Sugar Esters of Fatty Acids: Chemo-Enzymatic Synthesis and Biological Activity
Abstract
1. Introduction
2. Synthesis
2.1. Chemical Synthesis
| Sugar | Substrate | Solvent | Catalyst | Product | CMC | DS/HLB | Yield [%] | Reaction condition | Purity [%] | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Sucrose | Methyl palmitate | H2O (1:1 v/v) | KOH | Sucrose palmitate ester | 0.8757 g/cm3 | 5.47 | 39 | 100 °C, 2 h | 90 | [55,62] |
| Ethyl laurate | DMSO (2:1 v/v) | K2CO3 | Sucrose laurate | 0.067 mol/L | 0.364–6 | 78 | 70 °C, 2 h | 92 | [63] | |
| Acetic anhydride, coconut oil | DMF | K2CO3 | - | 38.6 dyne/cm | - | 98 | 120–140 °C, 2–6 h | - | [64] | |
| Ethyl palmitate | DMSO (2:1 v/v) | K2CO3 | Sucrose palmitate ester | 1.5 × 10−5 mol/L | 10–16 | 73 | 70 °C, 2 h | 92 | [55,63,65] | |
| Acetonitrile | DMSO (60/40 v/v) | K2CO3 | - | - | - | 40 | 140 °C | 68 | [66] | |
| 3-Laurylthiazolidine-2-thione | Pyridine | NaH | - | - | - | 72 | 22 °C, 15 h | - | [55] | |
| Palmitic acid | Methyl alcohol | CH3Ona | Sucrose palmitate ester | 0.181 × 10−3 mol/L | - | 76 | 65 °C, 2 h | 90 | [67] | |
| Methyl laurate | DMSO | Diaion PA306S | Sucrose laurate | - | - | 98 | 90 °C, 3 h | 70 | [68] | |
| Palmitic benzoic anhydrite | Cyclohexane | Resin Amerlyst 15 | Sucrose palmitate ester | - | - | 63 | 4355 °C, 12 h | - | [69] | |
| Methyl stearate | DMF | KOMe | Sucrose stearate | - | - | 81.6 | 120 °C, 20 min | - | [70] | |
| Vinyl palmitate | Bmim [dca]/2-methylbutan-2-ol (2M2B) | [Bu4N][Ac] | Sucrose palmitate ester | - | - | 42 | 60 °C, 5 days | - | [71] | |
| Methyl laurate | DMSO | K2CO3 | Sucrose laurate | - | - | 69.89–73.28 | 80 °C | - | [72] | |
| Glucose | Vinyl laureate | 2M2B | [Bu4N][Ac] | Glucose laurate | - | - | 33 | 40 °C, 30 h | - | [72,73] |
2.2. Enzymatic Methods
2.2.1. Enzymatic Catalytic System
2.2.2. Solvent-Free System
2.2.3. Synthesis in the Presence of DES
2.3. Analytical Approaches for Structural Characterization and Quantification
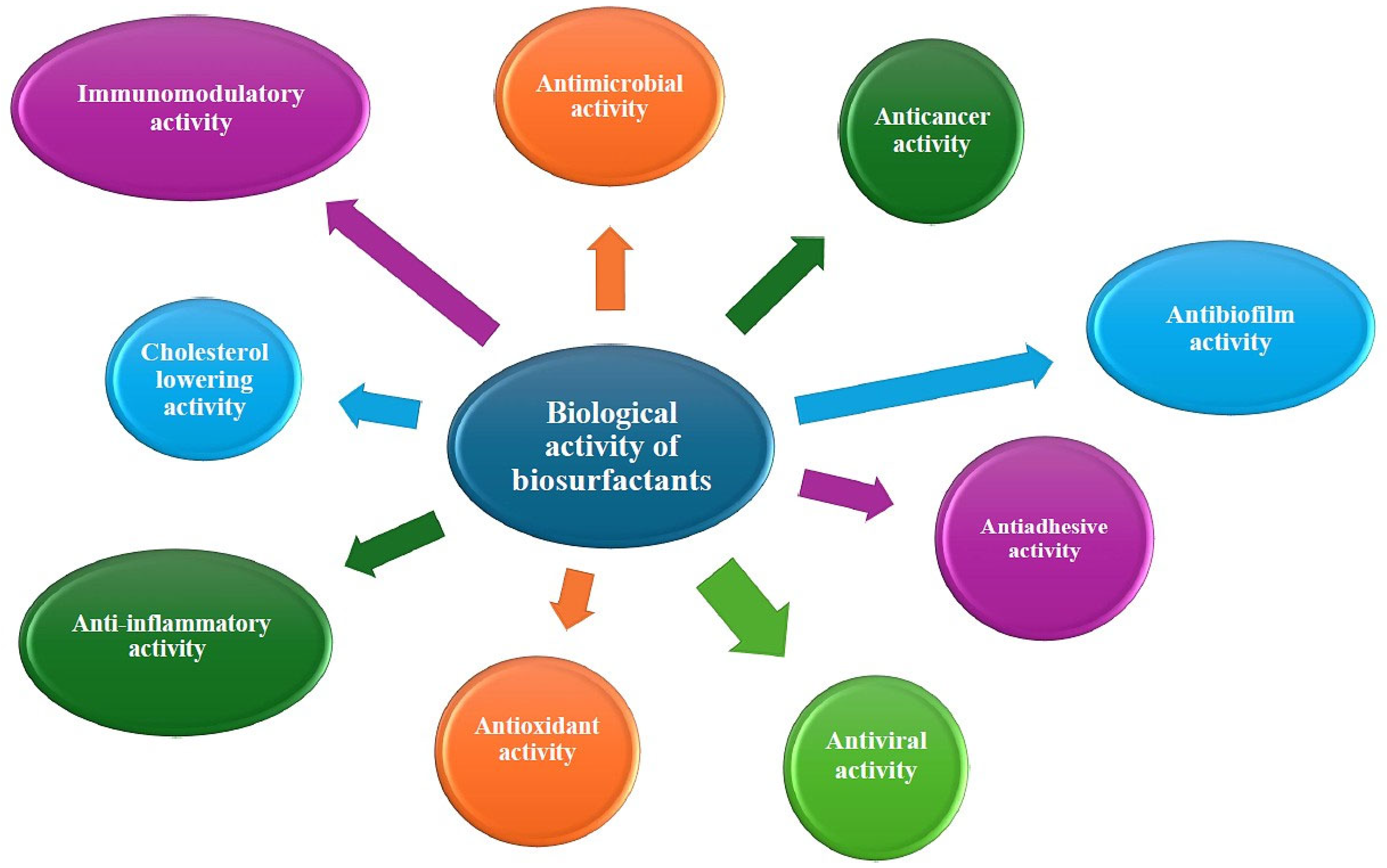
3. Biological Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.B.; Mishra, A.; Clark, J.; Farmer, T. Synthesis, chemistry, physicochemical properties and industrial applications of amino acid surfactants: A review. Comptes Rendus Chim. 2018, 21, 112–130. [Google Scholar] [CrossRef]
- Moldes, A.B.; Rodríguez-López, L.; Rincón-Fontán, M.; López-Prieto, A.; Vecino, X.; Cruz, J.M. Synthetic and Bio-Derived Surfactants Versus Microbial Biosurfactants in the Cosmetic Industry: An Overview. Int. J. Mol. Sci. 2021, 22, 2371. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Hernández, L.; Meléndez-Ortiz, H.I.; Cortez-Mazatan, G.Y.; Vaillant-Sánchez, S.; Peralta-Rodríguez, R.D. Gemini and Bicephalous Surfactants: A Review on Their Synthesis, Micelle Formation, and Uses. Int. J. Mol. Sci. 2022, 23, 1798. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.; Adamsson, M.; Dave, G. Toxicity and detoxification of Swedish detergents and softener products. Chemosphere 2000, 41, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Arora, U.; Khuntia, H.K.; Chanakya, H.N.; Kapley, A. Surfactants: Combating the fate, impact, and aftermath of their release in the environment. Int. J. Environ. Sci. Technol. 2023, 20, 11551–11574. [Google Scholar] [CrossRef]
- Mesnage, R.; Benbrook, C.; Antoniou, M.N. Insight into the confusion over surfactant co-formulants in glyphosate-based herbicides. Food Chem. Toxicol. 2019, 128, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Di Corcia, A. Characterization of surfactants and their biointermediates by liquid chromatography–mass spectrometry. J. Chromatogr. A 1998, 794, 165–185. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, M.; Kasomo, R.M.; Li, H.; Zheng, H.; Song, S.; Luo, H.; He, D. Depression effect of Al(III) and Fe(III) on rutile flotation using dodecylamine polyxyethylene ether as collector. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125269. [Google Scholar] [CrossRef]
- Gaudin, T.; Lu, H.; Fayet, G.; Berthauld-Drelich, A.; Rotureau, P.; Pourceau, G.; Wadouachi, A.; Van Hecke, E.; Nesterenko, A.; Pezron, I. Impact of the chemical structure on amphiphilic properties of sugar-based surfactants: A literature overview. Adv. Colloid Interface Sci. 2019, 270, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Geoghegan, C.; Brayden, D.J. Safety of surfactant excipients in oral drug formulations. Adv. Drug Deliv. Rev. 2023, 202, 115086. [Google Scholar] [CrossRef] [PubMed]
- Barra Caracciolo, A.; Cardoni, M.; Pescatore, T.; Patrolecco, L. Characteristics and environmental fate of the anionic surfactant sodium lauryl ether sulphate (SLES) used as the main component in foaming agents for mechanized tunnelling. Environ. Pollut. 2017, 226, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Zakharova LYa Pashirova, T.N.; Doktorovova, S.; Fernandes, A.R.; Sanchez-Lopez, E.; Silva, A.M.; Souto, S.B.; Souto, E.B. Cationic Surfactants: Self-Assembly, Structure-Activity Correlation and Their Biological Applications. Int. J. Mol. Sci. 2019, 20, 5534. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Pal, A.; Rakshit, A.; Saha, B. Properties and applications of amphoteric surfactant: A concise review. J. Surfactants Deterg. 2021, 24, 709–730. [Google Scholar] [CrossRef]
