Cori Ester as the Ligand for Monovalent Cations
Abstract
1. Introduction
2. Results and Discussion
2.1. Complexes in Solid Phase
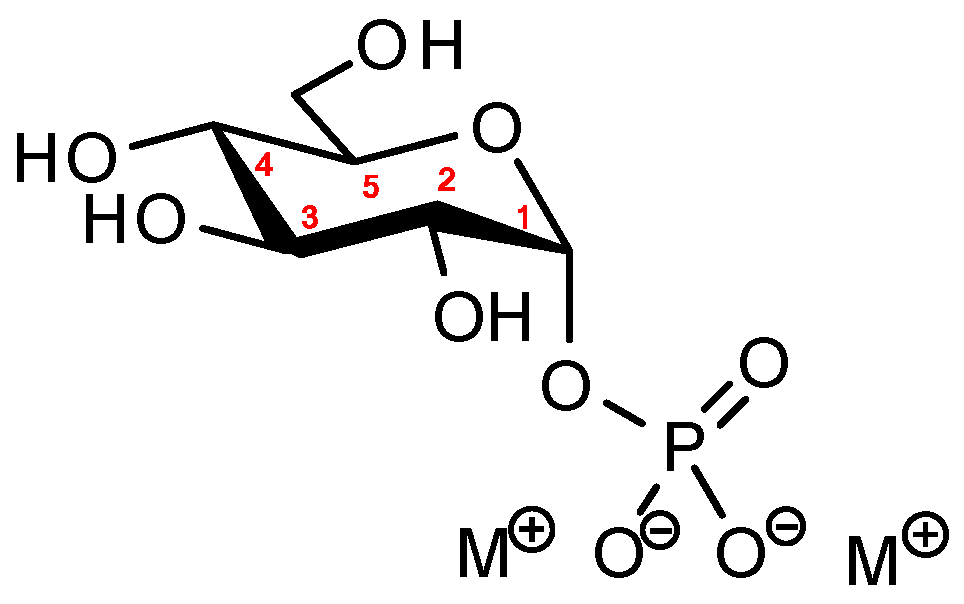
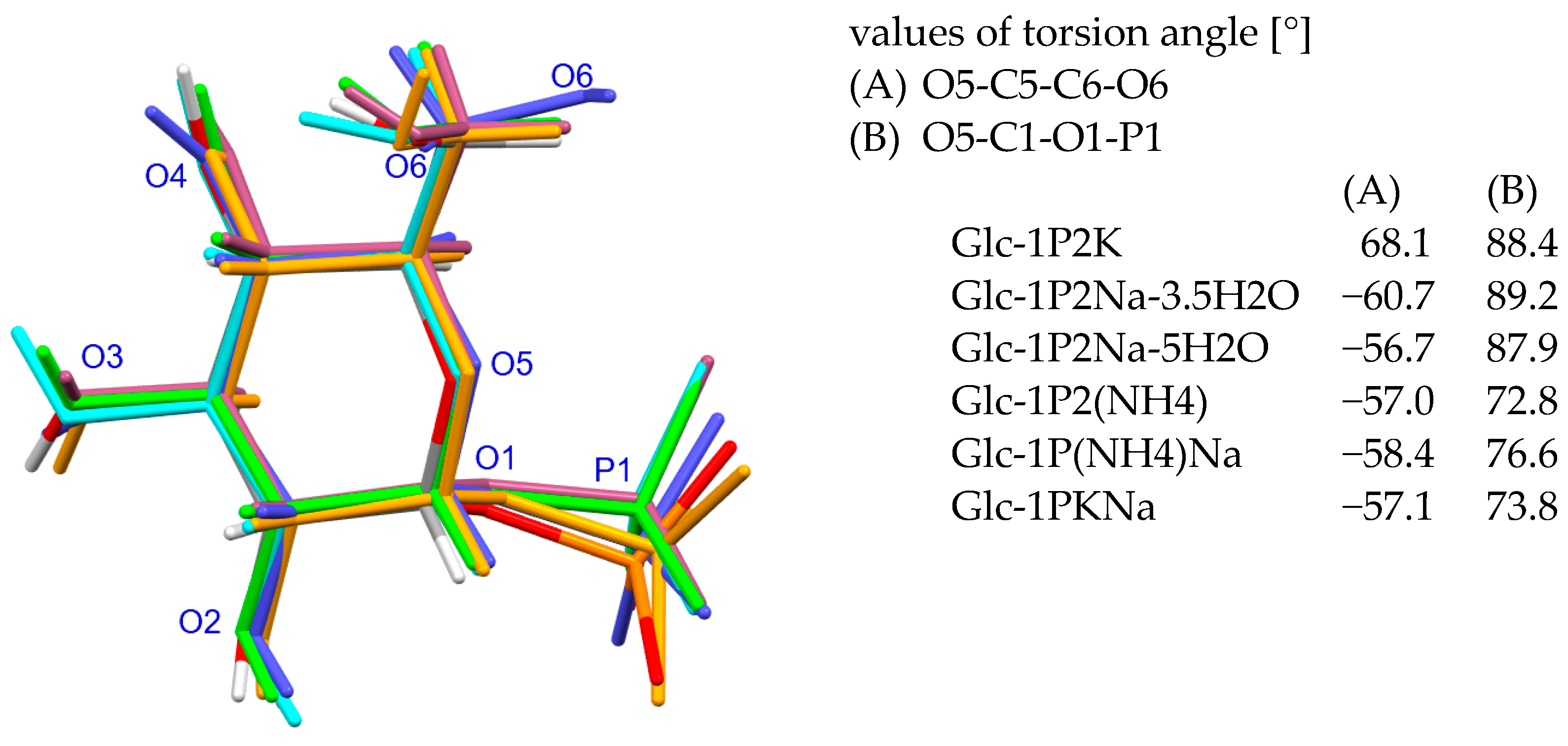
2.2. Structure of α-D-Glucopyranosyl-1-Phosphate Dianion
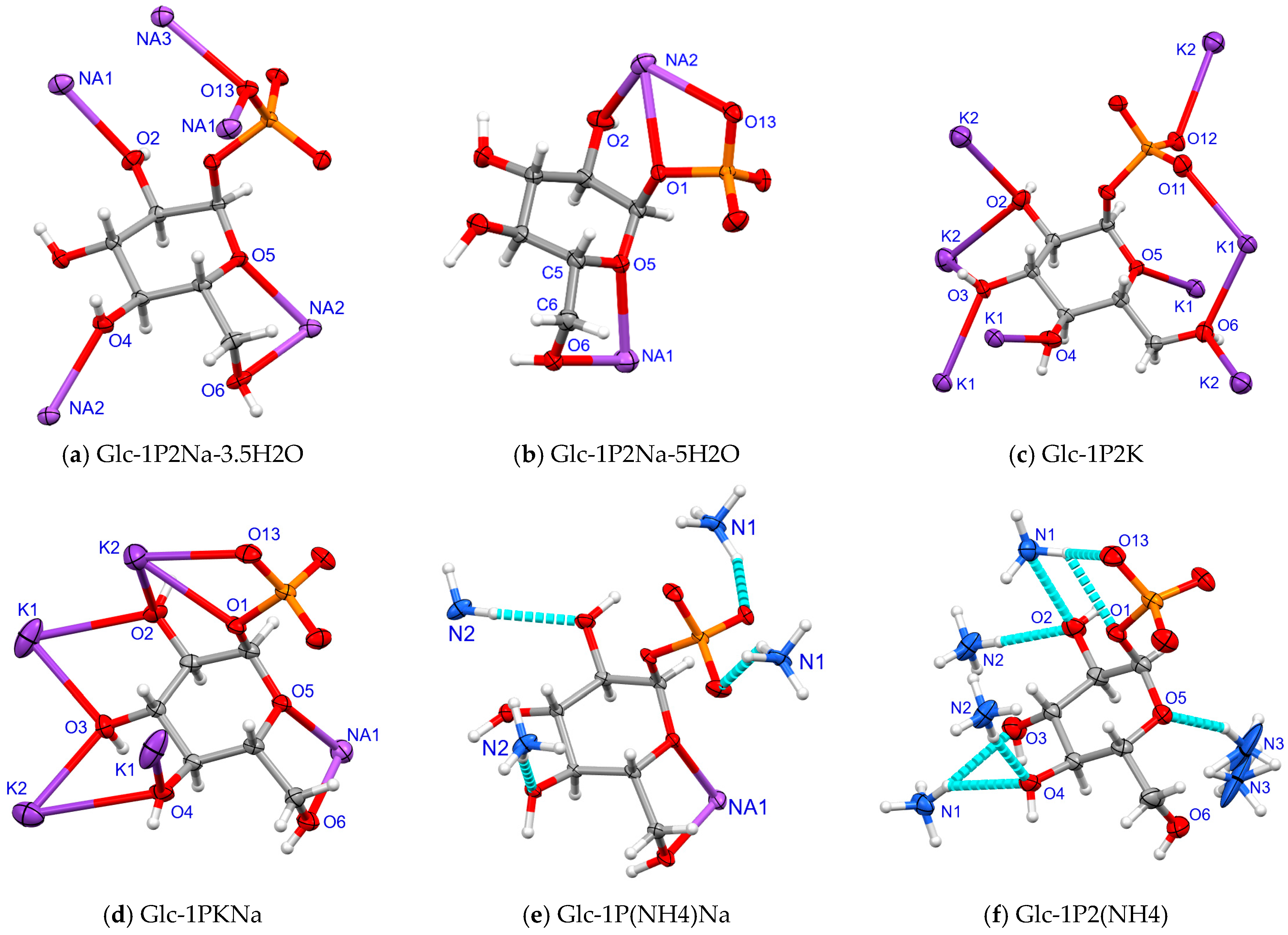
2.3. Environment of α-D-Glucopyranosyl-1-Phosphate Dianion
