Complete Characterization of Degradation Byproducts of Bemotrizinol and Degradation Pathway Associated with Sodium Hypochlorite Treatment
Abstract
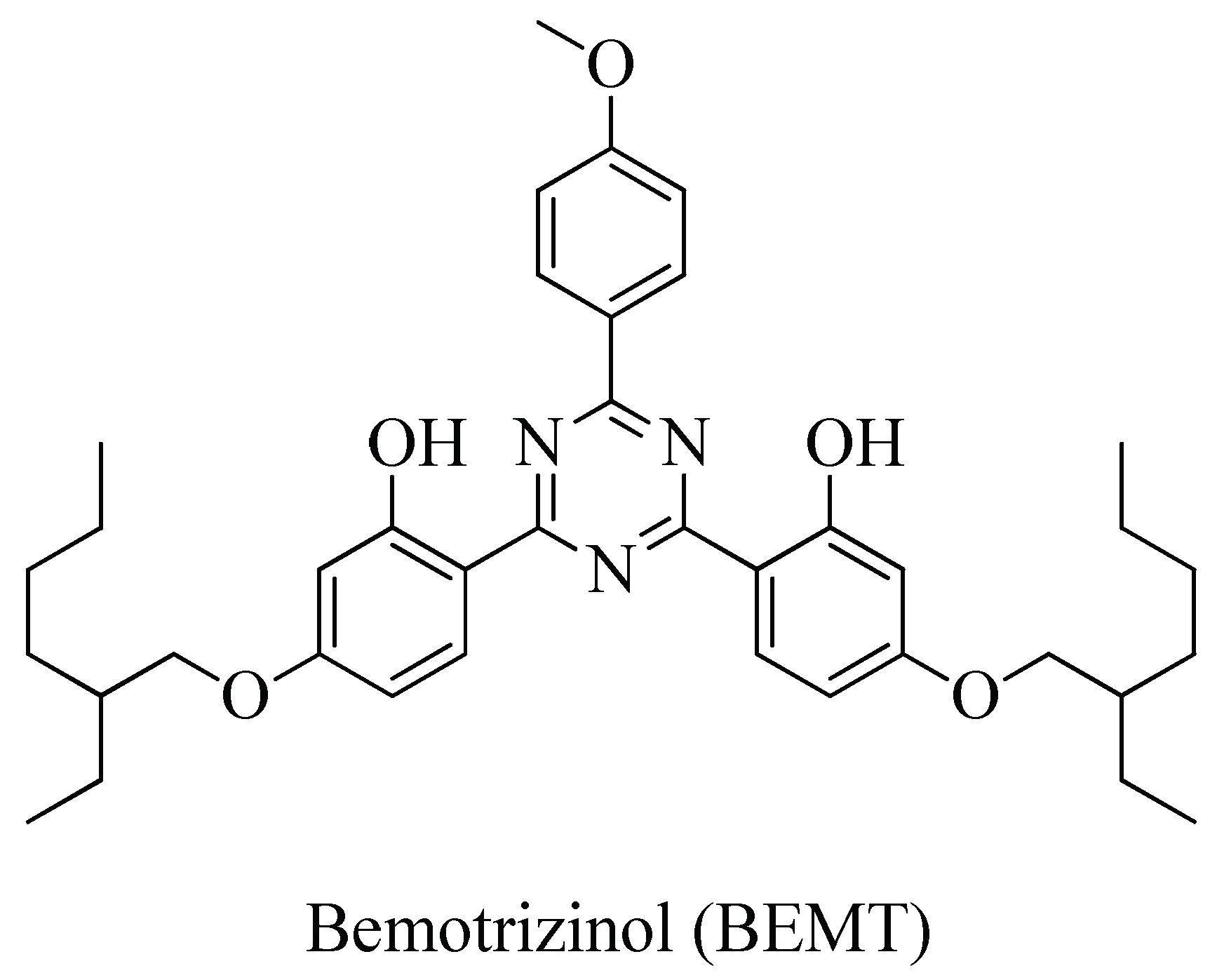
1. Introduction
2. Results and Discussion
2.1. Degradation Experiments
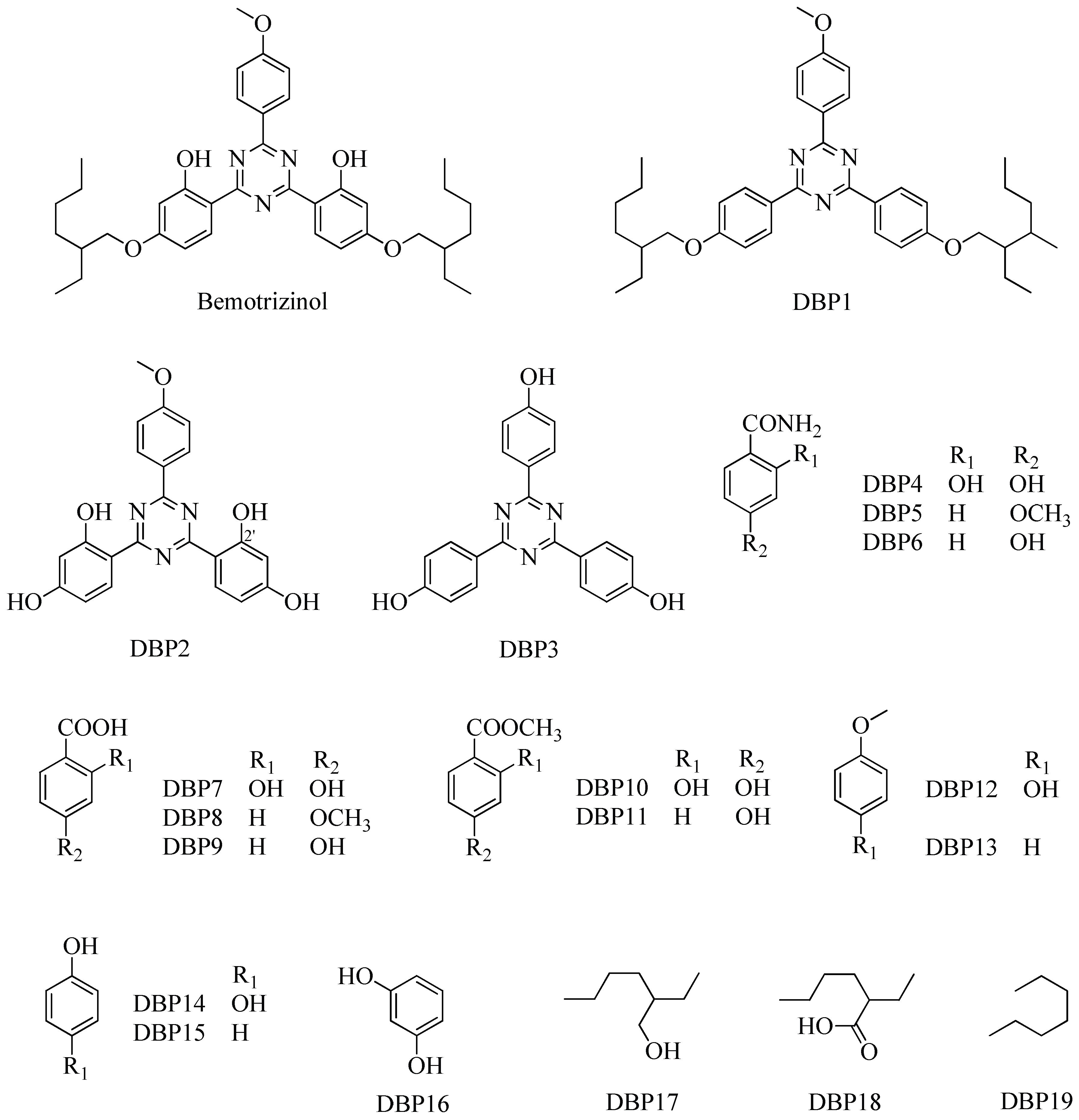
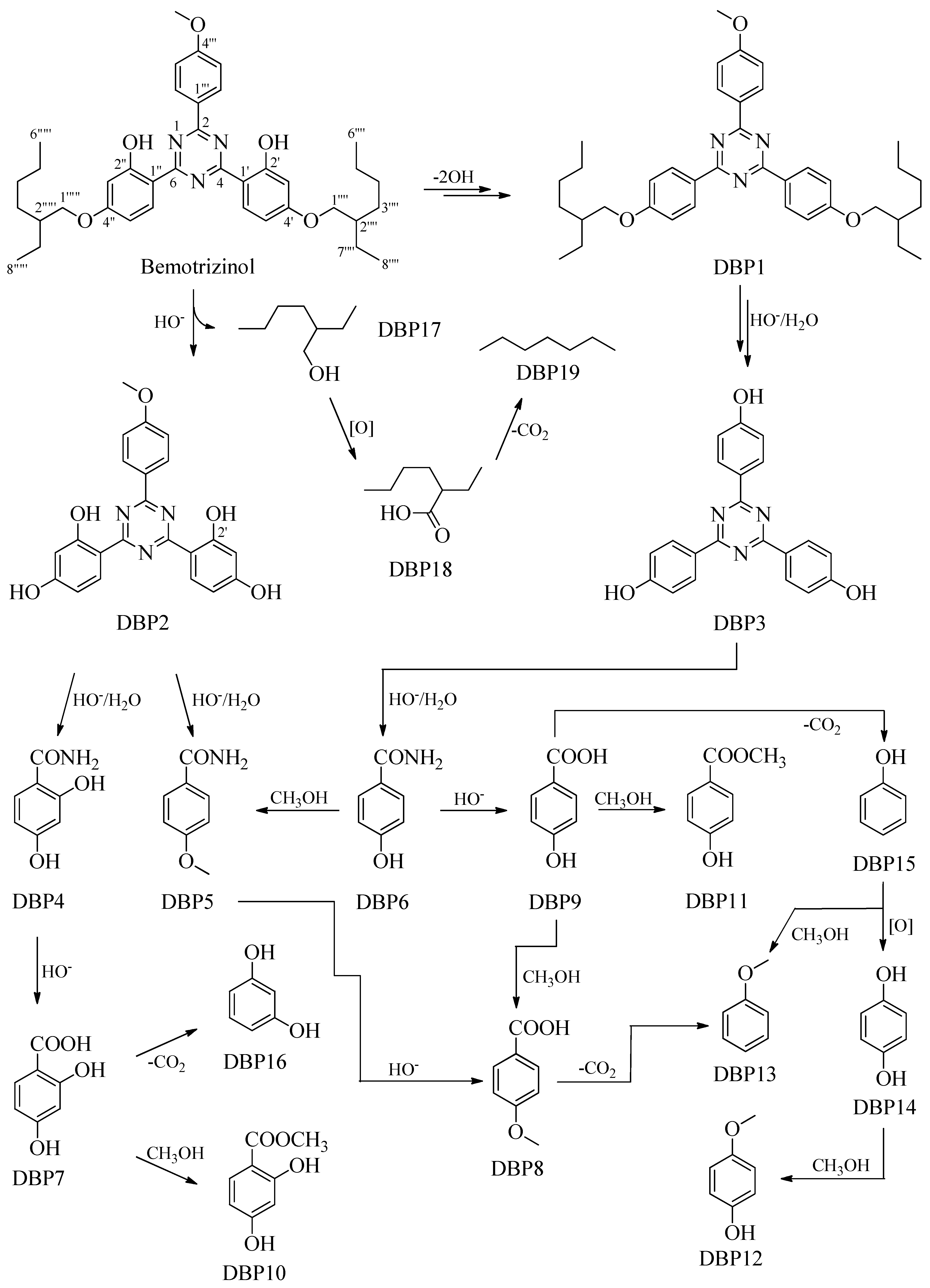
2.2. Proposed Mechanism for the Formation of Degradation Byproducts DBP1–DBP19