- Crepaldi, E.L.; Pavan, P.C.; Tronto, J.; Valim, J.B. Chemical, Structural, and Thermal Properties of Zn(II)–Cr(III) Layered Double Hydroxides Intercalated with Sulfated and Sulfonated Surfactants. J. Colloid Interface Sci. 2002, 248, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Lake, I.R.; Swift, L.; Catling, L.A.; Abubakar, I.; Sabel, C.E.; Hunter, P.R. Effect of water hardness on cardiovascular mortality: An ecological time series approach. J. Public Health 2010, 32, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Alwadani, N.; Fatehi, P. Synthetic and lignin-based surfactants: Challenges and opportunities. Carbon Resour. Convers. 2018, 1, 126–138. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y. Structure–activity relationship of cationic surfactants as antimicrobial agents. Curr. Opin. Colloid Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Blagojević, S.N.; Blagojević, S.M.; Pejić, N.D. Performance and Efficiency of Anionic Dishwashing Liquids with Amphoteric and Nonionic Surfactants. J. Surfactants Deterg. 2016, 19, 363–372. [Google Scholar] [CrossRef]
- Jesus, C.F.; Alves, A.A.S.; Fiuza, S.M.; Murtinho, D.; Antunes, F.E. Mini-review: Synthetic methods for the production of cationic sugar-based surfactants. J. Mol. Liq. 2021, 342, 117389. [Google Scholar] [CrossRef]
- Nagtode, V.S.; Cardoza, C.; Yasin, H.K.A.; Mali, S.N.; Tambe, S.M.; Roy, P.; Singh, K.; Goel, A.; Amin, P.D.; Thorat, B.R.; et al. Green Surfactants (Biosurfactants): A Petroleum-Free Substitute for Sustainability─Comparison, Applications, Market, and Future Prospects. ACS Omega 2023, 8, 11674–11699. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.S.; Alvarado, J.G.; Zambrano, F.; Marquez, R. Surfactants produced from carbohydrate derivatives: A review of the biobased building blocks used in their synthesis. J. Surfactants Deterg. 2022, 25, 147–183. [Google Scholar] [CrossRef]
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A review on natural surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Patowary, K.; Das, M.; Patowary, R.; Kalita, M.C.; Deka, S. Recycling of Bakery Waste as an Alternative Carbon Source for Rhamnolipid Biosurfactant Production. J. Surfactants Deterg. 2019, 22, 373–384. [Google Scholar] [CrossRef]
- De Clercq, V.; Roelants, S.L.K.W.; Castelein, M.G.; De Maeseneire, S.L.; Soetaert, W.K. Elucidation of the Natural Function of Sophorolipids Produced by Starmerella bombicola. J. Fungi 2021, 7, 917. [Google Scholar] [CrossRef] [PubMed]
- Juma, A.; Lemoine, P.; Simpson, A.B.J.; Murray, J.; O’Hagan, B.M.G.; Naughton, P.J.; Dooley, J.G.; Banat, I.M. Microscopic Investigation of the Combined Use of Antibiotics and Biosurfactants on Methicillin Resistant Staphylococcus aureus. Front. Microbiol. 2020, 11, 1477. [Google Scholar] [CrossRef] [PubMed]
- Twigg, M.S.; Baccile, N.; Banat, I.M.; Déziel, E.; Marchant, R.; Roelants, S.; Van Bogaert, I.N.A. Microbial biosurfactant research: Time to improve the rigour in the reporting of synthesis, functional characterization and process development. Microb. Biotechnol. 2021, 14, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Singha, L.P.; Shukla, P. Biotechnological potential of microbial bio-surfactants, their significance, and diverse applications. FEMS Microbes 2023, 4, xtad015. [Google Scholar] [CrossRef] [PubMed]
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. [Google Scholar] [CrossRef] [PubMed]
- Inamuddin; Ahamed, M.I.; Prasad, R. (Eds.) Microbial Biosurfactants; Springer: Singapore, 2021. [Google Scholar]
- Marchut-Mikołajczyk, O.; Drożdżyński, P.; Polewczyk, A.; Smułek, W.; Antczak, T. Biosurfactant from endophytic Bacillus pumilus 2A: Physicochemical characterization, production and optimization and potential for plant growth promotion. Microb. Cell Fact. 2021, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Han, F.; Abdelaziz, I.I.M.; Liu, X.; Ghazali, K.H.; Mishra, P. Application of biosurfactant tea saponin in flotation separation for ternary plastic mixtures: Statistical optimization and mechanism analysis. J. Clean. Prod. 2019, 232, 499–507. [Google Scholar] [CrossRef]
- Kaur, G.; Wang, H.; To, M.H.; Roelants, S.L.K.W.; Soetaert, W.; Lin, C.S.K. Efficient sophorolipids production using food waste. J. Clean. Prod. 2019, 232, 1–11. [Google Scholar] [CrossRef]
- Roy, A. A Review on the Biosurfactants: Properties, Types and its Applications. J. Fundam. Renew. Energy Appl. 2017, 08, 1000248. [Google Scholar] [CrossRef]
- Sałek, K.; Euston, S.R.; Janek, T. Phase Behaviour, Functionality, and Physicochemical Characteristics of Glycolipid Surfactants of Microbial Origin. Front. Bioeng. Biotechnol. 2022, 10, 816613. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.M.; Kügler, J.H.; Henkel, M.; Gerlitzki, M.; Hörmann, B.; Pöhnlein, M.; Syldatk, C.; Hausmann, R. Rhamnolipids—Next generation surfactants. J. Biotechnol. 2012, 162, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Hu, C.; Sun, G.; Ji, B.; Yan, K. Antimicrobial finish of cotton fabrics treated by sophorolipids combined with 1,2,3,4-butanetetracarboxyic acid. Cellulose 2020, 27, 2859–2872. [Google Scholar] [CrossRef]
- Im, J.H.; Nakane, T.; Yanagishita, H.; Ikegami, T.; Kitamoto, D. Mannosylerythritol lipid, a yeast extracellular glycolipid, shows high binding affinity towards human immunoglobulin G. BMC Biotechnol 2001, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Lou, H.; Wei, T.; Liu, X.; Chen, Q. Contributions of Glycolipid Biosurfactants and Glycolipid-Modified Materials to Antimicrobial Strategy: A Review. Pharmaceutics 2021, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Arcens, D.; Grau, E.; Grelier, S.; Cramail, H.; Peruch, F. 6-O-glucose palmitate synthesis with lipase: Investigation of some key parameters. Mol. Catal. 2018, 460, 63–68. [Google Scholar] [CrossRef]
