Methylsteric Effects Enhance Fluorescence in Diphenylfumaronitrile AIEgens
Abstract
1. Introduction
2. Results and Discussion
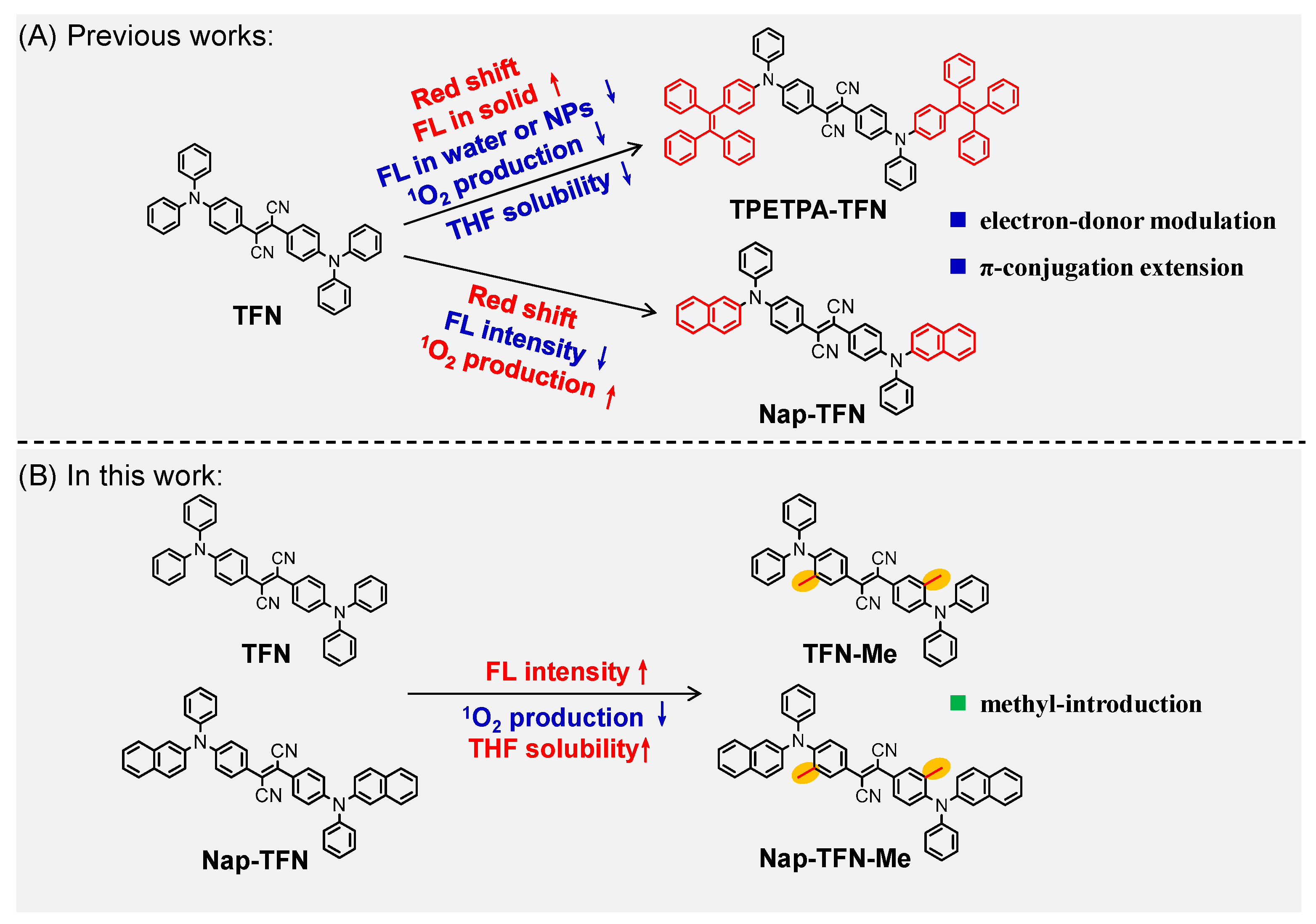
2.1. Synthesis and Characterization of Fluorophores
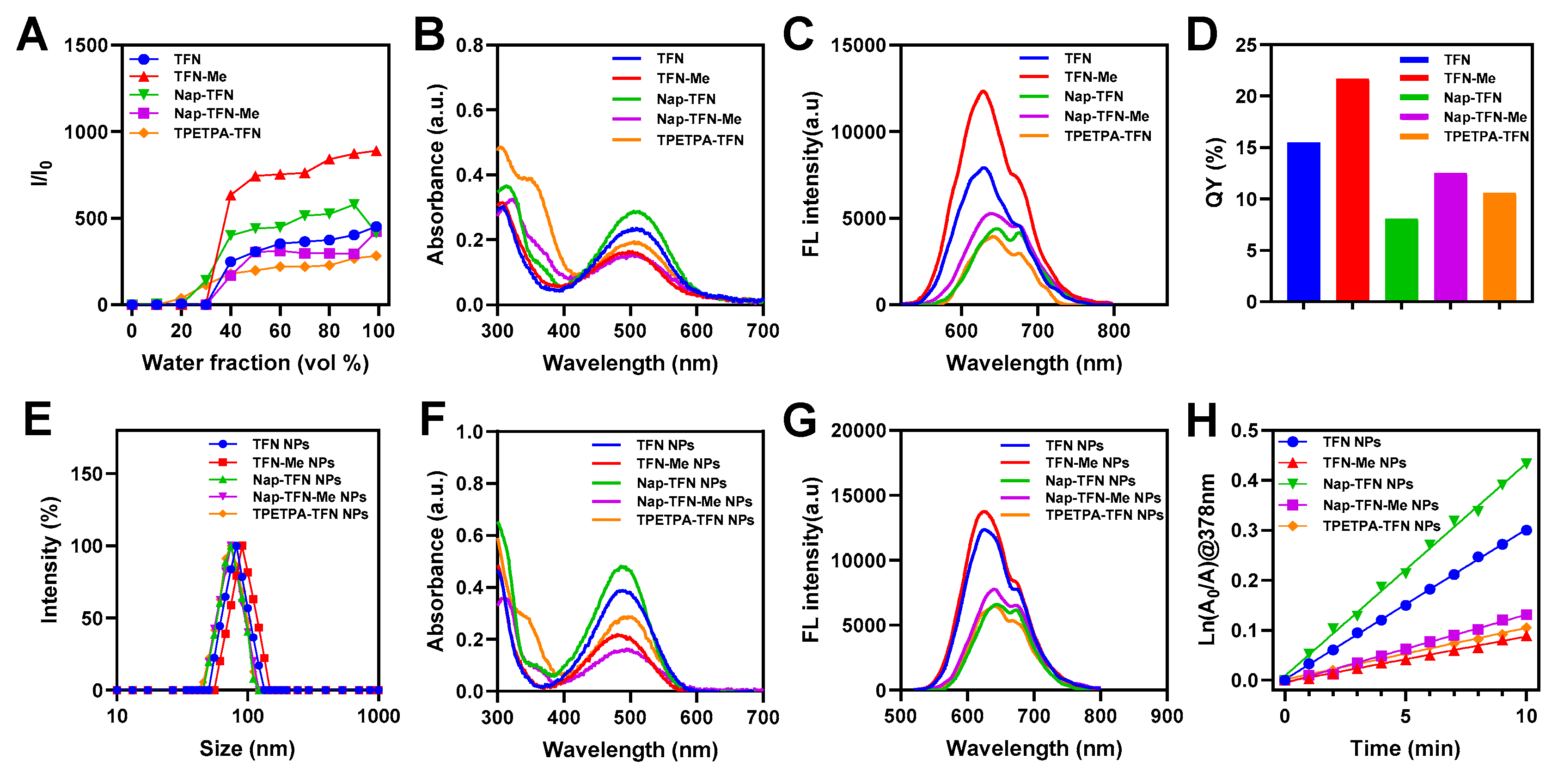
2.2. Preparation and Characterization of NPs
2.3. Photophysical Performance of NPs In Vitro
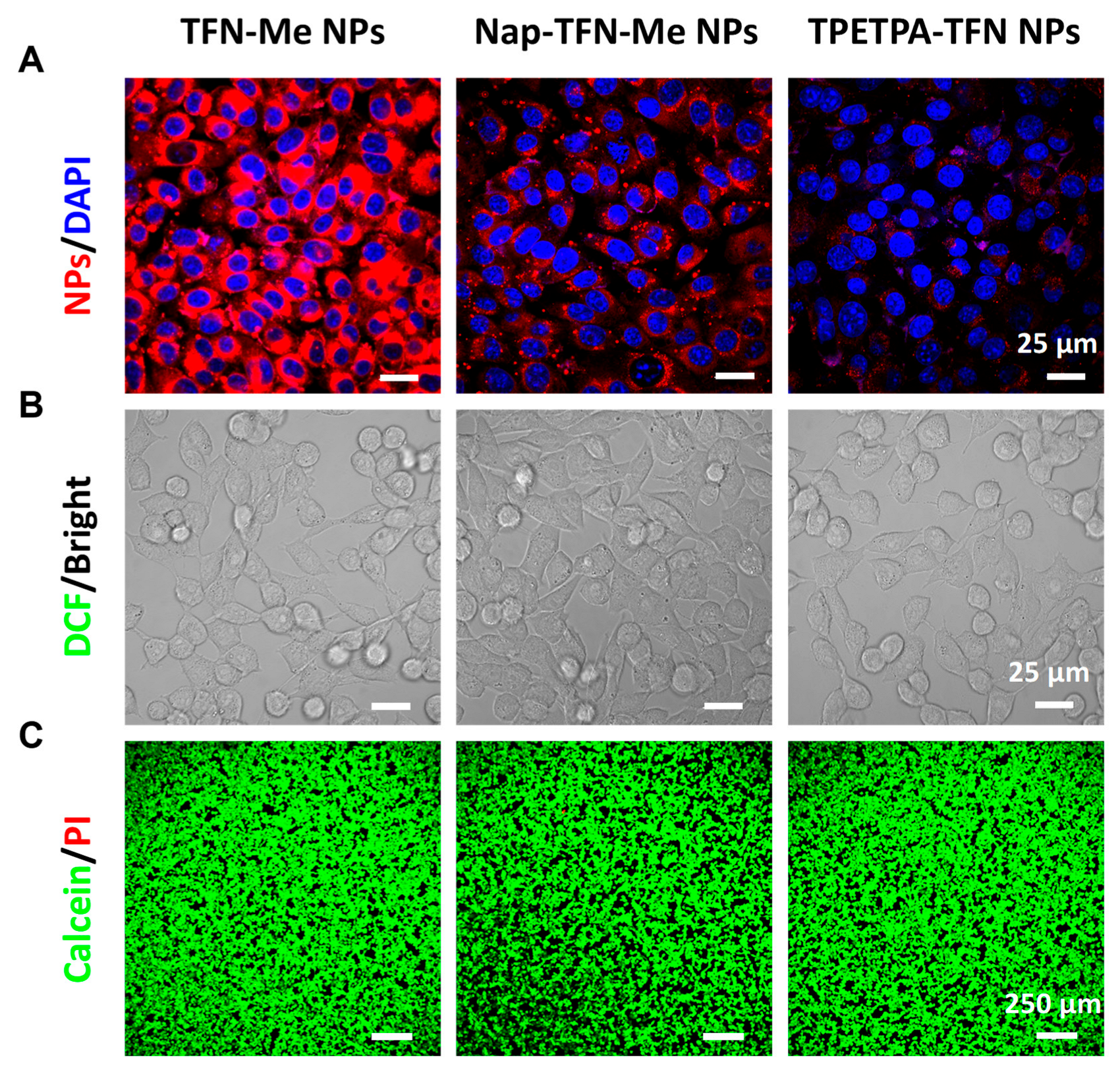
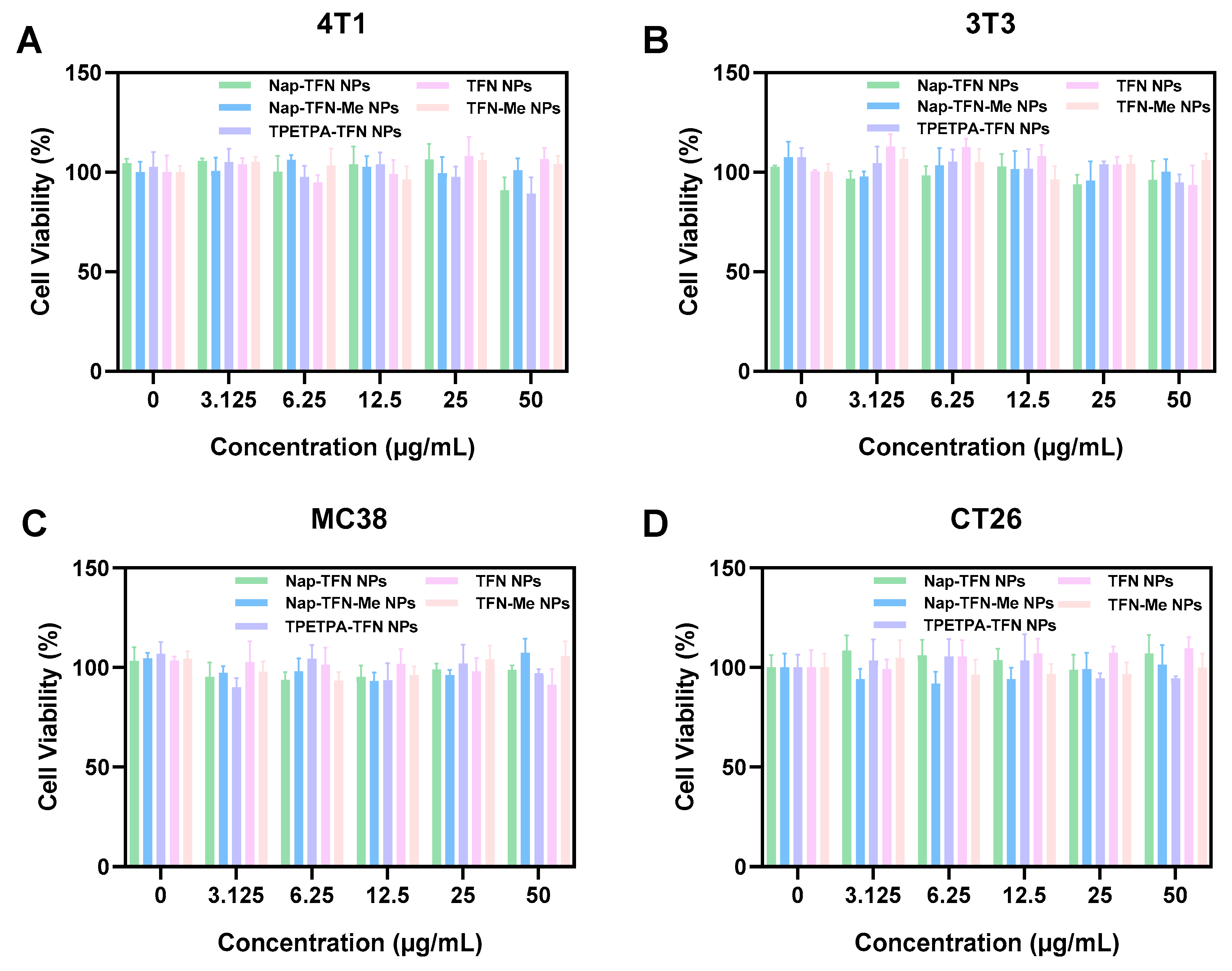
2.4. Fluorescence Imaging of Tumor Cells In Vivo
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Guo, R.; Chen, X.Y.; Yang, Y.S.; Qiao, L.Q.; Wang, M.L. Multifunctional lysosome-targetable fluorescent probe for imaging peroxynitrite in acute liver injury model. Chem. Eng. J. 2023, 455, 140491. [Google Scholar] [CrossRef]
- Han, T.H.; Mu, S.; Huang, Q.; Sun, J.X.; Lv, X.; Zhang, Y.X.; Liu, X.Y.; Guo, W.; Zhang, H.X. In vivo imaging tool: High-brightness NIR-II “J-aggregates” based on TICT suppression strategy. Chem. Eng. J. 2025, 515, 163858. [Google Scholar] [CrossRef]
- Xia, T.P.; Xia, Z.R.; Tang, P.C.; Fan, J.L.; Peng, X.J. Light-Driven Mitochondrion-to-Nucleus DNA Cascade Fluorescence Imaging and Enhanced Cancer Cell Photoablation. J. Am. Chem. Soc. 2024, 146, 12941–12949. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, F.P.; Zheng, B.B.; Yang, Y.X.; Yang, J.Y.; Xiong, H. Electron-Withdrawing Substituents Enhance the Type I PDT and NIR-II Fluorescence of BODIPY J Aggregates for Bioimaging and Cancer Therapy. Nano Lett. 2024, 24, 8287–8295. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Q.L.; Du, Y.H.; Zhou, X.B.; Du, X.C.; Wu, Q.Y.; Lin, L.Y.; Song, Y.L.; Li, F.Y.; Yang, C.Y.; et al. Metabolic Labeling of Peptidoglycan with NIR-II Dye Enables In Vivo Imaging of Gut Microbiota. Angew. Chem. Int. Ed. 2020, 59, 2628–2633. [Google Scholar] [CrossRef]
