Abstract
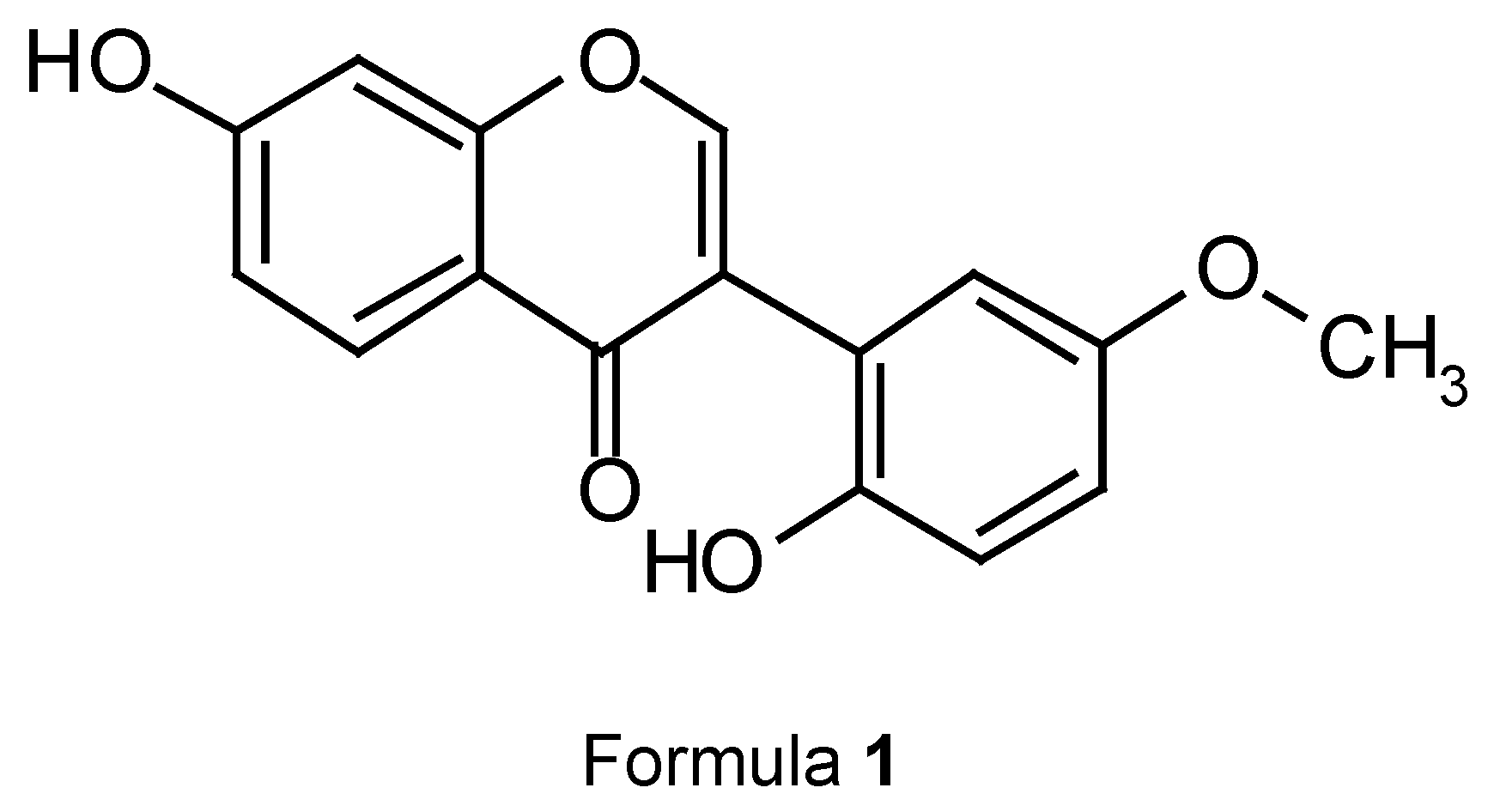
A new isoflavone, 7,6’-dihydroxy-3’-methoxyisoflavone, has been isolated from the roots of Smilax glabra. The structure was determined by 2D-NMR techniques.
Introduction
Smilax glabra Roxb. (Liliaceae), which was called ”Tu Fu Ling”, is widely distributed in east and southwest regions of China. In traditional chinese medicine, the rhizome of S. glabra has been used clinically to prevent leptospirosis, and to treat syphilis, acute bacterial dysentery, acute and chronic nephritis, etc. [1].