- Lucarini, S.; Fagioli, L.; Cavanagh, R.; Liang, W.; Perinelli, D.; Campana, M.; Stolnik, S.; Lam, J.; Casettari, L.; Duranti, A. Synthesis, Structure–Activity Relationships and In Vitro Toxicity Profile of Lactose-Based Fatty Acid Monoesters as Possible Drug Permeability Enhancers. Pharmaceutics 2018, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Gu, J.; Zhao, T.; Liu, L.; Wang, N.; Gu, F. Laboratory Study on the Oil Displacement Properties of Sugar Amine Sulfonate Surfactant. J. Surfactants Deterg. 2017, 20, 1037–1049. [Google Scholar] [CrossRef]
- Lu, H.; Pourceau, G.; Briou, B.; Wadouachi, A.; Gaudin, T.; Pezron, I.; Drelich, A. Sugar-Based Surfactants: Effects of Structural Features on the Physicochemical Properties of Sugar Esters and Their Comparison to Commercial Octyl Glycosides. Molecules 2024, 29, 2338. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.J.; Shah, N.N.; Singhal, R.S. Enzymatic synthesis of fatty acid esters of trehalose: Process optimization, characterization of the esters and evaluation of their bioactivities. Bioorg. Chem. 2020, 94, 103460. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.J.; Dedhia, N.; Singhal, R.S. Esterification of sugars and polyphenols with fatty acids: Techniques, bioactivities, and applications. Curr. Opin. Food Sci. 2022, 43, 163–173. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Liang, M.-Y.; Qing, C.; Teng, Y.; White LVBanwell, M.G.; Li, A.; Wang, Y.; Lan, P. Investigation of the Emulsifying and In Vitro Digestive Properties of High-Purity Sucrose Monostearate Esters. ACS Food Sci. Technol. 2022, 2, 1662–1671. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Almeida, F.C.G.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Soares da Silva Rde, C.F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Rebello, S.; Asok, A.K.; Mundayoor, S.; Jisha, M.S. Surfactants: Chemistry, Toxicity and Remediation; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer International Publishing: Cham, Switzerland, 2013; Volume 4, pp. 277–320. [Google Scholar]
- Jimoh, A.A.; Lin, J. Biosurfactant: A new frontier for greener technology and environmental sustainability. Ecotoxicol. Environ. Saf. 2019, 184, 109607. [Google Scholar] [CrossRef] [PubMed]
- Taddese, T.; Anderson, R.L.; Bray, D.J.; Warren, P.B. Recent advances in particle-based simulation of surfactants. Curr. Opin. Colloid Interface Sci. 2020, 48, 137–148. [Google Scholar] [CrossRef]
- Panpipat, W.; Chaijan, M. Ionic Liquids in Lipid Processing and Analysis; Xu, X., Guo, Z., Cheong, L.-Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 347–371. [Google Scholar]
- Pérez, B.; Anankanbil, S.; Guo, Z. Fatty Acids; Ahmad, M.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 329–354. [Google Scholar]
- Zheng, Y.; Zheng, M.; Ma, Z.; Xin, B.; Guo, R.; Xu, X. Polar Lipids; Ahmad, M.U., Xu, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 215–243. [Google Scholar]
- Otache, M.A.; Duru, R.U.; Ozioma, A.; Abayeh, J.O. Catalytic Methods for the Synthesis of Sugar Esters. Catal. Ind. 2022, 14, 115–130. [Google Scholar] [CrossRef]
- Koumba Ibinga, S.K.; Fabre, J.-F.; Bikanga, R.; Mouloungui, Z. Atypical Reaction Media and Organized Systems for the Synthesis of Low-Substitution Sugar Esters. Front. Chem. 2019, 7, 587. [Google Scholar] [CrossRef] [PubMed]
- Arellano, H.; Nardello-Rataj, V.; Szunerits, S.; Boukherroub, R.; Fameau, A.-L. Saturated long chain fatty acids as possible natural alternative antibacterial agents: Opportunities and challenges. Adv. Colloid Interface Sci. 2023, 318, 102952. [Google Scholar] [CrossRef] [PubMed]
- Feuge, R.O.; Zeringue, H.J.; Weiss, T.J.; Brown, M. Preparation of sucrose esters by interesterification. J. Am. Oil Chem. Soc. 1970, 47, 56–60. [Google Scholar] [CrossRef]
- Soares Ade, S.; Augusto, P.E.D.; Leite Júnior BRde, C.; Nogueira, C.A.; Vieira, É.N.R.; de Barros, F.A.R.; Stringheta, P.C.; Ramos, A.M. Ultrasound assisted enzymatic hydrolysis of sucrose catalyzed by invertase: Investigation on substrate, enzyme and kinetics parameters. LWT 2019, 107, 164–170. [Google Scholar] [CrossRef]
- Teng, Y.; Stewart, S.G.; Hai, Y.-W.; Li, X.; Banwell, M.G.; Lan, P. Sucrose fatty acid esters: Synthesis, emulsifying capacities, biological activities and structure-property profiles. Crit. Rev. Food Sci. Nutr. 2021, 61, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Staroń, J.; Dąbrowski, J.M.; Cichoń, E.; Guzik, M. Lactose esters: Synthesis and biotechnological applications. Crit. Rev. Biotechnol. 2018, 38, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Agatha, S.B.; Ariviani, S.; Yuliatun, S. Production of Fatty Acid Methyl Ester Surfactants using Palm Oil with Various Reaction Temperatures and Duration. agriTECH 2024, 44, 144. [Google Scholar] [CrossRef]
- Huang, D.; Jiang, X.; Zhu, H.; Fu, X.; Zhong, K.; Gao, W. Improved synthesis of sucrose fatty acid monoesters under ultrasonic irradiation. Ultrason. Sonochem. 2010, 17, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.S.; Deshpande, T.D.; Kulkarni, R.D.; Mahulikar, P.P. Synthesis of Sucrose–Coconut Fatty Acids Esters: Reaction Kinetics and Rheological Analysis. Ind. Eng. Chem. Res. 2013, 52, 15024–15033. [Google Scholar] [CrossRef]
- Gupta, R.K.; James, K.; Smith, F.J. Sucrose esters and sucrose ester/glyceride blends as emulsifiers. J. Am. Oil Chem. Soc. 1983, 60, 862–869. [Google Scholar] [CrossRef]
