Fabrication of Human Milk Fat Substitute: Based on the Similarity Evaluation Model and Computer Software
Abstract
1. Introduction
2. Results and Discussion
2.1. Fatty Acid Profile of Vegetable Oils
2.2. Triacylglycerol Profile of Vegetable Oils
2.3. Formula of Mixed Vegetable Oils
2.4. Results of In Vivo Experiment
2.4.1. Influence on the Growth, Blood Routine and Serum Biochemical Indices of Sprague-Dawley Mice
2.4.2. Fatty Acid Profile of Intestinal Contents and Feces
2.4.3. Concentration of Calcium and Magnesium in Feces
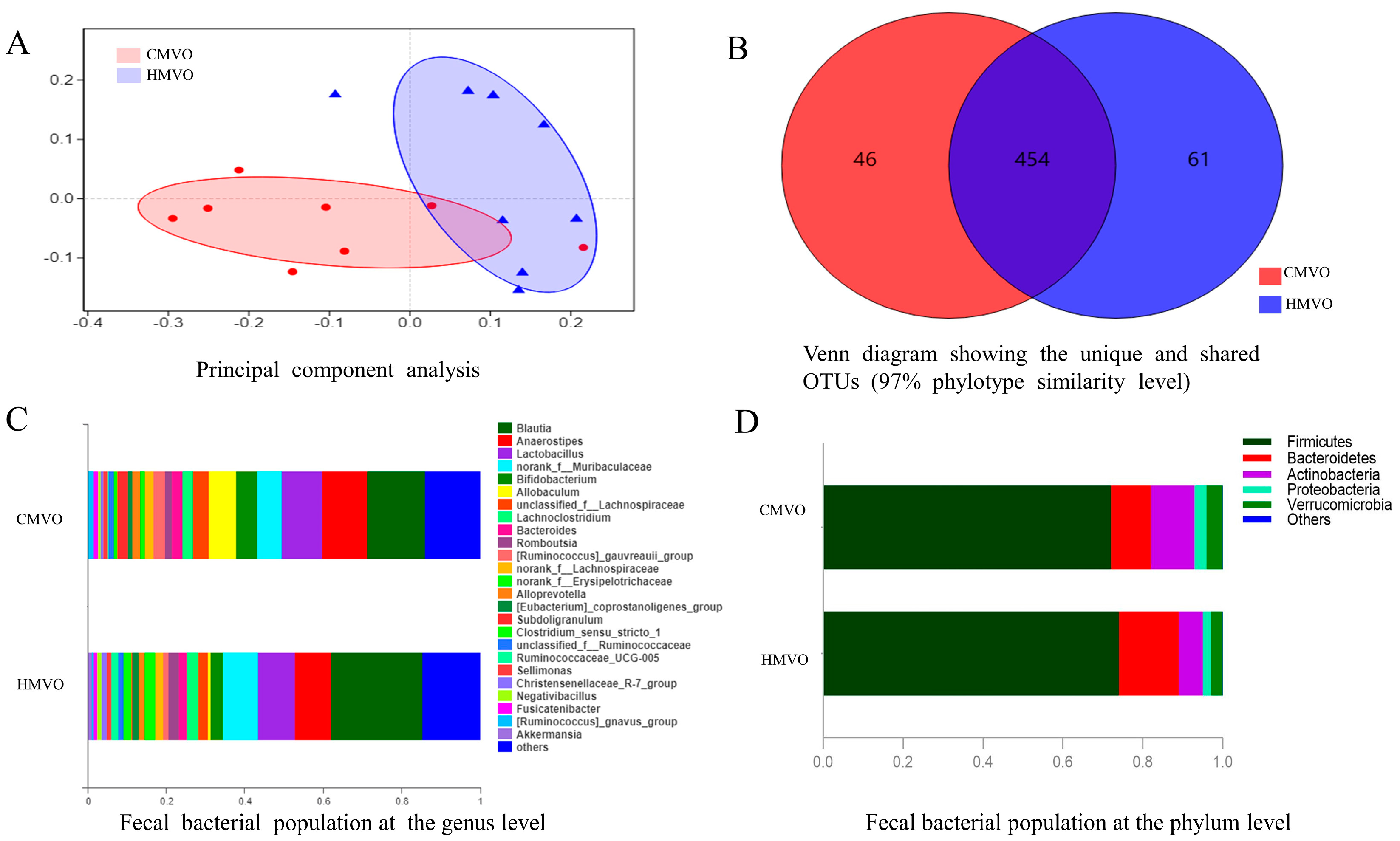
2.5. The Influence on Intestinal Flora
2.5.1. Alpha (α) and Beta Diversity Analysis
2.5.2. Species Composition Analysis
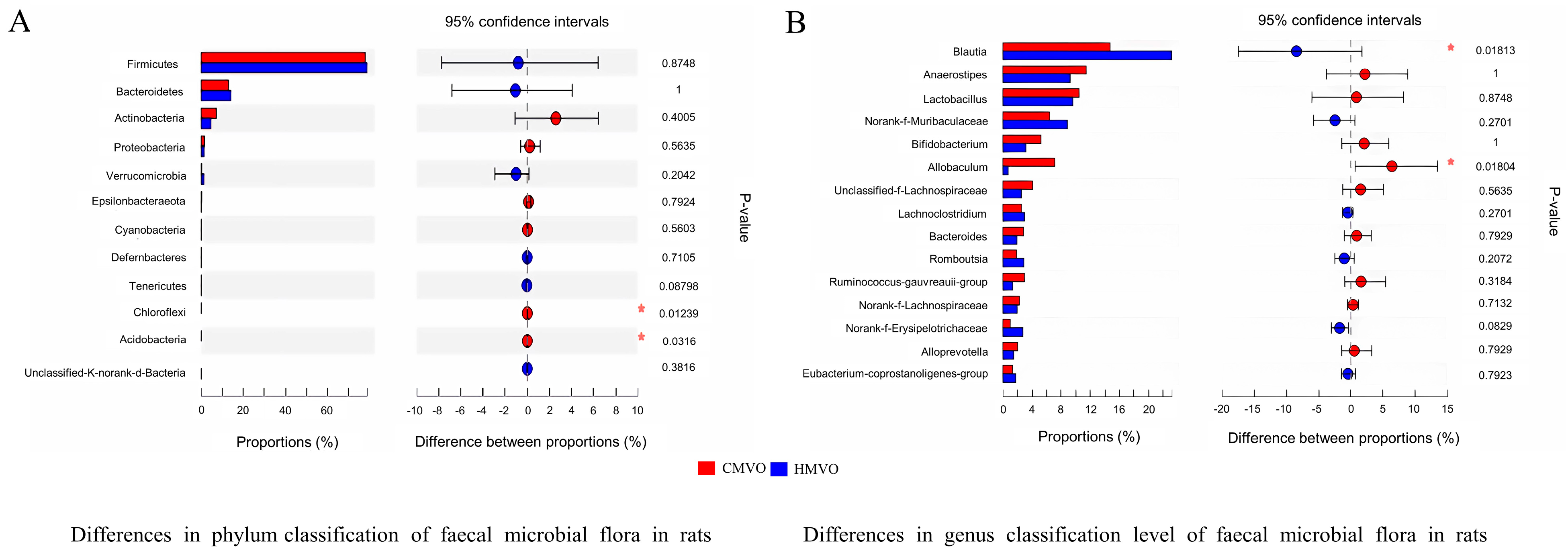
2.5.3. Species Difference Analysis
3. Materials and Methods
3.1. Materials
3.2. Fatty acid Analysis
3.3. Triacylglycerol Analysis
3.4. Establishment of Evaluation Model
3.4.1. Determination of the Evaluation Conditions
3.4.2. Establishment of Evaluation Model
3.4.3. Establishment of the Computer Software
3.5. In Vivo Evaluation of Nutritional Function
3.5.1. Animals, Experiment Design and Treatments
3.5.2. Blood Routine and Serum Biochemical Indices
3.5.3. FA Profile of Intestinal Contents and Feces
3.5.4. Determination of Calcium in Feces
3.5.5. Metagenomic Detection of Intestinal Flora
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thum, C.; Wall, C.; Day, L.; Szeto, I.M.Y.; Li, F.; Yan, Y.; Barnett, M.P.G. Changes in Human Milk Fat Globule Composition Throughout Lactation: A Review. Front. Nutr. 2022, 9, 835856. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, J.; Liu, Y.; Qiao, W.; Jiang, T.; Liu, Y.; Yu, X.; Chen, L. Advances in analysis, metabolism and mimicking of human milk lipids. Food Chem. 2022, 393, 133332. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, S.; Liu, L.; Pang, X.; Yang, Y.; Lu, J.; Lv, J. Differences in the triacylglycerol and fatty acid compositions of human colostrum and mature milk. J. Agric. Food Chem. 2018, 66, 4571–4579. [Google Scholar] [CrossRef]
- Kim, K.-M.; Park, T.-S.; Shim, S.-M. Optimization and validation of HRLC-MS method to identify and quantify triacylglycerol molecular species in human milk. Anal. Methods 2015, 7, 4362–4370. [Google Scholar] [CrossRef]
- Kallio, H.; Nylund, M.; Boström, P.; Yang, B. Triacylglycerol regioisomers in human milk resolved with an algorithmic novel electrospray ionization tandem mass spectrometry method. Food Chem. 2017, 233, 351–360. [Google Scholar] [CrossRef]
- Yuan, T.; Qi, C.; Dai, X.; Xia, Y.; Sun, C.; Sun, J.; Yu, R.; Zhou, Q.; Jin, Q.; Wei, W. Triacylglycerol composition of breast milk during different lactation stages. J. Agric. Food Chem. 2019, 67, 2272–22787. [Google Scholar] [CrossRef]
