Breast Cancer Cytochromes P450: Chemopreventive and/or Therapeutic Targets for Naturally Occurring Phytochemicals
Abstract
1. Introduction
2. Characteristics of the Major CYPs Involved in Estrogen Synthesis and Catabolism
2.1. CYPs and Estrogen Biosynthesis
2.2. CYP-Mediating Estrogen Catabolism
2.3. Orphan CYPs and Breast Cancer
2.4. Structures of Major CYPs Involved in Estrogen Synthesis and Catabolism
3. Targeting Breast Cancer Cytochromes P50 by Naturally Occurring and Modified Phytochemicals
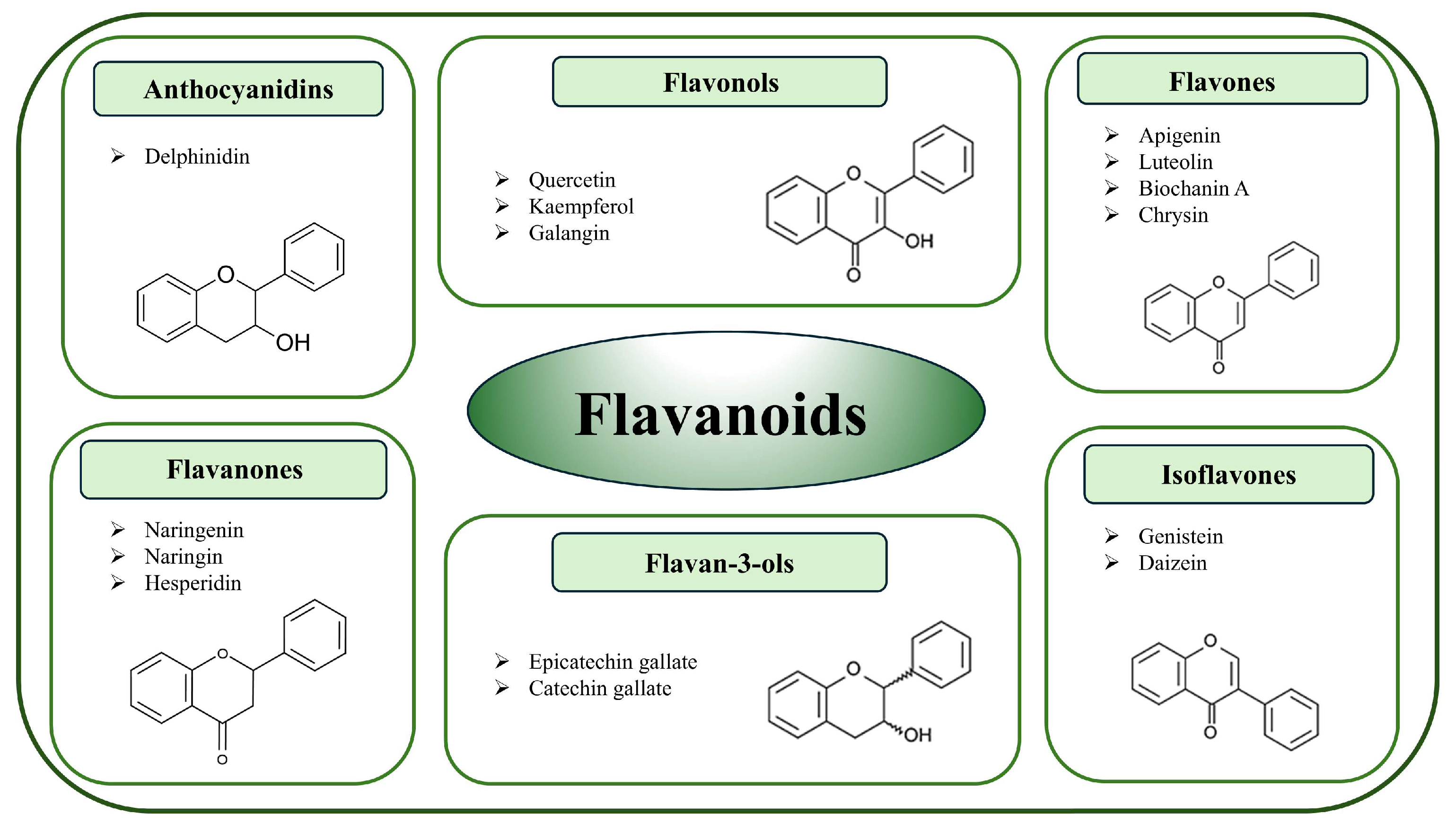
3.1. Flavonoids
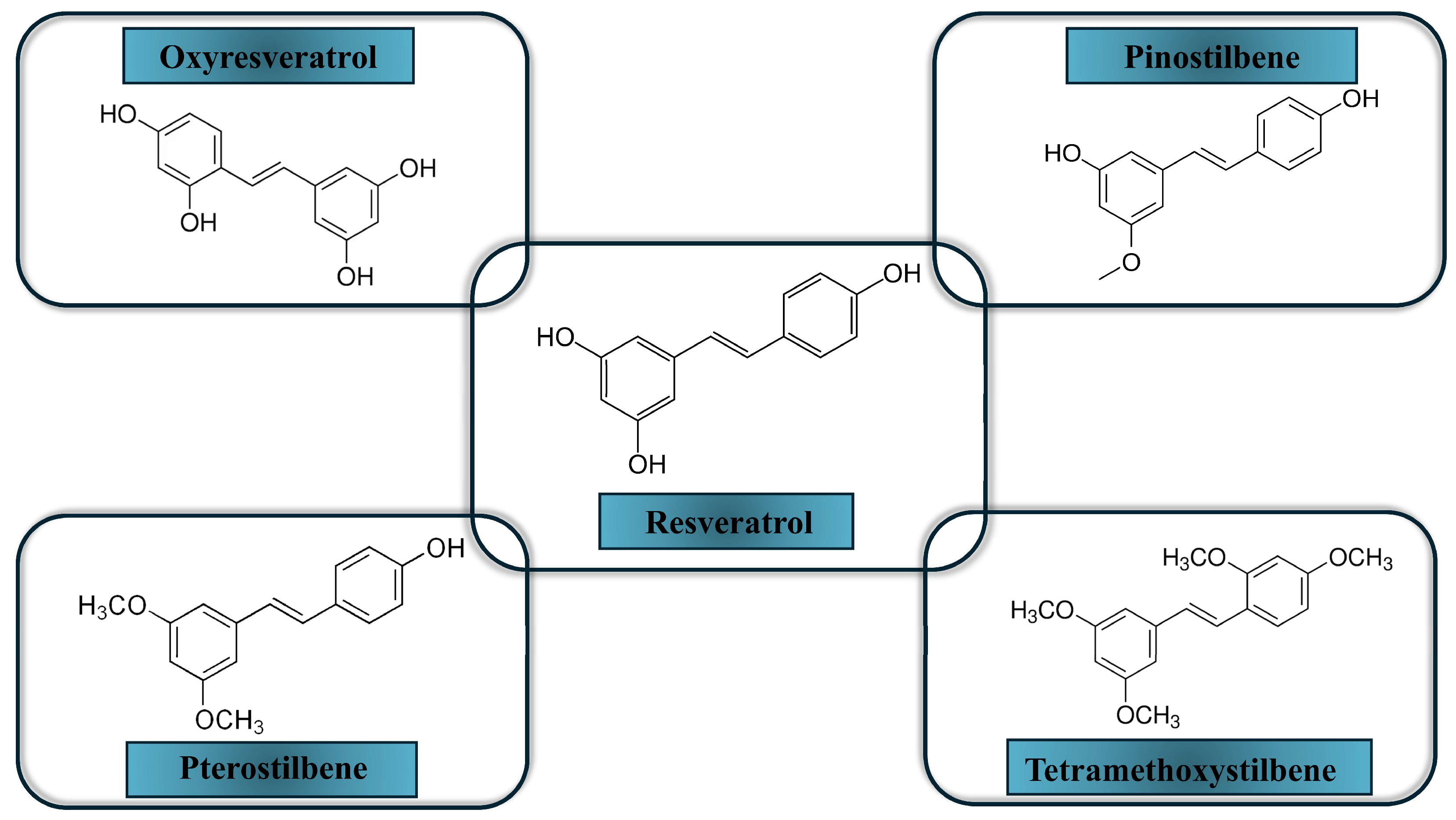
3.2. Resveratrol and Its Derivatives
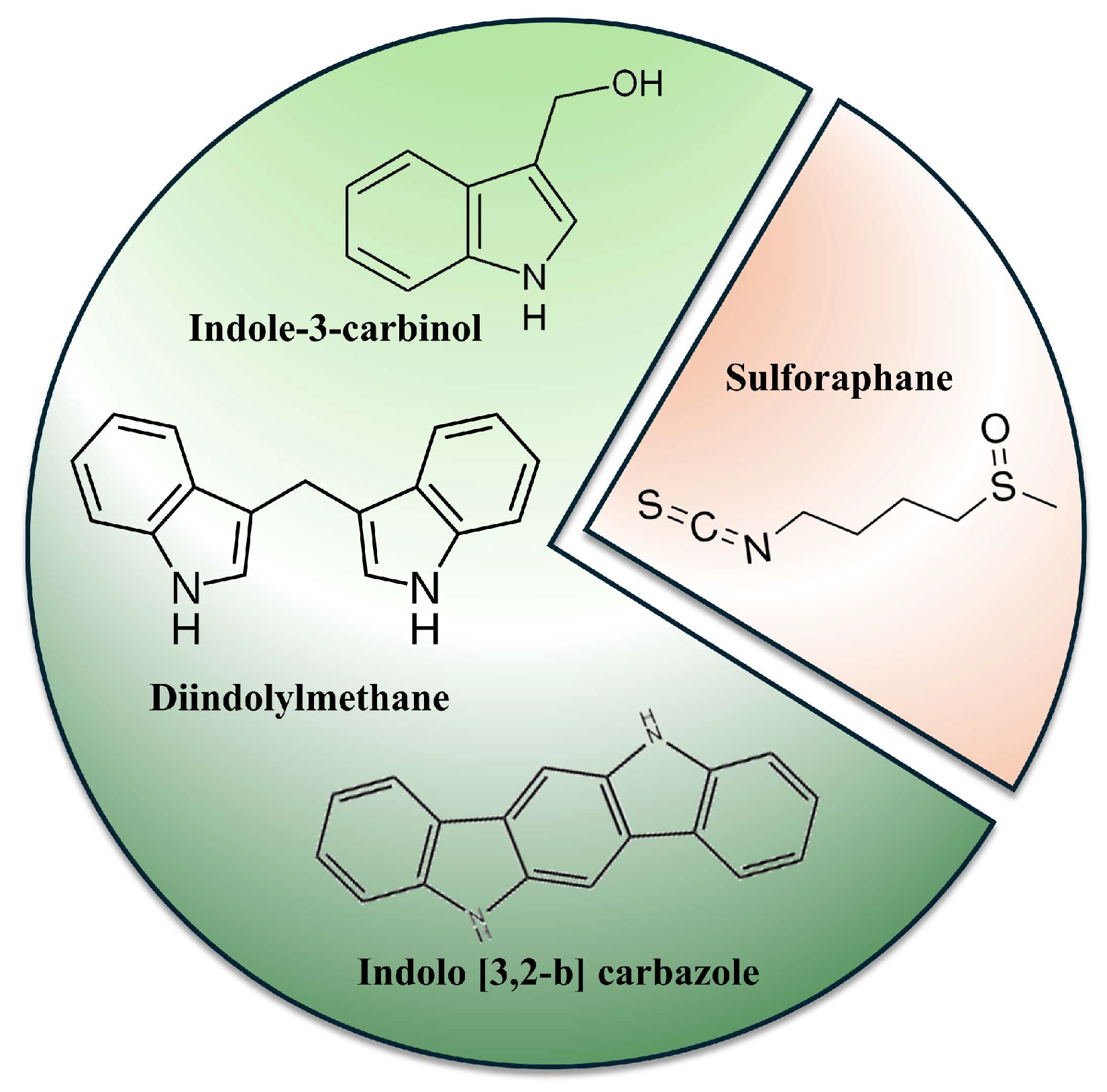
3.3. Glucosinolates Breakdown Products
3.4. Other Phytochemicals with Breast CYP Modification Potential
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AhR | aryl hydrocarbon receptor |
| COMT | catechol O-methyltransferase |
| DHEA | dehydroepiandrosterone |
| DIM | 3,3′-diindolomethane |
| EETs | epoxyeicosatrienoic acids |
| ER(+) | estrogen receptor positive |
| GLS | glucosinolates |
| HETE | hydroxyeicosatetraenoic acid |
| HER2 | human epidermal growth factor receptor 2 positive |
| I3C | indole-3-carbinol |
| ITCs | isothiocyanates |
| PR(+) | progesterone receptor positive |
| TCDD | 2,3,7,8-tetrachlorodibenzo-p-dioxin |
| TMS | 2,3′,4,5′- tetramethoxy-trans-stilbene |
| TNBC | triple negative breast cancer |
| XRE | xenobiotic response elements |
References
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast cancer statistics 2024. CA Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.K.; Castiglione-Gertsch, M. Estrogen receptor modulators and down regulators: Optimal use in postmenopausal women with breast cancer. Drugs 2007, 67, 2335–2353. [Google Scholar] [CrossRef] [PubMed]
- Clusan, L.; Ferrière, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 6834. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Au, C.C.; Benito-Martin, A.; Ladumor, H.; Oshchepkova, S.; Moges, R.; Brown, K.A. Estrogens and breast cancer: Mechanisms involved in obesity-related development, growth and progression. J. Steroid Biochem. Mol. Biol. 2019, 189, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Treeck, O.; Schüler-Toprak, S.; Ortmann, O. Estrogen Actions in Triple-Negative Breast Cancer. Cells 2020, 9, 2358. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Lin, C.Y. Oestrogen receptors in breast cancer: Basic mechanisms and clinical implications. Ecancermedicalscience 2013, 7, 370. [Google Scholar] [CrossRef] [PubMed]
- Cusack, L.; Brennan, M.; Baber, R.; Boyle, F. Menopausal symptoms in breast cancer survivors: Management update. Br. J. Gen. Pract. 2013, 63, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Estabrook, R.W. A passion for P450s (rememberances of the early history of research on cytochrome P450). Drug Metab. Dispos. 2003, 31, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Yan, D.; Yan, H.; Yuan, J. Cytochrome P450: Implications for human breast cancer. Oncol. Lett. 2021, 22, 548. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Weise, A.M.; Falany, J.L.; Falany, C.N.; Thibodeau, B.J.; Miller, F.R.; Kocarek, T.A.; Runge-Morris, M. Expression of estrogenicity genes in a lineage cell culture model of human breast cancer progression. Breast Cancer Res. Treat. 2010, 120, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Tateishi, Y.; McCarty, K.D.; Yoshimoto, F.K. Updates on Mechanisms of Cytochrome P450 Catalysis of Complex Steroid Oxidations. Int. J. Mol. Sci. 2024, 25, 9020. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Nakata, T.; Suzuki, T.; Darnel, A.D.; Moriya, T.; Kaneko, C.; Hidaka, K.; Shiotsu, Y.; Kusaka, H.; Sasano, H. Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues. J. Clin. Endocrinol. Metab. 2002, 87, 5760–5768. [Google Scholar] [CrossRef] [PubMed]
