Abstract
The demand for biomass has been growing in recent years for several reasons, related to environmental, economic, and social trends. In the context of global climate changes and the depletion of natural resources, the recycling of plant biomass waste is a promising strategy for sustainable development that contributes to minimizing waste, improving resource efficiency, and achieving the goal of creating a circular economy. One of the highly demanded products of agricultural waste recycling is glucose. Glucose is an important organic substrate that allows a number of value-added products to be obtained. In this review, we focused on the commercially significant products of glucose oxidation: gluconic and glucaric acids. This review summarized the latest available data on the scope of the application of each product as well as the methods of their production. The capabilities and limitations of currently used methods of synthesis were highlighted.
1. Introduction
In recent years, the demand for biomass has been growing worldwide due to the need to transition to a low-carbon bioeconomy. Renewable sources of raw materials are an alternative to fossil resources and have good prospects since they can be used for obtaining valuable products in the chemical industry. Over the last century, the world population has grown significantly; meanwhile, the global production and consumption of plant food have also increased [1]. Consequently, the generation of biomass waste, produced by the agro-industrial sector, increases annually, and its combustion leads to greenhouse gas emissions, which have serious consequences for the environment [2,3]. Biomass of plant origin represents a prospective renewable and sustainable source of energy, chemicals, and various carbon-containing materials [4,5,6,7]. The recycling of plant biomass waste is especially beneficial in terms of reducing economic expenditures, preventing waste burial, and, as a result, improving the environmental situation [8,9,10,11]. In addition, the use of renewable energy sources promotes energy security, reducing resource and energy expenditures and preventing environmental pollution [12,13]. Non-food plant crops, agricultural waste (seed and cereal husks, potato peelings, beet peels, sugar cane bagasse, corn husks, peanut shells, rice husks, wheat straw, etc.), and waste from the pulp and paper industry are of particular interest for recycling [1,14,15,16,17]. Over the past 10 years, the need for the recycling of plant waste has increased due to the growing demand for waste-free production and the development of new approaches to the utilization of raw materials (Figure 1).
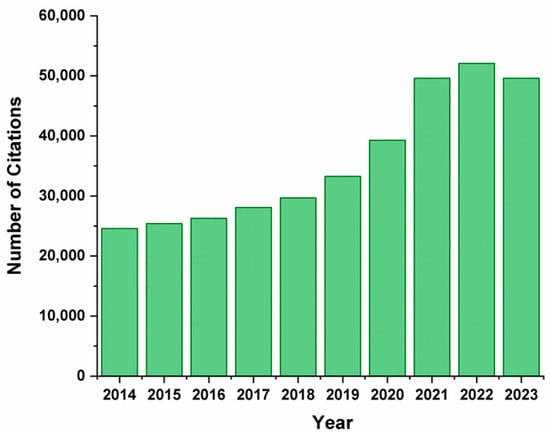
Figure 1.
The number of citations on the recycling of plant-based waste into value-added products over the past decade.
The market for the use of chemicals of biological origin is also growing [2]. Particular attention is paid to strategies for the development of new technologies for the recycling of lignocellulose into high-value-added bio-products [14]. Renewable resources and by-products of plant-growing have great potential in terms of recycling for obtaining biofuels as well as products of special interest to industry [18,19,20]. Biomass and plant waste are rich in three main components: cellulose, hemicellulose, and lignin [21]. One of the most important products, obtained as a result of the hydrolysis of natural polysaccharides (cellulose and hemicellulose), is glucose (Figure 2) [22,23,24]. Potato peelings and corn husks are waste products of plant origin and contain starch, which is the raw material for obtaining glucose [24].
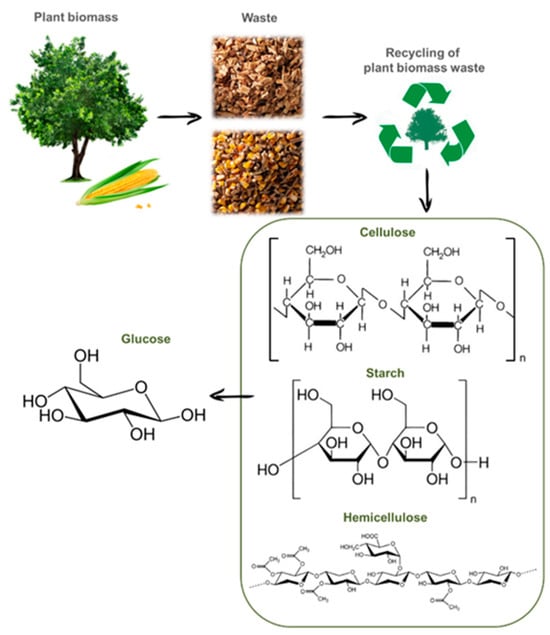
Figure 2.
The scheme of the recycling of plant biomass into glucose.
Glucose is a predecessor of a wide range of value-added products that are used in different spheres of the industrial sector [25]. The industrial glucose market was valued at 47.17 billion US dollars in 2024. The annual growth rate of the global glucose market is forecasted to increase by 4.8%, and it will reach nearly 80.4 billion US dollars by 2032 [26].
The most significant ways to convert glucose into value-added products are presented in Figure 3.
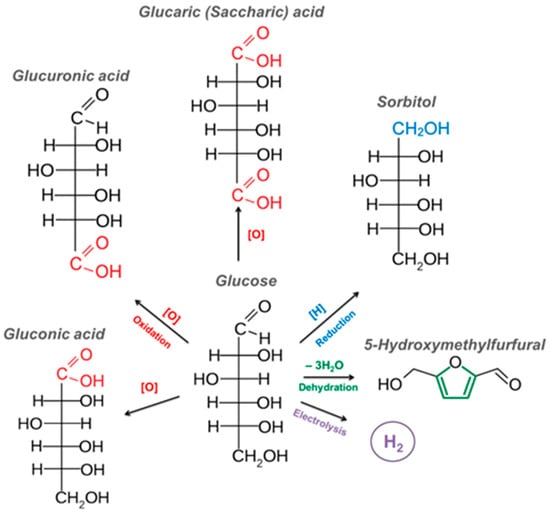
Figure 3.
Industrially significant products obtained from glucose.
The valuable products, obtained from glucose, include gluconic, glucuronic, and glucaric acids, sorbitol, 5-hydroxymethylfurfural, and hydrogen [27,28,29,30,31,32]. In this work, we will dwell at length on the significance and methods of obtaining commercially valuable products of glucose oxidation, gluconic and glucaric acids, based on the analysis of the relevant information on this topic over the last years.
2. Gluconic Acid
2.1. Product Significance
Gluconic acid (2,3,4,5,6-pentahydroxyhexane acid) is a non-toxic, non-corrosive, mild organic acid, possessing functional properties, with a molecular formula of C6H12O7 [33]. Gluconic acid and its derivatives are found in nature, mainly in plants, fruit juice, grapes, apples, meat, juice, wine, and honey [34].
Gluconic acid and its derivatives have a wide scope of applications and are used in food products as a preserving agent, a marinade, a leavening agent, and a pH control agent (E574-579 food additives). Due to the high ability to form complexes, gluconates are used as a component of juice and fruit puree to prevent turbidity and the formation of calcium and iron phosphates. D-gluconic acid improves the taste of food products, giving them a fresher and less bitter taste. In the dairy industry, gluconic acid prevents the deposition of milkstone in technological equipment and glass storage containers.
D-gluconic acid in the form of calcium (and less often potassium, zinc, and other) salts is widely used in medical practice. Gluconic acid compounds are also used in the production of concrete, cosmetic preparations, agricultural industries, and a number of other industries [35]. D-gluconic acid and its derivatives are used as prebiotics and tend to exhibit antioxidant properties. The chelating properties of gluconates make them a soft and environmentally friendly cleaning agent and degreaser. Glucono-δ-lactone is actively used in cosmetic products [36].
The volume of the gluconic acid market was estimated at 0.2 billion US dollars in 2024. The gluconic acid industry is forecasted to reach 0.28 billion US dollars by 2034, with a combined annual growth rate of 3.5% during the forecast period (2025–2034) [37].
According to Kirimura and colleagues [38], the shares of the use of gluconic acid and its derivatives worldwide by application area are, approximately, construction—45%, food—35%, medical—10%, and other—10% of the industry. Among gluconic acid derivatives in 2023, the largest market segment was occupied by glucono-delta-lactone (40.1%), followed in descending order by sodium gluconate, calcium gluconate, and potassium gluconate [39]. According to the author of the 2025 market research [37], among all segments of the global market, gluconic acid occupies a dominant position in the market, and the fastest growing market segment is glucono-delta-lactone, which is used as an acidifier, leavening agent, and sequestrant in the food industry. The demand for gluconic acid is expected to grow further owing to the increased demand for cosmetics and products containing natural and safe ingredients, including bioacids [39]. In addition, there is growing use of gluconic acid in the agricultural sector as a chelating agent to improve the nutrient uptake by plants and in water treatment to remove heavy metals. The fields of application of gluconic acid and its derivatives are presented in more detail in Table 1.

Table 1.
Applications of gluconic acid and its derivatives in various fields of industry.
2.2. Gluconic Acid Production
The industrial method of obtaining gluconic acid is currently the microbiological oxidation of glucose using cell cultures [44]. However, it is also worth highlighting other methods of obtaining gluconic acid from glucose:
- -
- Catalytic, using noble metals;
- -
- Electrocatalytic, using electrodes based on noble metals;
- -
- Photocatalytic;
- -
- Enzymatic, using immobilized enzymes.
2.2.1. Microbiological Synthesis
The biotechnological method is a widely used industrial method of producing gluconic acid using living cell cultures. Various microorganisms and molds capable of producing the enzyme glucose oxidase have been studied in this process: Aspergillus niger, Aureobasidium pullulans, Penicillum spp., bacteria of the Pseudomonas, Gluconobacter, Zymomonas mobilis species, etc. Both pure glucose and a complex nutrient medium (breadfruit hydrolysate, sugarcane molasses, grape must, corncob enzymatic hydrolysate, and others) can be selected as a carbon source for microorganisms [86,87,88]. Figure 4 presents a scheme of the enzymatic conversion of glucose in the presence of Aspergillus niger. Table 2 shows the most commonly used microorganisms for producing gluconic acid and the process characteristics [89].
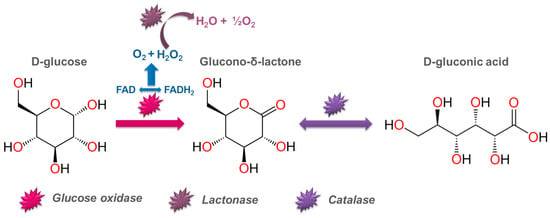
Figure 4.
Enzymatic conversion of glucose by means of Aspergillus niger.

Table 2.
Various microorganisms, process conditions, and gluconic acid yield.
Microbiological synthesis is the main industrial method for obtaining gluconic acid. It is based on the catalytic activity of the enzyme glucose oxidase, expressed by various microorganisms, which oxidizes D-glucose with atmospheric oxygen to glucono-δ-lactone. Glucono-δ-lactone is subsequently hydrolyzed into gluconic acid.
- Key producers:
- –
- Aspergillus niger: The most widely used fungus, showing high productivity (yields up to 311 g/L [89]) on various substrates.
- –
- Gluconobacter oxydans: A bacterium known for its fast kinetics and high efficiency, especially at certain pH and with the use of neutralizing agents [60,88].
- –
- Penicillium spp.: Various species are also capable of synthesis, adapting to different substrates and conditions [95,96,97].
- –
- Others: Aureobasidium pullulans [91], Zymomonas mobilis (including immobilized forms) [98], Klebsiella pneumoniae [94], etc., have also been studied.
- Carbon sources:
- –
- Pure glucose: Used to achieve high titers (yield) but increases the cost of the process.
- –
- Complex media: Hydrolysates (breadfruit, corn cobs), molasses, must (grape, banana), puree, etc., reduce the cost but can complicate the purification.
- Fermentation conditions and modes:
- –
- Parameters: Typically mesophilic temperature (≈30–39 °C), controlled pH (often 5.0–6.5, strain-dependent), aeration (1–3 vvm), and agitation.
- –
- Modes: Batch, fed-batch (often gives best results in terms of gluconic acid yield >140 g/L for A. niger [87]), submerged fermentation.
- Product yields vary widely (from ~15 g/L to >300 g/L). Glucose conversion efficiencies are often high (>85–95% of theoretical).
The microbiological synthesis of gluconic acid is a well-studied and industrially developed process. Aspergillus niger and Gluconobacter oxydans remain key producers due to their high efficiency. The use of a variety of substrates, including low-cost ones, and optimized fermentation regimes (especially fed-batch) allow high titers of the product to be achieved. However, as mentioned earlier [27,99,100,101,102] and confirmed by the data in Table 2, there remain challenges associated with the relatively slow reaction rate compared to chemical methods, such as long fermentation times in many cases, low yield per time unit, the generation of significant wastewater volumes, difficulties in isolating the product from complex culture media, and the utilization of waste biomass. Ongoing research is aimed at selecting or engineering more productive strains, optimizing cultivation conditions, and developing more efficient downstream processing methods to overcome these limitations.
