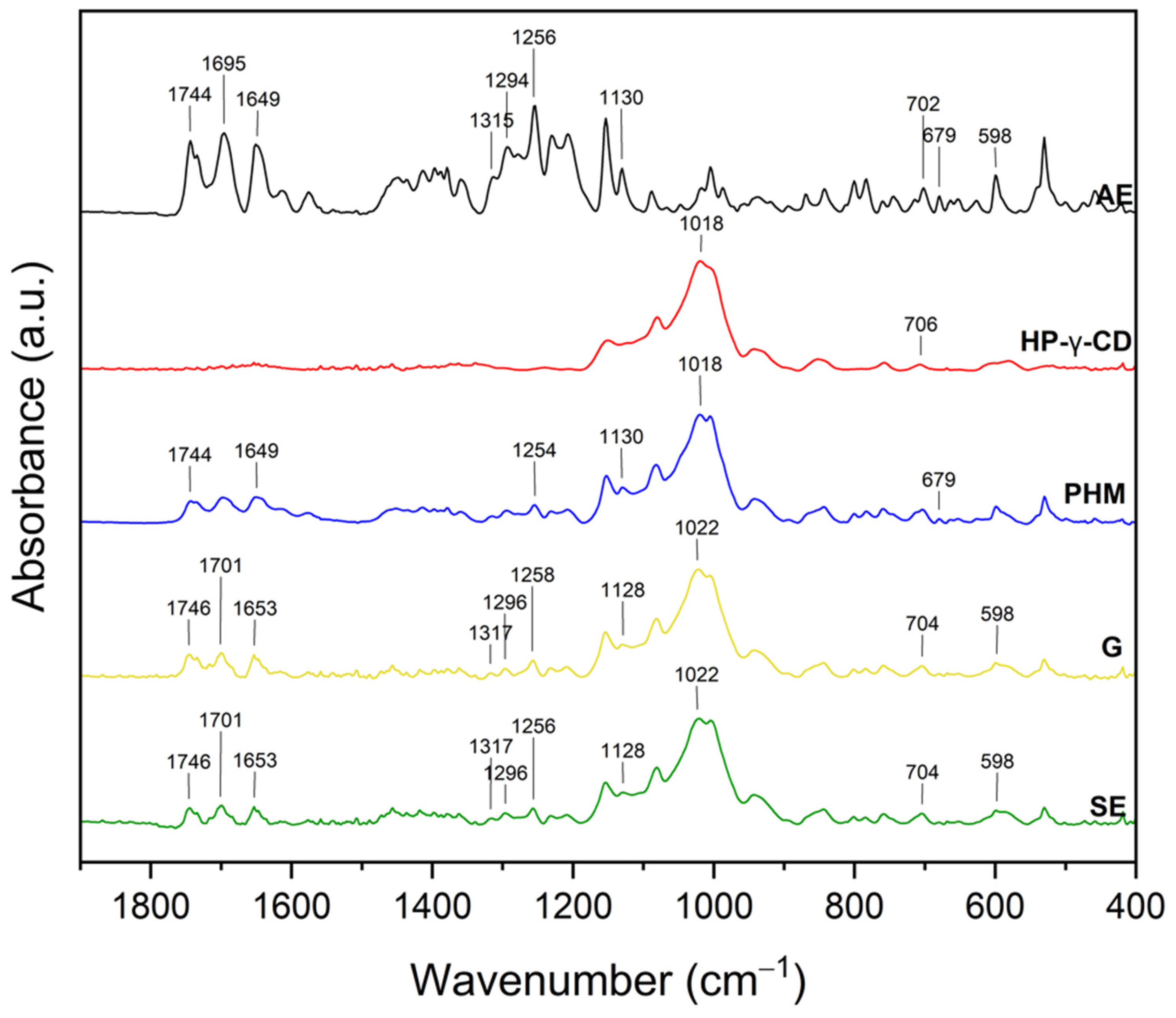
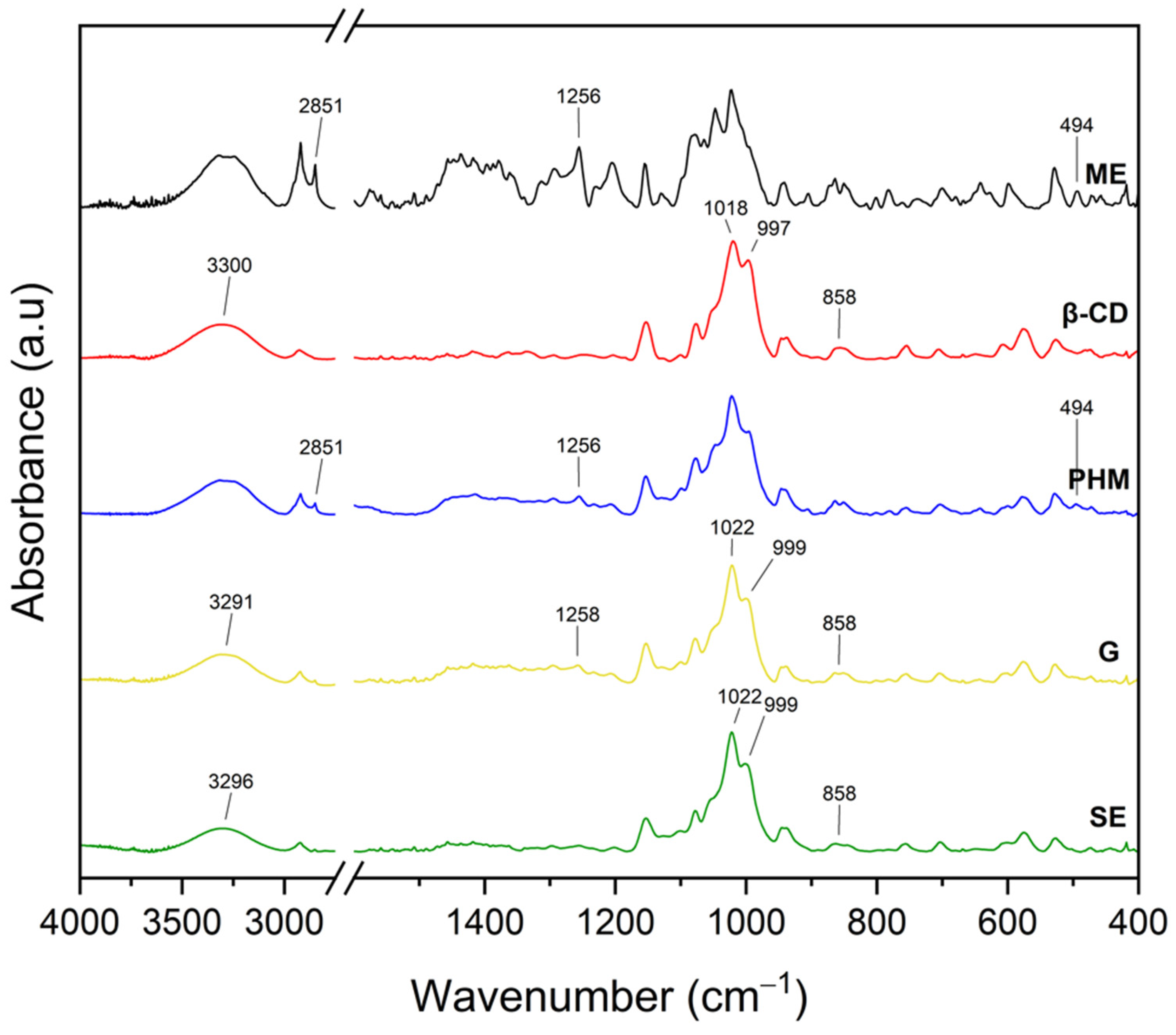


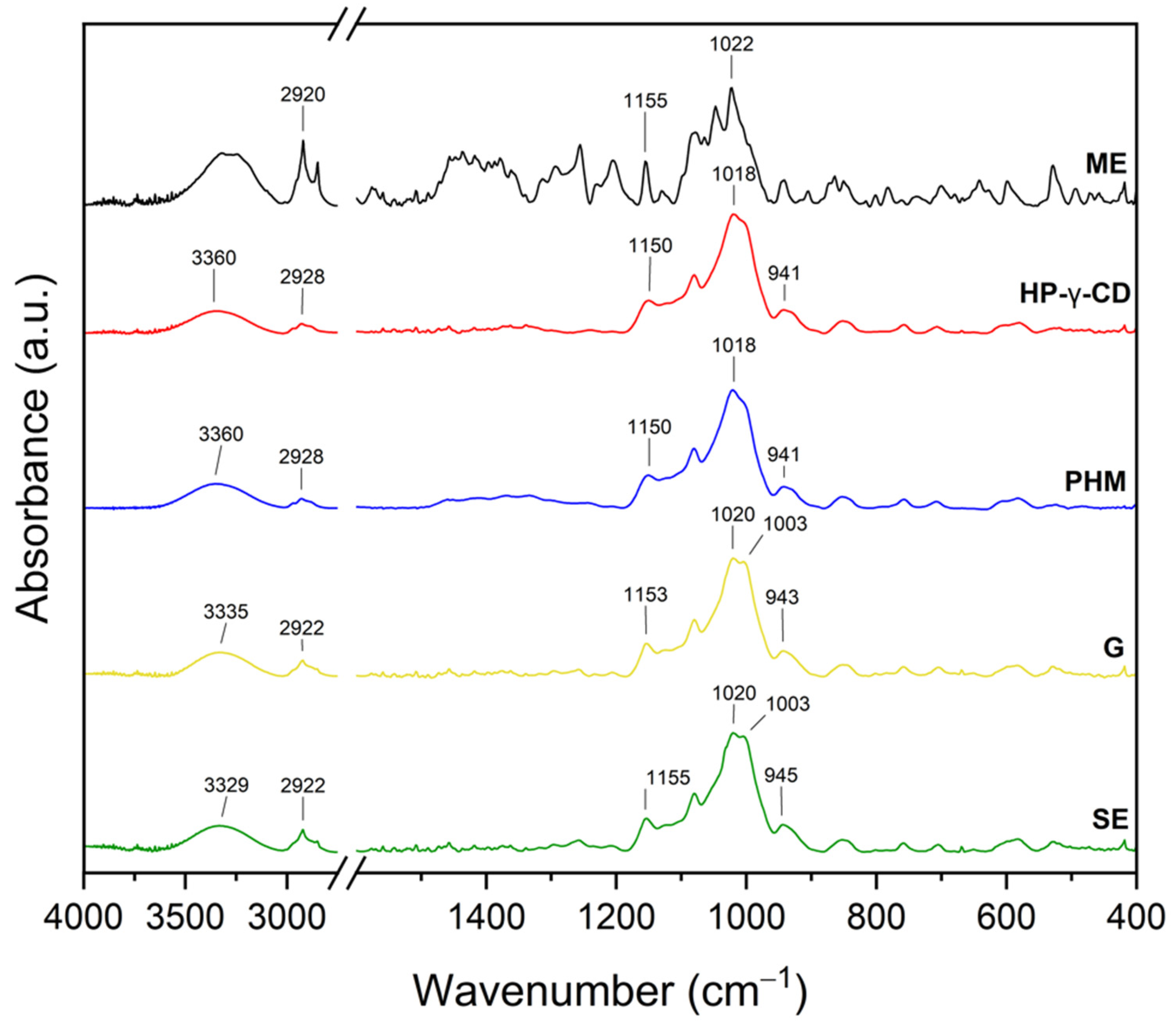
| Extract | Rt (min.) | Compounds | % of Total | MW | Formula |
|---|---|---|---|---|---|
| Acetone extract | 18.468 | Tricyclo [5.4.3.0(1,8)]tetradecan-6-one, 4-ethenyl-3-hydroxy-2,4,7,14-tetramethyl | 1.019 | 304.46 | C20H32O2 |
| 19.512 | Hexadecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | 1.876 | 568.91 | C35H68O5 | |
| 20.837 | Cholestan-3-one, cyclic 1,2-ethanediyl aetal, (5ß)- | 5.149 | 430.70 | C29H50O2 | |
| 21.142 | 12-Methyl-E,E-2,13-octadecadien-1-ol | 2.354 | 280.49 | C19H36O | |
| 21.242 | Oleic Acid | 6.710 | 282.46 | C18H34O2 | |
| 21.496 | 7,8-Epoxylanostan-11-ol, 3-acetoxy- | 4.006 | 502.76 | C32H54O4 | |
| 21.723 | Oxiraneoctanoic acid, 3-octyl-, cis- | 7.108 | 298.46 | C18H34O3 | |
| 21.979 | 8-Hexadecenal, 14-methyl-, (Z)- | 64.164 | 252.43 | C17H32O | |
| 23.735 | 15,17,19,21-Hexatriacontatetrayne | 7.615 | 490.84 | C36H58 | |
| Total | 100.00 | ||||
| Methanol extract | 18.117 | 14-Octadecenal | 1.21 | 266.46 | C18H34O |
| 19.438 | n-Hexadecanoic acid | 5.99 | 256.42 | C16H32O2 | |
| 20.688 | 7,10-Octadecadienoic acid, methyl ester | 6.23 | 294.47 | C19H34O2 | |
| 21.070 | Linoleic acid | 32.88 | 280.44 | C18H32O2 | |