- Chen, Y.J.; Zhou, X.H.; Han, B.; Li, S.M.; Xu, T.; Yi, H.X.; Liu, P.; Zhang, L.W.; Li, Y.Y.; Jiang, S.L.; et al. Composition analysis of fatty acids and stereo-distribution of triglycerides in human milk from three regions of China. Food Res. Int. 2020, 133, 109196. [Google Scholar] [CrossRef]
- Karrar, E.; Albakry, Z.; Mohamed Ahmed, I.A.; Chen, C.; Yan, Y.; Brennan, C.S.; Li, J. The composition, characterisation, and health impacts of human milk lipid medium- and long-chain TAGs. Int. J. Food Sci. Technol. 2023, 58, 6192–6201. [Google Scholar] [CrossRef]
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid Quality in Infant Nutrition: Current Knowledge and Future Opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17. [Google Scholar] [CrossRef]
- Wei, W.; Sun, C.; Jiang, W.; Zhang, X.; Hong, Y.; Jin, Q.; Tao, G.; Wang, X.; Yang, Z. Triacylglycerols fingerprint of edible vegetable oils by ultra-performance liquid chromatography-Q-ToF-MS. LWT 2019, 112, 108261. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, L.; Liu, D.; Du, W. Progress and perspectives of enzymatic preparation of human milk fat substitutes. Biotechnol. Biofuels Bioprod. 2022, 15, 118. [Google Scholar] [CrossRef]
- Ghide, M.K.; Li, K.; Wang, J.; Abdulmalek, S.A.; Yan, Y. Effective Production of Human Milk Fat Substitutes Rich in 1,3-Dioleoyl-2-palmitoyl Glycerol (OPO) viaa New Strategy. Food Biophys. 2022, 17, 495–507. [Google Scholar] [CrossRef]
- Wan, J.; Hu, S.; Jacoby, J.J.; Liu, J.; Zhang, Y.; Yu, L.L. The impact of dietary sn-2 palmitic triacylglycerols in combination with docosahexaenoic acid or arachidonic acid on lipid metabolism and host faecal microbiota composition in Sprague Dawley rats. Food Funct. 2017, 8, 1793–1802. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, Q.; Jiang, C.; Evivie, S.E.; Cao, T.; Wang, Z.; Zhao, L.; Liang, S.; Li, B.; Huo, G. Infant formula supplemented with 1,3-olein-2-palmitin regulated the immunity, gut microbiota, and metabolites of mice colonized by feces from healthy infants. J. Dairy Sci. 2022, 105, 6405–6421. [Google Scholar] [CrossRef]
- Yaron, S.; Shachar, D.; Abramas, L.; Riskin, A.; Bader, D.; Litmanovitz, I.; Bar-Yoseph, F.; Cohen, T.; Levi, L.; Lifshitz, Y.; et al. Effect of high β-palmitate content in infant formula on the intestinal microbiota of term infants. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 376–381. [Google Scholar] [CrossRef]
- Qiao, B.; Li, X.; Zheng, T.; Tan, Z. Different Effects of Lard and Vegetable Blend Oil on Intestinal Microorganisms, Enzyme Activity and Blood Routine in Mice. J. Oleo Sci. 2022, 71, 301–310. [Google Scholar] [CrossRef]
- Wang, Y.; Mai, Q.; Qin, X.; Yang, B.; Wang, Z.; Chen, H. Establishment of an evaluation model for human milk fat substitutes. J. Agric. Food Chem. 2010, 58, 642–649. [Google Scholar] [CrossRef]
- Hokkanen, S.; Frey, A.D.; Yang, B.; Linderborg, K.M. Similarity Index for the Fat Fraction between Breast Milk and Infant Formulas. J. Agric. Food Chem. 2022, 70, 6191–6201. [Google Scholar] [CrossRef]