- Orjuela, A.; Gutierrez, M.F.; Suaza, A.; Rivera, J.L. Production of sucroesters using solvent-free reactive systems containing emulsifiers. Ing. E Investig. 2018, 38, 16–23. [Google Scholar] [CrossRef]
- Vassilev, D.; Petkova, N.; Koleva, M.; Denev, P. Optimization of ultrasound synthesis of sucrose esters by selection of a suitable catalyst and reaction conditions. J. Chem. Technol. Metall. 2021, 56, 268–274. [Google Scholar]
- Sasayama, T.; Kamikanda, Y.; Shibasaki-Kitakawa, N. Process design for green and selective production of bio-based surfactant with heterogeneous resin catalyst. Chem. Eng. J. 2018, 334, 2231–2237. [Google Scholar] [CrossRef]
- Trabelsi, I.; Essid, K.; Frikha, M.H. Synthesis of Sucrose Fatty Acid Esters by Using Mixed Carboxylic-fatty Anhydrides. J. Oleo. Sci. 2020, 69, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Kondamudi, N.; McDougal, O.M. Microwave-Assisted Synthesis and Characterization of Stearic Acid Sucrose Ester: A Bio-Based Surfactant. J. Surfactants Deterg. 2019, 22, 721–729. [Google Scholar] [CrossRef]
- Choi, J.; Nidetzky, B. Ionic liquid as dual-function catalyst and solvent for efficient synthesis of sucrose fatty acid esters. Mol. Catal. 2022, 526, 112371. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, M.; Jin, T.; Chong, W.; Zhang, Z.; Nian, B.; Hu, Y. Computer-aided engineering of lipases solvent tolerance enhanced their applications in sugar esters synthesis: State of the art. Trends. Food Sci. Technol. 2024, 144, 104323. [Google Scholar] [CrossRef]
- Lin, X.; Zou, Y.; Zhao, K.; Yang, T.; Halling, P.; Yang, Z. Tetraalkylammonium Ionic Liquids as Dual Solvents–Catalysts for Direct Synthesis of Sugar Fatty Acid Esters. J. Surfactants Deterg. 2016, 19, 511–517. [Google Scholar] [CrossRef]
- Nowicki, J.; Muszynski, M. Ionic Liquids as Catalysts and Reaction Media in Oleochemical Raw Materials Processing: A Review. Curr. Org. Chem. 2014, 18, 2797–2807. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Ratti, R.; Phoon, B.L.; Batagarawa, S.M.; Din, I.U.; Selvaraj, M.; Ramalingam, R.J. A review of the recent progress on heterogeneous catalysts for Knoevenagel condensation. Dalton Trans. 2021, 50, 4445–4469. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, M. Ionic Liquids Toxicity—Benefits and Threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, I.I.I.; Ferreira, M.L.; Alba, C.G.; Bahamon, D.; Llovell, F.; Pereiro, A.B.; Araújo, J.M.M.; Abu-Zahra, M.R.M.; Vega, L.F. Screening of Ionic Liquids and Deep Eutectic Solvents for Physical CO 2 Absorption by Soft-SAFT Using Key Performance Indicators. J. Chem. Eng. Data 2020, 65, 5844–5861. [Google Scholar] [CrossRef]
- Henkel, M.; Müller, M.M.; Kügler, J.H.; Lovaglio, R.B.; Contiero, J.; Syldatk, C.; Hausmann, R. Rhamnolipids as biosurfactants from renewable resources: Concepts for next-generation rhamnolipid production. Process Biochem. 2012, 47, 1207–1219. [Google Scholar] [CrossRef]
- Vecino, X.; Barbosa-Pereira, L.; Devesa-Rey, R.; Cruz, J.M.; Moldes, A.B. Optimization of liquid–liquid extraction of biosurfactants from corn steep liquor. Bioprocess Biosyst. Eng. 2015, 38, 1629–1637. [Google Scholar]
- López-Prieto, A.; Martínez-Padrón, H.; Rodríguez-López, L.; Moldes, A.B.; Cruz, J.M. Isolation and characterization of a microorganism that produces biosurfactants in corn steep water. CyTA - J. Food 2019, 17, 509–516. [Google Scholar] [CrossRef]
- Rincón-Fontán, M.; Rodríguez-López, L.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Study of the synergic effect between mica and biosurfactant to stabilize Pickering emulsions containing Vitamin E using a triangular design. J. Colloid Interface Sci. 2019, 537, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, L.; Rincón-Fontán, M.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Study of biosurfactant extract from corn steep water as a potential ingredient in antiacne formulations. J. Dermatol. Treat. 2022, 33, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, L.; Rincón-Fontán, M.; Vecino, X.; Moldes, A.B.; Cruz, J.M. Biodegradability Study of the Biosurfactant Contained in a Crude Extract from Corn Steep Water. J. Surfactants Deterg. 2020, 23, 79–90. [Google Scholar] [CrossRef]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Gou, L.; Zhao, X.; Zhao, C.; Guo, H.; Shan, Y. Catalytic esterification performance of protease in micro-aqueous system. Biotechnol. Lett. 2020, 42, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Oulkhir, A.; Lyamlouli, K.; Bounaga, A.; Danouche, M.; Etahiri, A.; Benhida, R. Green innovations in mineral flotation for enhancing sustainability through enzymatically synthesized sugar and fatty alcohol biosurfactants – A comprehensive review. Miner. Eng. 2025, 230, 109415. [Google Scholar] [CrossRef]
- Spalletta, A.; Joly, N.; Martin, P. Latest Trends in Lipase-Catalyzed Synthesis of Ester Carbohydrate Surfactants: From Key Parameters to Opportunities and Future Development. Int. J. Mol. Sci. 2024, 25, 3727. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.-H.; Chen, J.; Ye, R.; Hayes, D.G. Biobased Surfactants, 2nd ed.; Hayes, D.G., Solaiman, D.K.Y., Ashby, R.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 325–363. [Google Scholar]