2.3. Spectral Data
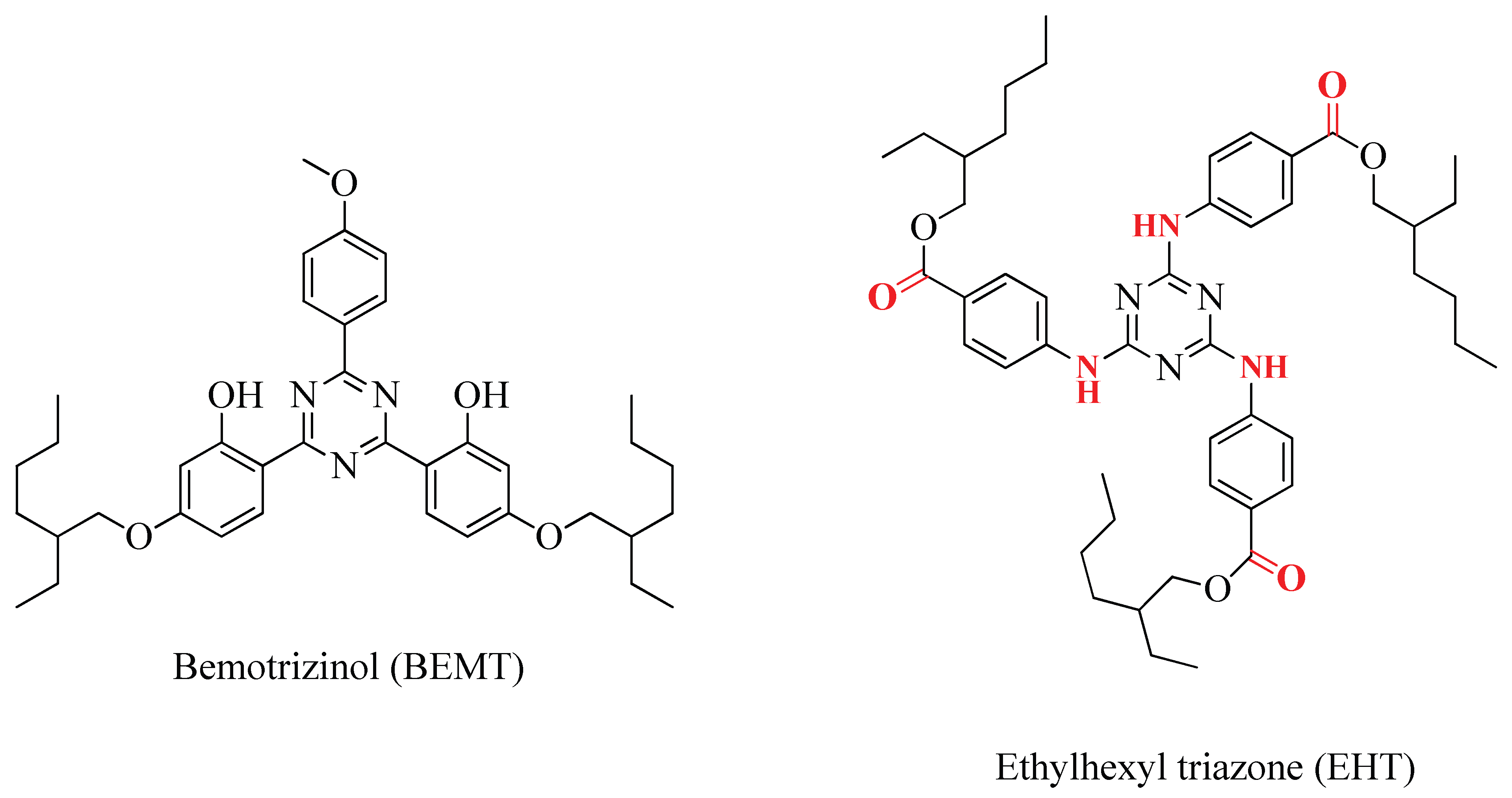
2.4. Degradation of Bemotrizinol vs. Ethylhexyltriazone
3. Materials and Methods
3.1. Drug and Reagents
3.2. Sodium Hypochlorite Reaction
3.2.1. Apparatus and Equipment
3.2.2. Analytical and Preparative Experiments
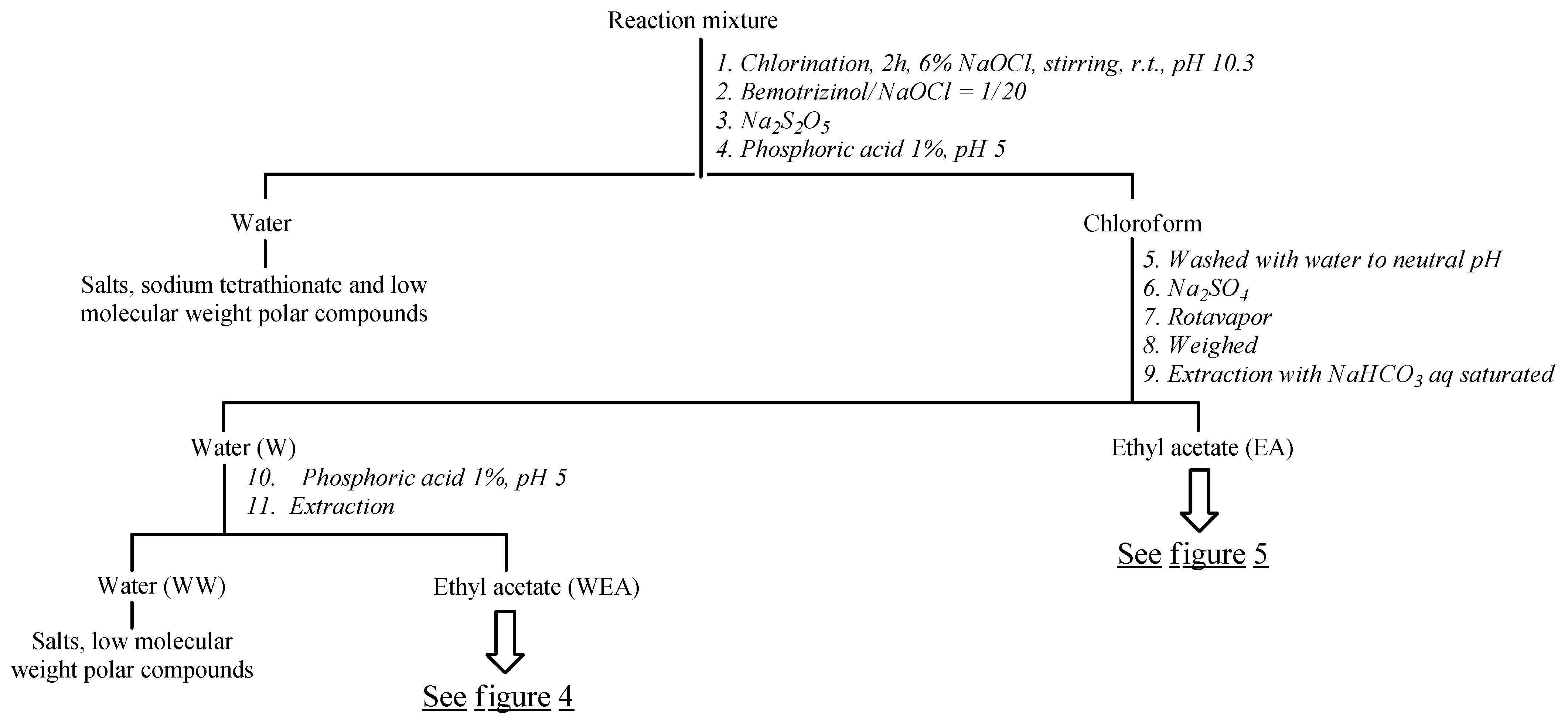
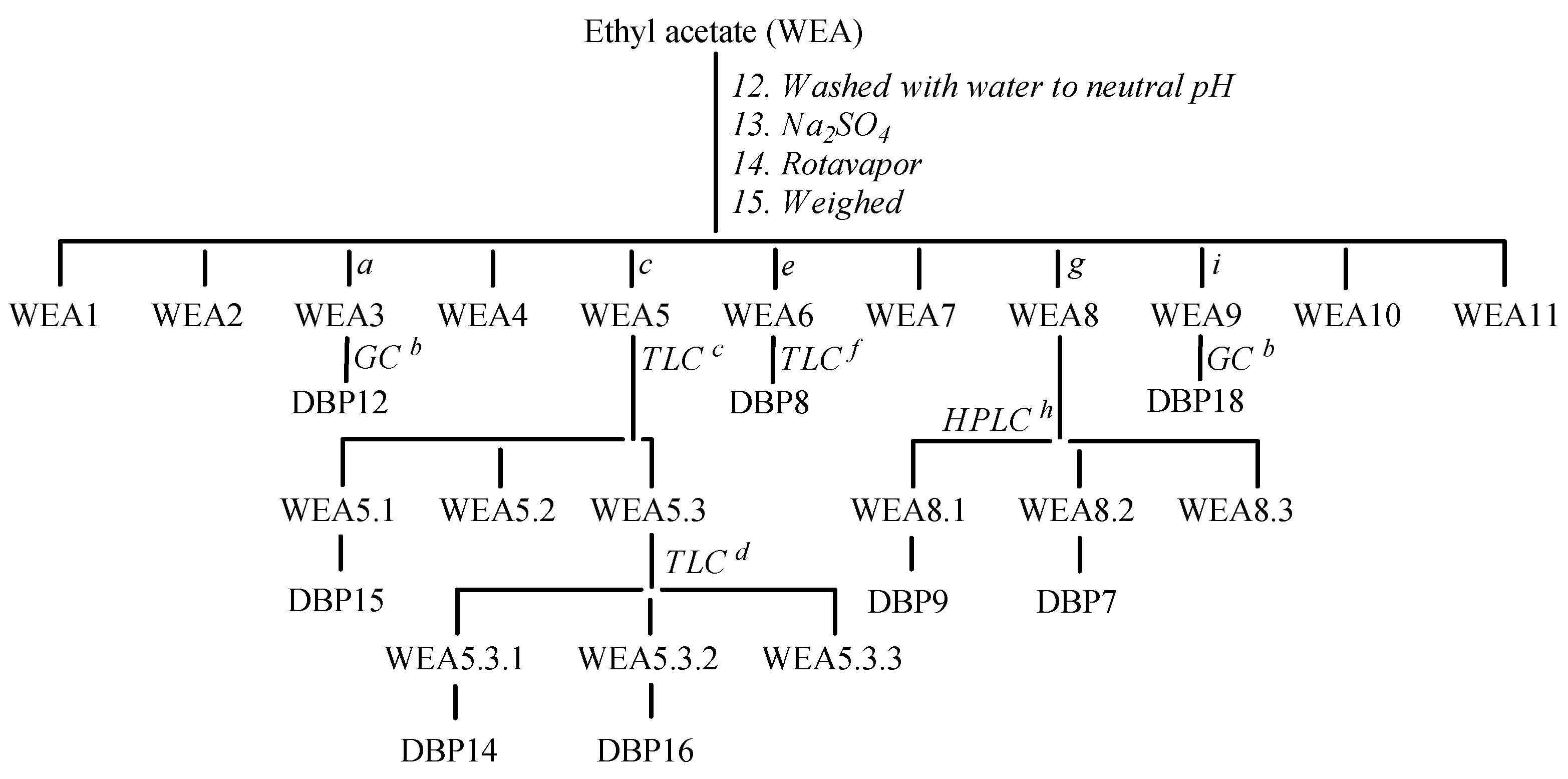
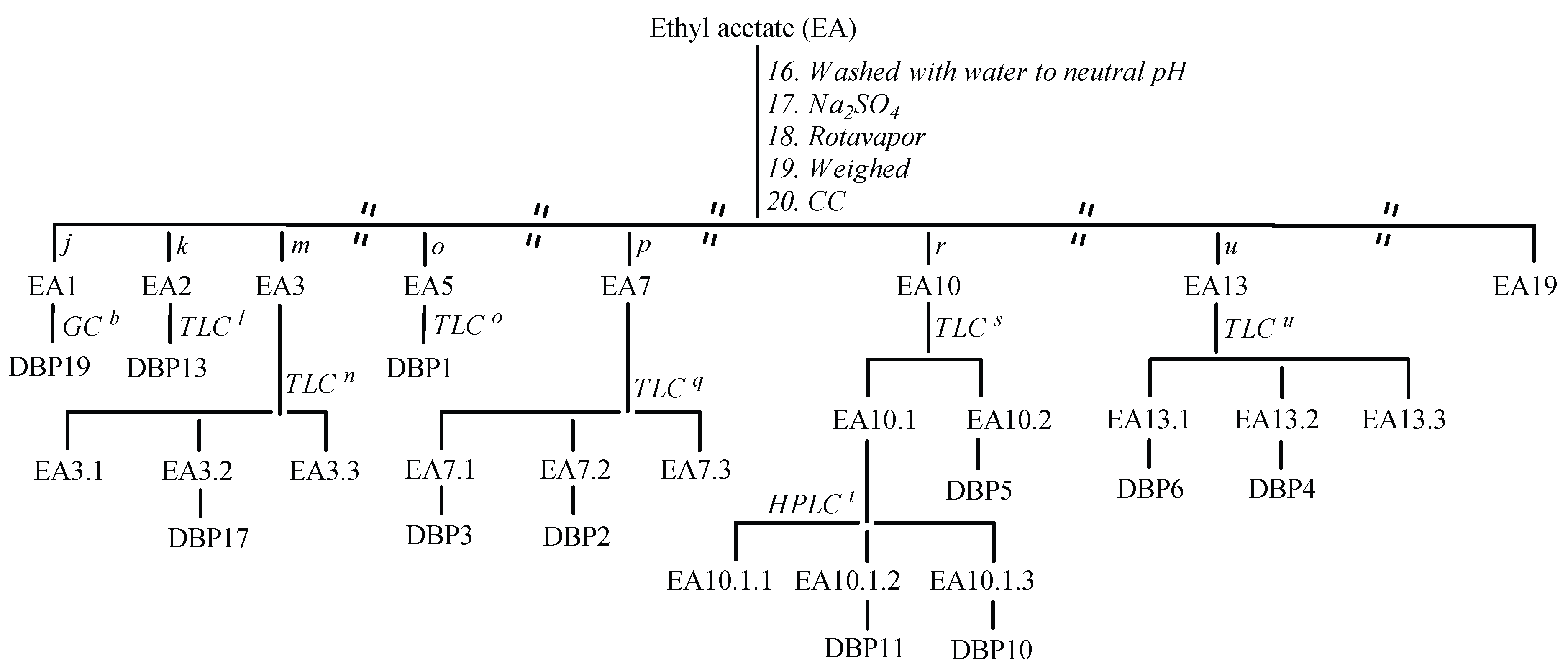