| 21.495 | Methyl kauran-18-oate | 4.76 | 318.49 | C21H34O2 | |
| 21.973 | 2-Methyl-Z,Z-3,13-octadecadienol | 24.29 | 280.49 | C19H36O | |
| 22.221 | Oxiraneoctanoic acid, 3-octyl-, cis- | 3.92 | 298,461 | C18H34O3 | |
| 23.252 | Cholesta-5,7,9(11)-trien-3-ol acetate | 5.34 | 424.66 | C29H44O2 | |
| 23.747 | Olean-12-ene-3,15,16,21,22,28-hexol, (3ß,15a,16a,21ß,22a)- | 4.07 | 506.71 | C30H50O6 | |
| 24.902 | Pregna-4,6-diene-3,20-dione, 17-(acetyloxy)-6,16-dimethyl-, (16a)- | 11.31 | 398.53 | C25H34O4 | |
| Total | 100.00 |
| CI (%) | ||||
|---|---|---|---|---|
| raw | SE | G | ||
| AE/β-CD | β-CD | 55.52 | 33.46 | 47.17 |
| AE | 56.96 | 32.45 | 19.20 | |
| AE/γ-CD | γ-CD | 84.16 | 27.33 | 65.02 |
| AE | 56.96 | 27.13 | 15.63 | |
| AE/HP-β-CD | AE | 56.96 | 7.74 | 19.94 |
| AE/HP-γ-CD | AE | 56.96 | 22.83 | 6.14 |
| CI (%) | ||||
|---|---|---|---|---|
| raw | SE | G | ||
| ME/β-CD | β-CD | 55.52 | 34.74 | 2.79 |
| ME | 72.32 | 19.65 | 63.43 | |
| ME/γ-CD | γ-CD | 84.16 | 36.13 | 72.05 |
| ME | 72.32 | 25.36 | 5.11 | |
| ME/HP-β-CD | ME | 72.32 | 1.88 | 6.78 |
| ME/HP-γ-CD | ME | 72.32 | 8.61 | 4.34 |
| Tested Sample | Total Polyphenol Content (GAE mg/g) | ||||
|---|---|---|---|---|---|
| UE | G | SE | PHM | ||