- Liang, M.-Y.; Chen, Y.; Banwell, M.G.; Wang, Y.; Lan, P. Enzymatic Preparation of a Homologous Series of Long-Chain 6- O -Acylglucose Esters and Their Evaluation as Emulsifiers. J. Agric. Food Chem. 2018, 66, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Inprakhon, P.; Wongthongdee, N.; Amornsakchai, T.; Pongtharankul, T.; Sunintaboon, P.; Wiemann, L.O.; Durand, A.; Sieber, V. Lipase-catalyzed synthesis of sucrose monoester: Increased productivity by combining enzyme pretreatment and non-aqueous biphasic medium. J. Biotechnol. 2017, 259, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.; Dejonghe, W.; Cauwenberghs, L.; Maesen, M.; Vanbroekhoven, K.; Satyawali, Y. Enzymatic Synthesis of Glucose- and Xylose Laurate Esters Using Different Acyl Donors, Higher Substrate Concentrations, and Membrane Assisted Solvent Recovery. Eur. J. Lipid Sci. Technol. 2021, 123, 2000225. [Google Scholar] [CrossRef]
- Oliveira, H.; Correia, P.; Bessa, L.J.; Guimarães, M.; Gameiro, P.; Freitas Vde Mateus, N.; Cruz, L.; Fernandes, I. Cyanidin-3-glucoside Lipophilic Conjugates for Topical Application: Tuning the Antimicrobial Activities with Fatty Acid Chain Length. Processes 2021, 9, 340. [Google Scholar] [CrossRef]
- Guimarães, M.; Mateus, N.; de Freitas, V.; Cruz, L. Improvement of the Color Stability of Cyanidin-3-glucoside by Fatty Acid Enzymatic Acylation. J. Agric. Food Chem. 2018, 66, 10003–10010. [Google Scholar] [CrossRef] [PubMed]
- Gérard, D.; Méline, T.; Muzard, M.; Deleu, M.; Plantier-Royon, R.; Rémond, C. Enzymatically-synthesized xylo-oligosaccharides laurate esters as surfactants of interest. Carbohydr. Res. 2020, 495, 108090. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, H.A.; Elazzazy, A.M.; Saleh, T.S.; Dourou, M.; Mahyoub, J.A.; Baeshen, M.N.; Madian, H.R.; Aggelis, G. Enzymatic Synthesis of Glucose Fatty Acid Esters Using SCOs as Acyl Group-Donors and Their Biological Activities. Appl. Sci. 2021, 11, 2700. [Google Scholar] [CrossRef]
- Jocquel, C.; Muzard, M.; Plantier-Royon, R.; Rémond, C. An Integrated Enzymatic Approach to Produce Pentyl Xylosides and Glucose/Xylose Laurate Esters From Wheat Bran. Front. Bioeng. Biotechnol. 2021, 9, 647442. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.C.; Nguyen, M.T.T.; Kim, J.-H.; Hong, S.-T.; Kim, H.-L.; Park, J.-T. A novel maltoheptaose-based sugar ester having excellent emulsifying properties and optimization of its lipase-catalyzed synthesis. Food Chem. 2021, 352, 129358. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-R.; Banwell, M.G.; Yan, R.; Lan, P. Comparative Study of the Emulsifying Properties of a Homologous Series of Long-Chain 6′- O -Acylmaltose Esters. J. Agric. Food Chem. 2018, 66, 8832–8840. [Google Scholar] [CrossRef] [PubMed]
- De Lima, L.; Mendes, A.; Fernandez-Lafuente, R.; Tardioli, P.; Giordano, R. Performance of Different Immobilized Lipases in the Syntheses of Short- and Long-Chain Carboxylic Acid Esters by Esterification Reactions in Organic Media. Molecules 2018, 23, 766. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Mai, N.L.; Bae, S.-W.; Koo, Y.-M. Enhanced lipase-catalyzed synthesis of sugar fatty acid esters using supersaturated sugar solution in ionic liquids. Enzyme. Microb. Technol. 2019, 126, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Barros, E.L.S.; Rebelatto, E.A.; Mayer, D.A.; Wancura, J.H.C.; Oliveira, J.V. Lipase-catalyzed Production of Sugar Esters in Pressurized Fluid Media: A Review. Chem. Eng. Process. Process Intensif. 2023, 191, 109480. [Google Scholar] [CrossRef]
- García-Oliva, C.; Perona, A.; Rumbero, Á.; Hoyos, P.; Hernáiz, M.J. Enzymatic Synthesis and Molecular Modelling Studies of Rhamnose Esters Using Lipase from Pseudomonas stutzeri. Int. J. Mol. Sci. 2022, 23, 2239. [Google Scholar] [CrossRef] [PubMed]
- Bento, H.B.S.; Carvalho, A.K.F.; Reis, C.E.R.; De Castro, H.F. Microbial biodiesel production: From sucrose-based carbon sources to alkyl esters via enzymatic transesterification. Process Saf. Environ. Prot. 2019, 121, 349–356. [Google Scholar] [CrossRef]
- Shao, S.-Y.; Shi, Y.-G.; Wu, Y.; Bian, L.-Q.; Zhu, Y.-J.; Huang, X.-Y.; Pan, Y.; Zeng, L.-Y.; Zhang, R.-R. Lipase-Catalyzed Synthesis of Sucrose Monolaurate and Its Antibacterial Property and Mode of Action against Four Pathogenic Bacteria. Molecules 2018, 23, 1118. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.C.P.; Cansian, A.B.M.; Tardioli, P.W.; Saville, B.A. Production of sugars from mixed hardwoods for use in the synthesis of sugar fatty acid esters catalyzed by immobilized-stabilized derivatives of Candida antarctica lipase B. Biofuels Bioprod. Biorefining 2023, 17, 1236–1250. [Google Scholar] [CrossRef]
- Kumar, R.; Maikhuri, V.K.; Mathur, D.; Kumar, M.; Singh, N.; Prasad, A.K. Novozyme-435: Perfect catalyst for chemo- and regio-selective synthesis of modified carbohydrates – a review. Biocatal. Biotransform. 2024, 42, 19–33. [Google Scholar] [CrossRef]
- Ogawa, S.; Endo, A.; Kitahara, N.; Yamagishi, T.; Aoyagi, S.; Hara, S. Factors determining the reaction temperature of the solvent-free enzymatic synthesis of trehalose esters. Carbohydr. Res. 2019, 482, 107739. [Google Scholar] [CrossRef] [PubMed]
- Sousa RRde Silva ASda Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Simplified Method to Optimize Enzymatic Esters Syntheses in Solvent-Free Systems: Validation Using Literature and Experimental Data. Catalysts 2021, 11, 1357. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R. Immobilization of Lipases—A Review. Part I: Enzyme Immobilization. ChemBioEng Rev. 2019, 6, 157–166. [Google Scholar] [CrossRef]
- Xie, M.-F.; White, L.V.; Banwell, M.G.; Wang, Y.; Lan, P. Solvent-Free Synthesis of High-Purity Sucrose Fatty Acid Monoesters and a Comparison of Their Properties with Those of Their Commercial Counterparts. ACS Food Sci. Technol. 2021, 1, 1550–1560. [Google Scholar] [CrossRef]