- Yan, C.X.; Zhang, Y.T.; Guo, Z.Q. Recent progress on molecularly near-infrared fluorescent probes for chemotherapy and phototherapy. Coord. Chem. Rev. 2021, 427, 213556. [Google Scholar] [CrossRef]
- Wang, Y.W.; Liao, J.H.; Lyu, Y.T.; Guo, Q.Q.; Zhu, Z.R.; Wu, X.P.; Yu, J.H.; Wang, Q.; Zhu, W.H. An AIE Photosensitizer with Simultaneous Type I and Type II ROS Generation: Efficient Bacterial Elimination and Hypoxic Tumor Ablation. Adv. Funct. Mater. 2023, 33, 2301692. [Google Scholar] [CrossRef]
- Zeng, Y.Q.; Zhou, S.Y.; Yang, Y.C.; Tang, B.; Wei, W.J.; Huang, G.; Wu, C.F.; Fang, X.F. Dual-Functional Nanobody Optical Probes for In Vivo Fluorescence Imaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2025, 17, 11764–11773. [Google Scholar] [CrossRef]
- Gao, L.; Dalapati, R.; Gao, B.B.; Huang, X.Y.; Zhao, D.; Wang, F.; Zang, L. Mitochondrial STED Imaging and Membrane Potential Monitoring with a Cationic Molecular Probe. Small Methods 2024, 8, 2400525. [Google Scholar] [CrossRef]
- Hong, Y.N.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 4332–4353. [Google Scholar] [CrossRef]
- Luo, J.D.; Xie, Z.L.; Lam, J.W.Y.; Cheng, L.; Chen, H.Y.; Qiu, C.F.; Kwok, H.S.; Zhan, X.W.; Liu, Y.Q.; Zhu, D.B.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Ryan, T.K.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Wu, T.L.; Yu, Y.J.; Xin, Y.T.; Lu, H.G.; Zhao, S.K.; Su, M.H.; Ga, L.; Dong, A.; Mahmoud, E.; et al. A Peptide-Derived Aggregation-Induced Emission Nanobioprobe: Unlocking Selective Detection of Fusobacterium nucleatum and Noninvasive Screening of Colorectal Cancer. Aggregate 2025, 6, e740. [Google Scholar] [CrossRef]