- Guerrero-Esperanza, M.; Wrobel, K.; Wrobel, K.; Ordaz-Ortiz, J.J. Determination of fatty acids in vegetable oils by GC-MS, using multiple-ion quantification (MIQ). J. Food Compos. Anal. 2023, 115, 104963. [Google Scholar] [CrossRef]
- Lu, C.H.; Huang, X.Y.; Shao, J.; Pei, D. A review: Role of mass spectrometry in the quality and authenticity identification of olive oil. Int. J. Food Sci. Technol. 2022, 57, 7592–7606. [Google Scholar] [CrossRef]
- Hussain, M.; Sun, Y.; Pan, Y.; Liu, L.; Zhang, X.; Wang, Q.; Lin, S.; Qayum, A.; Hussain, K.; Li, X. Formulation, invitro digestive study, and comparative fatty acid analysis of walnut oil-based infant formula, with human milk, animal milk, and commercial infant formula. Innov. Food Sci. Emerg. Technol. 2023, 84, 103279. [Google Scholar] [CrossRef]
- Li, H.; Han, J.; Zhao, Z.; Tian, J.; Fu, X.; Zhao, Y.; Wei, C.; Liu, W. Roasting treatments affect oil extraction rate, fatty acids, oxidative stability, antioxidant activity, and flavor of walnut oil. Front. Nutr. 2023, 9, 1077081. [Google Scholar] [CrossRef]
- Campos, J.R.; Severino, P.; Ferreira, C.S.; Zielinska, A.; Santini, A.; Souto, S.B.; Souto, E.B. Linseed Essential Oil—Source of Lipids as Active Ingredients for Pharmaceuticals and Nutraceuticals. Curr. Med. Chem. 2019, 26, 4537–4558. [Google Scholar] [CrossRef] [PubMed]
- Khalid, W.; Gill, P.; Arshad, M.S.; Ali, A.; Ranjha, M.M.A.N.; Mukhtar, S.; Afzal, F.; Maqbool, Z. Functional behavior of DHA and EPA in the formation of babies brain at different stages of age, and protect from different brain-related diseases. Int. J. Food Prop. 2022, 25, 1021–1044. [Google Scholar] [CrossRef]
- Leskinen, H.; Suomela, J.P.; Kallio, H. Quantification of triacylglycerol regioisomers in oils and fat using different mass spectrometric and liquid chromatographic methods. Rapid Commun. Mass Spectrom. RCM 2007, 21, 2361–2373. [Google Scholar] [CrossRef]
- Indelicato, S.; Bongiorno, D.; Pitonzo, R.; Di Stefano, V.; Calabrese, V.; Indelicato, S.; Avellone, G. Triacylglycerols in edible oils: Determination, characterization, quantitation, chemometric approach and evaluation of adulterations. J. Chromatogr. A 2017, 1515, 1–16. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Yu, X.; Gao, J.-M. A mini review of nervonic acid: Source, production, and biological functions. Food Chem. 2019, 301, 125286. [Google Scholar] [CrossRef]
- Guo, Q.; Hou, X.; Cui, Q.; Li, S.; Shen, G.; Luo, Q.; Wu, H.; Chen, H.; Liu, Y.; Chen, A.; et al. Pectin mediates the mechanism of host blood glucose regulation through intestinal flora. Crit. Rev. Food Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef]