| Acetone extract | AE | 7.0 ± 0.1 h | - | - | - |
| β-CD | - | 7.7 ± 0.1 f,g | 10.7 ± 0.1 c | 7.5 ± 0.1 g | |
| γ-CD | - | 7.6 ± 0.2 f,g | 7.7 ± 0.3 f,g | 7.9 ± 0.3 f | |
| HP-β-CD | - | 9.6 ± 0.3 d | 13.8 ± 0.4 a | 7.9 ± 0.3 f | |
| HP-γ-CD | - | 12.1 ± 0.2 b | 13.6 ± 0.2 a | 9.0 ± 0.2 e | |
| Methanol extract | ME | 17.9 ± 0.5 e | - | - | - |
| β-CD | - | 18.7 ± 0.6 d | 16.8 ± 0.6 f | 16.4 ± 0.3 f | |
| γ-CD | - | 18.6 ± 0.4 d,e | 16.7 ± 0.9 f | 18.1 ± 1.0 d,e | |
| HP-β-CD | - | 21.2 ± 0.4 b | 20.2 ± 0.6 c | 17.9 ± 0.2 e | |
| HP-γ-CD | - | 21.3 ± 0.5 b | 22.9 ± 0.2 a | 21.6 ± 0.2 b | |
| Tested Sample | Antioxidant Activity (%) | |||||
|---|---|---|---|---|---|---|
| UE | G | SE | PHM | CD | ||
| Acetone extract | AE | 66.0 ± 1.3 c | - | - | - | - |
| β-CD | - | 68.9 ± 2.6 c | 62.8 ± 0.9 d | 68.9 ± 1.9 c | na | |
| γ-CD | - | 56.8 ± 3.3 e | 66.1 ± 5.5 c | 62.0 ± 1.9 d | na | |
| HP-β-CD | - | 73.9 ± 2.5 b | 78.9 ± 1.4 a | 62.6 ± 1.4 d | na | |
| HP-γ-CD | - | 67.5 ± 1.2 c | 73.7 ± 0.7 b | 52.1 ± 0.9 f | na | |
| Methanol extract | ME | 93.2 ± 0.2 b,c | - | - | - | - |
| β-CD | - | 93.1 ± 2.0 b,c | 88.1 ± 0.4 d | 92.5 ± 0.2 c | na | |
| γ-CD | - | 82.0 ± 2.1 f | 87.6 ± 1.3 d | 85.4 ± 0.8 e | na | |
| HP-β-CD | - | 93.6 ± 0.1 a,b,c | 92.8 ± 0.3 b,c | 94.5 ± 0.2 a | na | |