2.2.2. Heterogeneous Catalytic Oxidation of Glucose
Another method that allows the disadvantages of the traditional method of obtaining gluconic acid to be overcome is the liquid-phase oxidation of glucose with molecular oxygen in the presence of nanosized heterogeneous catalysts based on noble metals (platinum or palladium) [27,103,104,105]. However, during catalysis in the presence of air or molecular oxygen, these systems are subject to oxidative poisoning; therefore, a modifying metal (Cu, Bi, Te, etc.) is introduced as an additive [106,107,108,109,110,111]. A promoter prevents palladium oxidation and increases the gluconic acid yield owing to the electron interaction between an active metal and the promoter metal. This metal is capable of improving the catalytic characteristics of the material [112,113,114]. Activated carbon, titanium oxide, aluminum oxide, silica gel, cerium oxide, etc., are used as support [103,104,105,109,115]. I. Delidovich and colleagues achieved significant success in obtaining gluconic acid using Au/Al2O3. Even with a relatively low catalyst loading (the molar ratio of glucose to an active component was 17,000: 1), the glucose conversion (97%) and selectivity for the desired product (96%) were close to 100%. The catalysts and reaction conditions of the catalytic process are presented in more detail in Table 3.

Table 3.
Reaction conditions and heterogeneous catalysts used for the glucose oxidation process.
Glucose oxidation in the presence of heterogeneous catalysts containing noble metals allows for a significant reduction in the amount of industrial waste, eliminates the use of aggressive chemical compounds, increases environmental safety, and simplifies the process of separating catalysts from the liquid reaction medium for repeated use. Despite the undoubted advantages of using heterogeneous catalysts, they have a number of disadvantages. The limiting factor in their use at present is the high cost of these materials. The diffusion of reactants to active centers and the diffusion of products from them can be limited, which slows down the reaction rate. A serious problem limiting their use is catalyst deactivation. Even when using a support, the leaching of active metals into the solution can reduce the overall efficiency of the catalytic system [110,121,122,123,124,125]. Strict control of the pH of the reaction medium is also necessary since the formation of gluconic acid proportionally reduces the acidity of the medium. Thus, the catalytic process of glucose oxidation in the presence of heterogeneous supported catalysts is complicated. With a significant increase in the acidity of the medium to pH 11–12, the base-catalyzed reaction of glucose isomerization occurs, as well as the destruction of the reaction products, which leads to a significant decrease in selectivity for the target product (sodium gluconate) [111,126,127].
Gold supported on metal oxides, carbon materials, or as a bimetallic nanoparticle together with other noble metals with the addition of Bi, Sn, or other promoters is generally more stable, while Pt or Pd on carbon supports or some metal oxides may be less stable. The correct choice of support, synthesis method, use of bimetallic catalysts, and optimization of reaction conditions are important conditions for obtaining highly active and stable catalysts. Despite the fact that there is already a lot of information about the reaction of the heterogeneous selective oxidation of glucose, research on optimizing the characteristics of the catalyst to achieve high efficiency and stability in this process is still relevant.
2.2.3. Glucose Electrooxidation
Glucose electrooxidation is a process that is primarily aimed at generating electricity in a low-temperature glucose fuel cell [128]. However, the formation of gluconic acid in the electrode cell allows the electrolytic oxidation of glucose to be considered as a potentially possible method for producing gluconic acid [129]. Gluconic acid is most often not the only product of glucose electrolysis; it is formed together with glucaric acid. Electrodes, based on noble metals (Au, Pt, Pd), as well as electrodes that do not contain noble metals (for instance, MnO2/Ti), were studied as electrocatalysts for the process of producing gluconic acid (Table 4) [100,129,130,131].

Table 4.
Reaction conditions and electrocatalysts used for the glucose oxidation process.
Despite the high conversion of glucose in a short reaction time and the relative environmental friendliness and simplicity of the process, this method is characterized by low selectivity for the target product (Table 4) [100].
2.2.4. Photocatalytic Oxidation of Glucose
Heterogeneous photocatalysis is a new and promising strategy for the green synthesis of gluconic acid, which attracts much attention because this process can efficiently proceed under the action of UV and visible light in mild conditions at temperatures not exceeding 303 K [121,132,133,134,135]. Photocatalysts are used as substitutes for glucose oxidase, which induces glucose oxidation [132].
In recent years, metallothioporphyrazines (MPzs) have been considered biomimetic photocatalysts due to their strong absorption in the visible region [136]. These systems exhibit unique photocatalytic activity for the activation of hydrogen peroxide or oxygen in visible light owing to the presence of delocalized π-electrons [137]. Such compounds have proven themselves in selective organic transformations in mild conditions [138]. The studies of Cheng M. noted that the application of cobalt thioporphyrazine (0.5%) on ZnO increased the conversion of glucose compared to pure ZnO, 4.4 times in 5 h of the reaction. In relation to gluconic acid, the selectivity increased up to 15% as compared to 7%, achieved when using pure ZnO. At the same time, the total selectivity was below 100%, probably due to the glucose mineralization up to CO2 and H2O [128]. Yin J. and colleagues obtained a photocomposite material by modifying TiO2 with HPW (phosphotungstic acid) and CoPz (cobalt tetra(2-hydroxymethyl-1,4-dithiin)porphyrazine), which demonstrated a selectivity for gluconic acid of 65.5% with a total glucose conversion of 22.2%. In this case, the total selectivity was 80.4%, which also confirms the complete oxidation of glucose to carbon dioxide and water [139]. In the work of Q. Znang, the SnO2/FePz(SBu)8 composite was obtained by immobilizing tetra(2,3-bis(butylthio)maleonitrile)porphyrazine of iron (FePz(SBu)8) on the SnO2 surface. The study of this material in the photocatalytic process of glucose oxidation to gluconic acid showed a conversion of 34.2% with a selectivity for the target product equal to 32.9% [140]. In another work, Q. Zhang et al. synthesized the g-C3N4/CoPz composites by applying cobalt tetra(2,3-bis(butylthio)maleonitrile)porphyrazine onto the surface of graphite-like carbon nitride. The selectivity of glucose in the presence of g-C3N4/CoPz was 65% with a glucose conversion of 65% and a light intensity of 2 W/cm2 [141]. R. Chen et al. applied tetra(2,3-bis(butylthio)maleonitrile)porphyrazine (FePz(SBu)8) on a Na-ZSM support in the amount of 5%. The glucose conversion reached 21.7% with a selectivity for gluconic acid of 34.1%. However, the total selectivity for all the products was only 65.7% [136].
X. Bai reported the efficiency of a metal-free photocatalyst consisting of nitrogen-deficient carbon nitride (BNCN) and chlorin e6 (Ce6) during the oxidation of glucose to gluconic acid. The resulting photocatalyst showed a glucose conversion of 62.3% with a selectivity for gluconic acid of 59%. The total selectivity for arabinose and gluconic and glucaric acids did not exceed 71% [142]. TiO2-based photocatalysts are used in photocatalytic processes of oxidation of organic substances, including glucose, owing to the peculiarities of the TiO2 band structure [143]. J. C. Colmenares and co-authors succeeded in increasing the total selectivity of the obtained TiO2 in relation to gluconic and glucaric acids and arabinose (Stotal = 71.3%) as compared to the commercial sample of Degussa P-25 (Stotal = 17.2%) [135]. The authors attributed the improved performance of the photocatalyst to the physicochemical properties (for example, to the high specific surface area, nanostructured anatase phase, and visible light absorption) of the new TiO2 materials and the reaction conditions [135]. K. Roongraung and colleagues studied the influence of applying 20% TiO2 on Y-type zeolites with different ratios of SiO2:Al2O3. The strength and number of acid sites have been shown to influence the catalytic properties of various catalysts [134]. An increase in the strength of acid sites with increasing aluminum content at SiO2:Al2O3 = 10 led to an increase in the yield and selectivity (29%) of gluconic acid, whereas at SiO2:Al2O3 ratios of 100 and 500, the selectivity was at the level of 11% and 10%, respectively. In addition, L Da Vià and colleagues studied different concentrations of Ag deposited on the photoactive TiO2 material during glucose oxidation. The selectivity for gluconic acid was 15–18% with the highest glucose conversion of 11.5% [144]. B. Zhou and coauthors found that Au/TiO2 contributed to an increase in the gluconic acid yield in both the visible light region (99%) and the UV radiation region (99%) in an aqueous Na2CO3 solution with a glucose conversion of 99% in both cases [145]. The authors attributed the increased photoactivity of the catalyst in the visible spectrum to the plasmon resonance of Au nanoparticles. In UV light, Au nanoparticles enhance the photoexcitation of the TiO2 band gap, promoting an increase in activity and, as a result, an increase in the gluconic acid yield. Na2CO3 acts as an inhibitor of reactive oxygen species with a strong oxidizing ability under UV light (for instance, hydroxyl radicals and singlet oxygen). Figure 5 shows the proposed mechanism of glucose photooxidation in the UV and visible regions of the spectrum [145].
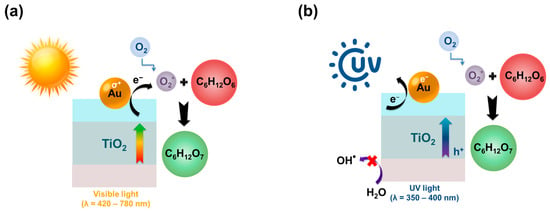
Figure 5.
The scheme of highly selective glucose oxidation into gluconic acid under the action of (a) visible light and (b) UV radiation.
Therefore, despite the various approaches to the photocatalytic production of gluconic acid from glucose used by the researchers, the main limitations of this method are still low selectivity for the desired product, losses associated with partial oxidation of glucose to CO2 and H2O, and the use of expensive gold catalysts [146].
2.2.5. Glucose Oxidation in the Presence of Immobilized Glucose Oxidase
A relatively new method of obtaining gluconic acid is using glucose oxidase immobilized on supports, including magnetically separated ones. In this review, emphasis was placed on the use of immobilized enzymes, since the use of enzymes in their native form is often hampered by a number of limitations, such as high cost, low functional stability, inactivation by physical and chemical factors, and a lack of recovery or reuse [147,148,149,150,151,152]. Therefore, in recent years, many researchers have considered immobilization as an effective tool to overcome these drawbacks and improve the catalytic properties of enzymes, such as activity, selectivity, specificity, and resistance to inhibitors [153]. A biocatalytic method for the oxidation of D-glucose to D-gluconic acid using glucose oxidase immobilized on inorganic supports and magnetically separated systems is a promising direction in the field of biotechnology. Over the past decade, there has been a significant increase in the attention of researchers to magnetic nanoparticles (MNPs) and materials based on them. Firstly, due to the nano-size of the particles, a large surface area of the biocatalyst is achieved, and this significantly increases the probability of contact between the support and the enzyme. In turn, this feature allows the biocatalyst to achieve indicators close to the native enzyme. Secondly, the obtained magnetically separable systems can be easily separated by a magnet and reused for successive cycles [154].
The synthesis of the biocatalyst involves treating a mesoporous support (SiO2, Al2O3, ZrO2) with iron nitrate (III) and calcination to form Fe3O4 nanoparticles [155,156]. The mixed support is functionalized with 3-aminopropyltriethoxysilane (APTES) to modify the support with amino groups, then glutaraldehyde is used for the covalent crosslinking of glucose oxidase with the support surface. Jaquish R. and co-authors found that the treatment of mesoporous SiO2 and Al2O3 with the iron nitrate (III) solution not only imparts magnetic properties but also increases relative activity during gluconic acid production, using the biocatalyst in all 10 consecutive cycles [155]. The improvement in catalytic activity is associated with the enzyme-like properties of iron oxide. However, SiO2-based samples showed higher activity compared to that of Al2O3 used as a support. The authors explain this phenomenon by the larger pores in Fe3O4-SiO2 as compared to those of Fe3O4-Al2O3 and stronger Brønsted acid sites. A. K. Haskell and colleagues showed that the highest relative activity of the conversion of glucose into gluconic acid in the presence of the obtained Fe3O4/ZrO2/GOx biocatalyst was 98% when the pH was 6–7 and the temperature was 313–318 K [156]. The research of V. Matveeva et al. described a method of obtaining a biocatalyst that is based on glucose oxidase immobilized on a magnetically separable Fe3O4/SiO2 support [154]. The Fe3O4/SiO2/GOx biocatalyst demonstrated the highest yield of gluconic acid (88%) at an initial glucose concentration of 3.68 mmol/L (the pH was 5, T was 313 K) for 1 h of the reaction process. O. V. Grebennikova and colleagues found that Fe3O4/SiO2/GOx not only has a relative activity of 95% but also exhibits stability in 10 consecutive cycles of glucose oxidation [157]. The work [157] also established that the use of aluminum oxide for the preparation of a magnetically separable support led to a decrease in the relative activity, which in the 10th cycle of the reaction process was equal to ~80%. The combined scheme of the process of preparing the magnetically separable support, the immobilization of glucose oxidase, and the oxidation of glucose to gluconic acid is presented in Figure 6 [155,156,157].
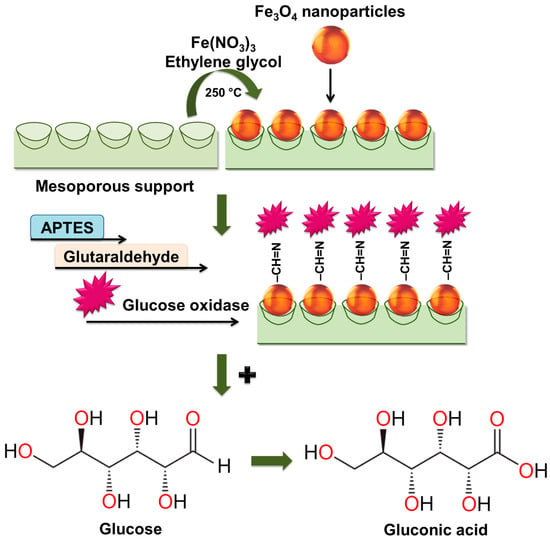
Figure 6.
The scheme of the synthesis of the biocatalyst based on glucose oxidase immobilized onto the magnetically separable support and the oxidation of glucose to gluconic acid.