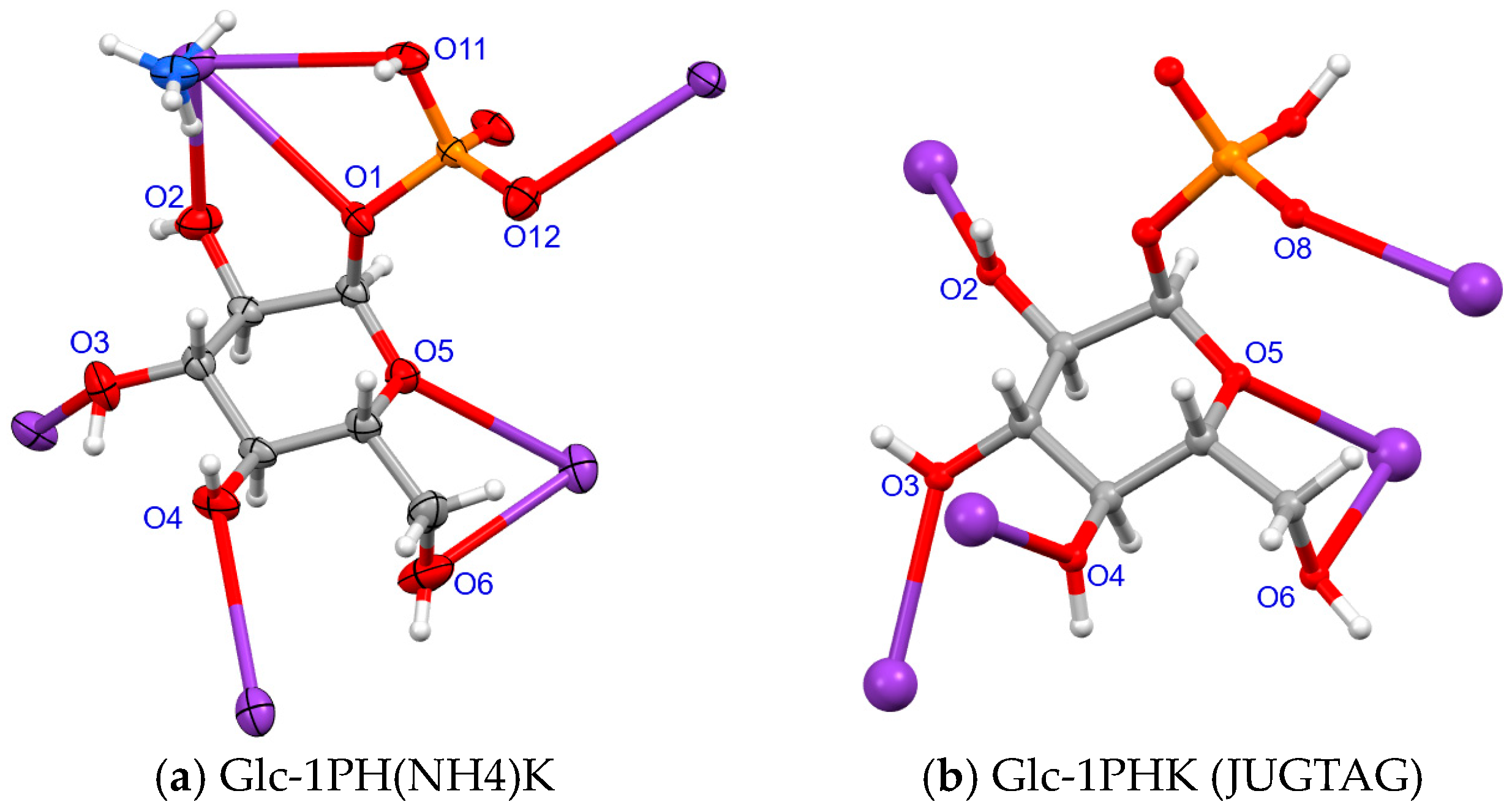
2.4. Environment of α-D-Glucopyranosyl-1-Hydrogenphosphate Anion
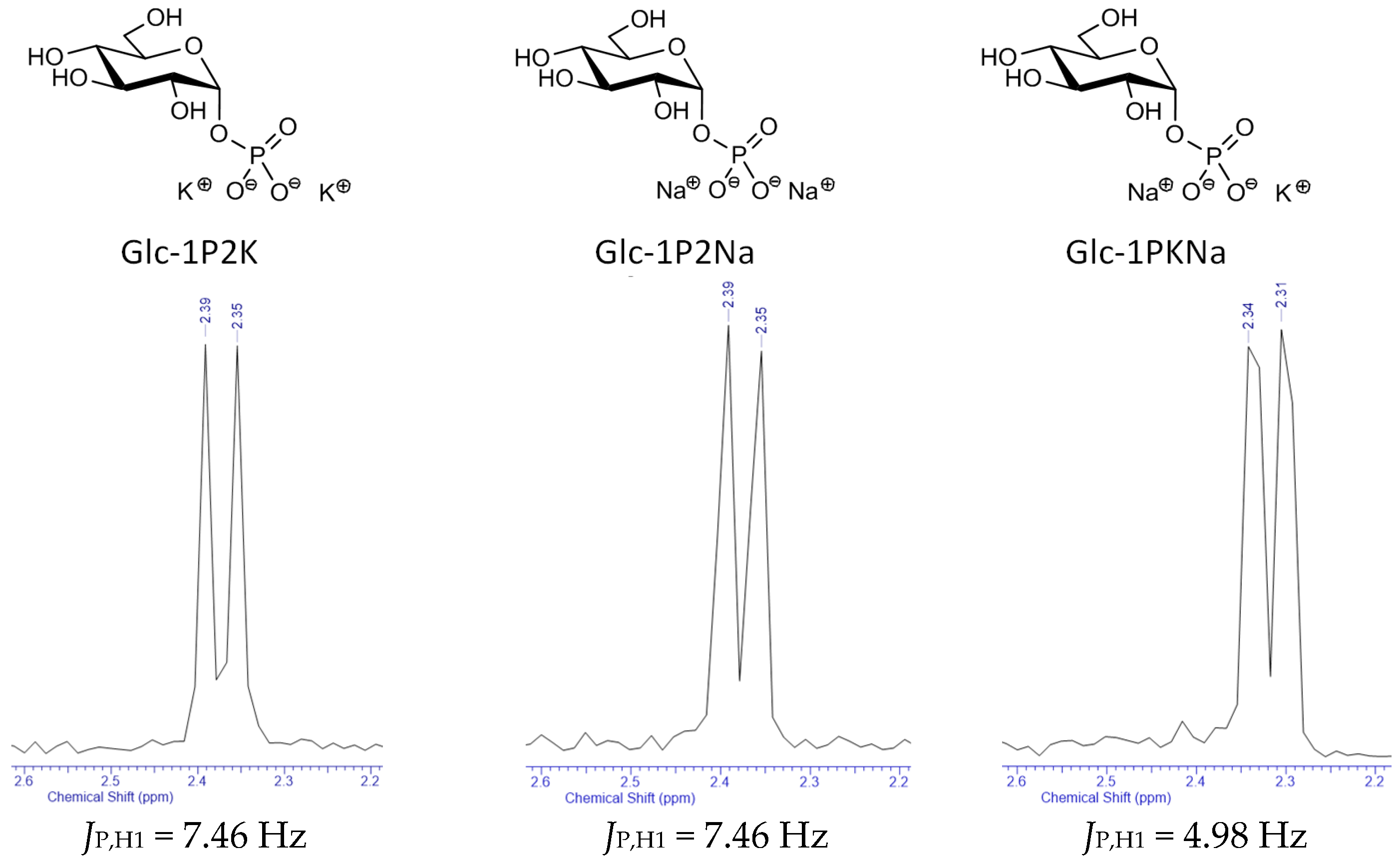
2.5. Nuclear Magnetic Resonance Studies
3. Materials and Methods
3.1. General Information
3.1.1. Syntheses
Preparation of the Glc-1P2(NH4) Complex
Preparation of the Glc-1PKNa Complex