3.2.3. Protocol for the Degradation and Isolation of Degradation Byproducts/Preparative Experiment
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Häder, D.P.; Banaszak, A.T.; Villafañe, V.E.; Narvarte, M.A.; González, R.A.; Helbling, E.W. Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Sci. Total Environ. 2020, 713, 136586. [Google Scholar] [CrossRef]
- Ogidi, O.I.; Akpan, U.M. Aquatic biodiversity loss: Impacts of pollution and anthropogenic activities and strategies for conservation. In Biodiversity in Africa: Potentials, threats and conservation; Springer: Singapore, 2022. [Google Scholar]
- Tovar-Sanchez, A.; Sanchez-Quiles, D.; Basterretxea, G.; Benede, J.L.; Chisvert, A.; Salvador, A.; Moreno-Garrido, I.; Blasco, J. Sunscreen products as emerging pollutants to coastal waters. PLoS ONE 2013, 8, e65451. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.M.P.; Leung, H.W.; Wai, T.C.; Yamashita, N.; Taniyasu, S.; Liu, W.; Lam, P.K.S.; Murphy, M.B. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countries. Water Res. 2014, 67, 55–65. [Google Scholar] [CrossRef]
- Tsui, M.M.P.; Leung, H.W.; Lam, P.K.S.; Murphy, M.B. Seasonal occurrence, removal efficiencies and preliminary risk assessment of multiple classes of organic UV filters in wastewater treatment plants. Water Res. 2014, 53, 58–67. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and fate of emerging pollutants in water environment and options for their removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- EU Monitor. Available online: https://www.eumonitor.eu/9353000/1/j9vvik7m1c3gyxp/vibn2mp7slr0 (accessed on 5 April 2025).