In all the considered cases of using immobilized enzymes applied onto magnetically separable supports, the process was characterized by 100% selectivity. The main advantages of using immobilized enzymes applied onto magnetically separable supports are high enentio-, regio- and chemoselectivity, a high rate of the conversion of glucose into gluconic acid, and the possibility for the rapid separation of the biocatalyst from the reaction medium for its subsequent multiple uses [155,156,157].
Covalent binding is one of the most common and interesting methods of enzyme immobilization for industrial applications. The main disadvantages of the method include the labor-intensive nature of the biocatalyst production and the likelihood of enzyme inactivation in some cases [153]. It should be noted that the cost of most industrial enzymes is often only a minor component in the overall economics of the process. Therefore, in these cases, the additional costs associated with the immobilization of enzymes and the process are often not justified, which limits the widespread use of immobilized enzymes for now [158].
3. Glucaric Acid
3.1. Product Significance
D-glucaric acid (2,3,4,5-tetrahydroxyadipic acid) is a dibasic acid obtained directly by the oxidation of D-glucose. Glucaric acid is not a widely available chemical product, and the available information on its production and market trends is limited. In recent years, there has been growing interest in glucaric acid as a renewable and eco-friendly chemical feedstock, which may contribute to its increased production in the future. From 2004, Glucaric acid was classified as a “top value-added chemical from biomass” by the United States Department of Energy because of its potential applications as a material for making biodegradable detergents and biodegradable polymers such as nylons and plastics [159], and its market value will be 1.4 billion US dollars by 2028 according to some estimations [27]. However, the selective production of glucaric acid is difficult; therefore, the market for glucaric acid is underdeveloped due to limited availability and high prices [160].
Glucaric acid has chelating properties owing to the presence of two carboxyl groups. This peculiarity of glucaric acid allows it to extract heavy metals, such as Cd, Cr, Cu, Ni, Pb, and Zn, from soil contaminated with wastewater [161]. Glucaric acid is used to create biodegradable polymers for the “green” industrial production of compounds, based on heavy metals and their derivatives after the extraction from contaminated aquatic environments [162]. The chelating ability of glucaric acid allows it to be used for the degradation of organic pollutants [163]. Glucaric acid also serves as a monomer for synthesizing organic biopolymers, such as polyglucaramides, hyperbranched polyethers, and chemically soluble cross-linked gels [164,165,166,167,168]. D-glucaric acid is a non-toxic compound that is produced in small quantities by mammals and some plants. D-glucaric acid is a natural metabolite of D-glucuronic acid conversion in mammal organisms [169]. This feature allowed glucaric acid to be used as a biomarker for early cancer diagnostics and the noninvasive imaging of tumor necrosis [170,171,172,173,174]. Glucaric acid is used as a dietary supplement to regulate the human endocrine profile, improve the immune response, treat diabetes, lower cholesterol, and reduce canceration risk [159,175,176]. Glucaric acid is known to be used as an anticancer chemotherapy [173]. The anticarcinogenic properties of glucaric acid and its lactones are related to the fact that D-glucarate inhibits the action of β-glucuronidase and promotes the elimination of potentially toxic compounds that increase the risk of developing various types of cancer [177,178].
Sodium and calcium glucarates are used as detergents and cleaning agents, while magnesium and calcium salts of glucaric acid are used in hard water treatment and in preventing soap formation [27]. Glucaric acid is a bio-based building block for the synthesis of adipinic acid by the catalytic reduction with hydrogen as well as 2,5-furandicarbonic acid [27,179,180]. Adipinic acid is a precursor to nylons and is used in coatings and detergents [27], while 2,5-furandicarbonic acid is a promising bioplastic monomer that is a potential replacement for terephthalic acid in polyethylene terephthalate [180].
Glucaric acid has anticorrosive, antiplasticizing properties and can be used as a deicing agent. Liquid deicing chemicals usually contain aggressive chloride-based components that can cause significant damage to both road surfaces and the vehicles in which they are used. Phosphates were traditionally used as corrosion inhibitors in deicing mixtures, but their use was hampered by growing environmental concerns [177]. Replacing environmentally harmful phosphates and chlorides with gluconic acid has contributed to the growth of the commercial production of deicing mixtures, based on glucaric acid. The anticorrosive effect is achieved even at sufficiently low concentrations of glucaric acid [27,177,181]. The main areas of using glucaric acid are presented in Figure 7.
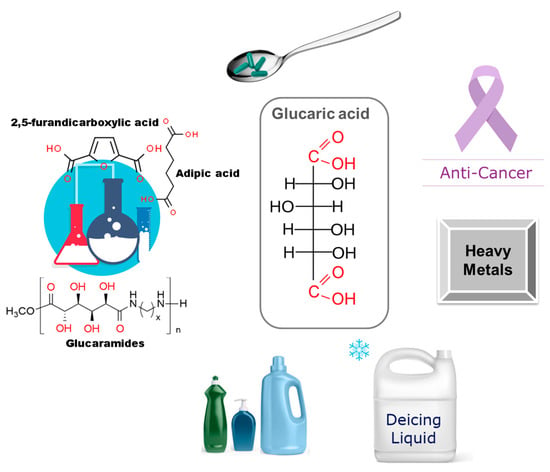
Figure 7.
Main applications of glucaric acid: a chemical platform for the synthesis of new compounds, a pharmaceutical food additive, cancer diagnosis and treatment, a heavy metal accumulator, anti-corrosion deicing fluids, and a cleaning agent.
3.2. Glucaric Acid Production
The synthesis of glucaric acid, as well as gluconic acid, from glucose or glucose-containing raw materials can be accomplished using chemical, catalytic, electrochemical, or biochemical methods. However, only two methods have been practically applied at present [27,182,183]: the chemical oxidation of glucose using nitric acid as an oxidizing agent and oxidation using palladium or platinum catalysts.
3.2.1. Biotechnological Methods of Obtaining Glucaric Acid
The process of the biotransformation of glucose into glucaric acid (glucaric acid = GRA) proceeds with low selectivity. This method of obtaining glucaric acid is characterized by difficulties associated with the separation of products (large volumes of microbial biomass and hundreds of by-products with similar properties are formed) and is not used for industrial production [32].
There are two main routes for glucaric acid biosynthesis. The first involves the introduction of heterologous glucaric acid synthesis pathways into Escherichia coli or yeast cells, and the second involves the use of multienzyme biocatalytic methods in vitro [184].
Innovative approaches to the biotechnological production of GRA using Escherichia coli and Pseudomonas syringae are developing, demonstrating the growing capabilities of modern metabolic engineering and synthetic biology [185,186,187,188]. Thus, a synthetic method was developed to produce glucaric acid from glucose in recombinant E. coli using enzymes from three different sources: Ino1, MIOX, and Udh [185]. Ino1, isolated from Saccharomyces cerevisiae, converts glucose-6-phosphate to myo-inositol-1-phosphate, which is then dephosphorylated to myo-inositol. MIOX from mice catalyzes the formation of D-glucuronic acid from myo-inositol, which is then converted to D-glucaric acid by Udh from Pseudomonas syringae. The activity of this recombinant enzyme was more than two orders of magnitude higher than that of Ino1 and MIOX, allowing the glucaric acid to be obtained at concentrations exceeding 1 g/L. The main limiting factor is the activity of MIOX and the need to optimize enzyme expression [185]. To improve the efficiency of the GRA production by this method, the authors [186] proposed using polypeptide scaffolds from domains of the protein–proteinous interaction to colocalize all three enzymes in a designed complex to synthetically increase the effective concentration of myo-inositol. Since the catalytic activity of myo-inositol oxygenase (MIOX) is highly dependent on the concentration of the myo-inositol substrate, the scaffolds directly increased the specific activity of MIOX and the glucaric acid titers correlated with MIOX activity. An approximately fivefold increase in GRA titers was observed as compared to the scaffold-free control and a 50% increase as compared to the previously reported highest titers. To increase the productivity of this pathway, protein fusion tags that increase the solubility of MIOX and directed evolution to increase the activity of MIOX were studied [187]. The fusion of the N-terminal fragment of SUMO to MIOX increased the final titers of D-glucaric acid to 4.85 g/L from 10.8 g/L of myo-inositol and the increase in the production of glucaric acid from myo-inositol was 75%. The expression of a small fragment of manXmRNA allowed the final titer of D-glucaric acid to reach 4.58 g/L with an increase in glucaric acid production of 65%. Using systematic metabolic engineering, the E. coli GA10 strain [188] was developed, which allowed a titer of 5.35 g/L to be achieved. Since E. coli is not considered a completely safe strain, and with the aim of increasing the yield of GRA, the researchers demonstrated the use of modified Pichia pastoris as a platform for the production of glucaric acid from myo-inositol [189]. Glucaric acid with the highest concentration of 6.61 ± 0.30 g/L was obtained synthetically in Pichia pastoris by the coexpression of murine myo-inositol oxygenase (mMIOX) and uronate dehydrogenase (Udh) from Pseudomonas putida KT2440 during fermentation in a nutrient medium of a mixed substrate containing glucose and myo-inositol. These studies on the use of scaffolds in E. coli and the protein fusion strategy in P. pastoris provide the basis for further research on increasing the titer of glucaric acid. Attempts were also made to use Saccharomyces cerevisiae to obtain D-glucaric acid [178,190,191]. The paper [191] presents a table with the results for 2009–2023 on the production of D-glucaric acid using E. coli, S. cerevisiae yeast, or P. pastoris in vivo. The highest titer of D-glucaric acid at a level of 11–13 g/L was shown to be achieved using Saccharomyces cerevisiae. Tables with the results of using biocatalytic methods can also be found in the works Chen L.-Z. et al. and Zhao Y. et al. [159,184]. The development of biotechnological methods for the production of glucaric acid promises to make it an affordable and environmentally friendly alternative to chemical methods.
3.2.2. Chemical Methods of Obtaining Glucaric Acid
When exposed to strong oxidizing agents, both the aldehyde group of glucose and the primary alcohol group undergo oxidation. In this case, dibasic glucaric acid is formed (Figure 8).
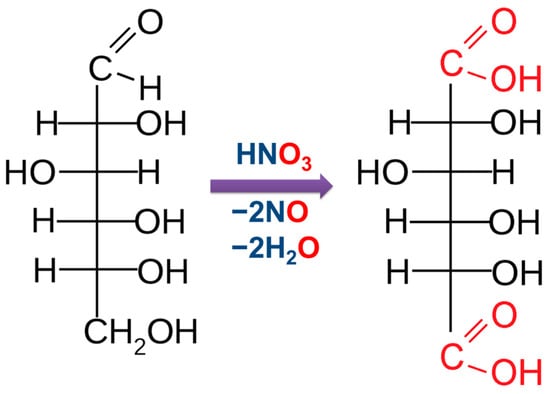
Figure 8.
The obtainment of glucaric acid by glucose oxidation.
The oxidation of glucose with nitric acid in the absence of catalysts, followed by the obtainment of glucaric acid at temperatures from plus 328 K to plus 348 K, is described in the work [192,193]. Since D-glucaric acid is not easily crystallized, it is convenient to isolate salts (calcium or potassium glucarates) by neutralizing the bases with glucaric acid. Despite its commercial potential, the large-scale production of D-glucaric acid by the oxidation of D-glucose with nitric acid is difficult, mainly because of competing side reactions, resulting in a low degree of conversion into D-glucaric acid, and the rapid and highly exothermic nature of the oxidation [194]. This method remains attractive for commercialization owing to its relative simplicity, since nitric acid serves as both a solvent and an oxidizing agent in the synthesis, and because of the low cost of the oxidizing agent, although the product yield is only 40–45%. In the process, implemented by Rivertop Renewable, nitric acid is oxidized at temperatures from 298 to 313 K and a total pressure from 1.25 to 1.5 bar, with a yield of 35 to 40% [27]. Significant disadvantages of this method are significant costs in organizing the production and the formation of toxic by-products and inorganic salts, which worsen the ecology [194].
3.2.3. D-Glucose Oxidation to Glucaric Acid Using Catalysts
There are known works [195,196] reporting on the homogeneous catalyzed oxidation of glucose by means of HNO3 and NaNO2. Studying the glucose oxidation process in the temperature range from plus 298 K to plus 313 K, the authors of [194] showed that nitric acid is, in fact, a catalyst in this process, and oxygen is consumed for oxidation. Sodium nitrite catalyzes the sequential oxidation of D-glucose with oxygen in the strongly acidic solution of HClO4-H2O-sulfolane to D-gluconic and then to D-glucaric acids [195].
Other catalytic systems are also being developed. D-glucose can be oxidized with sodium hypochlorite (potassium hypochlorite) in the presence of sodium (potassium) bromide using 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) or 4-acetamido-2,6,6-tetramethylpiperidine-1-oxyl (4-acetamido-TEMPO) as a catalyst [196,197]. When the pH values range from 11.4 to 11.6 and the temperature is below 5 °C, the D-glucose oxidation, initiated by nitroxide, produces glucaric acid salts with high selectivity and a good yield of more than 85%.
In 2001, a work appeared on the catalyzed 4-acetylamino-2,2,6,6-tetramethyl-1-piperidinyloxy (4-AcNH-TEMPO) oxidation of D-glucose to D-glucaric acid using elemental chlorine or bromine as a final oxidizer at a temperature of 273–278 K and a pH of 11.5 [198]. Glucarate yields of over 90% were reported. Unfortunately, 4-AcNH-TEMPO is expensive to produce and there are problems with its utilization, which hinders the widespread use of this catalyst [199]. The disadvantages of such processes, apart from using corrosive and hazardous reagents, also include difficulties in separating and utilizing homogeneous catalysts from the products. Gluconic, glucaric, and 2-keto-gluconic acids and their salts can be obtained by the oxidation of aqueous glucose solutions with oxygen, air, or hydrogen peroxide [200] in the presence of metal catalysts on supports. During the synthesis of gluconic acid, the catalysts containing gold are preferable as catalysts, and catalysts containing platinum on various supports (TiO2, ZrO2, C) are desirable in the case of glucaric acid synthesis [27].