Several chemical constituents have been isolated from this plant in our laboratory [2,3,4,5]. In the present work, silica gel chromatography of the EtOAc part of the EtOH extracts of the roots of S. glabra has led to the isolation of a new isoflavone. It was elucidated as 7,6’-dihydroxy-3’-methoxy-isoflavone (1) based on MS and 1H-and 13C-NMR spectroscopy, including 1H-1H COSY, COLOC and NOEDS techniques. In this paper we report the structural elucidation of the new isoflavone 1.
Results and Discussions
Compound 1, pale yellow needles (acetone), mp 250~252. Gave positive Mg-HCl (reddish) and FeCl3 (greenish-brown) colour tests. In the 1H-NMR spectrum, the singlet was observed at δ 7.7(s, 1H). In the UV spectrum, the maximum absorption was at 255 nm. Thus, 1 was deduced to be an isoflavone which was also confirmed by the 13C-NMR(DEPT) spectra (see Table 1).

Table 1.
Correlated 13C-and 1H-Data and COSY for compound 1.
The EI-MS of 1 exhibited [M]+ at m/z 284.0672 for C16H12O5 (Calc. 284.0679) which is in accord with an isoflavone containing two hydroxyl groups and a methoxy group. In order to determine the substituted positions, 2D-NMR techniques were used. The COLOC spectrum showed a correlation between H-2 and C-9, H-8 and C-9, C-7. This correlation confirmed that the hydroxyl group in ring A was at the C-7 position.
Furthermore, the ion m/z 148 observed in the EIMS was derived from ring B and suggested that it carried one hydroxyl group and a methoxy group. In the 1H-NMR, there are two groups of peaks of B ring, δ6.5(1H, d, J=2Hz) and δ6.3(2H, m). The position of methoxy group at C-3’ was deduced from the NOEDS of 1 that showed an enhancement of the peaks of methoxy and H-2 on irradiation of H-2’. In the 1H-1H-COSY, the correlations of H-2 and H-2’, H-2 and MeO were shown. The position of the hydroxyl group at C-6’ was confirmed by 1H-1H-COSY which displayed the correlation between methoxy and H-4’.
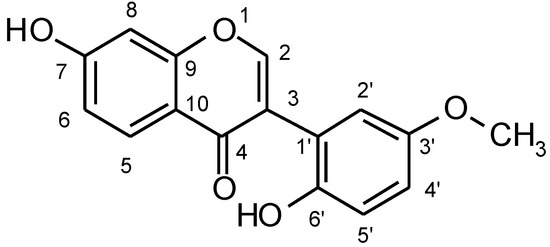
Figure 1.
Atomic numbering of compound 1.
The structure of 1 was fully in accordance with its NMR spectra including COLOC, DEPT and 1H-1H-COSY spectra. Thus, 1 was deduced to be 7,6’-dihydroxy, 3’-methoxy isoflavone.
Experimental Section
Melting point was uncorrected. The IR spectrum was measured on a Necolet IR-2000, 1HNMR and 13CNMR spectra were recorded on an ACF-300, DMSO-d6 as solvent and TMS as int. standard. MS was recorded on a Finnigan FTMS-2000.
The dried roots of Smilax glabra were extracted with 95% EtOH under reflux. After evaporating the solvent, the crude extract was extracted with Et2O and EtOAc, respectively to give 30g of EtOAc extract. Successive column chromatography using a CHCl3-MeOH solvent system with increasing polarity, and repeated column chromatography of series (CHCl3-MeOH, 20:1) afforded compound 1.
Compound 1 is pale yellow crystal, mp 250-252 °C. IR λmax 3420, 1640 cm-1. UV (MeOH) λmax 227, 255, 290, 308 nm. EIMS m/z 284 (M+), 269, 253, 241, 213, 148, 137, 105. For 1HNMR, 13CNMR, 1H-1H COSY, COLOC data, see Table 1.
Acknowledgement
We acknowledge with gratitude the financial support of National Natural Science Foundation of China.
References and Notes
- Jiangsu New College of Medicine. A Dictionary of Traditional Chinese Drugs; People’s Press: Shanghai, 1977; pp. 91–93. [Google Scholar]
- Cao, Z. Z.; Yi, Y. J.; Hong, W. Q.; Ling, Y. L. Zhongcaoyao 1993, 24, 234.
- Cao, Z. Z.; Yi, Y. J.; Yang, D. L. Natural Product R & D 1994, 6(2), 33.
- Cao, Z. Z.; Yi, Y. J.; Cao, Y.; Leng, Z. K. Chin. Chem. Lett. 1995, 6, 587.
- Yi, Y. J.; Cao, Z. Z.; Yang, W. H.; Hong, W. Q.; Cao, Y.; Leng, Z. K. Yaoxue Xuebao 1995, 30, 718.
- Sample Availability: available from MDPI.
© 1998 MDPI. All rights reserved. Molecules http://www.mdpi.org/molecules/