- Shi, H.B.; Ryan, T.K.K.; Liu, J.Z.; Xing, B.G.; Tang, B.Z.; Liu, B. Real-Time Monitoring of Cell Apoptosis and Drug Screening Using Fluorescent Light-Up Probe with Aggregation-Induced Emission Characteristics. J. Am. Chem. Soc. 2012, 134, 17972–17981. [Google Scholar] [CrossRef]
- Ma, F.L.; Gao, Z.Y.; Jia, Q.; Yang, Y.; Wang, B.Z.; Zhang, J.Y.; Deng, Z.W.; Mo, R.F.; Ding, Z.Y.; Xing, G.C.; et al. Intramolecular Repulsive Interactions Enable High Efficiency of NIR-II Aggregation-Induced Emission Luminogens for High-Contrast Glioblastoma Imaging. ACS Nano 2025, 19, 1676–1688. [Google Scholar] [CrossRef]
- Ma, F.L.; Jia, Q.; Deng, Z.W.; Wang, B.Z.; Zhang, S.W.; Jiang, J.H.; Xing, G.C.; Wang, Z.L.; Qiu, Z.J.; Zhao, Z.; et al. Boosting Luminescence Efficiency of Near-Infrared-II Aggregation-Induced Emission Luminogens via a Mash-Up Strategy of π-Extension and Deuteration for Dual-Model Image-Guided Surgery. ACS Nano 2024, 18, 9431–9442. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Wang, W.J.; Yang, X.R.; An, X.M.; Liu, X.Y.; Zhao, W.W.; Zhu, L.N.; Wang, T.; Wang, Y.; Chen, Y.; et al. Molecular Diagnosis and Therapy of Dental Caries by Oral Microbiome-Selective Aggregation-Induced Photosensitivity. Aggregate 2025, 6, e733. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.Q.; Li, G.C.; Pan, W.; Li, Y.H.; Li, N.; Tang, B. AIE-Active Probe for Unveiling the Role of Protein Sulfenylation In Vivo: Specific Imaging and Therapeutic Insights. Anal. Chem. 2025, 97, 11190–11201. [Google Scholar] [CrossRef]
- Fan, X.X.; Xia, Q.M.; Zhang, Y.Y.; Li, Y.R.; Feng, Z.; Zhou, J.; Qi, J.; Tang, B.Z.; Qian, J.; Lin, H. Aggregation-Induced Emission (AIE) Nanoparticles-Assisted NIR-II Fluorescence Imaging-Guided Diagnosis and Surgery for Inflammatory Bowel Disease (IBD). Adv. Healthc. Mater. 2021, 10, 2101043. [Google Scholar] [CrossRef]