Preparation of the Glc-1P (NH4)Na and Glc-1P (NH4)K Complexes
3.1.2. NMR Data of α-D-Glucose 1-Phosphate Complexes
Dipotassium α-D-Glucose 1-Phosphate (Glc-1P2K)
Disodium α-D-Glucose 1-Phosphate (Glc-1P2Na)
Potassium Sodium α-D-Glucose 1-Phosphate (Glc-1PKNa)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garrett, R.H.; Grisham, C.M. Biochemistry, 3rd ed.; Saunders College Publishing: Philadelphia, PA, USA, 1999. [Google Scholar]
- Mathews, C.K.; van Holde, K.E.; Ahern, K.G. Biochemistry, 2nd ed.; Benjamin Cummings: San Francisco, CA, USA, 2000. [Google Scholar]
- Cori, C.F.; Cori, G.T. Polysaccharide phosphorylase. Nobel Lect. 1947. Available online: https://www.nobelprize.org/prizes/medicine/1947/cori-gt/lecture/ (accessed on 5 April 2024).
- Ginsberg, J. Carl and Gerty Cori and Carbohydrate Metabolism; American Chemical Society National Historic Chemical Landmarks: Washington, DC, USA, 2004; Available online: http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/carbohydratemetabolism.html (accessed on 5 April 2024).