Glucose is oxidized to gluconic acid faster than gluconic acid is oxidized to glucaric acid. For instance, the work [201] noted that after 10 min, a high conversion of glucose (about 64%) with gluconic acid as the main product is achieved when using a Pt/C catalyst with a selectivity of about 81%. After 10 h of glucose oxidation, its conversion is observed with a selectivity for glucaric and gluconic acids of 65% and 19%, respectively. The hydroxyl group (-OH) is oxidized on C6 of gluconic acid, accompanied by the formation of an intermediate product (glucuronic acid), which is then oxidized to form glucaric acid. The yield of glucaric acid is lower than that of gluconic acid because the oxidation of the -OH-group of gluconic acid is thermodynamically less favorable than the oxidation of the aldehyde group of glucose [28]. The selectivity in relation to glucaric acid is influenced by side and consecutive reactions of the C−C bond cleavage.
In view of this, the reaction of the direct conversion of glucose into glucaric acid is still a relevant technological task due to the formation of a number of undesirable products that reduce the glucaric acid yield. Based on the analysis of the literature data, a recent review article [27] provides a table containing the catalyst type used by researchers in the reaction to obtain glucaric acid and the process conditions, taking into account the obtainment of the final product in one reactor. The best results were obtained using the 5% Pt/CNT catalyst (the conversion was 100%, the selectivity was 82%, and the glucaric acid yield was 82%). The catalysts that contained gold and gold–palladium showed the worst results. For instance, for the 3.5% Au−3.45% Pt/ZrO2 catalyst, during the conversion of 100%, the selectivity and the yield of glucaric acid were only 50%.
The degree of conversion, the selectivity of the process, and the glucaric acid yield depend on the conditions of the oxidation process, such as temperature, the partial pressure of oxygen (PO2), the initial concentration of glucose, the time, and the pH of the reaction medium.
The highest yield of glucaric acid is observed when the reaction proceeds in a neutral or slightly alkaline medium. The assessment of the influence of the partial pressure of oxygen on the yield of glucaric and gluconic acids in the reaction of glucose oxidation on the various catalysts under review [27] showed an increase in the yield of gluconic and glucaric acids when the partial pressure increases from 1 to 13.8 bar. When obtaining glucaric acid in sequential operation reactors, the first stage of the reaction can be conducted at a lower pressure, which increases the process efficiency.
As for the temperature of the process, it varied in the studies of different researchers from 333 to 353 K, and the highest yield of 82% was recorded at 333 K [202].
In comparison to chemical methods, glucose oxidation in the presence of heterogeneous catalysts containing noble metals seems to be preferable. When using this method, the amount of industrial waste is significantly reduced. There is no need to use aggressive chemical compounds, environmental safety is increased, and the process of separating the catalysts from the liquid reaction medium and their repeated use is simplified. Despite the fact that there is already much information on the reaction of the heterogeneous selective oxidation of glucose, the problem of optimizing the catalyst composition to achieve its high efficiency and stability in this process still remains unresolved.
Using the photocatalytic method of glucose oxidation to obtain gluconic and glucaric acids and a number of other products is known [139,140]. This review examines the latest published works on this topic.
Using SnO2/FePz(SBu)8 as a photocatalyst, the photocatalytic oxidation of glucose in water in aerated conditions upon exposure to light of the visible spectrum was reported [140]. Metallothioporphyrazines (MPzs), which include sulfur-containing groups at the periphery of the porphyrinic macrocycle, have an extensive system of delocalized π-electrons and are characterized by strong absorption in the visible region of light, which can improve the photocatalytic activity of the catalysts. When conducting the oxidation process with SnO2, the glucose conversion was only 6.4% with a selectivity of 28.7% for gluconic acid, while, when using FePz(SBu)8, the conversion was less than 0.1% and trace amounts of the products were observed. It turned out that a synergistic effect was observed when using SnO2/FePz(SBu)8, and it was possible to achieve a 34.2% conversion of glucose with a selectivity of 32.9% for gluconic acid and 12.9% for glucaric acid. In addition to these acids, formic acid and other organic acids were also formed. To achieve highly selective oxidation of glucose to gluconic and glucaric acids, the authors of [139] prepared a new composite TiO2/HPW/CoPz photocatalyst by modifying TiO2 by means of HPW (phosphotungstic acid) and CoPz (cobalt tetra (2-hydroxymethyl-1,4-dithiine)porphyrazine). It has been shown that for the photocatalyst of the TiO2/HPW(29%)/CoPz(1%) composition, the glucose conversion degree in the oxidation process under the action of light in the visible region of the spectrum is 22.2%, and the selectivity for gluconic acid is 63.5% and 16.9% for glucaric acid.
For the selective oxidation of glucose to glucaric acid, a photoanode was developed, which is a single-atom Pt anchored on defective TiO2 nanorod arrays. The high selectivity of the glucose oxidation process is achieved by optimizing the oxygen vacancies of the defective TiO2 photoanode. Using such a photoanode allowed an 84.3% yield of glucaric acid to be achieved in 5.5 h during a glucose conversion of 98.8% and a photocurrent density of 1.91 mAcm−2 [203].
Summarizing the review of the works devoted to using photocatalysts for the production of gluconic and glucaric acids during glucose oxidation, one can note that using solar energy, inexpensive, non-toxic metal oxide semiconductors, and mild conditions for the glucose oxidation process in an aqueous medium seems attractive. The latest results allow hope for the commercialization of these developments, but the search for an effective photocatalyst for this process is still an urgent task.
3.2.4. The Electrochemical Method of D-Glucose Oxidation to Glucaric Acid
During the electrochemical oxidation of glucose, both glucaric acid and gluconic acid are typically present in the reaction products, and the process is conducted using catalytic systems of various compositions.
To carry out the electrooxidation of glucose to gluconic acid and glucaric acid, Bin et al. [100] used an electrocatalytic reactor with a tubular porous titanium anode coated with nanosized MnO2 (MnO2/Ti electrode) and a stainless steel mesh as a cathode. The experiments were performed at a glucose concentration in water of 50.5 mol/L, a temperature of 303 K, a current density of 4 mA cm−2, and a pH of 7, using 4.98 mass. The percentage of MnO loading showed that, during the oxidation time (19 min), it was possible to achieve 98% glucose conversion and 98% total selectivity for gluconic and glucaric acids (43% selectivity for gluconic acid and 55% selectivity for glucaric acid), which was obtained during electrocatalysis. Increasing the current density up to 6 mA cm–2 allowed the conversion degree to be increased up to 99%, the total selectivity up to 99%, and the selectivity for glucaric acid up to 84%. At temperatures above plus 303 K, the selectivity and total conversion of glucose significantly decreased. The authors explain the achieved good results of controlled glucose oxidation by the mass transfer, enhanced by convection, as well as by the timely removal of the desired products from the reactor.
The use of TEMPO in the indirect electrochemical oxidation of D-glucose to glucaric acid in a cell with a graphite felt anode in combination with a stainless steel cathode was reported [204]. It was noted that, by optimizing such parameters as pH (12.2), temperature (plus 278 K), anode type, and the amount of the catalyst, glucaric acid can be obtained with a yield of 85%.
High glucose conversion and glucaric acid yield in 2 h of the process (98.3% and 83.3%, respectively) were obtained using complex catalytic systems based on nickel and iron (NiFeOx-NF). Faraday’s efficiency in this process was 87% [32]. According to the authors, the electrochemical oxidation of glucose proceeded in several stages: the oxidation of glucose to gluconic acid, in which two electrons are involved, and the oxidation of gluconic acid to glucaric acid, in which four electrons are involved (Figure 9) through an intermediate product (guluronic acid) [32].
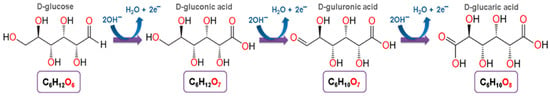
Figure 9.
The scheme of the electrochemical oxidation of glucose to gluconic, glucuronic, and glucaric acids.
The technical and economic analysis conducted by the authors [32] showed that such electrochemical production of glucaric acid is 54% cheaper than production requiring the application of currently used chemical methods.
The two-stage synthesis of D-glucaric acid through D-gluconic acid by the electrocatalytic oxidation of D-glucose on a gold electrode (in a batch operation cell) allowed D-glucaric acid to be obtained with a selectivity of 89.5%. Such high indicators of similar electrocatalytic systems indicate that such oxidation can be conducted effectively, which will be economically advantageous [202].
The parameters of the glucose electrooxidation on the various most promising catalysts, considered above, are provided in Table 5.

Table 5.
The summary of the glucose electrooxidation to glucaric acid.
Among the considered widely used methods (biochemical, catalytic) of glucose oxidation, electrochemical methods are, in our opinion, the most promising for obtaining glucaric acid, since they avoid the use of aggressive and toxic reagents and reduce the amount of waste. At present, electrochemical technologies have received a new impetus for development, since they satisfy most of the postulates of “green” chemistry, being a clean and carbon-neutral way to stimulate chemical transformations, and they can potentially use peak surpluses of renewable electric energy.
4. Conclusions
The processing of biomass waste into glucose and its subsequent conversion into valuable chemical products is a pressing issue in green chemistry. Both gluconic and glucaric acids have unique properties that put them in demand in a wide range of industries—from the food industry and pharmaceuticals to the production of polymers and cleaning agents. Glucaric acid, recognized as a “top value-added chemical”, has particularly high potential, although its market is still less developed.
The production of gluconic acid by biotechnology (Aspergillus niger, Gluconobacter oxydans) is a traditional industrial process. However, existing methods have limitations in the rate of the biotechnological process, are characterized by the complexity of product isolation, and lead to the formation of wastewater. There are alternative ways to obtain gluconic acid, such as heterogeneous catalysis, electrocatalysis, and photocatalysis, which can potentially increase the rate and simplify the process of obtaining gluconic acid as well as reduce the environmental burden. The main problems are the high cost of catalysts based on noble metals, their stability, and control of the selectivity of the target process. The immobilization of enzymes, such as glucose oxidase, will ensure high selectivity and the possibility of repeated use, especially when immobilized on the surface of magnetic supports. The key limitations are the cost of immobilization and the preservation of enzyme activity under industrial conditions.
The selective production of glucaric acid is much more difficult than gluconic acid. The traditional method of oxidation with nitric acid is ineffective and environmentally unsafe. A promising method for producing glucaric acid is the use of heterogeneous electro- and photocatalysis. However, selectivity remains the main problem due to competing reactions and the breaking of C-C bonds. Nevertheless, the development of catalysts (based on Pt, Au, NiFe, and MnO2 photocatalysts) will allow the achievement of high efficiency and potential economic benefits.
Key issues and promising research areas:
1. Increasing selectivity, especially in the synthesis of glucaric acid and using catalytic/photocatalytic methods, is a priority.
2. It is necessary to develop cheaper, more stable, and active catalytic systems (including base metals) and biocatalysts with enzymes (with improved stability and immobilization efficiency).
3. Promising laboratory developments (electro- and photocatalysis, enzymatic catalysis) require scaling and the assessment of technical and economic feasibility. The integration of different approaches is possible (for example, combining electro- or photocatalytic processes with enzymatic ones).
4. It is necessary to adapt the processes to work with real, less pure biomass hydrolysates and develop effective, cost-effective methods for isolating and purifying target products from complex mixtures.
5. Further research in the field of green chemistry, waste minimization, the use of renewable energy sources (especially for electro- and photocatalysis), and the evaluation of the process cycle are integral parts of future developments.
Thus, the conversion of glucose from biomass to gluconic and glucaric acids represents a dynamically developing field with great potential for the sustainable production of valuable chemicals. Overcoming the existing challenges requires an interdisciplinary approach that combines the efforts of chemists, biologists, and engineers, which will allow the full potential of these acids to be realized for the needs of modern industry.