- Hu, Y.C.; Gao, X.; Ma, J.L.; Shangguan, Z.; Chen, L.L.; Zhang, G.X.; Zhang, X.S.; Li, C.; Li, Y.B.; Zhang, D.Q. New AIE Emitters from the Unexpected Boron Tribromide/Boron Trichloride-mediated Cyclization Reaction and Application for Fluorescence Imaging of Lipid Droplets. Aggregate 2025, 6, e735. [Google Scholar] [CrossRef]
- Wang, E.J.; Zhao, E.G.; Hong, Y.N.; Jacky, W.Y.L.; Tang, B.Z. A highly selective AIE fluorogen for lipid droplet imaging in live cells and green algae. J. Mater. Chem. B 2014, 2, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Wu, S.F.; Li, S.H.; Yuan, Y.; Wu, F.P.; Kumar, S.; Jiang, Z.Q.; Fung, M.K.; Liao, L.S. D–A–A-Type Emitter Featuring Benzo[c][1,2,5]thiadiazole and Polar CN Bond as Tandem Acceptor for High-Performance Near-Infrared Organic Light-Emitting Diodes. Adv. Opt. Mater. 2017, 5, 1700566. [Google Scholar] [CrossRef]
- Hu, X.K.; Jia, Q.; Fang, Q.Y.; Song, C.Q.; Zhang, R.L.; Liang, Y.; Yang, Z.L.; Wu, J.T.; Li, H.B.; Zhao, T.X.; et al. Synchronously Manipulating the D−A Interaction and Planarity in Semiconducting Polymers to Achieve 84.7% Photothermal Conversion Efficiency for NIR-II Imaging-Guided Tumor Therapy. Adv. Funct. Mater. 2024, 34, 2407100. [Google Scholar] [CrossRef]
- Yang, S.P.; Zhang, J.Y.; Zhang, Z.J.; Zhang, R.Y.; Ou, X.W.; Xu, W.L.; Kang, M.; Li, X.; Yan, D.Y.; Ryan, T.K.K.; et al. More Is Better: Dual-Acceptor Engineering for Constructing Second Near-Infrared Aggregation-Induced Emission Luminogens to Boost Multimodal Phototheranostics. J. Am. Chem. Soc. 2023, 145, 22776–22787. [Google Scholar] [CrossRef]