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorg. Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Fent, K.; Zenker, A.; Rapp, M. Widespread occurrence of estrogenic UV-filters in aquatic ecosystems in Switzerland. Environ. Poll. 2010, 158, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Giokas, D.L.; Sakkas, V.A.; Albanis, T.A.; Lampropoulou, D.A. Determination of UV-filter residues in bathing waters by liquid chromatography UV-diode array and gas chromatography–mass spectrometry after micelle mediated extraction-solvent back extraction. J. Chromatogr. A 2005, 1077, 19–27. [Google Scholar] [CrossRef]
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. A review of organic UV-filters in wastewater treatment plants. Environ. Int. 2016, 86, 24–44. [Google Scholar] [CrossRef]
- Pirotta, G. The encyclopedia allowed sunfilters in the world. Skin. Care-Househ. Pers. Care Today 2016, 11, 19–21. [Google Scholar]
- Downs, C.A.; Kramarsky-Winter, E.; Fauth, J.E.; Segal, R.; Bronstein, O.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; Pennington, P.; Kushmaro, A.; et al. Toxicological effects of the sunscreen UV filter, benzophenone-2, on planulae and in vitro cells of the coral, Stylophora pistillata. Ecotox 2014, 23, 175–191. [Google Scholar] [CrossRef]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar] [CrossRef]
- Rainieri, S.; Barranco, A.; Primec, M.; Langerholc, T. Occurrence and toxicity of musks and UV filters in the marine environment. Food Chem. Toxicol. 2017, 104, 57–68. [Google Scholar] [CrossRef]
- Molins-Delgado, D.; Távora, J.; Díaz-Cruz, M.S.; Barceló, D. UV filters and benzotriazoles in urban aquatic ecosystems: The footprint of daily use products. Sci. Total Environ. 2017, 601, 975–986. [Google Scholar] [CrossRef]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Are sunscreens a new environmental risk associated with coastal tourism? Environ. Int. 2015, 83, 158–170. [Google Scholar] [CrossRef]
- Just-Sarobé, M. Sunscreens and their impact on human health and the environment: A review. Int. J. Dermatol. 2025. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep. 2017, 4, 245–259. [Google Scholar] [CrossRef]
- He, T.; Tsui, M.M.; Mayfield, A.B.; Liu, P.J.; Chen, T.H.; Wang, L.H.; Fan, T.-Y.; Lam, P.K.S.; Murphy, M.B. Organic ultraviolet filter mixture promotes bleaching of reef corals upon the threat of elevated seawater temperature. Sci. Total Environ. 2023, 876, 162744. [Google Scholar] [CrossRef]
- Miller, I.B.; Pawlowski, S.; Kellermann, M.Y.; Petersen-Thiery, M.; Moeller, M.; Nietzer, S.; Schupp, P.J. Toxic effects of UV filters from sunscreens on coral reefs revisited: Regulatory aspects for “reef safe” products. Environ. Sci. Eur. 2021, 33, 74. [Google Scholar] [CrossRef]
- Fent, K.; Kunz, P.Y.; Gomez, E. UV filters in the aquatic environment induce hormonal effects and affect fertility and reproduction in fish. Chimia 2008, 62, 368. [Google Scholar] [CrossRef]
- Chatzigianni, M.; Pavlou, P.; Siamidi, A.; Vlachou, M.; Varvaresou, A.; Papageorgiou, S. Environmental impacts due to the use of sunscreen products: A mini-review. Ecotoxicol. 2022, 31, 1331–1345. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Malcolm Shick, J.; Yamamoto, Y. Sunscreens, oxidative stress and antioxidant functions in marine organisms of the Great Barrier Reef. Redox Rep. 1999, 4, 301–306. [Google Scholar] [CrossRef]
- Moeller, M.; Pawlowski, S.; Petersen-Thiery, M.; Miller, I.B.; Nietzer, S.; Heisel-Sure, Y.; Kellermann, M.Y.; Schupp, P.J. Challenges in current coral reef protection–possible impacts of UV filters used in sunscreens, a critical review. Front. Mar. Sci. 2021, 8, 665548. [Google Scholar] [CrossRef]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef]
- Neale, P.J.; Banaszak, A.T.; Jarriel, C.R. Ultraviolet sunscreens in Gymnodinium sanguineum (Dinophyceae): Mycosporine-like amino acids protect against inhibition of photosynthesis. J. Phycol. 1998, 34, 928–938. [Google Scholar] [CrossRef]
- Karsten, U.; Lembcke, S.; Schumann, R. The effects of ultraviolet radiation on photosynthetic performance, growth and sunscreen compounds in aeroterrestrial biofilm algae isolated from building facades. Planta 2007, 225, 991–1000. [Google Scholar] [CrossRef]