- Ahmed, T.B.; Eggesbø, M.; Criswell, R.; Uhl, O.; Demmelmair, H.; Koletzko, B. Total Fatty Acid and Polar Lipid Species Composition of Human Milk. Nutrients 2022, 14, 158. [Google Scholar] [CrossRef]
- Dao, X.; Zhang, D.; Wang, L.; Wang, L. Analysis of human milk fatty acid composition and its correlation with diet pattern (A study in Tibetan population gathering area). J. Food Compos. Anal. 2023, 116, 105046. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
| Fatty Acid | Walnut Oil | Plam Oil | Linseed Oil | Soybean Oil | Rapeseed Oil | Sunflower Oil | Maize Oil |
|---|---|---|---|---|---|---|---|
| C4:0 | 0.074 ± 0.016 | 0.665 ± 0.021 | 0.072 ± 0.024 | 0.096 ± 0.021 | 0.035 ± 0.008 | 0.037 ± 0.003 | 0.069 ± 0.016 |
| C6:0 | 0.202 ± 0.026 | 0.161 ± 0.016 | 0.138 ± 0.032 | 0.176 ± 0.023 | 0.195 ± 0.027 | 0.153 ± 0.020 | 0.152 ± 0.020 |
| C8:0 | 2.130 ± 0.087 | 1.857 ± 0.423 | 2.558 ± 0.360 | 2.174 ± 0.093 | 2.381 ± 0.120 | 2.512 ± 0.121 | 2.509 ± 0.113 |
| C10:0 | 5.805 ± 1.886 | 4.536 ± 0.988 | 4.928 ± 2.116 | 5.049 ± 1.639 | 4.666 ± 1.533 | 4.897 ± 1.601 | 5.447 ± 1.774 |
| C11:0 | 0.097 ± 0.006 | 0.075 ± 0.015 | 0.023 ± 0.004 | 0.069 ± 0.004 | 0.061 ± 0.004 | - | 0.106 ± 0.007 |
| C12:0 | 0.064 ± 0.002 | 0.193 ± 0.047 | - | 0.078 ± 0.003 | 0.015 ± 0.001 | - | - |
| C13:0 | 0.041 ± 0.001 | 0.055 ± 0.014 | 0.096 ± 0.011 | 0.063 ± 0.002 | 0.057 ± 0.002 | 0.302 ± 0.009 | 0.031 ± 0.001 |
| C14:0 | 0.091 ± 0.002 | 1.088 ± 0.271 | 0.048 ± 0.006 | 0.076 ± 0.002 | 0.066 ± 0.002 | 0.115 ± 0.003 | 0.087 ± 0.002 |
| C14:1 | 0.041 ± 0.001 | - | - | - | - | - | - |
| C15:0 | 0.022 ± 0.000 | 0.046 ± 0.014 | - | 0.034 ± 0.000 | 0.023 ± 0.000 | 0.055 ± 0.001 | - |
| C16:0 | 9.508 ± 0.034 | 37.093 ± 10.59 | 6.117 ± 0.560 | 10.634 ± 0.013 | 4.670 ± 0.031 | 6.576 ± 0.028 | 12.923 ± 0.011 |
| C16:1 | 0.112 ± 0.000 | 0.219 ± 0.063 | 0.114 ± 0.011 | 0.117 ± 0.000 | 0.175 ± 0.002 | 0.075 ± 0.000 | 0.154 ± 0.001 |
| C17:0 | 0.092 ± 0.005 | 0.136 ± 0.027 | 0.112 ± 0.016 | 0.163 ± 0.009 | 0.075 ± 0.005 | 0.030 ± 0.002 | 0.132 ± 0.007 |
| C17:1 | 0.118 ± 0.002 | 0.033 ± 0.010 | 0.042 ± 0.004 | 0.070 ± 0.001 | 0.055 ± 0.000 | 0.051 ± 0.001 | 0.024 ± 0.000 |
| C18:0 | 3.655 ± 0.067 | 4.397 ± 1.356 | 4.100 ± 0.308 | 4.315 ± 0.071 | 2.034 ± 0.017 | 3.866 ± 0.043 | 1.915 ± 0.028 |
| C18:1n9t | 0.028 ± 0.002 | 0.050 ± 0.020 | 0.023 ± 0.001 | 0.025 ± 0.002 | 0.025 ± 0.002 | 0.058 ± 0.004 | 0.103 ± 0.007 |
| C18:1n9c | 21.285 ± 0.867 | 37.63 ± 15.567 | 19.692 ± 9.669 | 19.483 ± 0.740 | 53.225 ± 1.581 | 18.917 ± 0.609 | 26.843 ± 0.961 |
| C18:2n6t | - | - | 0.870 ± 0.113 | 1.377 ± 0.049 | 3.338 ± 0.144 | - | - |
| C18:2n6c | 49.671 ± 1.147 | 10.704 ± 3.366 | 15.736 ± 1.121 | 48.459 ± 0.994 | 20.063 ± 0.249 | 61.268 ± 0.913 | 48.049 ± 0.883 |
| C20:0 | 0.155 ± 0.039 | 0.230 ± 0.154 | 0.517 ± 0.084 | 0.211 ± 0.052 | 0.465 ± 0.109 | 0.163 ± 0.038 | 0.234 ± 0.057 |
| C18:3n6 | 6.041 ± 0.121 | 0.263 ± 0.066 | 44.235 ± 5.098 | 6.624 ± 0.147 | 6.307 ± 0.188 | 0.142 ± 0.003 | 0.600 ± 0.015 |