- Rodrigues de Sousa, R.; Vitor Belo Pazutti, L.; Zamith Leal Dalmaso, G.; Frauches Siqueira, D.; Sant’Ana da Silva, A.; Ferreira-Leitão, V.S. A practical approach to obtain high yield lipase-mediated synthesis of octyl caprylate with Novozym 435. Biocatal. Biotransform. 2020, 38, 293–303. [Google Scholar] [CrossRef]
- Serrano-Arnaldos, M.; Montiel, M.C.; Ortega-Requena, S.; Máximo, F.; Bastida, J. Development and economic evaluation of an eco-friendly biocatalytic synthesis of emollient esters. Bioprocess Biosyst. Eng. 2020, 43, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Nieto, S.; Villa, R.; Donaire, A.; Lozano, P. Ultrasound-assisted enzymatic synthesis of xylitol fatty acid esters in solvent-free conditions. Ultrason. Sonochem. 2021, 75, 105606. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Guo, J.; Liang, C. Sequential Dy(OTf) 3 -Catalyzed Solvent-Free Per- O -Acetylation and Regioselective Anomeric De- O -Acetylation of Carbohydrates. Chem. Asian. J. 2017, 12, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.K.; Gour, R.; Kartha, K.P.R. Diazepinium perchlorate: A neutral catalyst for mild, solvent-free acetylation of carbohydrates and other substances. RSC Adv. 2017, 7, 13653–13667. [Google Scholar] [CrossRef]
- Traboni, S.; Bedini, E.; Vessella, G.; Iadonisi, A. Solvent-Free Approaches in Carbohydrate Synthetic Chemistry: Role of Catalysis in Reactivity and Selectivity. Catalysts 2020, 10, 1142. [Google Scholar] [CrossRef]
- Liu, P.; Hao, J.-W.; Mo, L.-P.; Zhang, Z.-H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv. 2015, 5, 48675–48704. [Google Scholar] [CrossRef]
- Chevé-Kools, E.; Choi, Y.H.; Roullier, C.; Ruprich-Robert, G.; Grougnet, R.; Chapeland-Leclerc, F.; Hollmann, F. Natural deep eutectic solvents (NaDES): Green solvents for pharmaceutical applications and beyond. Green Chem. 2025, 27, 8360–8385. [Google Scholar] [CrossRef] [PubMed]
- Grüninger, J.; Delavault, A.; Ochsenreither, K. Enzymatic glycolipid surfactant synthesis from renewables. Process Biochem. 2019, 87, 45–54. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Kumar, S.; Bahadur, I.; Singh, T.; Varma, R.S. Deep eutectic solvents as reusable catalysts and promoter for the greener syntheses of small molecules: Recent advances. J. Mol. Liq 2023, 371, 121013. [Google Scholar] [CrossRef]
- Mgxadeni, N.; Mmelesi, O.; Kabane, B.; Bahadur, I. Influence of hydrogen bond donor on zinc chloride in separation of binary mixtures: Activity coefficients at infinite dilution. J. Mol. Liq. 2022, 351, 118596. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, N.S.; Sayed, M.; Muhammad, N.; Rehman, S.-; Khan, J.A.; Haq Khan, Z.U.; Howari, F.M.; Nazzal, Y.; Xavier, C.; et al. Deep eutectic solvent-mediated synthesis of ceria nanoparticles with the enhanced yield for photocatalytic degradation of flumequine under UV-C. J. Water Process Eng. 2020, 33, 101012. [Google Scholar] [CrossRef]
- Pätzold, M.; Siebenhaller, S.; Kara, S.; Liese, A.; Syldatk, C.; Holtmann, D. Deep Eutectic Solvents as Efficient Solvents in Biocatalysis. Trends Biotechnol. 2019, 37, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Nhivekar, G.S.; Rathod, V.K. Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system. Green Process. Synth. 2019, 8, 30–37. [Google Scholar] [CrossRef]
- Hollenbach, R.; Ochsenreither, K.; Syldatk, C. Enzymatic Synthesis of Glucose Monodecanoate in a Hydrophobic Deep Eutectic Solvent. Int. J. Mol. Sci. 2020, 21, 4342. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.; Li, Q. Microbial production of rhamnolipids: Opportunities, challenges and strategies. Microb. Cell Fact. 2017, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Bjerk, T.R.; Severino, P.; Jain, S.; Marques, C.; Silva, A.M.; Pashirova, T.; Souto, E.B. Biosurfactants: Properties and Applications in Drug Delivery, Biotechnology and Ecotoxicology. Bioengineering 2021, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Essghaier, B.; Mallat, N.; Khwaldia, K.; Mottola, F.; Rocco, L.; Hannachi, H. Production and Characterization of New Biosurfactants/Bioemulsifiers from Pantoea alhagi and Their Antioxidant, Antimicrobial and Anti-Biofilm Potentiality Evaluations. Molecules 2023, 28, 1912. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, K.; Lotfabad, T.B.; Jabeen, F.; Mohammad Ganji, S. Cytotoxic effects of mono- and di-rhamnolipids from Pseudomonas aeruginosa MR01 on MCF-7 human breast cancer cells. Colloids Surf. B Biointerfaces 2019, 181, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Kashif, A.; Rehman, R.; Fuwad, A.; Shahid, M.K.; Dayarathne, H.N.P.; Jamal, A.; Aftab, M.N.; Mainali, B.; Choi, Y. Current advances in the classification, production, properties and applications of microbial biosurfactants – A critical review. Adv. Colloid Interface Sci. 2022, 306, 102718. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, R.; Jones, P.; Somani, B.K. Role of D-Mannose in the Prevention of Recurrent Urinary Tract Infections: Evidence from a Systematic Review of the Literature. Eur. Urol. Focus 2021, 7, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Hao, J.; Tang, Q.; Chandarajoti, K.; Ye, W.; Fan, C.; Wang, X.; Wang, C.; Zhang, K.; Han, X.; et al. Antimicrobial activity and structure–activity relationships of molecules containing mono- or di- or oligosaccharides: An update. Bioorg. Chem. 2024, 148, 107406. [Google Scholar] [CrossRef] [PubMed]
- Ankulkar, R.; Chavan, M. Characterisation and Application Studies of Sophorolipid Biosurfactant by Candida tropicalis RA1. J. Pure. Appl. Microbiol. 2019, 13, 1653–1665. [Google Scholar] [CrossRef]