- Anhui Yingtemei Technology Co., Ltd. China. Preparation of Bemotrizinol. CN115772133 A, 10 March 2023. [Google Scholar]
- Mitchelmore, C.L.; Burns, E.E.; Conway, A.; Heyes, A.; Davies, I.A. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ. Toxicol. Chem. 2021, 40, 967–988. [Google Scholar] [CrossRef]
- Fagervold, S.K.; Rodrigues, A.M.S.; Rohée, C.; Roe, R.; Bourrain, M.; Stien, D.; Lebaron, P. Occurrence and environmental distribution of 5 UV filters during the summer season in different water bodies. Water Air Soil. Pollut. 2019, 230, 172. [Google Scholar] [CrossRef]
- Gandar, A.; Giraudo, M.; Perion, T.; Houël, E.; Noguer, T.; Rodrigues, A.M.; Calas-Blanchard, C.; Stien, D. Targeted and untargeted discovery of UV filters and emerging contaminants with environmental risk assessment on the Northwestern Mediterranean coast. Mar. Pollut. Bull. 2025, 212, 117567. [Google Scholar] [CrossRef]
- Ge, J.; Li, J.; Han, X.; Du, B.; Guo, Y.; Zhu, H.; Zeng, L. Triazine-Derived Ultraviolet Filters and Flame Retardants: A Nationwide Co-Occurrence and Prevalence Study in China. Environ. Sci. Technol. Lett. 2023, 10, 909–915. [Google Scholar] [CrossRef]
- Stein, D.; Suzuki, M.; Rodrigues, A.M.S.; Yvin, M.; Clergeaud, D.; Thorel, E.; Lebaron, P. A unique approach to monitor stress in coral exposed to emerging pollutants. Sci. Rep. 2020, 10, e9601. [Google Scholar] [CrossRef] [PubMed]
- Thorel, E.; Clergeaud, F.; Jaugeon, L.; Rodrigues, A.M.S.; Lucas, J.; Stien, D.; Lebaron, P. Effect of 10 UV filters on the brine shrimp Artemia salina and the marine microalga Tetraselmis sp. Toxics 2020, 8, 29. Toxics 2020, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Luongo, G.; Previtera, L.; Ladhari, A.; Di Fabio, G.; Zarrelli, A. Peracetic acid vs. sodium hypochlorite: Degradation and transformation of drugs in wastewater. Molecules 2020, 25, 2294. [Google Scholar] [CrossRef]
- Lavorgna, M.; Medici, A.; Russo, C.; Orlo, E.; Di Fabio, G.; Luongo, G.; De Nisco, M.; Isidori, M.; Zarrelli, A. Ethylhexyl triazone sunscreen and its disinfection byproducts obtained after chlorine treatment: Ecofriendliness or ecotoxicity? Sci. Total Environ. 2024, 955, 177279. [Google Scholar] [CrossRef] [PubMed]
- Jana, K.; Mahanti, B. Challenges of HPLC Method development and validation for the assay of Bemotrizinol from complex matrix in cosmeceutical preparation. Int. J. Pharm. Qual. Assur. 2020, 11, 310–316. [Google Scholar] [CrossRef]
- Romanucci, V.; Siciliano, A.; Guida, M.; Libralato, G.; Saviano, L.; Luongo, G.; Previtera, L.; Di Fabio, G.; Zarrelli, A. Disinfection byproducts and ecotoxic risk associated with hypochlorite treatment of irbesartan. Sci. Total Environ. 2020, 712, 135625. [Google Scholar] [CrossRef]
- Zarrelli, A.; DellaGreca, M.; Parolisi, A.; Iesce, M.R.; Cermola, F.; Temussi, F.; Isidori, M.; Lavorgna, M.; Passananti, M.; Previtera, L. Chemical fate and genotoxic risk associated with hypochlorite treatment of nicotine. Sci. Total Environ. 2012, 426, 132–138. [Google Scholar] [CrossRef]
| % BEMT | No. DBPs | %DBPs | %DBP1 | Mineralization | |
|---|---|---|---|---|---|
| BEMT–NaClO aq. di 1:100 | 2 | 19 | 51 | 46 | 47 |
| BEMT–NaClO aq. di 1:80 | 20 | 42 | |||
| BEMT–NaClO aq. di 1:25 | 91 | 1 | |||
| % EHT | Num. DBPs | %DBPs | Mineralization | ||
| EHT–NaClO aq. di 1:25 | 62 | 12 | 25 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarrelli, A. Complete Characterization of Degradation Byproducts of Bemotrizinol and Degradation Pathway Associated with Sodium Hypochlorite Treatment. Molecules 2025, 30, 2935. https://doi.org/10.3390/molecules30142935
Zarrelli A. Complete Characterization of Degradation Byproducts of Bemotrizinol and Degradation Pathway Associated with Sodium Hypochlorite Treatment. Molecules. 2025; 30(14):2935. https://doi.org/10.3390/molecules30142935
Chicago/Turabian StyleZarrelli, Armando. 2025. "Complete Characterization of Degradation Byproducts of Bemotrizinol and Degradation Pathway Associated with Sodium Hypochlorite Treatment" Molecules 30, no. 14: 2935. https://doi.org/10.3390/molecules30142935
APA StyleZarrelli, A. (2025). Complete Characterization of Degradation Byproducts of Bemotrizinol and Degradation Pathway Associated with Sodium Hypochlorite Treatment. Molecules, 30(14), 2935. https://doi.org/10.3390/molecules30142935