Author Contributions
M.P.S.-S.—conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing. E.P.M.—conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing. S.A.G.—conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing. A.K.K.—project administration, resources, supervision, conceptualization, funding acquisition. R.K.—project administration, resources, supervision, conceptualization, funding acquisition. A.K.S.—formal analysis, investigation, methodology. S.S.—formal analysis, investigation, methodology. I.A.K.—project administration, resources, supervision, conceptualization, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education (MSHE) of the Russian Federation, grant No. 075-15-2023-468 and the Government of India, grant No. DST/INT/MSHE/P-02/2022(G), for providing the financial support of the India–Russia joint grant.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sharma, G.; Kaur, M.; Punj, S.; Singh, K. Biomass as a sustainable resource for value-added modern materials: A review. Biofuels Bioprod. Biorefining 2020, 14, 673–695. [Google Scholar] [CrossRef]
- Popp, J.; Kovács, S.; Oláh, J.; Divéki, Z.; Balázs, E. Bioeconomy: Biomass and biomass-based energy supply and demand. New Biotechnol. 2021, 60, 76–84. [Google Scholar] [CrossRef]
- Sri Shalini, S.; Palanivelu, K.; Ramachandran, A.; Raghavan, V. Biochar from biomass waste as a renewable carbon material for climate change mitigation in reducing greenhouse gas emissions—A review. Biomass Conv. Bioref 2021, 11, 2247–2267. [Google Scholar] [CrossRef]
- Cai, W.; Luo, Z.; Zhou, J.; Wang, Q. A review on the selection of raw materials and reactors for biomass fast pyrolysis in China. Fuel Process. Technol. 2021, 221, 106919. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Liao, Y.; Koelewijn, S.-F.; Van den Bossche, G.; Van Aelst, J.; Van den Bosch, S.; Renders, T.; Navare, K.; Nicolaï, T.; Van Aelst, K.; Maesen, M.; et al. A sustainable wood biorefinery for low–carbon footprint chemicals production. Science 2020, 367, 1385–1390. [Google Scholar] [CrossRef]
- Pang, S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sarsaiya, S.; Patel, A.; Juneja, A.; Singh, R.P.; Yan, B.; Awasthi, S.K.; Jain, A.; Liu, T.; Duan, Y.; et al. Refining biomass residues for sustainable energy and bio-products: An assessment of technology, its importance, and strategic applications in circular bio-economy. Renew. Sustain. Energy Rev. 2020, 127, 109876. [Google Scholar] [CrossRef]
- Sherwood, J. The significance of biomass in a circular economy. Bioresour. Technol. 2020, 300, 122755. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Tun, M.M.; Juchelkova, D.; Win, M.M.; Thu, A.M.; Puchor, T. Biomass Energy: An Overview of Biomass Sources, Energy Potential, and Management in Southeast Asian Countries. Resources 2019, 8, 81. [Google Scholar] [CrossRef]
- Tun, M.M.; Juchelková, D. Biomass Sources and Energy Potential for Energy Sector in Myanmar: An Outlook. Resources 2019, 8, 102. [Google Scholar] [CrossRef]
- Duque, A.; Álvarez, C.; Doménech, P.; Manzanares, P.; Moreno, A.D. Advanced Bioethanol Production: From Novel Raw Materials to Integrated Biorefineries. Processes 2021, 9, 206. [Google Scholar] [CrossRef]
- Álvarez, C.; Mullen, A.M.; Pojić, M.; Hadnađev, T.D.; Papageorgiou, M. Classification and Target Compounds. In Food Waste Recovery; Academic Press: Cambridge, MA, USA, 2021; pp. 21–49. [Google Scholar]
- Galanakis, C.M. Food Waste Recovery: Processing Technologies and Industrial Techniques; Academic Press: Amsterdam, The Netherlands, 2020; p. 560. [Google Scholar]
- Singh, R.; Das, R.; Sangwan, S.; Rohatgi, B.; Khanam, R.; Peera, S.K.P.G.; Das, S.; Lyngdoh, Y.A.; Langyan, S.; Shukla, A.; et al. Utilisation of agro-industrial waste for sustainable green production: A review. Environ. Sustain. 2021, 4, 619–636. [Google Scholar] [CrossRef]
- Kukharets, V.; Juočiūnienė, D.; Hutsol, T.; Sukmaniuk, O.; Čėsna, J.; Kukharets, S.; Piersa, P.; Szufa, S.; Horetska, I.; Shevtsova, A. An Algorithm for Managerial Actions on the Rational Use of Renewable Sources of Energy: Determination of the Energy Potential of Biomass in Lithuania. Energies 2023, 16, 548. [Google Scholar] [CrossRef]
- Freitas, L.C.; Barbosa, J.R.; da Costa, A.L.C.; Bezerra, F.W.F.; Pinto, R.H.H.; de Carvalho Junior, R.N. From waste to sustainable industry: How can agro-industrial wastes help in the development of new products? Resour. Conserv. Recycl. 2021, 169, 105466. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, R.; Zicari, S. Integrated Processing Technologies for Food and Agricultural By-Products; Academic Press: London, UK; San Diego, CA, USA, 2019. [Google Scholar]
- Mansor, A.M.; Lim, J.S.; Ani, F.N.; Hashim, H.; Ho, W.S. Characteristics of Cellulose, Hemicellulose and Lignin of MD2 Pineapple Biomass. Chem. Eng. Trans. 2019, 72, 79–84. [Google Scholar] [CrossRef]
- Rusanen, A.; Lappalainen, K.; Kärkkäinen, J.; Tuuttila, T.; Mikola, M.; Lassi, U. Selective hemicellulose hydrolysis of Scots pine sawdust. Biomass Convers. Biorefinery 2019, 9, 283–291. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green. Chem. 2013, 15, 1095. [Google Scholar] [CrossRef]
- Meenakshi, A.; Kumaresan, R. Ethanol production from corn, potato peel waste and its process development. Int. J. Chemtech Res. 2014, 6, 2843. [Google Scholar]
- Gharib-Bibalan, S. High Value-added Products Recovery from Sugar Processing By-products and Residuals by Green Technologies: Opportunities, Challenges, and Prospects. Food Eng. Rev. 2018, 10, 95–111. [Google Scholar] [CrossRef]
- Industrial Glucose Global Market Report. 2025. Available online: https://www.thebusinessresearchcompany.com/report/industrial-glucose-global-market-report (accessed on 26 February 2025).
- Machado, J.V.; Silva, M.L.A.; Silva, C.L.S.; Correia, M.C.G.; Ruy, A.D.S.; Pontes, L.A.M. Catalysts and processes for gluconic and glucaric acids production: A comprehensive review of market and chemical routes. Catal. Commun. 2023, 182, 106740. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, Z.; Yu, I.K.M.; Tsang, D.C.W. Sustainable production of high-value gluconic acid and glucaric acid through oxidation of biomass-derived glucose: A critical review. J. Clean. Prod. 2021, 312, 127745. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, C.; Liu, K.; Wang, Y.; Fan, G.; Sun, S.; Xu, J.; Zhu, Y.; Li, Y. Aqueous-phase hydrogenolysis of glucose to value-added chemicals and biofuels: A comparative study of active metals. Biomass Bioenergy 2015, 72, 189–199. [Google Scholar] [CrossRef]
- Lee, J.; Jung, S.; Kim, Y.T.; Kim, H.J.; Kim, K.-H. Catalytic and electrocatalytic conversion of glucose into value-added chemicals. Renew. Sustain. Energy Rev. 2023, 181, 113337. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Roncal, T. Production of Sorbitol from Biomass. In Production of Platform Chemicals from Sustainable Resources. Biofuels and Biorefineries; Fang, Z., Smith, R., Jr., Qi, X., Eds.; Springer: Singapore, 2017; pp. 265–309. [Google Scholar] [CrossRef]
- Liu, W.-J.; Xu, Z.; Zhao, D.; Pan, X.-Q.; Li, H.-C.; Hu, X.; Fan, Z.-Y.; Wang, W.-K.; Zhao, G.-H.; Jin, S.; et al. Efficient electrochemical production of glucaric acid and H2 via glucose electrolysis. Nat. Commun. 2020, 11, 265. [Google Scholar] [CrossRef]
- Anastassiadis, S.; Morgunov, I.G. Gluconic Acid Production. Recent. Pat. Biotechnol. 2007, 1, 167. [Google Scholar] [CrossRef]
- Cañete-Rodríguez, A.M.; Santos-Dueñas, I.M.; Jiménez-Hornero, J.E.; Ehrenreich, A.; Liebl, W.; García-García, I. Gluconic acid: Properties, production methods and applications—An excellent opportunity for agro-industrial by-products and waste bio-valorization. Process Biochem. 2016, 51, 1891–1903. [Google Scholar] [CrossRef]
- Wulaningsih, T.I. Gluconolactone in Cosmetic (Review Article). KESANS Int. J. Health Sci. 2023, 2, 730–745. [Google Scholar] [CrossRef]
- Ataman Chemicals. Gluconic Acid. Available online: https://www.atamanchemicals.com/gluconic-acid_u32471/ (accessed on 26 February 2025).
- Market Research Future, Gluconic Acid Market Overview. Available online: https://www.marketresearchfuture.com/reports/gluconic-acid-market-21714 (accessed on 26 February 2025).
- Kirimura, K.; Yoshioka, I. Gluconic and Itaconic Acids. In Comprehensive Biotechnol; Elsevier: Amsterdam, The Netherlands, 2019; pp. 166–171. [Google Scholar]
- Gluconic Acid Market Size By Application, By Downstream Potential Analysis, Share, Growth Forecast, 2024–2032. 2024. Available online: https://www.gminsights.com/industry-analysis/gluconic-acid-market (accessed on 26 February 2025).
- Kornecki, J.F.; Carballares, D.; Tardioli, P.W.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Enzyme production of D-gluconic acid and glucose oxidase: Successful tales of cascade reactions. Catal. Sci. Technol. 2020, 10, 5740–5771. [Google Scholar] [CrossRef]
- Hustede, H.; Haberstroh, H.; Schinzig, E. Gluconic Acid. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2000; Volume 15, pp. 211–216. [Google Scholar] [CrossRef]
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic Acid: Properties, Applications and Microbial Production. Food Technol. Biotechnol. 2006, 44, 185–195. Available online: http://ir.niist.res.in:8080/jspui/handle/123456789/2172 (accessed on 26 February 2025).
- Choi, I.; Zhong, Q. Gluconic acid as a chelator to improve clarity of skim milk powder dispersions at pH 3.0. Food Chem. 2021, 344, 128639. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Kumar, R.; Banerjee, S. Manufacture of gluconic acid: A review towards process intensification for green production. Chem. Eng. Process. Process Intensif. 2016, 104, 160–171. [Google Scholar] [CrossRef]
- Ramachandran, S.; Nair, S.; Larroche, C.; Pandey, A. Gluconic Acid. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 577–599. [Google Scholar] [CrossRef]
- Jungbunzlauer Suisse AG. Glucono-delta-Lactone. Available online: https://www.jungbunzlauer.com/en/products/gluconates/glucono-delta-lactone (accessed on 26 February 2025).
- Yadav, P.; Chauhan, A.K.; Singh, R.B.; Khan, S.; Halabi, G. Organic Acids: Microbial Sources, Production, and Applications. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Academic Press: Cambridge, MA, USA, 2022; pp. 325–337. [Google Scholar] [CrossRef]
- Mycielska, M.E.; Mohr, M.T.J.; Schmidt, K.; Drexler, K.; Rümmele, P.; Haferkamp, S.; Schlitt, H.J.; Gaumann, A.; Adamski, J.; Geissler, E.K. Potential Use of Gluconate in Cancer Therapy. Front. Oncol. 2019, 9, 522. [Google Scholar] [CrossRef]
- Guo, Y.; Messner, F.; Beck, S.E.; Lozano, M.I.; Schwelberger, H.; Zhang, Y.; Kammers, K.; Oh, B.C.; Greene, E.D.; Brandacher, G.; et al. Gluconate-Lactobionate-Dextran Perfusion Solutions Attenuate Ischemic Injury and Improve Function in a Murine Cardiac Transplant Model. Cells 2022, 11, 1653. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, Y.; Chensny, L.; Shepherd, R.E.; Zhang, S.; Kortes, R.; John, K. Lactobionic and gluconic acid complexes of FeII and FeIII; control of oxidation pathways by an organ transplantation preservant. J. Inorg. Biochem. 1993, 49, 23–48. [Google Scholar] [CrossRef]
- Mossad, S.B.; Macknin, M.L.; Mendendorp, S.V.; Mason, P. Zinc gluconate lozenges for treating the common cold. A randomized, double-blind, placebo-controlled study. Ann. Intern. Med. 1996, 125, 81–88. [Google Scholar] [CrossRef]
- Roxas, M.; Jurenka, J. Colds and Influenza: A Review of Diagnosis and Conventional, Botanical, and Nutritional Considerations. Altern. Med. Rev. 2007, 12, 25–48. Available online: https://anaturalhealingcenter.com/documents/Thorne/articles/Influenza12-1.pdf (accessed on 16 April 2025).