| C20:1n9 | 0.205 ± 0.000 | 0.140 ± 0.000 | 0.177 ± 0.012 | 0.171 ± 0.004 | 1.147 ± 0.018 | 0.119 ± 0.002 | 0.202 ± 0.004 |
| C18:3n3 | 0.061 ± 0.001 | - | 0.069 ± 0.000 | 0.026 ± 0.000 | 0.061 ± 0.002 | - | 0.084 ± 0.001 |
| C21:0 | 0.017 ± 0.000 | 0.052 ± 0.015 | - | 0.047 ± 0.000 | 0.057 ± 0.000 | 0.023 ± 0.001 | 0.026 ± 0.000 |
| C20:2 | 0.166 ± 0.000 | 0.035 ± 0.04 | 0.120 ± 0.056 | 0.223 ± 0.122 | 0.162 ± 0.087 | 0.359 ± 0.193 | 0.056 ± 0.031 |
| C22:0 | - | - | - | - | 0.194 ± 0.000 | - | - |
| C20:3n6 | 0.021 ± 0.001 | - | - | - | - | - | 0.057 ± 0.000 |
| C22:1 | - | 0.077 ± 0.012 | - | 0.027 ± 0.002 | - | - | - |
| C20:3n6 | - | - | 0.082 ± 0.001 | - | 0.114 ± 0.012 | - | - |
| C20:4n6 | 0.063 ± 0.000 | 0.054 ± 0.015 | 0.020 ± 0.002 | 0.058 ± 0.001 | 0.027 ± 0.001 | 0.051 ± 0.001 | 0.017 ± 0.000 |
| C24:0 | 0.121 ± 0.005 | 0.098 ± 0.020 | 0.112 ± 0.015 | 0.156 ± 0.006 | 0.166 ± 0.008 | 0.231 ± 0.010 | 0.155 ± 0.006 |
| C24:1 | 0.038 ± 0.000 | - | - | - | 0.141 ± 0.002 | - | - |
| C22:6 | 0.078 ± 0.002 | 0.113 ± 0.027 | - | - | - | - | 0.026 ± 0.001 |
| TAG | Abbreviation | Walnut Oil | Plam Oil | Linseed Oil | Soybean Oil | Rapeseed Oil | Sunflower Oil | Maize Oil |
|---|---|---|---|---|---|---|---|---|
| 12:0/10:0/8:0 | LaCCy | 0.002 ± 0.000 a | 0.002 ± 0.001 | - | - | - | - | - |
| 12:0/6:0/12:0 | LaCaLa | - | 0.002 ± 0.000 | - | - | - | - | - |
| 12:0/8:0/12:0 | LaCyLa | - | 0.019 ± 0.012 | - | - | - | - | - |
| 12:0/6:0/14:0 | LaCaM | 0.001 ± 0.000 | 0.002 ± 0.000 | - | - | - | - | - |
| 12:0/8:0/14:0 | LaCyM | 0.008 ± 0.003 | 0.013 ± 0.003 | 0.006 ± 0.001 | - | 0.004 ± 0.001 | - | - |
| 12:0/10:0/12:0 | LaCLa | 0.005 ± 0.001 | 0.016 ± 0.003 | - | - | - | - | - |
| 12:0/12:0/12:0 | LaLaLa | 0.025 ± 0.005 | 0.203 ± 0.050 | 0.019 ± 0.004 | 0.003 ± 0.001 | 0.009 ± 0.003 | 0.002 ± 0.000 | 0.002 ± 0.000 |
| 12:0/10:0/14:0 | LaCM | 0.004 ± 0.001 | 0.011 ± 0.002 | - | - | 0.003 ± 0000 | - | - |
| 14:0/8:0/14:0 | MCyM | 0.005 ± 0.000 | 0.014 ± 0.003 | 0.008 ± 0.002 | - | 0.003 ± 0.001 | - | - |
| 12:0/14:0/12:0 | LaMLa | 0.018 ± 0.004 | 0.115 ± 0.013 | 0.007 ± 0.001 | - | 0.007 ± 0.001 | 0.001 ± 0.000 | - |
| 12:0/14:0/14:0 | LaMM | 0.01 ± 0.001 | 0.033 ± 0.007 | 0.010 ± 0.003 | - | 0.006 ± 0.001 | - | - |
| 12:0/16:0/12:0 | LaPLa | 0.008 ± 0.002 | 0.031 ± 0.007 | 0.014 ± 0.003 | - | 0.009 ± 0.002 | - | - |
| 14:0/16:0/12:0 | MPLa | 0.004 ± 0.001 | 0.019 ± 0.003 | - | - | 0.005 ± 0000 | - | - |
| 12:0/18:0/12:0 | LaSLa | 0.004 ± 0.000 | 0.009 ± 0.001 | - | - | 0.002 ± 0000 | - | - |
| 14:0/14:0/14:0 | MMM | 0.005 ± 0.001 | 0.02 ± 0.004 | - | - | 0.006 ± 0000 | - | - |
| 14:0/16:0/14:0 | MPM | - | 0.024 ± 0.002 | - | - | - | - | - |
| 16:0/12:0/16:0 | PLaP | - | 0.037 ± 0.004 | - | - | - | - | - |
| 16:0/14:0/16:0 | PMP | - | 0.096 ± 0.01 | - | - | - | - | - |