- Acimovic, J.; Goyal, S.; Kosir, R.; Golicnik, M.; Perse, M.; Belic, A.; Urlep, Z.; Guengerich, F.P.; Rozman, D. Cytochrome P450 metabolism of the post-lanosterol intermediates explains enigmas of cholesterol synthesis. Sci. Rep. 2016, 6, 28462. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Li, W.; Yi, A.K.; Postlethwaite, A.; Tuckey, R.C. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 2014, 144, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.I.; Zhao, J.; Khan, I.A.; Walker, L.A.; Dasmahapatra, A.K. Potential utility of natural products as regulators of breast cancer-associated aromatase promoters. Reprod. Biol. Endocrinol. 2011, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Chumsri, S.; Thompson, E.A. Carryover effects of aromatase inhibitors in prevention. Lancet 2020, 395, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, M.; Forbes, J.F.; Bradley, R.; Ingle, J.; Aihara, T.; Bliss, J.; Boccardo, F.; Coates, A.; Coombes, R.C.; Cuzick, J.; et al. Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 2015, 386, 1341–1352. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Nakajima, M.; Yoko, T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005, 227, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Cai, M.X.; Thomas, P.E.; Conney, A.H.; Zhu, B.T. Characterization of the oxidative metabolites of 17b-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology 2003, 144, 3382–3398. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, K.; Martin-Hirsch, P.L.; Martin, F.L. CYP1B1 and hormone-induced cancer. Cancer Lett. 2012, 324, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.I.; Taylor, M.C.; McFadyen, M.C.; McKay, J.A.; Greenlee, W.F.; Burke, M.D.; Melvin, W.T. Tumor-specific expression of cytochrome P450 CYP1B1. Cancer Res. 1997, 57, 3026–3031. [Google Scholar] [PubMed]
- Vaclavikova, R.; Hubackova, M.; Stribrna-Sarmanova, J.; Kodet, R.; Mrhalova, M.; Novotny, J.; Gut, I.; Soucek, P. RNA expression of cytochrome P450 in breast cancer patients. Anticancer Res. 2007, 27, 4443–4450. [Google Scholar] [PubMed]
- Aziz, A.A.; Md Salleh, M.S.; Mohamad, I.; Krishna Bhavaraju, V.M.; Mazuwin Yahya, M.; Zakaria, A.D.; Hua Gan, S.; Ankathil, R. Single-nucleotide polymorphisms and mRNA expression of CYP1B1 influence treatment response in triple negative breast cancer patients undergoing chemotherapy. J. Genet. 2018, 97, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Szaefer, H.; Licznerska, B.; Baer-Dubowska, W. The Aryl Hydrocarbon Receptor and Its Crosstalk: A Chemopreventive Target of Naturally Occurring and Modified Phytochemicals. Molecules 2024, 29, 4283. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Cheng, Q. Orphan in the human cytochrome P450 superfamily: Approaches to discovering functions and relevance in pharmacology. Pharmacol. Rev. 2011, 63, 684–699. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.I.; Patimalla, S.; Stewart, K.N.; Miller, I.D.; Heys, S.D. Profiling the expression of cytochrome P450 in breast cancer. Histopathology 2010, 57, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, J.P.; Rettie, A.E. There and Back Again: A Perspective on 20 Years of CYP4Z1. Drug Metab. Dispos. 2024, 52, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Molina-Ortiz, D.; Torres-Zárate, C.; Santes-Palacios, R. Human Orphan Cytochromes P450: An Update. Curr. Drug Metab. 2022, 23, 942–963. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Chai, H.; Li, Y.; Zhao, H.; Xie, X.; Zheng, H.; Wang, C.; Wang, X.; Yang, G.; Cai, X.; et al. Increased expression of CYP4Z1 promotes tumor angiogenesis and growth in human breast cancer. Toxicol. Appl. Pharmacol. 2012, 264, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Fekry, M.I.; Xiao, Y.; Berg, J.Z.; Guengerich, F.P. A role for the orphan human cytochrome P450 2S1 in polyunsaturated fatty acid omega-1 hydroxylation using an untargeted metabolomic approach. Drug Metab. Dispos. 