- Zeichner, J.A. Cosmeceuticals for the Treatment of Acne Vulgaris In Cosmeceuticals and Cosmetic Practice; John Wiley & Sons: Chichester, UK, 2013; pp. 209–217. [Google Scholar] [CrossRef]
- Morelli, V.; Calmet, E.; Jhingade, V. Alternative Therapies for Common Dermatologic Disorders, Part 2. Prim. Care Clin. Off. Pr. 2010, 37, 285–296. [Google Scholar] [CrossRef]
- Mycielska, M.E.; Dettmer, K.; Rümmele, P.; Schmidt, K.; Prehn, C.; Milenkovic, V.M.; Jagla, W.; Madej, M.G.; Lantow, M.; Schladt, M.; et al. Extracellular citrate affects critical elements of cancer cell metabolism and supports cancer development in vivo. Cancer Res. 2018, 78, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Monosaccharides, Disaccharides, and Related Ingredients as Used in Cosmetics. Int. J. Toxicol. 2019, 38, 5S–38S. [Google Scholar] [CrossRef]
- Woźniak, B.; Chudzińska, J.; Szczyglewska, P.; Nowak, I.; Feliczak-Guzik, A. Optimization of the Composition of a Cosmetic Formulation Containing Tremella fuciformis Extract (Fungi). Cosmetics 2023, 10, 82. [Google Scholar] [CrossRef]
- Green, B.A. Cosmeceutical Uses and Benefits of Alpha, Poly and Bionic Hydroxy Acids. In Cosmeceuticals and Cosmetic Practice; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 67–80. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; Lu, C.; Huang, Z. Effects of sodium gluconate on early hydration and mortar performance of Portland cement-calcium aluminate cement-anhydrite binder. Constr. Build. Mater. 2017, 157, 1065–1073. [Google Scholar] [CrossRef]
- Dai, L.; Lian, Z.; Zhang, R.; Nawaz, A.; ul Haq, I.; Zhou, X.; Xu, Y. Multi-strategy in production of high titer gluconic acid by the fermentation of concentrated cellulosic hydrolysate with Gluconobacter oxydans. Ind. Crops Prod. 2022, 189, 115748. [Google Scholar] [CrossRef]
- Qi, H.; Ma, B.; Tan, H.; Li, C.; Zhi, Z.; Wang, H.; Liu, X.; Yang, Q. A review of zinc-based battery from alkaline to acid. J. Mol. Model. 2020, 26, 45. [Google Scholar] [CrossRef]
- Seymour, D.J.; Sanz-Fernandez, M.V.; Daniel, J.B.; Martín-Tereso, J.; Doelman, J. Effects of supplemental calcium gluconate embedded in a hydrogenated fat matrix on lactation, digestive, and metabolic variables in dairy cattle. J. Dairy Sci. 2021, 104, 7845–7855. [Google Scholar] [CrossRef]
- McKnight, L.L.; Doelman, J.; Carson, M.; Waterman, D.F.; Metcalf, J.A. Effect of hydrogenated fat-embedded calcium gluconate on lactation performance in dairy cows. Can. J. Anim. Sci. 2019, 99, 563–569. [Google Scholar] [CrossRef]
- Leonhard-Marek, S.; Becker, G.; Breves, G.; Schröder, B. Chloride, Gluconate, Sulfate, and Short-Chain Fatty Acids Affect Calcium Flux Rates Across the Sheep Forestomach Epithelium. J. Dairy Sci. 2007, 90, 1516–1526. [Google Scholar] [CrossRef]
- Doelman, J.; McKnight, L.L.; Carson, M.; Nichols, K.; Waterman, D.F.; Metcalf, J.A. Postruminal infusion of calcium gluconate increases milk fat production and alters fecal volatile fatty acid profile in lactating dairy cows. J. Dairy Sci. 2019, 102, 1274–1280. [Google Scholar] [CrossRef]
- Kislyakova, E.M.; Vorobyova, S.L.; Kokonov, S.I. Influence of innovative calcium-containing additive on growth and development of heifer replacement. IOP Conf. Ser. Earth Environ. Sci. 2019, 315, 062020. [Google Scholar] [CrossRef]
- Zheng, L.; Hao, X.; Liu, Z.; Peng, H.; Chen, J.; Huang, W.; Yu, F. Foliar spraying of potassium gluconate promotes the synthesis of the seed oil of Styrax tonkinensis. J. Am. Oil Chem. Soc. 2023, 100, 623–634. [Google Scholar] [CrossRef]
- Magalhães Júnior, A.I.; Soccol, C.R.; Camara, M.C.; Aulestia, D.T.M.; de Souza Vandenberghe, L.P.; de Carvalho, J.C. Challenges in the production of second-generation organic acids (potential monomers for application in biopolymers). Biomass Bioenergy 2021, 149, 106092. [Google Scholar] [CrossRef]
- Saulnier, B.; Coudane, J.; Garreau, H.; Vert, M. Hydroxyl-bearing poly(α-hydroxy acid)-type aliphatic degradable polyesters prepared by ring opening (co)polymerization of dilactones based on glycolic, gluconic and l-lactic acids. Polymer 2006, 47, 1921–1929. [Google Scholar] [CrossRef]
- Saulnier, B.; Ponsart, S.; Coudane, J.; Garreau, H.; Vert, M. Lactic acid-based functionalized polymers via copolymerization and chemical modification. Macromol. Biosci. 2004, 4, 232–237. [Google Scholar] [CrossRef]
- Abtew, E.; Ezra, A.; Basu, A.F.; Domb, A.J. Biodegradable Poly(Acetonide Gluconic Acid) for Controlled Drug Delivery. Biomacromolecules 2019, 20, 2934–2941. [Google Scholar] [CrossRef]
- Lim, H.Y.; Dolzhenko, A.V. Gluconic acid aqueous solution: A bio-based catalytic medium for organic synthesis. Sustain. Chem. Pharm. 2021, 21, 100443. [Google Scholar] [CrossRef]
- Contreras-Esquivel, J.C.; Vasquez-Mejia, M.-J.; Sañudo-Barajas, A.; Vazquez-Vuelvas, O.F.; Galindo-Musico, H.; Velez-de-la-Rocha, R.; Perez-Cruz, C.; Balagurusamy, N. Gluconic Acid as a New Green Solvent for Recovery of Polysaccharides by Clean Technologies. In Alternative Solvents for Natural Products Extraction, Green Chemistry and Sustainable Technology; Chemat, F., Abert Vian, M., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2014; pp. 237–251. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, J.; Li, M.; Gu, Y. Gluconic acid aqueous solution as a sustainable and recyclable promoting medium for organic reactions. Green. Chem. 2011, 13, 2204. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, B.; Li, M.; Gu, Y. Gluconic acid aqueous solution: A task-specific bio-based solvent for ring-opening reactions of dihydropyrans. Tetrahedron 2013, 69, 1057–1064. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- CORE. Gluconic Acid and Its Derivatives—OECD SIDS. Available online: https://core.ac.uk/download/pdf/24065748.pdf (accessed on 26 February 2025).
- Mao, Y.; Jie, Y.; Zhi-hua, S.; Hui, C. Ferrous-gluconic acid compound tanning: A cleaner chrome-free tanning system. J. Am. Leather Chem. Assoc. 2013, 108, 257–265. [Google Scholar]
- Rezanezhad, S.; Nazarnezhad, N.; Resalati, H.; Zabihzadeh, S.M. The use of gluconic acid as an additive in magnetic paper. BioResources 2021, 17, 342–354. [Google Scholar] [CrossRef]
- Survila, A.; Mockus, Z.; Kanapeckaite, S.; Pileckiene, J.; Stalnionis, G. Cathodic processes in copper (II) solutions containing gluconic acid. Russ. J. Electrochem. 2011, 47, 129–135. [Google Scholar] [CrossRef]
- Talawat, S.; Ahantharik, P.; Laohawiwattanakul, S.; Premsuk, A.; Ratanapo, S. Considerations on the in-vitro inhibitor effect of kombucha on pseudomonas aeruginosa isolates from female urethral and periurethral area. J. Nat. Sci. 2006, 40, 925–933. [Google Scholar]
- Kaewkod, T.; Bovonsombut, S.; Tragoolpua, Y. Efficacy of kombucha obtained from green, oolong, and black teas on inhibition of pathogenic bacteria, antioxidation, and toxicity on colorectal cancer cell line. Microorganisms 2019, 7, 700. [Google Scholar] [CrossRef]
- Li, R.; Xu, Y.; Chen, J.; Wang, F.; Zou, C.; Yin, J. Enhancing the proportion of gluconic acid with a microbial community reconstruction method to improve the taste quality of Kombucha. LWT—Food Sci. Technol. 2022, 155, 112937. [Google Scholar] [CrossRef]
- Ma, J.-L.; Chen, H.-J.; Chung, K.-C.; Wu, Y.-F.; Yen, C.-Y.; Li, W.J.; Lin, H.-C. Effect of sodium gluconate addition on anomalous codeposition of electroplated nickel-iron alloys. Thin Solid. Film. 2024, 794, 140292. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Shi, D.; Fugen, S. Synthesis and application of low-cost layered double hydroxides intercalated by gluconic acid anion for flame retardancy and tensile strength conservation of high filling epoxy resin. J. Colloid. Interface Sci. 2021, 594, 791–801. [Google Scholar] [CrossRef]
- Ajala, E.O.; Ajala, M.A.; Ogunniyi, D.S.; Sunmonu, M.O. Kinetics of gluconic acid production and cell growth in a batch bioreactor by Aspergillus niger using breadfruit hydrolysate. J. Food Process Eng. 2016, 40, e12461. [Google Scholar] [CrossRef]
- Fernandes, S.; Dias, B.; Belo, I.; Lopes, M. Enhancement of gluconic acid production by Aspergillus niger from by-products as glucose source using pressurized air conditions. J. Chem. Technol. Biotechnol. 2023, 98, 2146–2153. [Google Scholar] [CrossRef]
- Dai, L.; Lian, Z.; Fu, Y.; Zhou, X.; Xu, Y.; Zhou, X.; Kuznetsov, B.N.; Jiang, K. Low pH Stress Enhances Gluconic Acid Accumulation with Enzymatic Hydrolysate as Feedstock Using Gluconobacter oxydans. Fermentation 2023, 9, 278. [Google Scholar] [CrossRef]
- Lu, F.; Ping, K.; Wen, L.; Zhao, W.; Wang, Z.; Chu, J.; Zhuang, Y. Enhancing Gluconic Acid Production by Controlling the Morphology of Aspergillus niger in Submerged Fermentation. Process Biochem. 2015, 50, 1342–1348. [Google Scholar] [CrossRef]
- Dowdells, C.; Jones, R.L.; Mattey, M.; Benčina, M.; Legiša, M.; Mousdale, D.M. Gluconic acid production by Aspergillus terreus. Lett. Appl. Microbiol. 2010, 51, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Belo, I.; Lopes, M. Highly aerated cultures boost gluconic acid production by the yeast-like fungus Aureobasidium pullulans. Biochem. Eng. J. 2021, 175, 108133. [Google Scholar] [CrossRef]
- Fernandes, S.; Dias, B.; Gonçalves, D.A.; Nobre, C.; Belo, I.; Lopes, M. Co-production of Gluconic Acid and Fructo-oligosaccharides by Aureobasidium pullulans from Sugarcane Molasses: Effect of Oxygen Transfer Rate in Stirred Tank and Airlift Bioreactors. Food Bioprocess. Technol. 2023, 17, 1321–1334. [Google Scholar] [CrossRef]
- Cañete-Rodríguez, A.M.; Santos-Dueñas, I.M.; Torija-Martínez, M.J.; Mas, A.; Jiménez-Hornero, J.E.; García-García, I. Preparation of a pure inoculum of acetic acid bacteria for the selective conversion of glucose in strawberry purée into gluconic acid. Food Bioprod. Process. 2015, 96, 35–42. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Wei, D.; Shi, J.; Kim, C.H.; Jiang, B.; Han, Z.; Hao, J. Gluconic acid production by gad mutant of Klebsiella pneumoniae. World J. Microbiol. Biotechnol. 2016, 32, 132. [Google Scholar] [CrossRef]
- Purane, N.K.; Sharma, S.K.; Topre, S.D.; Panikar, S.S.; Labade, D.S. To study the various parameters for bioconversion of glucose to gluconic acid by Penicillium chrysogenum in submerged culture. Recent. Res. Sci. Technol. 2011, 3, 88–91. Available online: https://core.ac.uk/download/pdf/236009230.pdf (accessed on 26 February 2025).