- Fontoura ICCda Saikawa, G.I.A.; Silveira, V.A.I.; Pan, N.C.; Amador, I.R.; Baldo, C.; Rocha SPDda Celligoi, M.A.P.C. Antibacterial Activity of Sophorolipids from Candida bombicola Against Human Pathogens. Braz. Arch. Biol. Technol. 2020, 63, e20180568. [Google Scholar] [CrossRef]
- Bae, I.; Lee, E.S.; Yoo, J.W.; Lee, S.H.; Ko, J.Y.; Kim, Y.J.; Lee, T.R.; Kim, D.; Lee, C.S. Mannosylerythritol lipids inhibit melanogenesis via suppressing ERK-CREB-MiTF-tyrosinase signalling in normal human melanocytes and a three-dimensional human skin equivalent. Exp. Dermatol. 2019, 28, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Hutton, S.; Lajarin-Cuesta, M.; Heaton, R.; Hargreaves, I.; Fracchia, L.; De Rienzo, M.A.D. Production of Mannosylerythritol Lipids (MELs) to be Used as Antimicrobial Agents Against S. aureus ATCC 6538. Curr. Microbiol. 2020, 77, 1373–1380. [Google Scholar] [PubMed]
- Ndlovu, T.; Rautenbach, M.; Vosloo, J.A.; Khan, S.; Khan, W. Characterisation and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express 2017, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Saini, N.K.; Thakur, V.K.; Gupta, V.K.; Saini, R.V.; Saini, A.K. Rhamnolipid the Glycolipid Biosurfactant: Emerging trends and promising strategies in the field of biotechnology and biomedicine. Microb. Cell Fact. 2021, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Sood, U.; Singh, D.N.; Hira, P.; Lee, J.-K.; Kalia, V.C.; Lal, R.; Shakarad, M. Rapid and solitary production of mono-rhamnolipid biosurfactant and biofilm inhibiting pyocyanin by a taxonomic outlier Pseudomonas aeruginosa strain CR1. J. Biotechnol. 2020, 307, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Silveira, V.A.I.; Nishio, E.K.; Freitas, C.A.U.Q.; Amador, I.R.; Kobayashi, R.K.T.; Caretta, T.; Macedo, F.; Celligoi, M.A.P.C. Production and antimicrobial activity of sophorolipid against Clostridium perfringens and Campylobacter jejuni and their additive interaction with lactic acid. Biocatal. Agric. Biotechnol. 2019, 21, 101287. [Google Scholar] [CrossRef]
- Hipólito, A.; Alves da Silva, R.A.; Caretta Tde, O.; Silveira, V.A.I.; Amador, I.R.; Panagio, L.A.; Borsato, D.; Celligoi, M.A.P.C. Evaluation of the antifungal activity of sophorolipids from Starmerella bombicola against food spoilage fungi. Biocatal. Agric. Biotechnol. 2020, 29, 101797. [Google Scholar] [CrossRef]
- de OCaretta, T.; ISilveira, V.A.; Andrade, G.; Macedo, F.; PCCelligoi, M.A. Antimicrobial activity of sophorolipids produced by Starmerella bombicola against phytopathogens from cherry tomato. J. Sci. Food Agric. 2022, 102, 1245–1254. [Google Scholar]
- Semkova, S.; Antov, G.; Iliev, I.; Tsoneva, I.; Lefterov, P.; Christova, N.; Nacheva, L.; Stoineva, I.; Kabaivanova, L.; Staneva, G.; et al. Rhamnolipid Biosurfactants—Possible Natural Anticancer Agents and Autophagy Inhibitors. Separations 2021, 8, 92. [Google Scholar] [CrossRef]
- Ceresa, C.; Tessarolo, F.; Maniglio, D.; Tambone, E.; Carmagnola, I.; Fedeli, E.; Caola, I.; Nollo, G.; Chiono, V.; Allegrone, G.; et al. Medical-Grade Silicone Coated with Rhamnolipid R89 Is Effective against Staphylococcus spp. Biofilms. Mol. 2019, 24, 3843. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Black, W.; Sawyer, T. Application of Environment-Friendly Rhamnolipids against Transmission of Enveloped Viruses Like SARS-CoV2. Viruses 2021, 13, 322. [Google Scholar] [CrossRef] [PubMed]
- Daverey, A.; Dutta, K.; Joshi, S.; Daverey, A. Sophorolipid: A glycolipid biosurfactant as a potential therapeutic agent against COVID-19. Bioengineered 2021, 12, 9550–9560. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Teruel, J.A.; Espuny, M.J.; Marqués, A.; Aranda, F.J.; Manresa, Á.; Ortiz, A. Modulation of the physical properties of dielaidoylphosphatidylethanolamine membranes by a dirhamnolipid biosurfactant produced by Pseudomonas aeruginosa. Chem. Phys. Lipids 2006, 142, 118–127. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Ferreira, J.; Vieira, E.A.; Nitschke, M. The antibacterial activity of rhamnolipid biosurfactant is pH dependent. Food Res. Int. 2019, 116, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Diaz De Rienzo, M.A.; Banat, I.M.; Lajarin Cuesta, M.; Williams, M.; Fracchia, L. Evaluation of the activity of sophorolipids against microbial biofilms on medical-grade silicone. New Biotechnol. 2018, 44, S13. [Google Scholar] [CrossRef]
- Ceresa, C.; Fracchia, L.; Williams, M.; Banat, I.M.; Díaz De Rienzo, M.A. The effect of sophorolipids against microbial biofilms on medical-grade silicone. J. Biotechnol. 2020, 309, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Twigg, M.S.; Adu, S.A.; Sugiyama, S.; Marchant, R.; Banat, I.M. Mono-Rhamnolipid Biosurfactants Synthesized by Pseudomonas aeruginosa Detrimentally Affect Colorectal Cancer Cells. Pharmaceutics 2022, 14, 2799. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, Z.; Li, S.; Han, N.; Zhai, J.; Yin, J. Novel glycolipids from Potentilla anserina L. rhizomes: Anti-inflammatory, hepatoprotective activities, and structure-activity relationship studies. Food Biosci. 2025, 64, 105894. [Google Scholar] [CrossRef]
- Lai, A.C.-Y.; González-Cuesta, M.; Ho, C.-H.; Chi, P.-Y.; Wu, K.-C.; Rocha, G.; Muñoz-García, J.C.; Angulo, J.; García Fernández, J.M.; Chang, Y.-J.; et al. α-GalCer sp2-iminoglycolipid analogs as CD1d-dependent iNKT modulators: Evaluation of their immunotherapeutic potential in murine models of asthma and autoimmune hepatitis. Eur. J. Med. Chem. 2025, 282, 117060. [Google Scholar] [CrossRef] [PubMed]