| HP-γ-CD | - | 93.7 ± 0.2 a,b | 93.3 ± 0.2 b,c | 93.5 ± 0.3 a,b,c | na | |
| Tested Sample | AChE Inhibition (%) | BChE Inhibition (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| UE | G | SE | PHM | CD | UE | G | SE | PHM | CD | ||
| Acetone extract | AE | 3.1 ± 1.2 d,e,f | - | - | - | - | 9.8 ± 1.9 b,c | - | - | - | - |
| β-CD | - | 4.0 ± 0.3 d,e | 4.6 ± 1.6 d | 8.9 ± 1.3 b,c | 2.6 ± 2.7 e,f,g | - | 5.8 ± 1.0 d | 5.6 ± 0.3 d | 6.2 ± 1.7 d | 1.4 ± 0.7 h | |
| γ-CD | - | 3.6 ± 1.1 d,e | na | 1.3 ± 0.2 f,g,h | 0.24 ± 0.7 g | - | 3.5 ± 1.1 g | na | 3.5 ± 0.7 g | 0.5 ± 1.1 h | |
| HP-β-CD | - | 10.9 ± 0.3 a | 3.2 ± 1.5 d,e | 9.9 ± 0.6 a,b | na | - | 12.1 ± 1.7 a | 10.7 ± 1.0 a,b | 8.9 ± 0.7 c | na | |
| HP-γ-CD | - | 4.7 ± 1.4 d | 7.9 ± 0.6 c | 8.1 ± 1.3 b,c | 0.80 ± 0.5 f,g | - | 11.1 ± 1.1 a,b | 11.8 ± 0.7 a | 7.1 ± 0.9 d | 1.73 ± 1.0 h | |
| Methanol extract | ME | 3.9 ± 1.0 g | - | - | - | - | 7.2 ± 0.7 f | - | - | - | - |
| β-CD | - | 2.01 ± 0.7 h,i | 5.6 ± 0.4 f | 11.0 ± 0.4 c,d | 2.6 ± 2.7 g,h | - | 9.0 ± 1.3 d,e | 7.6 ± 1.4 e,f | 9.8 ± 0.6 d | 1.4 ± 0.7 h | |
| γ-CD | - | 3.6 ± 0.4 g | 1.6 ± 0.7 h,i,j | 10.0 ± 0.3 d | 0.24 ± 0.7 j | - | 3.9 ± 0.4 g | 5.1 ± 1.7 g | 7.8 ± 0.5 e,f | 0.5 ± 1.1 h | |