2019, 47, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Chen, C.; Wu, X.; Yan, D.; Chen, F.; Yu, X.; Yuan, J. Cytochrome P450 2U1 Is a Novel Independent Prognostic Biomarker in Breast Cancer Patients. Front. Oncol. 2020, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Nouri, K.; Pietrancosta, N.; Le Corre, L.; Dansette, P.M.; Mansuy, D.; Boucher, J.-L. Human Orphan Cytochrome P450 2U1 Catalyzes the ω-Hydroxylation of Leukotriene B4. Int. J. Mol. Sci. 2022, 23, 14615. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Jiang, W.; Lo, J.; Egbuta, C. Higher order organization of human placental aromatase. Steroids 2011, 76, 753–758. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lo, J.; Di Nardo, G.; Griswold, J.; Egbuta, C.; Jiang, W.; Gilardi, G.; Ghosh, D. Structural basis for the functional roles of critical residues in human cytochrome p450 aromatase. Biochemistry 2013, 52, 5821–5829. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Takemura, H.; Shimoi, K.; Yamamoto, K. A 3D model of CYP1B1 explains the dominant 4-hydroxylation of estradiol. J. Chem. Inf. Model. 2010, 50, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Chen, S. Phytochemicals for breast cancer prevention by targeting aromatase. Front. Biosci. 2009, 14, 3846–3863. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mou, Y.; Lu, S.; Xia, Y.; Cheng, B. Polymethoxylated flavonoids in citrusfruits: Absorption, metabolism, and anticancer mechanisms against breast cancer. Peer J. 2024, 12, e16711. [Google Scholar] [CrossRef] [PubMed]
- Chrzan, B.G.; Bradford, P. Phytoestrogens activate estrogen receptor beta1 and estrogenic responses in human breast and bone cancer cell lines. Mol. Nutr. Food Res. 2007, 51, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.N.; Zhao, W.Y.; Liao, G.R.; Choi, R.C.; Lo, C.K.; Dong, T.T.X.; Tsim, K.W.K. In vitro estrogenic activity of formononetin by two bioassay systems. Gynecol. Endocrinol. 2006, 22, 578–584. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Dell’istituto Super. Sanita 2007, 43, 348–361. [Google Scholar]
- Turati, F.; Carioli, G.; Bravi, F.; Ferraroni, M.; Serraino, D.; Montella, M.; Giacosa, A.; Toffolutti, F.; Negri, E.; Levi, F.; et al. Mediterranean Diet and Breast Cancer Risk. Nutrients 2018, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Kushwaha, S.; Luqman, S.; Meena, A. Flavonoids as Prospective Aromatase Inhibitors in Breast Cancer Prevention/ Therapy. Curr. Mol. Pharmacol. 2021, 14, 1112–1124. [Google Scholar] [CrossRef] [PubMed]
- Kellis, J.T., Jr.; Vickery, L.E. Inhibition of human estrogen synthetase (aromatase) by flavones. Science 1984, 225, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.C.; Zhou, C.; Sherman, M.; Chen, S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A site-directed mutagenesis study. Environ. Health Perspect. 1998, 106, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Shin, Y.G.; Pezzuto, J.M. Inhibition of aromatase activity by flavonoids. Arch. Pharm. Res. 1999, 22, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Balam, F.H.; Ahmadi, Z.S.; Ghorbani, A. Inhibitory effect of chrysin on estrogen biosynthesis by suppression of enzyme aromatase (CYP19): A systematic review. Heliyon 2020, 6, e03557. [Google Scholar] [CrossRef] [PubMed]