- Gao, H.Q.; Yao, Y.M.; Li, C.; Zhang, J.T.; Yu, H.Y.; Yang, X.D.; Shen, J.; Liu, Q.; Xu, R.T.; Gao, X.K.; et al. Fused Azulenyl Squaraine Derivatives Improve Phototheranostics in the Second Near-Infrared Window by Concentrating Excited State Energy on Non-Radiative Decay Pathways. Angew. Chem. Int. Ed. 2024, 63, e202400372. [Google Scholar]
- Wang, J.; Wu, Z.H.; Ma, X.X.; Huang, Z.H.; Dong, H.R.; Zhang, J.X.; Liu, X.M.; Zhang, P.F.; Yao, S.H. NIR-II emissive biohybrid nanovesicles as mild-temperature photothermal antibiofilm agents against acute bacterial skin and skin-structure infections. Interdiscip. Med. 2025, 3, e20240053. [Google Scholar] [CrossRef]
- Wang, Q.; Li, C.B.; Song, Y.C.; Shi, Q.K.; Li, H.; Zhong, H.; Wang, J.G.; Hu, F. Acene enlargement for absorption red-shifting and photosensitization enhancement of photosensitizers with aggregation-induced emission. Chem. Sci. 2023, 14, 684–690. [Google Scholar] [CrossRef]
- Wu, W.C.; Chen, C.Y.; Tian, Y.; Jang, S.H.; Hong, Y.; Liu, Y.; Hu, R.; Tang, B.Z.; Lee, Y.T.; Chen, C.T.; et al. Enhancement of Aggregation-Induced Emission in Dye-Encapsulating Polymeric Micelles for Bioimaging. Adv. Funct. Mater. 2010, 20, 1413–1423. [Google Scholar] [CrossRef]
- Wu, W.B.; Xu, S.D.; Qi, G.B.; Zhu, H.; Hu, F.; Liu, Z.T.; Zhang, D.Q.; Liu, B. A Cross-linked Conjugated Polymer Photosensitizer Enables Efficient Sunlight-Induced Photooxidation. Angew. Chem. Int. Ed. 2019, 58, 3062–3066. [Google Scholar] [CrossRef]
- Shi, Q.K.; Xu, J.Y.; Xu, H.H.; Wang, Q.; Huang, S.H.; Wang, X.R.; Wang, P.; Hu, F. Polystyrene-Based Matrix to Enhance the Fluorescence of Aggregation-Induced Emission Luminogen for Fluorescence-Guided Surgery. Small 2024, 20, 2309589. [Google Scholar] [CrossRef]