| 16:0/12:0/18:0 | PLaS | - | 0.007 ± 0.000 | - | - | - | - | - |
| 14:0/18:2/16:0 | MLP | 0.041 ± 0.005 | 0.346 ± 0.014 | 0.014 ± 0.001 | 0.059 ± 0.004 | 0.026 ± 0.002 | 0.039 ± 0.002 | 0.025 ± 0.002 |
| 16:0/18:0/16:0 | PSP | 0.040 ± 0.002 | 0.059 ± 0.001 | 0.113 ± 0.004 | 0.044 ± 0.002 | 0.019 ± 0.004 | 0.029 ± 0.001 | 0.033 ± 0.001 |
| 16:0/18:1/16:0 | POP | 1.738 ± 0.083 | 38.714 ± 1.722 | 1.737 ± 0.076 | 1.995 ± 0.062 | 0.930 ± 0.046 | 0.801 ± 0.024 | 3.073 ± 0.114 |
| 18:1/14:0/18:1 | OMO | 0.070 ± 0.003 | 0.664 ± 0.009 | 0.117 ± 0.006 | 0.078 ± 0.004 | 0.197 ± 0.013 | 0.056 ± 0.002 | 0.069 ± 0.004 |
| 16:0/18:2/16:0 | PLP | 4.051 ± 0.204 | 13.350 ± 1.626 | 1.723 ± 0.100 | 5.39 ± 0.214 | 0.87 ± 0.038 | 2.733 ± 0.151 | 6.74 ± 0.254 |
| 18:2/16:1/16:0 | LPoP | 0.324 ± 0.019 | 0.147 ± 0.006 | 1.884 ± 0.092 | 0.401 ± 0.013 | 0.193 ± 0.014 | 0.049 ± 0.002 | 0.116 ± 0.006 |
| 18:2/18:1/14:0 | LOM | 0.044 ± 0.002 | 0.085 ± 0.003 | 0.035 ± 0.002 | 0.046 ± 0.002 | 0.043 ± 0.009 | 0.058 ± 0.003 | 0.024 ± 0.001 |
| 16:0/18:3/16:0 | PLnP | 0.121 ± 0.005 | 0.063 ± 0.002 | 0.831 ± 0.042 | 0.155 ± 0.005 | 0.06 ± 0.008 | 0.003 ± 0.000 | 0.026 ± 0.001 |
| 18:0/16:0/18:0 | SPS | 0.022 ± 0.001 | - | 0.089 ± 0.003 | 0.034 ± 0.002 | 0.027 ± 0.011 | 0.019 ± 0.001 | 0.01 ± 0.001 |
| 18:1/12:0/18:1 | OLaO | - | 0.042 ± 0.002 | - | - | 0.040 ± 0.000 | - | - |
| 16:0/18:1/18:0 | POS | 0.505 ± 0.037 | 3.752 ± 0.268 | 0.917 ± 0.012 | 0.686 ± 0.031 | 0.243 ± 0.004 | 0.275 ± 0.018 | 0.312 ± 0.027 |
| 18:1/16:0/18:1 | OPO | 4.351 ± 0.159 | 21.965 ± 0.264 | 6.524 ± 0.273 | 4.226 ± 0.32 | 8.552 ± 0.167 | 2.841 ± 0.025 | 5.857 ± 0.316 |
| 18:2/18:0/16:0 | LSP | 0.764 ± 0.024 | 0.826 ± 0.038 | 0.474 ± 0.042 | 1.113 ± 0.059 | 0.145 ± 0.003 | 0.701 ± 0.039 | 0.451 ± 0.029 |
| 18:1/16:1/18:1 | OPoO | 5.739 ± 0.272 | 4.162 ± 0.348 | 3.383 ± 0.136 | 6.492 ± 0.488 | 3.52 ± 0.068 | 4.868 ± 0.048 | 8.432 ± 0.094 |
| 18:1/16:0/18:2 | OPL | 5.073 ± 0.081 | 3.652 ± 0.190 | 3.148 ± 0.605 | 5.802 ± 0.166 | 2.709 ± 0.076 | 4.563 ± 0.396 | 7.778 ± 0.358 |
| 18:2/16:0/18:2 | LPL | 11.273 ± 2.406 | 1.088 ± 0.031 | 3.353 ± 0.057 | 12.172 ± 1.369 | 1.309 ± 0.027 | 10.959 ± 0.222 | 12.538 ± 1.204 |
| 18:2/16:1/18:2 | LPoL | 1.325 ± 0.071 | 0.025 ± 0.003 | 4.647 ± 0.307 | 1.341 ± 0.057 | 0.416 ± 0.012 | 0.204 ± 0.012 | 0.263 ± 0.007 |
| 18:3/16:0/18:2 | LnPL | 1.372 ± 0.030 | 0.03 ± 0.002 | 5.576 ± 0.188 | 1.526 ± 0.015 | 0.398 ± 0.019 | 0.038 ± 0.003 | 0.173 ± 0.012 |
| 18:0/18:1/18:0 | SOS | 0.290 ± 0.010 | 0.402 ± 0.046 | 0.913 ± 0.054 | 0.416 ± 0.040 | 0.165 ± 0.012 | 0.163 ± 0.015 | 0.079 ± 0.008 |
| 20:0/18:1/16:0 | AOP | 0.103 ± 0.012 | 0.377 ± 0.059 | 0.124 ± 0.014 | 0.101 ± 0.010 | 0.101 ± 0.008 | 0.049 ± 0.004 | 0.112 ± 0.014 |
| 18:1/18:0/18:1 | OSO | 1.314 ± 0.147 | 1.840 ± 0.154 | 3.164 ± 0.273 | 1.460 ± 0.185 | 2.327 ± 0.135 | 1.021 ± 0.093 | 0.634 ± 0.023 |