- Crabb, E.; Moore, E. Metals and Life; RSC Publishing, The Open University: Cambridge UK, 2010. [Google Scholar]
- Groom, C.R.; Allen, F.H. The Cambridge Structural Database in Retrospect and Prospect. Angew. Chem. Int. Ed. 2014, 53, 662–671. [Google Scholar] [CrossRef]
- Adam, A.; Cirpus, V. Darstellung und Struktur der ersten gemischten Alkalimetallhydrogencarbonate NaA,[H(CO,),] 2 H20 mit A = K, Rb. Z. Anorg. Allg. Chem. 1996, 622, 2023–2030. [Google Scholar] [CrossRef]
- Styron, S.D.; French, A.D.; Friedrich, J.D.; Lake, C.H.; Kiely, D.E. MM3(96) Conformational Analysis of D-Glucaramide and X-Ray Crystal Structures of Three D-Glucaric Acid Derivatives—Models For Synthetic Poly(alkylene D-Glucaramides). J. Carbohydr. Chem. 2002, 21, 27–51. [Google Scholar] [CrossRef]
- Rammohan, A.; Kaduk, J.A. Sodium potassium hydrogen citrate, NaKHC6H5O7. Acta Cryst. 2016, E72, 170–173. [Google Scholar] [CrossRef]
- Rammohan, A.; Kaduk, J.A. Sodium dipotassium citrate, NaK2C6H5O7. Acta Cryst. 2016, E72, 403–406. [Google Scholar] [CrossRef]
- Egorova, A.E.; Ivanov, V.A.; Somov, N.V.; Portnov, V.N.; Chuprunov, E.V. Crystal Structure of Potassium Sodium Tartrate Trihydrate. Crystallogr. Rep. 2011, 56, 1038–1041. [Google Scholar] [CrossRef]
- Somov, N.V. KNATDL02: Catena-[(μ-aqua)-(μ-DL-tartrato)-triaqua-potassium-sodium]. CCDC 771649. Exp. Cryst. Struct. Determ. 2017. [Google Scholar] [CrossRef]
- Solans, X.; Gonzalez-Silgo, C.; Ruiz-Perez, C. A Structural Study on the Rochelle Salt. J. Solid State Chem. 1997, 131, 350–357. [Google Scholar] [CrossRef]
- Sharaya, S.S.; Zakharov, B.A.; Boldyreva, E.V. Structural changes in Rochelle salt on phase transitions revisited in a multi-temperature single-crystal X-ray diffraction study. Acta Cryst. 2024, B80, 94–104. [Google Scholar] [CrossRef]
- Narendra, N.; Seshadri, T.P.; Viswamitra, M.A. Structure of the disodium salt of glucose 1-phosphate hydrate, 2Na+.C6H11O9P2-.3.5H2O. Acta Cryst. 1984, C40, 1338–1340. [Google Scholar] [CrossRef]
- Kozioł, A.E. Crystal structure of disodium α-D-glucose 1-phosphate trihemihydrate. Reinvestigation. Pol. J. Chem. 1991, 65, 455–460. [Google Scholar]
- Sugawara, Y.; Iwasaki, H. Structure of disodium uridine diphosphoglucose dihydrate, C15H22N2O17P22-.2Na+.2H2O, and refinement of dipotassium glucose 1-phosphate dihydrate, C6H11O9P2-.2K+.2H2O (monoclinic form). Acta Cryst. 1984, C40, 389–393. [Google Scholar] [CrossRef]
- Lis, T. The crystal structure of α-D-glucopyranosyl potassium hydrogen-phosphate. Carbohydr. Res. 1992, 229, 33–39. [Google Scholar] [CrossRef]
- Tutton, A.E.H. The Monoclinic Double Sulphates Containing Ammonium. Philos. Trans. R. Soc. London. Ser. A Contain. Pap. Math. Phys. Character 1916, 216, 1–62. Available online: https://www.jstor.org/stable/91084 (accessed on 5 April 2024).
- Shiozaki, Y.; Sawaguchi, E.; Koh, S. Crystal structure of (NH4)2SO4—K2SO4 mixed crystal, J. Phys. Soc. Japan 1977, 43, 721–722. [Google Scholar] [CrossRef]
- Hahn, T.D. (Ed.) International Tables for Crystallography; Reidel Publishing Co.: Dordrecht, The Netherlands, 1987; Volume A, p. 195. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Beswick, L.; Ahmadipour, S.; Hofman, G.J.; Wootton, H.; Dimitriou, E.; Reynisson, J.; Field, R.A.; Linclau, B.; Miller, G.J. Exploring anomeric glycosylation of phosphoric acid: Optimisation and scope for non-native substrates. Carbohydr. Res. 2020, 488, 107896. [Google Scholar] [CrossRef] [PubMed]
- Oxford Diffraction Users Manual; Agilent Technologies Inc.: Yarnton, UK, 2014.
- CrysAlisPro 1.171.42.79a; Rigaku Oxford Diffraction: Tokyo, Japan, 2022.
- Gałdecki, Z.; Kowalski, A.; Kucharczyk, D.; Uszynski, I. KM-4 Software ver. KM4B8; Kuma Diffraction: Wrocław, Poland, 1999. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]

 .
.
 .
.