- Geng, J.L.; Li, K.; Qin, W.; Ma, L.; Gahik, G.G.; Tang, B.Z.; Liu, B. Eccentric Loading of Fluorogen with Aggregation-Induced Emission in PLGA Matrix Increases Nanoparticle Fluorescence Quantum Yield for Targeted Cellular Imaging. Small 2013, 9, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Bai, Q.; Yao, L.; Liu, H.C.; Gao, Y.; Li, J.Y.; Liu, L.Q.; Liu, Y.L.; Li, X.X.; Lu, P.; et al. Highly Efficient Solid-State Near-Infrared Emitting Material Based on Triphenylamine and Diphenylfumaronitrile with an EQE of 2.58% in Nondoped Organic Light-Emitting Diode. Adv. Funct. Mater. 2015, 25, 7521–7529. [Google Scholar] [CrossRef]
- Liu, S.J.; Zhang, H.K.; Li, Y.Y.; Liu, J.K.; Du, L.L.; Chen, M.; Ryan, T.K.K.; Jacky, W.Y.L.; Phillips, D.L.; Tang, B.Z. Strategies to Enhance the Photosensitization: Polymerization and the Donor–Acceptor Even–Odd Effect. Angew. Chem. Int. Ed. 2018, 57, 15189–15193. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Li, X.F.; Li, J.; Yang, Z.R.; Xu, B.J.; Chi, Z.; Xu, J.; Zhang, Y. Influence of cyano groups on the properties of piezofluorochromic aggregation-induced emission enhancement compounds derived from tetraphenylvinyl-capped ethane. J. Mater. Chem. C 2015, 3, 1225–1234. [Google Scholar] [CrossRef]
- Lu, X.C.; Li, W.L.; Nancy, R.S.; Jeffrey, S.M. Autonomous Damage Detection in Multilayered Coatings via Integrated Aggregation-Induced Emission Luminogens. ACS Appl. Mater. Interfaces 2018, 10, 40361–40365. [Google Scholar] [CrossRef]
- Li, K.; Ding, D.; Chandrasekharan, P.; Qin, W.; Yang, C.T.; Tang, B.Z.; Liu, B. Gadolinium-Functionalized Aggregation-Induced Emission Dots as Dual-Modality Probes for Cancer Metastasis Study. Adv. Healthc. Mater. 2013, 2, 1600–1605. [Google Scholar] [CrossRef]
- Takuya, Y.; Yusuke, O.; Tomataka, N.; Taniyuki, F.; Kenji, K.; Nagao, K. Phosphorus(V) Tetraazaporphyrin with an Intense, Broad CT Band in the Near-IR Region. Org. Lett. 2024, 26, 1931–1935. [Google Scholar]