| 18:0/18:2/18:0 | SLS | 1.250 ± 0.105 | 1.392 ± 0.131 | 2.584 ± 0.207 | 1.440 ± 0.176 | 1.709 ± 0.049 | 1.009 ± 0.122 | 0.530 ± 0.06 |
| 16:0/20:0/18:2 | PAL | 0.110 ± 0.005 | 0.081 ± 0.009 | 0.122 ± 0.015 | 0.188 ± 0.016 | 0.152 ± 0.012 | 0.071 ± 0.007 | 0.160 ± 0.013 |
| 18:1/18:1/18:1 | OOO | 4.164 ± 0.352 | 2.690 ± 0.451 | 8.485 ± 0.678 | 3.892 ± 0.508 | 26.833 ± 1.226 | 4.928 ± 0.205 | 4.257 ± 0.456 |
| 18:1/18:2/18:0 | OLS | 2.048 ± 0.044 | 0.524 ± 0.047 | 1.141 ± 0.074 | 2.466 ± 0.211 | 1.205 ± 0.108 | 1.918 ± 0.132 | 0.936 ± 0.104 |
| 18:1/18:2/18:1 | OLO | 10.805 ± 0.097 | 1.058 ± 0.162 | 13.194 ± 1.181 | 9.323 ± 0.898 | 22.025 ± 1.301 | 11.285 ± 0.717 | 12.551 ± 1.501 |
| 18:2/18:0/18:2 | LSL | 9.632 ± 1.027 | 0.672 ± 0.089 | 6.817 ± 0.465 | 9.284 ± 0.312 | 11.601 ± 1.000 | 11.598 ± 0.386 | 8.454 ± 0.589 |
| 18:2/18:1/18:2 | LOL | 10.012 ± 0.222 | 0.181 ± 0.013 | 5.649 ± 0.387 | 9.628 ± 0.450 | 4.149 ± 0.254 | 16.181 ± 1.053 | 12.001 ± 0.601 |
| 18:2/18:2/18:2 | LLL | 16.676 ± 0.18 | 0.070 ± 0.009 | 10.835 ± 1.571 | 14.753 ± 0.475 | 2.741 ± 0.227 | 20.694 ± 2.148 | 11.618 ± 0.099 |
| 18:2/18:3/18:2 | LLnL | 3.744 ± 0.303 | 0.010 ± 0.001 | 9.664 ± 1.086 | 3.284 ± 0.360 | 0.603 ± 0.020 | 0.078 ± 0.008 | 0.356 ± 0.025 |
| 22:0/18:1/16:0 | DOP | 0.107 ± 0.010 | 0.105 ± 0.019 | 0.202 ± 0.033 | 0.116 ± 0.020 | 0.096 ± 0.008 | 0.090 ± 0.014 | 0.070 ± 0.012 |
| 18:1/20:0/18:1 | OAO | 0.236 ± 0.031 | 0.287 ± 0.044 | 0.632 ± 0.068 | 0.251 ± 0.024 | 1.253 ± 0.131 | 0.207 ± 0.018 | 0.256 ± 0.037 |
| 16:0/22:0/18:2 | PDL | 0.155 ± 0.023 | 0.036 ± 0.006 | 0.122 ± 0.013 | 0.217 ± 0.024 | 0.133 ± 0.009 | 0.203 ± 0.025 | 0.064 ± 0.007 |
| 18:1/20:1/18:1 | OGO | 0.194 ± 0.022 | 0.085 ± 0.013 | 0.351 ± 0.040 | 0.215 ± 0.032 | 1.741 ± 0.185 | 0.141 ± 0.018 | 0.260 ± 0.033 |
| 18:2/18:1/20:0 | LOA | 0.168 ± 0.018 | 0.061 ± 0.008 | 0.117 ± 0.011 | 0.227 ± 0.034 | 0.330 ± 0.035 | 0.154 ± 0.018 | 0.206 ± 0.018 |
| 18:2/20:1/18:1 | LGO | 0.106 ± 0.014 | 0.024 ± 0.004 | 0.303 ± 0.031 | 0.133 ± 0.022 | 0.821 ± 0.112 | 0.081 ± 0.008 | 0.146 ± 0.013 |
| 18:2/20:0/18:2 | LAL | 0.285 ± 0.036 | 0.055 ± 0.006 | 0.199 ± 0.022 | 0.381 ± 0.061 | 0.645 ± 0.085 | 0.385 ± 0.037 | 0.400 ± 0.048 |
| 18:2/20:1/18:2 | LGL | 0.193 ± 0.023 | 0.010 ± 0.002 | 0.161 ± 0.020 | 0.200 ± 0.030 | 0.209 ± 0.020 | 0.195 ± 0.014 | 0.241 ± 0.018 |
| 24:0/16:0/18:1 | TPO | 0.037 ± 0.005 | 0.080 ± 0.017 | 0.148 ± 0.018 | 0.038 ± 0.008 | 0.032 ± 0.005 | 0.041 ± 0.007 | 0.051 ± 0.007 |
| 18:1/22:0/18:1 | ODO | 0.209 ± 0.033 | 0.161 ± 0.036 | 0.372 ± 0.052 | 0.220 ± 0.045 | 0.537 ± 0.077 | 0.416 ± 0.080 | 0.129 ± 0.017 |
| 18:2/24:0/16:0 | LTP | 0.069 ± 0.009 | 0.026 ± 0.005 | 0.080 ± 0.012 | 0.097 ± 0.020 | 0.035 ± 0.005 | 0.080 ± 0.015 | 0.055 ± 0.010 |