- Way, T.D.; Lee, H.H.; Kao, M.C.; Lin, J.K. Black tea polyphenol theaflavins inhibit aromatase activity and attenuate tamoxifen resistance in HER2/neu-transfected human breast cancer cells through tyrosine kinase suppression. Eur. J. Cancer 2004, 40, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Sakamoto, Y.; Ogata, A.; Nagai, F.; Mikuriya, H.; Numazawa, M.; Yamada, K.; Aoki, N. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oraladministration in rats. Food Chem. Toxicol. 2002, 40, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ye, L.; Lin, S.M.; Leung, L.K. Dietary flavones and flavonones display differential effects on aromatase (CYP19) transcription in the breast cancer cells MCF-7. Mol. Cell. Endocrinol. 2011, 344, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.R.; Patel, J.R.; Gupta, A.; Davidson, A.M.; Williams, C.C.; Payton-Stewart, F.; Boué, S.M.; Burow, M.E.; Khupse, R.; Tilghman, S.L. Glyceollins Trigger Anti-Proliferative Effects in Hormone-Dependent Aromatase-Inhibitor-Resistant Breast Cancer Cells through the Induction of Apoptosis. Int. J. Mol. Sci. 2022, 23, 2887. [Google Scholar] [CrossRef] [PubMed]
- El-Kersh, D.M.; Ezzat, S.M.; Salama, M.M.; Mahrous, E.A.; Attia, Y.M.; Ahmed, M.S.; Elmazar, M.M. Anti-estrogenic and anti-aromatase activities of citrus peels major compounds in breast cancer. Sci. Rep. 2021, 11, 7121. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.I.; Yu, J.S.; Zhang, Y.; Yoo, H.H. Evaluating flavonoids as potential aromatase inhibitors for breast cancer treatment: In vitro studies and in silico predictions. Chem. Biol. Interact. 2024, 392, 110927. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, H.P.; Wang, T.T.; Yeh, G.C. Diosmin and diosmetin are agonists of the aryl hydrocarbon receptor that differentially affect cytochrome P450 1A1 activity. Cancer Res. 1998, 58, 2754–2760. [Google Scholar] [PubMed]
- Ciolino, H.P.; Yeh, G.C. The flavonoid galangin is an inhibitor of CYP1A1 activity and an agonist/antagonist of the aryl hydrocarbon receptor. Br. J. Cancer 1999, 79, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, H.P.; Daschner, P.J.; Yeh, G.C. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem. J. 1999, 340, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Carrera, A.N.; Grant, M.K.O.; Zordoky, B.N. CYP1B1 as a therapeutic target in cardio-oncology. Clin. Sci. 2020, 134, 2897–2927. [Google Scholar] [CrossRef] [PubMed]
- Takemura, H.; Itoh, T.; Yamamoto, K.; Sakakibara, H.; Shimoi, K. Selective inhibition of methoxyflavonoids on human CYP1B1 activity. Bioorg. Med. Chem. 2010, 18, 6310–6315. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Gatchie, L.; Williams, I.S.; Jain, S.K.; Vishwakarma, R.A.; Chaudhuri, B.; Bharate, S.B. Glycyrrhiza glabra extract and quercetin reverses cisplatin resistance in triple-negative MDA-MB-468 breast cancer cells via inhibition of cytochrome P450 1B1 enzyme. Bioorg. Med. Chem. Lett. 2017, 27, 5400–5403. [Google Scholar] [CrossRef] [PubMed]
- Surichan, S.; Androutsopoulos, V.P.; Sifakis, S.; Koutala, E.; Tsatsakis, A.; Arroo, R.R.J.; Boarder, M.R. Bioactivation of the citrus flavonoid nobiletin by CYP1 enzymes in MCF7 breast adenocarcinoma cells. Food Chem. Toxicol. 2012, 50, 3320–3328. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Li, N.; Arroo, R.R. The methoxylated flavones eupatorin and cirsiliol induce CYP1 enzyme expression in MCF7 cells. J. Nat. Prod. 2009, 72, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Szaefer, H.; Licznerska, B.; Krajka-Kuźniak, V.; Bartoszek, A.; Baer-Dubowska, W. Modulation of CYP1A1, CYP1A2 and CYP1B1 expression by cabbage juices and indoles in human breast cell lines. Nutr. Cancer 2012, 64, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Surichan, S.; Arroo, R.R.; Tsatsakis, A.M.; Androutsopoulos, V.P. Tangeretin inhibits the proliferation of human breast cancer cells via CYP1A1/CYP1B1 enzyme induction and CYP1A1/CYP1B1-mediated metabolism to the product 4′ hydroxy tangeretin. Toxicol. In Vitro 2018, 50, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Wilsher, N.E.; Arroo, R.R.; Matsoukas, M.T.; Tsatsakis, A.M.; Spandidos, D.A.; Androutsopoulos, V.P. Cytochrome P450 CYP1 metabolism of hydroxylated flavones and flavonols: Selective bioactivation of luteolin in breast cancer cells. Food Chem. Toxicol. 