| Identification Code | Glc-1PKNa | Glc-1P2Na-5H2O | Glc-1P2Na-3.5H2O * | Glc-1P2K * |
|---|---|---|---|---|
| Formula | Glc-1PK1.5Na0.5·4H2O | Glc-1PNa2·5·H2O | Glc-1PNa2·3.5·H2O | Glc-1PK2·2H2O |
| Formula weight | 400.33 | 394.18 | 367.15 | 372.35 |
| Crystal system | orthorhombic | orthorhombic | monoclinic | monoclinic |
| Space group | P 21212 | P 212121 | C 2 | P 21 |
| Cell parameters | a = 16.8360(4) Å | a = 6.6347(5) Å | a = 8.4325(2) Å | a = 7.5234(3) Å |
| b = 13.4089(3) Å | b = 8.7585(9) Å | b = 10.1983(2) Å | b = 9.0643(3) Å | |
| c = 6.6729(1) Å | c = 27.129(2) Å | c = 16.5857(4) Å | c = 10.4588(4) Å | |
| β = 90° | β = 90° | β = 99.147(2)° | β = 110.576(5)° | |
| Volume | 1506.42(5) Å3 | 1576.5(2) Å3 | 1408.19(6) Å3 | 667.73(5) Å3 |
| Final R indices [I > 2σ(I)] | R1 = 0.0321 wR2 = 0.0852 | R1 = 0.0277 wR2 = 0.0613 | R1 = 0.0243 wR2 = 0.0635 | R1 = 0.0294 wR2 = 0.0774 |
| CCDC No | 2345577 | 2345578 | 2345575 | 2345574 |
| Identification Code | Glc-1P2(NH4) | Glc-1P(NH4)Na | Glc-1PH(NH4)K | Glc-1PHK [18] |
|---|---|---|---|---|
| Formula | Glc-1P(NH4)2·3H2O | Glc-1P(NH4)1.5Na0.5· 4H2O | Glc-1PH(NH4)0.69K0.31 | Glc-1PHK |
| Formula weight | 348.25 | 368.74 | 283.70 | 298.23 |
| Crystal system | orthorhombic | orthorhombic | orthorhombic | orthorhombic |
| Space group | P 21212 | P 21212 | P 212121 | P 212121 |
| Cell parameters | a = 13.756(7) Å | a = 16.9272(3) Å | a = 7.147(4) Å | a = 7.35(4) Å |
| b = 16.929(7) Å | b = 13.3171(3) Å | b = 12.036(5) Å | b = 9.666(6) Å | |
| c = 6.502(3) Å | c = 6.6436(1) Å | c = 12.524(5) Å | c = 15.230(7) Å | |
| Volume | 1514.2(12) Å3 | 1497.61(5) Å3 | 1077.3(9) Å3 | 1082.16(9) Å3 |
| Final R indices [I > 2σ(I)] | R1 = 0.0440 wR2 = 0.1193 | R1 = 0.0579 wR2 = 0.1565 | R1 = 0.0338 wR2 = 0.0848 | R1 = 0.0316 |
| CCDC No | 2345576 | 2345579 | 2345573 | JUGTAG [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępniak, K.; Lis, T.; Łastawiecka, E.; Kozioł, A.E. Cori Ester as the Ligand for Monovalent Cations. Molecules 2024, 29, 2133. https://doi.org/10.3390/molecules29092133
Stępniak K, Lis T, Łastawiecka E, Kozioł AE. Cori Ester as the Ligand for Monovalent Cations. Molecules. 2024; 29(9):2133. https://doi.org/10.3390/molecules29092133
Chicago/Turabian StyleStępniak, Krystyna, Tadeusz Lis, Elżbieta Łastawiecka, and Anna E. Kozioł. 2024. "Cori Ester as the Ligand for Monovalent Cations" Molecules 29, no. 9: 2133. https://doi.org/10.3390/molecules29092133
APA StyleStępniak, K., Lis, T., Łastawiecka, E., & Kozioł, A. E. (2024). Cori Ester as the Ligand for Monovalent Cations. Molecules, 29(9), 2133. https://doi.org/10.3390/molecules29092133