- Ahmed, A.S.; Farag, S.S.; Hassan, I.A.; Botros, H.W. Production of gluconic acid by using some irradiated microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 374–380. [Google Scholar] [CrossRef]
- Han, X.; Liu, G.; Pan, Y.; Song, W.; Qu, Y. Consolidated bioprocessing for sodium gluconate production from cellulose using Penicillium oxalicum. Bioresour. Technol. 2018, 251, 407–410. [Google Scholar] [CrossRef]
- Folle, A.B.; Baschera, V.M.; Vivan, L.T.; Carra, S.; Polidoro, T.A.; Malvessi, E.; da Silveira, M.M. Assessment of different systems for the production of aldonic acids and sorbitol by calcium alginate-immobilized Zymomonas mobilis cells. Bioprocess. Biosyst. Eng. 2017, 41, 185–194. [Google Scholar] [CrossRef]
- Zhang, H.; Toshima, N. Glucoseoxidation using Au-containing bimetallic and trimetallic nanoparticles. Catal. Sci. Technol. 2013, 3, 268–278. [Google Scholar] [CrossRef]
- Bin, D.; Wang, H.; Li, J.; Wang, H.; Yin, Z.; Kang, J.; He, B.; Li, Z. Controllable Oxidation of Glucose to Gluconic Acid and Glucaric Acid Using an Electrocatalytic Reactor. Electrochim. Acta 2014, 130, 170–178. [Google Scholar] [CrossRef]
- Okatsu, H.; Kinoshita, N.; Akita, T.; Ishida, T.; Haruta, M. Deposition of gold nanoparticles on carbons for aerobic glucose oxidation. Appl. Catal. A Gen. 2009, 369, 8–14. [Google Scholar] [CrossRef]
- Bao, J.; Furumoto, K.; Fukunaga, K.; Nakao, K. A Kinetic Study on Air Oxidation of Glucose Catalyzed by Immobilized Glucose Oxidase for Production of Calcium Gluconate. Biochem. Eng. J. 2001, 8, 91–102. [Google Scholar] [CrossRef]
- Liu, A.; Huang, Z.; Wang, X. Efficient Oxidation of Glucose into Gluconic Acid Catalyzed by Oxygen-Rich Carbon Supported Pd Under Room Temperature and Atmospheric Pressure. Catal. Lett. 2018, 148, 2019–2029. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Chi, Q.; Liu, X.; Chen, L. Cellulose-supported Pd nanoparticles: Effective for the selective oxidation of glucose into gluconic acid. Polym. Bull. 2020, 77, 1003–1014. [Google Scholar] [CrossRef]
- Jin, X.; Zhao, M.; Shen, J.; Yan, W.; He, L.; Thapa, P.S.; Ren, S.; Subramaniam, B.; Chaudhari, R.V. Exceptional performance of bimetallic Pt1Cu3/TiO2 nanocatalysts for oxidation of gluconic acid and glucose with O2 to glucaric acid. J. Catal. 2015, 330, 323–329. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on platinum metal catalysts in aqueous solutions. Catal. Today 1994, 19, 247–283. [Google Scholar] [CrossRef]
- Cattaneo, S.; Stucchi, M.; Villa, A.; Prati, L. Gold Catalysts for the Selective Oxidation of Biomass-Derived Products. ChemCatChem 2019, 11, 309–323. [Google Scholar] [CrossRef]
- Karski, S.; Paryjczak, T.; Witonñska, I. Selective Oxidation of Glucose to Gluconic Acid over Bimetallic Pd–Me Catalysts (Me = Bi, Tl, Sn, Co). Kinet. Catal. 2003, 44, 618–622. [Google Scholar] [CrossRef]
- Witońska, I.; Frajtak, M.; Karski, S. Selective oxidation of glucose to gluconic acid over Pd–Te supported catalysts. Appl. Catal. A Gen. 2011, 401, 73–82. [Google Scholar] [CrossRef]
- Sandu, M.P.; Kovtunov, M.A.; Baturin, V.S.; Oganov, A.R.; Kurzina, I.A. Influence of the Pd : Bi ratio on Pd-Bi/Al2O3 catalysts: Structure, surface and activity in glucose oxidation. Phys. Chem. Chem. Phys. 2021, 23, 14889–14897. [Google Scholar] [CrossRef]
- Sandu, M.P.; Kovtunov, M.A.; Gromov, N.V.; Kurzina, I.A. Effects of external parameters and mass-transfer on the glucose oxidation process catalyzed by Pd–Bi/Al2O3. New J. Chem. 2021, 45, 22289–22298. [Google Scholar] [CrossRef]
- Biswas, S.; Pal, A.; Pal, T. Supported metal and metal oxide particles with proximity effect for catalysis. RSC Adv. 2020, 10, 35449–35472. [Google Scholar] [CrossRef]
- Pu, T.; Zhang, W.; Zhu, M. Engineering Heterogeneous Catalysis with Strong Metal–Support Interactions: Characterization, Theory and Manipulation. Angew. Chem. Int. Ed. 2022, 62, e202212278. [Google Scholar] [CrossRef]
- Lang, R.; Du, X.; Huang, Y.; Jiang, X.; Zhang, Q.; Guo, Y.; Liu, K.; Qiao, B.; Wang, A.; Zhang, T. Single-Atom Catalysts Based on the Metal-Oxide Interaction. Chem. Rev. 2020, 120, 11986–12043. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Ivanova, S.; López-Cartes, C.; Centeno, M.A.; Odriozola, J.A. Gold catalysts screening in base-free aerobic oxidation of glucose to gluconic acid. Catal. Today 2017, 279, 148–154. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, X.; Iqbal, S.; Miedziak, P.J.; Edwards, J.K.; Armstrong, R.D.; Morgan, D.J.; Wang, J.; Hutchings, G.J. Base-free oxidation of glucose to gluconic acid using supported gold catalysts. Catal. Sci. Technol. 2016, 6, 107–117. [Google Scholar] [CrossRef]
- Qi, P.; Chen, S.; Chen, J.; Zheng, J.; Zheng, X.; Yuan, Y. Catalysis and Reactivation of Ordered Mesoporous Carbon-Supported Gold Nanoparticles for the Base-Free Oxidation of Glucose to Gluconic Acid. ACS Catal. 2015, 5, 2659–2670. [Google Scholar] [CrossRef]
- Delidovich, I.V.; Moroz, B.L.; Taran, O.P.; Gromov, N.V.; Pyrjaev, P.A.P.A.P.A.; Prosvirin, I.P.; Bukhtiyarov, V.I.; Parmon, V.N. Aerobic selective oxidation of glucose to gluconate catalyzed by Au/Al2O3 and Au/C: Impact of the mass-transfer processes on the overall kinetics. Chem. Eng. J. 2013, 223, 921–931. [Google Scholar] [CrossRef]
- Liu, M.; Jin, X.; Zhang, G.; Xia, Q.; Lai, L.; Wang, J.; Zhang, W.; Sun, Y.; Ding, J.; Yan, H.; et al. Bimetallic AuPt/TiO2 Catalysts for Direct Oxidation of Glucose and Gluconic Acid to Tartaric Acid in the Presence of Molecular O2. ACS Catal. 2020, 10, 10932–10945. [Google Scholar] [CrossRef]
- Zhuge, Y.; Fan, G.; Lin, Y.; Yang, L.; Li, F. A hybrid composite of hydroxyapatite and Ca–Al layered double hydroxide supported Au nanoparticles for highly efficient base-free aerobic oxidation of glucose. Dalton Trans. 2019, 48, 9161–9172. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, D.; Sun, Y.; Zhou, Z.; Du, Y.; Du, Y.; Li, Y.; Liu, M.; Zhang, Y.; Shen, J.; et al. Structural sensitivity of heterogeneous catalysts for sustainable chemical synthesis of gluconic acid from glucose. Chin. J. Catal. 2020, 41, 1320–1336. [Google Scholar] [CrossRef]
- Arias, P.L.; Cecilia, J.A.; Gandarias, I.; Iglesias, J.; Granados, M.L.; Mariscal, R.; Morales, G.; Moreno-Tost, R.; Maireles-Torres, P. Oxidation of lignocellulosic platform molecules to value-added chemicals using heterogeneous catalytic technologies. Catal. Sci. Technol. 2020, 10, 2721–2757. [Google Scholar] [CrossRef]
- Wenkin, M.; Ruiz, P.; Delmon, B.; Devillers, M. The role of bismuth as promoter in Pd–Bi catalysts for the selective oxidation of glucose to gluconate. J. Mol. Catal. A-Chem. 2002, 180, 141–159. [Google Scholar] [CrossRef]
- Khawaji, M.; Zhang, Y.; Loh, M.; Graça, I.; Ware, E.; Chadwick, D. Composition dependent selectivity of bimetallic Au-Pd NPs immobilised on titanate nanotubes in catalytic oxidation of glucose. Appl. Catal. B Environ. 2019, 256, 117799. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Bobadilla, L.F.; Ivanova, S.; Penkova, A.; Centeno, M.A.; Odriozola, J.A. Gold catalyst recycling study in base-free glucose oxidation reaction. Catal. Today 2018, 301, 72–77. [Google Scholar] [CrossRef]
- Delidovich, I. Toward understanding base-catalyzed isomerization of saccharides. ACS Catal. 2023, 13, 2250–2267. [Google Scholar] [CrossRef]
- Fischer, M.; Drabo, P.; Delidovich, I. Study of base-catalyzed isomerization of d-glucose with a focus on reaction kinetics. React. Kinet. Mech. Catal. 2022, 135, 2357–2377. [Google Scholar] [CrossRef]
- Antolini, E. External abiotic glucose fuel cells. Sustain. Energy Fuels 2021, 5, 5038–5060. [Google Scholar] [CrossRef]
- Schlegel, N.; Bagger, A.; Rossmeisl, J.; Arenz, M. Elucidating the Reaction Pathway of Glucose Electrooxidation to Its Valuable Products: The Influence of Mass Transport and Electrode Potential on the Product Distribution. J. Phys. Chem. 2023, 127, 18609–18618. [Google Scholar] [CrossRef]
- Rafaïdeen, T.; Baranton, S.; Coutanceau, C. Highly efficient and selective electrooxidation of glucose and xylose in alkaline medium at carbon supported alloyed PdAu nanocatalysts. Appl. Catal. 2019, 243, 641–656. [Google Scholar] [CrossRef]
- Moggia, G.; Kenis, T.; Daems, N.; Breugelmans, T. Electrochemical Oxidation of d-Glucose in Alkaline Medium: Impact of Oxidation Potential and Chemical Side Reactions on the Selectivity to d-Gluconic and d-Glucaric Acid. ChemElectroChem 2020, 7, 86–95. [Google Scholar] [CrossRef]
- Pomilla, F.R.; García-López, E.I.; Marcì, G.; Palmisano, L.; Parrino, F. Heterogeneous photocatalytic materials for sustainable formation of high-value chemicals in green solvents. Mater. Today Sustain. 2021, 13, 100071. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, Q.; Yang, C.; Zhang, B.; Deng, K. Photocatalytic oxidation of glucose in water to value-added chemicals by zinc oxide-supported cobalt thioporphyrazine. Catal. Sci. Technol. 2019, 9, 6909–6919. [Google Scholar] [CrossRef]
- Roongraung, K.; Chuangchote, S.; Laosiripojana, N. Enhancement of Photocatalytic Oxidation of Glucose to Value-Added Chemicals on TiO2 Photocatalysts by A Zeolite (Type Y) Support and Metal Loading. Catalysts 2020, 10, 423. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A.; Bielejewska, A. High-value chemicals obtained from selective photo-oxidation of glucose in the presence of nanostructured titanium photocatalysts. Bioresour. Technol. 2011, 102, 11254–11257. [Google Scholar] [CrossRef]
- Chen, R.; Yang, C.; Zhang, Q.; Zhang, B.; Deng, K. Visible-light-driven selective oxidation of glucose in water with H-ZSM-5 zeolite supported biomimetic photocatalyst. J. Catal. 2019, 374, 297–305. [Google Scholar] [CrossRef]
- Dubinina, T.V.; Borisova, N.E.; Sedova, M.V.; Tomilova, L.G.; Furuyama, T.; Kobayashi, N. Synthesis and spectral properties of nonclassical binuclear thienoporphyrazines. Dye. Pigment. 2015, 117, 1–6. [Google Scholar] [CrossRef]
- Li, H.; Cao, L.; Yang, C.; Zhang, Z.; Zhang, B.; Deng, K. Selective oxidation of benzyl alcohols to benzoic acid catalyzed by eco-friendly cobalt thioporphyrazine catalyst supported on silica-coated magnetic nanospheres. J. Environ. Sci. 2017, 60, 84–90. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, Q.; Yang, C.; Zhang, B.; Deng, K. Highly selective oxidation of glucose to gluconic acid and glucaric acid in water catalyzed by an efficient synergistic photocatalytic system. Catal. Sci. Technol. 2020, 10, 2231–2241. [Google Scholar] [CrossRef]
- Zhang, Q.; Ge, Y.; Yang, C.; Zhang, B.; Deng, K. Enhanced photocatalytic performance for oxidation of glucose to value-added organic acids in water using iron thioporphyrazine modified SnO2. Green. Chem. 2019, 21, 5019–5029. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiang, X.; Ge, Y.; Yang, C.; Zhang, B.; Deng, K. Selectivity enhancement in the g-C3N4-catalyzed conversion of glucose to gluconic acid and glucaric acid by modification of cobalt thioporphyrazine. J. Catal. 2020, 388, 11–19. [Google Scholar] [CrossRef]
- Bai, X.; Hou, Q.; Qian, H.; Nie, Y.; Xia, T.; Lai, R.; Yu, G.; Rehman, M.L.U.; Xie, H.; Ju, M. Selective oxidation of glucose to gluconic acid and glucaric acid with chlorin e6 modified carbon nitride as metal-free photocatalyst. Appl. Catal. B Environ. 2022, 303, 120895. [Google Scholar] [CrossRef]
- Zu, M.; Zhou, X.; Zhang, S.; Qian, S.; Li, D.-S.; Liu, X.; Zhang, S. Sustainable engineering of TiO2-based advanced oxidation technologies: From photocatalyst to application devices. J. Mater. Sci. Technol. 2021, 78, 202–222. [Google Scholar] [CrossRef]
- Da Vià, L.; Recchi, C.; Davies, T.E.; Greeves, N.; Lopez-Sanchez, J.A. Visible-Light-Controlled Oxidation of Glucose using Titania-Supported Silver Photocatalysts. ChemCatChem 2016, 8, 3475–3483. [Google Scholar] [CrossRef]
- Zhou, B.; Song, J.; Zhang, Z.; Jiang, Z.; Zhang, P.; Han, B. Highly selective photocatalytic oxidation of biomass-derived chemicals to carboxyl compounds over Au/TiO2. Green Chem. 2017, 19, 1075–1081. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, H.; Yu, X.; Hu, J.; Chen, Z. Glucose photorefinery for sustainable hydrogen and value-added chemicals coproduction. Chem. Synth. 2024, 4, 4. [Google Scholar] [CrossRef]
- Asgher, M.; Shahid, M.; Kamal, S.; Iqbal, H.M.N. Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial Biotechnol. J. Mol. Catal. Enzym. 2014, 101, 56–66. [Google Scholar] [CrossRef]