- Watchaputi, K.; Songdech, P.; Jayasekara, C.; Puttarak, P.; Lamping, E.; Cannon, R.D.; Soontorngun, N. Yeast-derived glycolipids disrupt Candida biofilm and inhibit expression of genes in cell adhesion. Sci. Rep. 2025, 15, 20405. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Hu, X.; Xia, Y.; Tang, J.; Yu, B.; Wan, J.; Zhou, Y.; Hong, L.; Yang, C. Rhamnolipid/Fullerene Nanocomposites as Potent Radical Scavenger for Acute Severe Ulcerative Colitis Prevention: A Low-Dose Therapeutic Approach. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Alyousif, N.A.; Al-luaibi, Y.Y.Y.; Al-tamimi, W.H. Evaluation of bacterial biosurfactant activities as an anticancer and antibiofilm agent. J. Appl. Nat. Sci. 2025, 17, 313–319. [Google Scholar] [CrossRef]

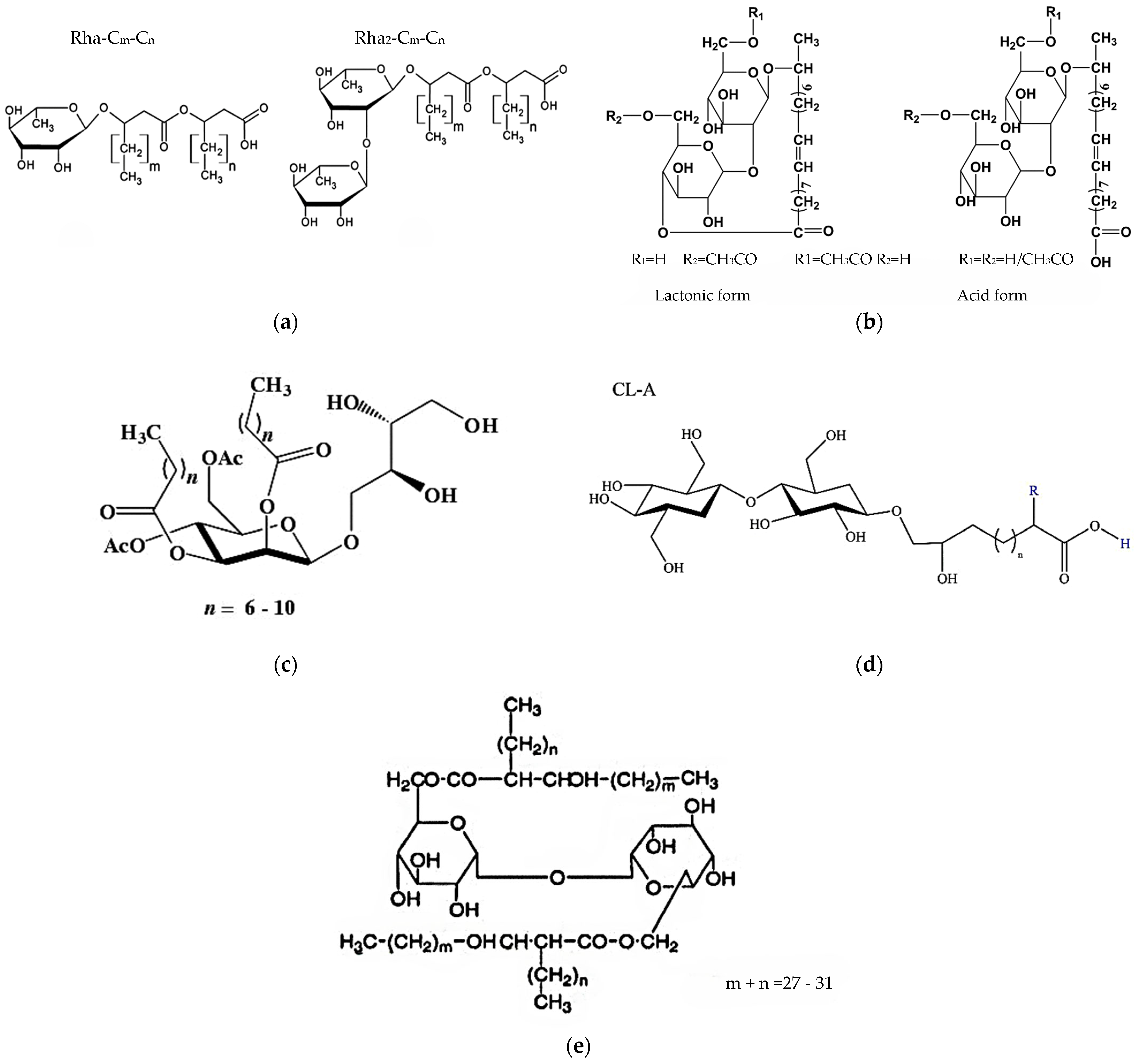
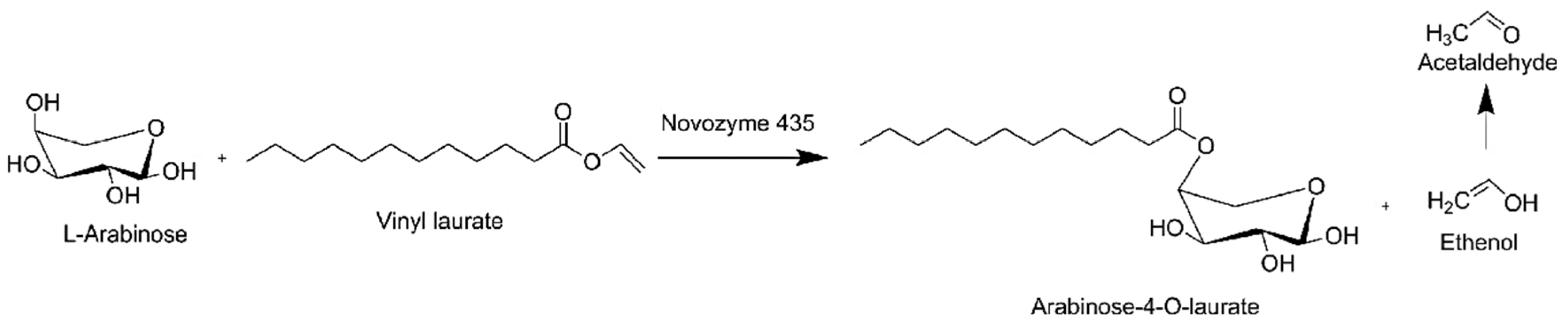
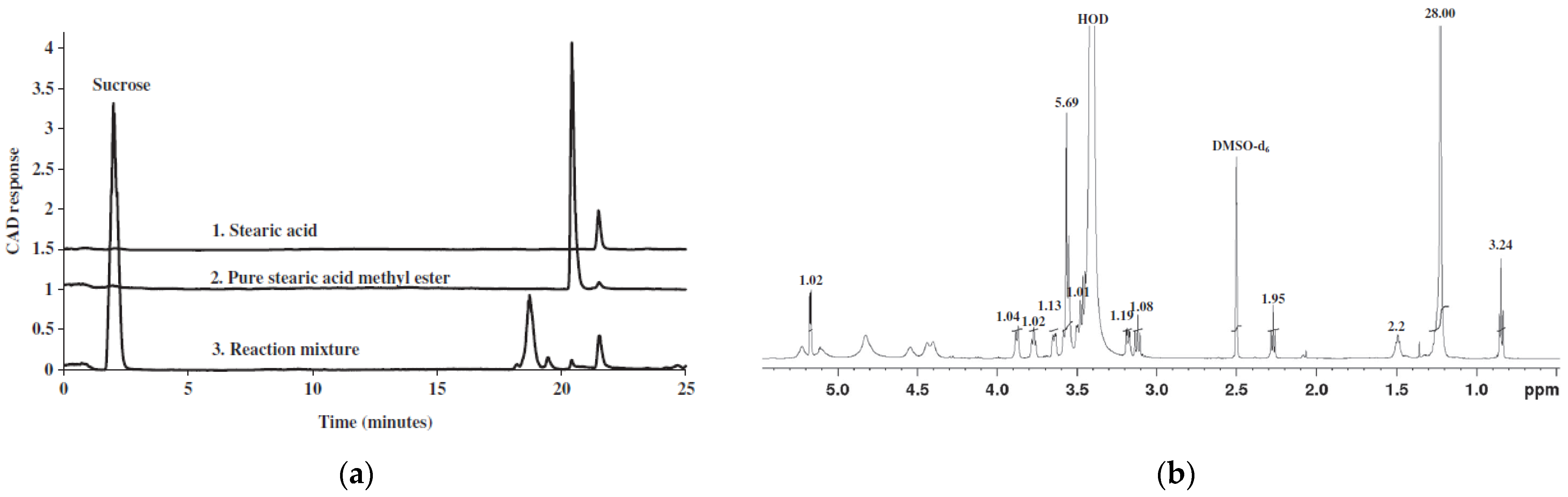
| Sugar | Substrate | Enzyme | Solvent | Product | Yield[%] | Purity [%] | Ref. |
|---|---|---|---|---|---|---|---|
| Trehalose | Palmitic, lauric, and caprylic acids | Candida antarctica lipase B (CALB 10000) | Acetone | Trehalose fatty acid esters (THFAE) | 34.55 | - | [45] |
| Glucose and xylose | Vinyl laurate | Novozyme 435 | t-amyl alcohol | Glucose laurate and xylose laurate | 87 | 96 | [92] |
| Cy3glc (from blueberry) | Fatty acid C-10 | CALB | t-amyl alcohol | Fatty acid esters of cyanidin-3-glucoside | 25–47 | 95 | [93,94] |
| Xylose | Vinyl laurate | Novozyme 435 | t-amyl alcohol | Xylobiose, xylotriose, xylotetraose laurate | 86 | - | [95] |
| Glucose | Fatty acid | CALB and Candida rugosa lipase (CRL) | DMSO: t-amyl alcohol (80:20 v/v) | Glucose fatty acid esters | 80.3 | - | [96] |
| Glucose, xylose | Lauric acid, methyl laurate | Novozyme 435 | t-amyl alcohol | D-glucose laurate and D-xylose laurate | 65 | - | [97] |
| Maltoheptaose | Palmitic acid, myristic acid, lauric acid, capric acid, and p-nitrophenyl laurate | CALB | 10% DMSO | Maltoheptaose palmitate | 64.60 | - | [98] |
| Sucrose | Vinyl caprate | Lipase AY | DMSO, Crown ether | Sucrose caprate | 68 | 22 | [72] |
| D-maltose | Vinyl laurate | Novozyme 435 | THF/pyridine (7:3 v/v) | 6ʹ-O-Lauroylmaltose | 80 | - | [99] |
| Fructose | Lauric acid | CALB | Tert-amyl alcohol | Fructose laurate | 70 | - | [100] |
| Glucose | Vinyl laurate | Novozyme 435 | [Bmim][Tf2N]/[Bmim][TfO] | Glucose laurate | 64 | - | [101] |
| Glucose | Vinyl laurate | CALB | [Bmim][BF4]/tert-BuOH (60:40%) | Glucose laurate | 96.4 | - | [72] |
| Galactose | Oleic acid | Lipozyme RMIM | DMSO: [Bmim][BF4] (1:20 v/v) | Galactose oleate ester | 77 | 87 | [72] |
| Glucose | Lauric acid | NZ435 | n-hexane SCCO2 | Glucose laurate | - | - | [102] |
| L-rhamnose | Vinyl laurate | Pseudomonas stutzeri lipase | Tetrahydrofuran | 4-O-lauroylrham | 58 | - | [103] |
| Sucrose | Erucic acid | Lipozyme TLIM | T-butanol, DMSO (4:1) | Sucrose erucate | 52.47 | 40 | [72] |
| Sucrose | Stearic acid | Novozyme 435 | Isooctane | Sucrose stearate | 97.1 | 95 | [104] |
| Sucrose | Vinyl laurate | Lipozyme TLIM | 2M2B: DMSO (4:1) | Sucrose laurate | - | - | [72] |
| Sucrose | Vinyl laurate | Lipozyme TLIM | [3CIM(EO)] [NTf2]/[2M2B] | Sucrose laurate | 70–90 | - | [105] |
| Sucrose | Oleic acid | CALB | Solvent- free | Sucrose oleate | 81–83 | 70 | [72] |
| Xylose | Oleic acid | Novozyme 435 | Methyl ethyl ketone | Xylose oleate | 80 | 16.3 | [106] |
| Surfactant/Biosurfactant | Biological Activity | Ref. |
|---|---|---|
| Mannosyl erythritol lipids | Anti-melanogenic | [136] |
| Mannosyl erythritol lipids | Antibacterial | [137] |
| Sophorolipids | Anti-biofilm | [137] |
| Mono- and di-rhamnolipids | Antimicrobial | [138] |
| Mono- and di-rhamnolipids | Cytotoxic effect on human breast cancer | [130] |
| Mono- and di-rhamnolipids | Cytotoxic effect on HL-60, SKW-3, JMSU-1, B-173 | [139] |
| Rhamnolipids | Antifungal activity against fungi such as Alternaria alternata, Mucor circinelloides, and Verticillium dahlia | [139] |
| Rhamnolipids | Antimicrobial, anti-biofilm against Bacillus subtilis | [140] |
| Sophorolipids | Antibacterial, antimicrobial, and anti-adhesive towards Escherichia coli, Bacillus subtilis, Staphylococcus aureus, and Campylobacter jejuni | [131,141] |
| Sophorolipids | Anti-phytopathogens, antifungal applications | [142,143] |
| Rhamnolipid | Anti-cancer and autophagy inhibitors | [144] |
| Rhamnolipid | Anti-bacterial effects on Staphylococcus aureus, Klebsiella pneumonia | [145] |
| Rhamnolipid | Anti-viral effects on herpesvirus, tobacco mosaic virus (crop viral infection), bovine coronavirus, SARS-CoV-2 | [146,147] |
| Sophorolipids | Anti-viral effects on HIV, Epstein–Barr virus, and influenza virus | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyla, K.; Janek, T. Sugar Esters of Fatty Acids: Chemo-Enzymatic Synthesis and Biological Activity. Molecules 2025, 30, 3123. https://doi.org/10.3390/molecules30153123
Hyla K, Janek T. Sugar Esters of Fatty Acids: Chemo-Enzymatic Synthesis and Biological Activity. Molecules. 2025; 30(15):3123. https://doi.org/10.3390/molecules30153123
Chicago/Turabian StyleHyla, Kinga, and Tomasz Janek. 2025. "Sugar Esters of Fatty Acids: Chemo-Enzymatic Synthesis and Biological Activity" Molecules 30, no. 15: 3123. https://doi.org/10.3390/molecules30153123
APA StyleHyla, K., & Janek, T. (2025). Sugar Esters of Fatty Acids: Chemo-Enzymatic Synthesis and Biological Activity. Molecules, 30(15), 3123. https://doi.org/10.3390/molecules30153123