| 18:1/22:1/18:1 | ODoO | 0.142 ± 0.017 | 0.038 ± 0.008 | 0.305 ± 0.037 | 0.207 ± 0.035 | 0.449 ± 0.082 | 0.338 ± 0.064 | 0.065 ± 0.012 |
| 22:0/18:1/18:2 | DOL | 0.148 ± 0.025 | - | - | 0.246 ± 0.034 | 0.182 ± 0.030 | 0.415 ± 0.073 | 0.065 ± 0.008 |
| 18:2/22:0/18:2 | LDL | 0.224 ± 0.025 | - | - | 0.329 ± 0.056 | 0.163 ± 0.021 | 0.793 ± 0.092 | 0.105 ± 0.013 |
| Conditions | Ci a | Ei | ∑Di | ∑Ei | G | |
|---|---|---|---|---|---|---|
| G 1 | 18:1/16:0/18:1 | 0.000 | 0.001 | 83.318 | 16.854 | 83.146 |
| 18:1/16:0/18:2 | 0.296 | 2.007 | ||||
| 18:1/18:1/18:1 | 0.000 | 0.000 | ||||
| 18:1/18:2/18:1 | 0.000 | 0.000 | ||||
| 18:2/16:0/18:2 | 0.000 | 0.000 | ||||
| 16:0/18:1/16:0 | 0.104 | 0.318 | ||||
| 18:2/18:1/18:2 | 0.000 | 0.000 | ||||
| 18:1/14:0/18:1 | 0.874 | 2.081 | ||||
| 16:0/18:2/16:0 | 0.135 | 0.279 | ||||
| 18:0/16:0/18:1 | 1.000 | 1.955 | ||||
| 18:1/12:0/18:1 | 0.977 | 1.444 | ||||
| 18:1/18:0/18:1 | 0.000 | 0.000 | ||||
| 18:1/16:0/12:0 | 1.000 | 1.404 | ||||
| 18:1/16:0/14:0 | 1.000 | 1.178 | ||||
| 18:2/18:2/18:2 | 0.000 | 0.000 | ||||
| 18:2/14:0/18:1 | 1.000 | 0.793 | ||||
| 18:1/16:0/16:1 | 1.000 | 0.682 | ||||
| 18:1/12:0/18:2 | 1.000 | 0.658 | ||||
| G 2 | C10:0 | 1.067 | 0.412 | 94.468 | ||
| C12:0 | 0.977 | 1.255 | ||||
| C14:0 | 0.834 | 0.822 | ||||
| C16:0 | 0.000 | 0.000 | ||||
| C18:0 | 0.245 | 0.341 | ||||
| C22:0 | 0.925 | 0.513 | ||||
| C16:1 | 0.854 | 0.41 | ||||
| C18:1 | 0.000 | 0.000 | ||||
| C18:2 | 0.000 | 0.000 | ||||
| C18:3 | 0.002 | 0.001 | ||||
| C20:4 | 0.000 | 0.000 | ||||
| C20:5 | 1.000 | 0.017 | ||||
| C22:6 | 0.753 | 0.067 | ||||
| G 3 | Sn-2 | 0.000 | 0.000 | |||
| Sn-2 | 0.000 | 0.000 | 183.917 | |||
| Sn-2 | 0.069 | 0.215 | ||||
| G 4 | UUU + USU | 0.000 | 0.000 | 152.43 | ||
| TAG with SFAs (carbon number < 14) at Sn-1/3 position | 0.000 | 0.000 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Zhao, P.; Wang, X.; Wang, Y.; Zhang, S.; Pang, X.; Lv, J. Fabrication of Human Milk Fat Substitute: Based on the Similarity Evaluation Model and Computer Software. Molecules 2024, 29, 2096. https://doi.org/10.3390/molecules29092096
Zhu H, Zhao P, Wang X, Wang Y, Zhang S, Pang X, Lv J. Fabrication of Human Milk Fat Substitute: Based on the Similarity Evaluation Model and Computer Software. Molecules. 2024; 29(9):2096. https://doi.org/10.3390/molecules29092096
Chicago/Turabian StyleZhu, Huiquan, Pu Zhao, Xiaodan Wang, Yunna Wang, Shuwen Zhang, Xiaoyang Pang, and Jiaping Lv. 2024. "Fabrication of Human Milk Fat Substitute: Based on the Similarity Evaluation Model and Computer Software" Molecules 29, no. 9: 2096. https://doi.org/10.3390/molecules29092096
APA StyleZhu, H., Zhao, P., Wang, X., Wang, Y., Zhang, S., Pang, X., & Lv, J. (2024). Fabrication of Human Milk Fat Substitute: Based on the Similarity Evaluation Model and Computer Software. Molecules, 29(9), 2096. https://doi.org/10.3390/molecules29092096