2017, 110, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Atherton, K.M.; Mutch, E.; Ford, D. Metabolism of the soyabean isoflavone daidzein by CYP1A2 and the extra-hepatic CYPs 1A1 and 1B1 affects biological activity. Biochem. Pharmacol. 2006, 72, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Jiang, L.; Lehmann, L. Phytoestrogens modulate the expression of 17alpha-estradiol metabolizing enzymes in cultured MCF-7 cells. Adv. Exp. Med. Biol. 2008, 617, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.Y.; Yung, L.H.; Poon, C.H.; Shi, G.; Lu, A.-L.; Leung, L.K. Genistein protects against polycyclic aromatic hydrocarbon-induced oxidative DNA damage in non-cancerous breast cells MCF-10A. Br. J. Nutr. 2009, 101, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Poschner, S.; Maier-Salamon, A.; Thalhammer, T.; Jäger, W. Resveratrol and other dietary polyphenols are inhibitors of estrogen metabolism in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2019, 190, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-H.; Hurh, Y.-J.; Na, H.-K.; Kim, J.-H.; Chun, Y.-J.; Kim, D.-H.; Kang, K.-S.; Cho, M.-H.; Surh, Y.-J. Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis 2004, 25, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, H.P.; Yeh, G.C. The effects of resveratrol on CYP1A1 expression and aryl hydrocarbon receptor function in vitro. Adv. Exp. Med. Biol. 2001, 492, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Q.; Wu, D.C.; Wang, X.W.; Sun, Y.; Chen, X.Y.; Zhang, K.L.; Li, H. Differential regulation of CYP1A1 and CYP1B1 expression in resveratrol-treated human medulloblastoma cells. Neurosci. Lett. 2004, 363, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lee, K.W.; Chan, F.L.; Chen, S.; Leung, L.K. The red wine polyphenol reseveratrol displays bilevel inhibition on aromatase in breast cancer cells. Toxicol. Sci. 2006, 92, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Chottanapund, S.; Van Duursen, M.B.M.; Navasumrit, P.; Hunsonti, P.; Timtavorn, S.; Ruchirawat, M.; Van den Berg, M. Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol. In Vitro 2014, 28, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Licznerska, B.; Szaefer, H.; Wierzchowski, M.; Sobierajska, H.; Baer-Dubowska, W. Resveratrol and its methoxy derivatives modulate the expression of estrogen metabolism enzymes in breast epithelial cells by AhR down-regulation. Mol. Cell. Biochem. 2017, 425, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Licznerska, B.; Szaefer, H.; Wierzchowski, M.; Mikstacka, R.; Papierska, K.; Baer-Dubowska, W. Evaluation of the effect of the new methoxy-stilbenes on expression of receptors and enzymes involved in estrogen synthesis in cancer breast cells. Mol. Cell. Biochem. 2018, 444, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, H.P.; Daschner, P.J.; Yeh, G.C. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998, 58, 5707–5712. [Google Scholar] [PubMed]
- Lee, J.E.; Safe, S. Involvement of a post-transcriptional mechanism in the inhibition of CYP1A1 expression by resveratrol in breast cancer cells. Biochem. Pharmacol. 2001, 62, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Beedanagari, S.R.; Bebenek, I.; Bui, P.; Hankinson, O. Resveratrol inhibits dioxin-induced expression of human CYP1A1 and CYP1B1 by inhibiting recruitment of the aryl hydrocarbon receptor complex and RNA polymerase II to the regulatory regions of the corresponding genes. Toxicol. Sci. 2009, 110, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Zahid, M.; Wang, C.; Saeed, M.; Cavalieri, E.L.; Rogan, E.G. Resveratrol prevents estrogen-DNA adduct formation and neoplastic transformation in MCF-10F cells. Cancer Prev. Res. 2008, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, L.; Matthews, J. Inhibition of aryl hydrocarbon receptor-dependent transcription by resveratrol or kaempferol is independent of estrogen receptor α expression in human breast cancer cells. Cancer Lett. 2010, 299, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Casper, R.F.; Quesne, M.; Rogers, I.M.; Shirota, T.; Jolivet, A.; Milgrom, E.; Savouret, J.F. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: Implications for prevention of dioxin toxicity. Mol. Pharmacol. 1999, 56, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.Y.; Yung, L.H.; Shi, G.; Lu, A.-L.; Leung, L.K. The red wine polyphenol resveratrol reduces polycyclic aromatic hydrocarbon-induced DNA damage in MCF-10A cells. Br. J. Nutr. 2009, 102, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Szaefer, H.; Licznerska, B.; Cykowiak, M.; Baer-Dubowska, W. Expression of CYP2S1 and CYP2W1 in breast cancer epithelial cells and modulation of their expression by synthetic methoxy stilbenes. Pharmacol. Rep. 2019, 71, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. 2010, 15, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Park, H.; Yue, W.; Wang, J.-P.; Atkins, K.A.; Zhang, Z.; Rogan, E.G.; Cavalieri, E.L.; Mohammad, K.S.; Kim, S.; et al. Tetra-methoxystilbene modulates ductal growth of the developing murine mammary gland. Breast Cancer Res. Treat. 2011, 126, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Wakimoto, R.; Ono, M.; Takeshima, M.; Higuchi, T.; Nakano, S. Differential an ticancer activity of pterostilbene against three subtypes of human breast cancer cells. Anticancer Res. 2017, 37, 6153–6159. [Google Scholar] [CrossRef] [PubMed]
- Van den Brand, A.D.; Villevoye, J.; Nijmeijer, S.M.; van den Berg, M.; van Duursen, M.B.M. Anti-tumor properties of methoxylated analogues of resveratrol in malignant MCF-7 but not in non-tumorigenic MCF-10A mammary epithelial cell lines. Toxicology 2019, 422, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Mikstacka, R.; Przybylska, D.; Rimando, A.M.; Baer-Dubowska, W. Inhibition of human recombinant cytochromes P450 CYP1A1 and CYP1B1 bytrans-resveratrol methyl ethers. Mol. Nutr. Food Res. 2007, 51, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.-J.; Lee, S.-K.; Kim, M.Y. Modulation of human cytochrome P450 1B1 expression by 2,4,3′,5′-tetramethoxystilbene. Drug Metab. Dispos. 2005, 33, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.; Wolk, A.; Persson, I.; Magnusson, C. Brassica vegetables and breast cancer risk. JAMA 2001, 285, 2975–2977. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; McMahon, M. Cross-talk between transcription factors AhR and Nrf2: Lessons for cancer chemoprevention from dioxin. Toxicol. Sci. 2009, 111, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Licznerska, B.E.; Szaefer, H.; Murias, M.; Bartoszek, A.; Baer-Dubowska, W. Modulation of CYP19 expression by cabbage juices and their active components: Indole-3-carbinol and 3,3’-diindolylmethene in human breast epithelial cell lines. Eur. J. Nutr. 2013, 52, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Licznerska, B.; Szaefer, H.; Matuszak, I.; Murias, M.; Baer-Dubowska, W. Modulating potential of L-sulforaphane in the expression of cytochrome p450 to identify potential targets for breast cancer chemoprevention and therapy using breast cell lines. Phytother. Res. 2015, 29, 93–99. [Google Scholar] [CrossRef] [PubMed]
- De Santi, M.; Carloni, E.; Galluzzi, L.; Diotallevi, A.; Lucarini, S.; Magnani, M.; Brandi, G. Inhibition of Testosterone Aromatization by the Indole-3-carbinol Derivative CTet in CYP19A1-overexpressing MCF-7 Breast Cancer Cells. Anticancer Agents Med. Chem. 2015, 15, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Jellinck, P.H.; Forkert, P.G.; Riddick, D.S.; Okey, A.B.; Michnovicz, J.J.; Bradlow, H.L. Ah receptor binding properties of indole carbinols and induction of hepatic estradiol hydroxylation. Biochem. Pharmacol. 1993, 45, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Leibelt, D.A.; Hedstrom, O.R.; Fischer, K.A.; Pereira, C.B.; Williams, D.E. Evaluation of chronic dietary exposure to indole-3-carbinol and absorption-enhanced 3,3′-diindolylmethane in sprague-dawley rats. Toxicol. Sci. 2003, 74, 10–21. [Google Scholar] [CrossRef] [PubMed]