| HP-β-CD | - | 10.8 ± 0.5 c,d | 7.7 ± 0.5 e | 11.5 ± 0.7 c | na | - | 13.3 ± 2.0 b,c | 14.2 ± 1.5 b | 14.3 ± 1.3 b | na | |
| HP-γ-CD | - | 11.9 ± 0.8 c | 23.9 ± 0.7 a | 20.9 ± 0.9 b | 0.80 ± 0.5 i,j | - | 12.6 ± 1.2 c | 16.7 ± 1.2 a | 14.3 ± 0.7 b | 1.73 ± 1.0 h | |
| Tested Sample | Tyrosinase Inhibition (%) | |||||
|---|---|---|---|---|---|---|
| UE | G | SE | PHM | CD | ||
| Acetone extract | AE | 18.8 ± 2.6 c | - | - | - | - |
| β-CD | - | 13.9 ± 2.0 d | 20.6 ± 2.0 b,c | 10.2 ± 1.1 e | 2.8 ± 0.5 f | |
| γ-CD | - | 13.8 ± 1.8 d | 22.3 ± 1.6 b | 14.9 ± 1.4 d | 4.3 ± 1.4 f | |
| HP-β-CD | - | 20.9 ± 1.5 b,c | 25.9 ± 1.6 a | 15.9 ± 4.1 d | na | |
| HP-γ-CD | - | 22.5 ± 0.3 b | 26.2 ± 0.7 a | 21.5 ± 0.4 b | 2.8 ± 0.7 f | |
| Methanol extract | ME | 25.3 ± 0.8 c,d | - | - | - | - |
| β-CD | - | 19.4 ± 1.5 h | 25.4 ± 0.8 c,d | 23.4 ± 0.7 e | 2.8 ± 0.5 j | |
| γ-CD | - | 21.0 ± 0.5 f,g | 24.9 ± 0.4 c,d | 19.9 ± 0.8 g,h | 4.3 ± 1.4 i | |
| HP-β-CD | - | 24.8 ± 0.7 d | 32.62 ± 2.2 b | 21.9 ± 0.7 f | na | |
| HP-γ-CD | - | 26.2 ± 1.0 c | 34.2 ± 0.5 a | 21.4 ± 0.6 f | 2.8 ± 0.7 j | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Studzińska-Sroka, E.; Cichoracka, K.; Rosiak, N.; Miklaszewski, A.; Szymański, M.; Cielecka-Piontek, J. Cyclodextrin-Based Systems of