- Wu, D.B.; Gong, W.J.; Yao, H.M.; Huang, L.M.; Lin, Z.H.; Ling, Q.D. Highly efficient solid-state emission of diphenylfumaronitriles with full-color AIE, and application in explosive sensing, data storage and WLEDs. Dye. Pigment. 2020, 172, 107829. [Google Scholar] [CrossRef]
- Geng, J.L.; Zhu, Z.S.; Qin, W.; Ma, L.; Hu, Y.; Gagik, G.G.; Tang, B.Z.; Liu, B. Near-infrared fluorescence amplified organic nanoparticles with aggregation-induced emission characteristics for in vivo imaging. Nanoscale 2014, 6, 939–945. [Google Scholar] [CrossRef]
- McNamara, L.E.; Melnychuk, C.; Boyn, J.-N.; Anferov, S.W.; Mazziotti, D.A.; Schaller, R.D.; Anderson, J.S. Elucidating Non-Radiative Decay in Near-Infrared Lumiphores: Leveraging New Design Principles to Develop a Telecom Band Organic Dye Laser. Chem 2024, 10, 2266–2282. [Google Scholar] [CrossRef]
- Tristan, A.G.; Ionela, G.; Giuseppe, I.; Francesco, P.; Andrea, P. Luminescent Solar Concentrators Based on Renewable Polyester Matrices. Chem. Asian J. 2019, 14, 877–883. [Google Scholar]
- Pierpaolo, M.; Giuseppe, I.; Giacomo, R.; Virgilio, M.; Andrea, P. Molecular Rotors with Aggregation-Induced Emission (AIE) as Fluorescent Probes for the Control of Polyurethane Synthesis of the article. Chemosensors 2021, 9, 3. [Google Scholar]
- Geng, J.L.; Li, K.; Qin, W.; Tang, B.Z.; Liu, B. Red-Emissive Chemiluminescent Nanoparticles with Aggregation-Induced Emission Characteristics for In Vivo Hydrogen Peroxide Imaging. Part. Part. Syst. Charact. 2014, 31, 1238–1243. [Google Scholar] [CrossRef]
- Dayane, B.T.; Mauricio, S.B. Photosensitizing nanoparticles and the modulation of ROS generation. Front. Chem. 2015, 3, 33. [Google Scholar]