- He, Y.H.; Smale, M.H.; Schut, H.A. Chemopreventive properties of indole-3-carbinol (I3C): Inhibition of DNA adduct formation of the dietary carcinogen, 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP), in female F344 rats. J. Cell Biochem. Suppl. 1997, 27, 42–51. [Google Scholar] [CrossRef]
- Chen, I.; Safe, S.; Bjeldanes, L. Indole-3-carbinol and diindolylmethane as aryl hydrocarbon (Ah) receptor agonists and antagonists in T47D human breast cancer cells. Biochem. Pharmacol. 1996, 51, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Dashwood, R.H. Indole-3-carbinol: Anticarcinogen or tumor promoter in brassica vegetables? Chem. Biol. Interact. 1998, 110, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ashok, B.T.; Chen, Y.; Liu, X.; Bradlow, H.L.; Mittelman, A.; Tiwari, R.K. Abrogation of estrogen-mediated cellular and biochemical effects by indole-3-carbinol. Nutr. Cancer 2001, 41, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Skupinska, K.; Misiewicz-Krzeminska, I.; Lubelska, K.; Kasprzycka-Guttman, T. The effect of isothiocyanates on CYP1A1 and CYP1A2 activities induced by polycyclic aromatic hydrocarbons in Mcf7 cells. Toxicol. In Vitro 2009, 23, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.S.; Bongiovanni, B.; Bralley, J.A. Estrogen metabolism and the dietcancer connection: Rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern. Med. Rev. 2002, 7, 112–129. [Google Scholar] [PubMed]
- Dalessandri, K.M.; Firestone, G.L.; Fitch, M.D.; Bradlow, H.L.; Bjeldanes, L.F. Pilot study: Effect of 3,3′-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr. Cancer 2004, 50, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.A.; Peterson, K.S.; Smith, H.J.; Gray, J.C.; Sullivan, D.K.; Mayo, M.S.; Crowell, J.A.; Hurwitz, A. A phase I study of indole-3-carbinol in women: Tolerability and effects. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- McCann, S.E.; Moysich, K.B.; Freudenheim, J.L.; Ambrosone, C.B.; Shields, P.G. The risk of breast cancer associated with dietary lignans differs by CYP17 genotype in women. J. Nutr. 2002, 132, 3036–3041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piller, R.; Verla-Tebit, E.; Wang-Gohrke, S.; Linseisen, J.; Chang-Claude, J. CYP17 genotype modifies the association between lignan supply and premenopausal breast cancer risk in humans. J. Nutr. 2006, 136, 1596–1603. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brooks, J.D.; Thompson, L.U. Mammalian lignans and genistein decrease the activities of aromatase and 17beta-hydroxysteroid dehydrogenase in MCF-7 cells. J. Steroid. Biochem. Mol. Biol. 2005, 94, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, T.; Wu, J.J.; Cao, Y.F.; Fang, Z.Z.; Sun, H.Z.; Zhu, Z.T.; Yang, K.; Liu, Y.Z.; Gonzalez, F.J.; et al. Inhibition of human CYP3A4 and CYP3A5 enzymes by gomisin C and gomisin G, two lignan analogs derived from Schisandra chinensis. Fitoterapia 2017, 119, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Kapucuoglu, N.; Coban, T.; Raunio, H.; Pelkonen, O.; Edwards, R.J.; Boobis, A.R.; Iscan, M. Expression of CYP3A4 in human breast tumour and non-tumour tissues. Cancer Lett. 2003, 202, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Modugno, F.; Kip, K.E.; Cochrane, B.; Kuller, L.; Klug, T.L.; Rohan, T.E.; Chlebowski, R.T.; Lasser, N.; Stefanick, M.L. Obesity, hormone therapy, estrogen metabolism and risk of postmenopausal breast cancer. Int. J. Cancer 2006, 118, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Haggans, C.J.; Hutchins, A.M.; Olson, B.A.; Thomas, W.; Martini, M.C.; Slavin, J.L. Effect of flaxseed consumption on urinary estrogen metabolites in postmenopausal women. Nutr. Cancer 1999, 33, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Cheng, Y.; Jiang, Z.; Alu, A.; Ma, X. Honokiol Exhibits Anti-Tumor Effects in Breast Cancer by Modulating the miR-148a-5p-CYP1B1 Axis. Am. J. Chin. Med. 2024, 52, 1843–1861. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Leung, L.K. The carotenoid lycopene differentially regulates phase I and II enzymes in dimethylbenz[a]anthracene-induced MCF-7 cells. Nutrition 2010, 26, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Aghapour, F.; Moghadamnia, A.A.; Nicolini, A.; Kani, S.N.M.; Barari, L.; Morakabati, P.; Rezazadeh, L.; Kazemi, S. Quercetin conjugated with silica nanoparticles inhibits tumor growth in MCF-7 breast cancer cell lines. Biochem. Biophys. Res. Commun. 2018, 500, 860–865. [Google Scholar] [CrossRef] [PubMed]

| Cytochrome P450 | Compound | Biological Effect | Experimental Model of Cells | References |
|---|---|---|---|---|
| CYP19 | Chrysin | Inhibition | MCF7 with fibroblasts co-culture | [49] |
| Naringenin | Inhibition | MCF7, T47D | [55] | |
| Naringin | Inhibition | MCF7, T47D | [55] | |
| Quercetin | Inhibition | MCF7, T47D | [55] | |
| Resveratrol | Inhibition | SK-BR-3 | [79] | |
| Inhibition | MCF7, MDA-MB-231 | [81] | ||
| Induction | MCF10A | [80] | ||
| Indole-3-carbinol | Inhibition | MCF7 | [98] | |
| 3,3′-diindolomethane | Inhibition | MCF7 | [98] | |
| Sulforaphane | Inhibition | MCF7 | [99] | |
| Induction | MDA-MB-231 | [99] | ||
| CYP1A1 | Galangin | Induction | MCF7 | [58] |
| Quercetin | Induction | MCF7 | [59] | |
| Resveratrol | Inhibition | MCF7 treated with TCDD * | [84] | |
| Inhibition | MCF7 and T47D treated with TCDD | [83] | ||
| Indole-3-carbinol | Induction | T47D treated with TCDD | [104] | |
| 3,3′-diindolomethane | Induction | T47D treated with TCDD | [104] | |
| Indole-3-carbinol | Induction | MCF7, MDA-MB-231, MCF10A | [98] | |
| 3,3′-diindolomethane | Induction | MCF7, MDA-MB-231, MCF10A | [98] | |
| Sulforaphane | Inhibition | MCF7, MDA-MB-231, MCF10A | [99] | |
| CYP1B1 | Quercetin | Inhibition | MDA-MB-468 | [62] |
| Eupatorin cirsiliol | Induction | MCF7 | [64] | |
| Resveratrol | Inhibition | MCF10F treated with TCDD | [85] | |
| Inhibition | MCF7, MDA-MB-231 | [81] | ||
| 2,4,3′,5′-tetramethoxystilbene | Inhibition | MCF7 and MF10A treated with TCDD | [95] | |
| Indole-3-carbinol | Induction | MCF7, MDA-MB-231, MCF10A | [98] | |
| 3,3′-diindolomethane | Induction | MCF7, MDA-MB-231, MCF10A | [98] | |
| Sulforaphane | Inhibition | MCF10A | [99] | |
| CYP2W1 | Resveratrol | Inhibition | MDA-MB-231 | [89] |
| CYP2S1 | Resveratrol | Inhibition | MDA-MB-231 | [89] |
| CYP17 | Lignans | Inhibition | MCF7 | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szaefer, H.; Licznerska, B.; Sobierajska, H.; Baer-Dubowska, W. Breast Cancer Cytochromes P450: Chemopreventive and/or Therapeutic Targets for Naturally Occurring Phytochemicals. Molecules 2025, 30, 3079. https://doi.org/10.3390/molecules30153079
Szaefer H, Licznerska B, Sobierajska H, Baer-Dubowska W. Breast Cancer Cytochromes P450: Chemopreventive and/or Therapeutic Targets for Naturally Occurring Phytochemicals. Molecules. 2025; 30(15):3079. https://doi.org/10.3390/molecules30153079
Chicago/Turabian StyleSzaefer, Hanna, Barbara Licznerska, Hanna Sobierajska, and Wanda Baer-Dubowska. 2025. "Breast Cancer Cytochromes P450: Chemopreventive and/or Therapeutic Targets for Naturally Occurring Phytochemicals" Molecules 30, no. 15: 3079. https://doi.org/10.3390/molecules30153079
APA StyleSzaefer, H., Licznerska, B., Sobierajska, H., & Baer-Dubowska, W. (2025). Breast Cancer Cytochromes P450: Chemopreventive and/or Therapeutic Targets for Naturally Occurring Phytochemicals. Molecules, 30(15), 3079. https://doi.org/10.3390/molecules30153079