Cetraria islandica Extracts: A Novel Approach to Improve Solubility and Biological Activity of Lichen-Derived Natural Products. Molecules 2025, 30, 3182. https://doi.org/10.3390/molecules30153182
Studzińska-Sroka E, Cichoracka K, Rosiak N, Miklaszewski A, Szymański M, Cielecka-Piontek J. Cyclodextrin-Based Systems of Cetraria islandica Extracts: A Novel Approach to Improve Solubility and Biological Activity of Lichen-Derived Natural Products. Molecules. 2025; 30(15):3182. https://doi.org/10.3390/molecules30153182
Chicago/Turabian StyleStudzińska-Sroka, Elżbieta, Karolina Cichoracka, Natalia Rosiak, Andrzej Miklaszewski, Marcin Szymański, and Judyta Cielecka-Piontek. 2025. "Cyclodextrin-Based Systems of Cetraria islandica Extracts: A Novel Approach to Improve Solubility and Biological Activity of Lichen-Derived Natural Products" Molecules 30, no. 15: 3182. https://doi.org/10.3390/molecules30153182
APA StyleStudzińska-Sroka, E., Cichoracka, K., Rosiak, N., Miklaszewski, A., Szymański, M., & Cielecka-Piontek, J. (2025). Cyclodextrin-Based Systems of Cetraria islandica Extracts: A Novel Approach to Improve Solubility and Biological Activity of Lichen-Derived Natural Products. Molecules, 30(15), 3182. https://doi.org/10.3390/molecules30153182