- Pastore, C. Size-dependent nano-bio interactions. Nat. Nanotechnol. 2021, 16, 1052. [Google Scholar] [CrossRef]
- Lahooti, B.; Akwii, R.G.; Zahra, F.T.; Sajib, M.S.; Lamprou, M.; Alobaida, A.; Lionakis, M.S.; Mattheolabakis, G.; Mikelis, C.M. Targeting endothelial permeability in the EPR effect. J. Control. Release 2023, 361, 212–235. [Google Scholar] [CrossRef]
- Entradas, T.; Waldron, S.; Volk, M. The detection sensitivity of commonly used singlet oxygen probes in aqueous environments. J. Photochem. Photobiol. B 2020, 204, 111787. [Google Scholar] [CrossRef]
- Pragti; Kundu, B.K.; Upadhyay, S.N.; Sinha, N.; Ganguly, R.; Grabchev, I.; Pakhira, S.; Mukhopadhyay, S. Pyrene-based fluorescent Ru(ii)-arene complexes for significant biological applications: Catalytic potential, DNA/protein binding, two photon cell imaging and in vitro cytotoxicity. Dalton Trans. 2022, 51, 3937–3953. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Ma, W.; Song, Y.; Tian, Y.; Hu, F.; Wu, W.; Cai, L. Methylsteric Effects Enhance Fluorescence in Diphenylfumaronitrile AIEgens. Molecules 2025, 30, 2898. https://doi.org/10.3390/molecules30142898
Xu Z, Ma W, Song Y, Tian Y, Hu F, Wu W, Cai L. Methylsteric Effects Enhance Fluorescence in Diphenylfumaronitrile AIEgens. Molecules. 2025; 30(14):2898. https://doi.org/10.3390/molecules30142898
Chicago/Turabian StyleXu, Zihao, Wenwen Ma, Yuchen Song, Yu Tian, Fang Hu, Wenbo Wu, and Liu Cai. 2025. "Methylsteric Effects Enhance Fluorescence in Diphenylfumaronitrile AIEgens" Molecules 30, no. 14: 2898. https://doi.org/10.3390/molecules30142898
APA StyleXu, Z., Ma, W., Song, Y., Tian, Y., Hu, F., Wu, W., & Cai, L. (2025). Methylsteric Effects Enhance Fluorescence in Diphenylfumaronitrile AIEgens. Molecules, 30(14), 2898. https://doi.org/10.3390/molecules30142898