- Amin, F.; Bhatti, H.N.; Bilal, M.; Asgher, M. Improvement of activity, thermo-stability and fruit juice clarification characteristics of fungal exo-polygalacturonase. Int. J. Biol. Macromol. 2017, 95, 974–984. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Parra-Saldivar, R. Immobilized ligninolytic enzymes: An innovative and environmental responsive technology to tackle dye-based industrial pollutants–a review. Sci. Total Environ. 2017, 576, 646–659. [Google Scholar] [CrossRef]
- Rehman, S.; Wang, P.; Bhatti, H.N.; Bilal, M.; Asgher, M. Improved catalytic properties of Penicillium notatum lipase immobilized in nanoscale silicone polymeric films. Int. J. Biol. Macromol. 2017, 97, 279–286. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.; Guo, S.; Hu, H.; Wang, W.; Zhang, X. State-of-the-art protein engineering approaches using biological macromolecules: A review from immobilization to implementation view point. Int. J. Biol. Macromol. 2018, 108, 893–901. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.; Cui, J. Smart chemistry and its application in peroxidase immobilization using different support materials. Int. J. Biol. Macromol. 2018, 119, 278–290. [Google Scholar] [CrossRef]
- Edet, E.; Ntekpe, M.; Omereji, S. Current Trend in Enzyme Immobilization: A Review. Int. J. Mod. Biochem. 2013, 2, 31–49. [Google Scholar]
- Matveeva, V.; Golikova, E.; Lakina, N.; Sulman, A.; Sidorov, A.; Doluda, V.; Karpenkov, A.Y.; Sulman, E. Magnetically separable biocatalyst of D-glucose oxidation. AIP Conf. Proc. 2018, 2022, 020008. [Google Scholar] [CrossRef]
- Jaquish, R.; Reilly, A.K.; Lawson, B.P.; Golikova, E.; Sulman, A.M.; Stein, B.D.; Lakina, N.V.; Tkachenko, O.P.; Sulman, E.M.; Matveeva, V.G.; et al. Immobilized glucose oxidase on magnetic silica and alumina: Beyond magnetic separation. Int. J. Biol. Macromol. 2018, 120, 896–905. [Google Scholar] [CrossRef]
- Haskell, A.K.; Sulman, A.M.; Golikova, E.P.; Stein, B.D.; Pink, M.; Morgan, D.G.; Lakina, N.V.; Karpenkov, A.Y.; Tkachenko, O.P.; Sulman, E.M.; et al. Glucose Oxidase Immobilized on Magnetic Zirconia: Controlling Catalytic Performance and Stability. ACS Omega 2020, 5, 12329–12338. [Google Scholar] [CrossRef]
- Grebennikova, O.V.; Sulman, A.M.; Sidorov, A.I.; Sulman, M.G.; Molchanov, V.P.; Tikhonov, B.B.; Matveeva, V.G. Magnetically separable biocatalysts based on glucose oxidase for d-glucose oxidation. Russ. Chem. Bull. 2022, 71, 524–530. [Google Scholar] [CrossRef]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Z.; Huang, S.-L.; Hou, J.; Guo, X.-P.; Wang, F.-S.; Sheng, J.-Z. Cell-based and cell-free biocatalysis for the production of d-glucaric acid. Biotechnol. Biofuels 2020, 13, 203. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green. Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Fischer, K.; Bipp, H.-P. Removal of Heavy Metals from Soil Components and Soils by Natural Chelating Agents. Part II. Soil Extraction by Sugar Acids. In Water, Air, & Soil Pollution; Springer Nature: Cham, Switzerland, 2002; Volume 138, pp. 271–288. [Google Scholar] [CrossRef]
- Javier, A.; Lim, P.J. Zeolite-like Arabinose-based Coordination Polymers: Synthesis and Characterization for Heavy-Metal Sequestration Applications. Winnower 2015, 2, e142709.97833. [Google Scholar] [CrossRef]
- Subramanian, G.; Madras, G. Introducing saccharic acid as an efficient iron chelate to enhance photo-Fenton degradation of organic contaminants. Water Res. 2016, 104, 168–177. [Google Scholar] [CrossRef]
- Johnston, E.R.; Smith, T.N.; Serban, M.A. Nanoparticulate Poly(glucaramide)-Based Hydrogels for Controlled Release Applications. Gels 2017, 3, 17. [Google Scholar] [CrossRef]
- Sakuta, R.; Nakamura, N. Production of Hexaric Acids from Biomass. Int. J. Mol. Sci. 2019, 20, 3660. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Ding, R.-H.; Ge, H.; Zhu, P.-L.; Ma, S.-S.; Zhang, B.; Song, X.-M. Solid-phase extraction of d-glucaric acid from aqueous solution. Sep. Purif. Technol. 2017, 175, 352–357. [Google Scholar] [CrossRef]
- Wu, Y.; Enomoto, Y.; Masaki, H.; Iwata, T. Synthesis of polyamides from sugar derived d-glucaric acid and xylylenediamines. J. Appl. Polym. Sci. 2019, 136, 47255. [Google Scholar] [CrossRef]
- Seo, J. D-Glucaric Acid Based Polymers and Crosslinker: Polyesters Bearing Pendent Hydroxyl Groups and Dissolvable Chemically Crosslinked Gels. Ph.D. Thesis, The University of Akron, Akron, OH, USA, 2019. [Google Scholar]
- Levvy, G.A.; Conchie, J. β-Glucuromdase and the Hydrolysis of Glucuronides. In Glucuronic Acid Free and Combined; Dutton, G.J., Ed.; Academic: New York, NY, USA, 1966; pp. 301–364. [Google Scholar]
- Zoltaszek, R.; Kowalczyk, P.; Kowalczyk, M.C.; Hanausek, M.; Kilianska, Z.M.; Slaga, T.J.; Walaszek, Z. Dietary D-glucarate effects on the biomarkers of inflammation during early post-initiation stages of benzo[a]pyrene-induced lung tumorigenesis in A/J mice. Oncol. Lett. 2011, 2, 145–154. [Google Scholar] [CrossRef]
- Simone, C.B.; Simone, N.L.; Pallante, M.; Simone, C.B. Cancer, Lifestyle Modification and Glucarate. J. Orthomol. Med. 2001, 16, 83–90. [Google Scholar]
- Khaw, B.-A.; Da Silva, J.; Petrov, A.; Hartner, W. Indium 111 antimyosin and Tc-99m glucaric acid for noninvasive identification of oncotic and apoptotic myocardial necrosis. J. Nucl. Cardiol. 2002, 9, 471–481. [Google Scholar] [CrossRef]
- Walaszek, Z.; Hanausek, M.; Szemraj, J.; Adams, A.K. D-glucaric acid as a prospective tumor marker. In Tumor Marker Protocols. Methods in Molecular Medicine™; Hanausek, M., Walaszek, Z., Eds.; Springer: Totowa, NJ, USA, 1998; Volume 14, pp. 487–495. [Google Scholar] [CrossRef]
- Li, J.; Beverly, L.; Gray, B.; Pak, K.; Ng, C. Effect of fructose and glucarate on 18F-fluoroglucaric acid uptake in cancer cells. J. Nucl. Med. 2020, 61, 1220. [Google Scholar]
- Su, H.; Guo, Z.; Wu, X.; Xu, P.; Li, N.; Zong, M.; Lou, W. Efficient Bioconversion of Sucrose to High-Value-Added Glucaric Acid by In Vitro Metabolic Engineering. ChemSusChem 2019, 12, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Zółtaszek, R.; Hanausek, M.; Kiliańska, Z.M.; Walaszek, Z. Biologiczna rola kwasu D-glukarowego i jego pochodnych; potencjalne zastosowanie w medycynie [The biological role of D-glucaric acid and its derivatives: Potential use in medicine]. Postepy Hig. Med. Dosw. 2008, 62, 451–462. Available online: https://www.medicinacomplementar.com.br/biblioteca/pdfs/Cancer/ca-1720.pdf (accessed on 26 February 2025).
- Mehtiö, T.; Toivari, M.; Wiebe, M.G.; Harlin, A.; Penttilä, M.; Koivula, A. Production and applications of carbohydrate-derived sugar acids as generic biobased chemicals. Crit. Rev. Biotechnol. 2015, 36, 904–916. [Google Scholar] [CrossRef]
- Brighten, J. The Benefits of Calcium D-Glucarate. Available online: https://drbrighten.com/benefits-of-calcium-d-glucarate/ (accessed on 26 February 2025).
- Diamond, G.M.; Murphy, V.; Boussie, T.R. Application of High Throughput Experimentation to the Production of Commodity Chemicals from Renewable Feedstocks. In Modern Applications of High Throughput R&D in Heterogeneous Catalysis; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; pp. 288–309. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.; Yoon, H.; Shin, J.H.; Park, S.; Kwak, H.W.; Ahn, M.-R.; Koo, B.; Choi, I.-G. Glucaric Acid Production from Miscanthus sacchariflorus via TEMPO-Mediated Oxidation with an Efficient Separation System. ACS Omega 2024, 9, 9432–9442. [Google Scholar] [CrossRef]
- Koefod, R.S. Corrosion-Inhibiting Deicer Composition. U.S. Patent 7658861B2, 9 February 2010. [Google Scholar]
- Kiely, D.E.; Hash, K.R. Method of Oxidation Using Nitric Acid. U.S. Patent US9162959B2, 20 October 2015. [Google Scholar]
- Jin, X.; Zhao, M.; Vora, M.; Shen, J.; Zeng, C.; Yan, W.; Thapa, P.S.; Subramaniam, B.; Chaudhari, R. Synergistic Effects of Bimetallic PtPd/TiO2 Nanocatalysts in Oxidation of Glucose to Glucaric Acid: Structure Dependent Activity and Selectivity. Ind. Eng. Chem. Res. 2016, 55, 2932–2945. [Google Scholar] [CrossRef]
- Zhao, Y.; Zuo, F.; Shu, Q.; Yang, X.; Deng, Y. Efficient Production of Glucaric Acid by Engineered Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2023, 6, e00535-23. [Google Scholar] [CrossRef]
- Moon, T.S.; Yoon, S.-H.; Lanza, A.M.; Roy-Mayhew, J.D.; Prather, K.L.J. Production of glucaric acid from a synthetic pathway in recombinant Escherichia coli. Appl. Environ. Microbiol. 2009, 75, 589–595. [Google Scholar] [CrossRef]
- Moon, T.S.; Dueber, J.E.; Shiue, E.; Prather, K.L. Use of modular, synthetic scaffolds for improved production of glucaric acid in engineered E. coli. Metab. Eng. 2010, 12, 298–305. [Google Scholar] [CrossRef]
- Shiue, E.; Prather, K.L.J. Improving D-glucaric acid production from myo-inositol in E. coli by increasing MIOX stability and myo-inositol transport. Metab. Eng. 2014, 22, 22–31. [Google Scholar] [CrossRef]
- Su, H.-H.; Peng, F.; Ou, X.-Y.; Zeng, Y.-J.; Zong, M.-H.; Lou, W.-Y. Combinatorial synthetic pathway fine-tuning and cofactor regeneration for metabolic engineering of Escherichia coli significantly improve production of D-glucaric acid. New Biotechnol. 2020, 59, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, X.; Wang, C.; Du, G.; Chen, J.; Kang, Z. Production of glucaric acid from myo-inositol in engineered Pichia pastor. Enzym. Microb. Technol. 2016, 91, 8–16. [Google Scholar] [CrossRef]
- Gupta, A.; Hicks, M.A.; Manchester, S.P.; Prather, J. Porting the synthetic D-glucaric acid pathway from Escherichia coli to Saccharomyces cerevisiae. Biotechnol. J. 2016, 11, 1201–1208. [Google Scholar] [CrossRef]
- Toivari, M.; Vehkomäki, M.-L.; Ruohonen, L.; Penttilä, M.; Wiebe, M.G. Production of d-glucaric acid with phosphoglucose isomerase-deficient Saccharomyces cerevisiae. Biotechnol. Lett. 2024, 46, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Mehltretter, C.L.; Rist, C.E. Oxidation, Saccharic and Oxalic Acids by the Nitric Acid Oxidation of Dextrose. J. Agric. Food Chem. 1953, 1, 779–783. [Google Scholar] [CrossRef]
- Mustakas, G.C.; Slotter, R.L.; Zipf, R.L. Pilot plant potassium acid saccharate by nitric acid oxidation of dextrose. Ind. Eng. Chem. 1954, 46, 427–434. [Google Scholar] [CrossRef]
- Smith, T.N.; Hash, K.; Davey, C.-L.; Mills, H.; Williams, H.; Kiely, D.E. Modifications in the nitric acid oxidation of D-glucose. Carbohydr. Res. 2012, 350, 6–13. [Google Scholar] [CrossRef]
- Grigor’eva, I.A.; Chernaya, S.S.; Trusov, S.R. Oxidation of D-Glucose to D-Gluconic and D-Glucaric Acids Catalyzed by Sodium Nitrite. Russ. J. Appl. Chem. 2001, 74, 2021–2026. [Google Scholar] [CrossRef]
- Merbouh, N.; Thaburet, J.F.; Ibert, M.; Marsais, F.; Bobbitt, J.M. Facile Nitroxide-mediated Oxidations of D-Glucose to D-Glucaric Acid. Carbohydr. Res. 2001, 336, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Thaburet, J.-F.; Merbouh, N.; Ibert, M.; Marsais, F.; Queguiner, G. TEMPO-mediated oxidation of maltodextrins and D-glucose: Effect of pH on the selectivity and sequestering ability of the resulting polycarboxylates. Carbohydrate Res. 2001, 330, 21–29. [Google Scholar] [CrossRef]
- Merbouh, N.; Bobbitt, J.M.; Brückner, C. 4-AcNH-TEMPO-Catalyzed oxidation of aldoses to aldaric acids using chlorine or bromine as terminal oxidants. J. Carbohydr. Chem. 2002, 21, 65–77. [Google Scholar] [CrossRef]
- Onda, A. Production of Platform Chemicals from Sustainable Resources; Biofuels and Biorefineries; Springer: Singapore, 2017; pp. 207–230. [Google Scholar]
- Besson, M.; Lahmer, F.; Gallezot, P.; Fuertès, P.; Flèche, G. Catalytic Oxidation of Glucose on Bismuth-Promoted Palladium Catalysts. J. Catal. 1995, 152, 116–121. [Google Scholar] [CrossRef]
- Lee, J.; Saha, B.; Vlachos, D.G. Pt catalysts for efficient aerobic oxidation of glucose to glucaric acid in water. Green. Chem. 2016, 18, 3815–3822. [Google Scholar] [CrossRef]
- Moggia, G.; Schalck, J.; Daems, N.; Breugelmans, T. Two-steps synthesis of D-glucaric acid via D-gluconic acid by electrocatalytic oxidation of D-glucose on gold electrode: Influence of operational parameters. Electrochim. Acta 2021, 374, 137852. [Google Scholar] [CrossRef]
- Tian, Z.; Da, Y.; Wang, M.; Dou, X.; Cui, X.; Chen, J.; Jiang, R.; Xi, S.; Cui, B.; Luo, Y.; et al. Selective photoelectrochemical oxidation of glucose to glucaric acid by single atom Pt decorated defective TiO2. Nat. Commun. 2023, 14, 142. [Google Scholar] [CrossRef]
- Ibert, M.; Fuertès, P.; Merbouh, N.; Fiol-Petit, C.; Feasson, C.; Marsais, F. Improved preparative electrochemical oxidation of d-glucose to d-glucaric acid. Electrochim. Acta 2010, 55, 3589–3594. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).