Abstract
Rhamnolipids (RLs) are widely used biosurfactants produced mainly by Pseudomonas aeruginosa and Burkholderia spp. in the form of mixtures of diverse congeners. The global transcriptional regulator gene irrE from radiation-tolerant extremophiles has been widely used as a stress-resistant element to construct robust producer strains and improve their production performance. A PrhlA-irrE cassette was constructed to express irrE genes in the Pseudomonas aeruginosa YM4 of the rhamnolipids producer strain. We found that the expression of irrE of Deinococcus radiodurans in the YM4 strain not only enhanced rhamnolipid production and the strain’s tolerance to environmental stresses, but also changed the composition of the rhamnolipid products. The synthesized rhamnolipids reached a maximum titer of 26 g/L, about 17.9% higher than the original, at 48 h. The rhamnolipid production of the recombinant strain was determined to be mono-rhamnolipids congener Rha–C10–C12, accounting for 94.1% of total products. The critical micelle concentration (CMC) value of the Rha–C10–C12 products was 62.5 mg/L and the air-water surface tension decreased to 25.5 mN/m. The Rha–C10–C12 products showed better emulsifying activity on diesel oil than the original products. This is the first report on the efficient production of the rare mono-rhamnolipids congener Rha–C10–C12 and the first report that the global regulator irrE can change the components of rhamnolipid products in Pseudomonas aeruginosa.
1. Introduction
Rhamnolipids are a glycolipid biosurfactant and can be applied in daily chemistry [1], food, agriculture [2], medicine [3], and microbial enhanced oil recovery (MEOR) [4]. At present, Pseudomonas aeruginosa is among the most potent and best characterized native rhamnolipids producers. The natural rhamnolipid products show rich structural diversity. More than 60 rhamnolipid congeners have been discovered. Among them, the difference in the number and length of the hydrophilic rhamnolipid groups and hydrophobic fatty acid chains makes the structure of rhamnolipids diverse [5]. In terms of rhamnolipids produced by Pseudomonas aeruginosa strains, they are mainly divided into four types: Rha–C10, Rha–C10–C10, Rha–Rha–C10, and Rha–Rha–C10–C10. The variation of congeners and contents of rhamnolipid products have significant influence on their physicochemical properties and subsequent application [6,7].
Rhamnolipids have remarkable tensioactive and emulsifying properties. Compared to chemical surfactants, rhamnolipids have the advantages of being non-toxic, environmentally friendly, and biodegradable. However, the use of rhamnolipids is limited. An important factor hindering the large-scale application of rhamnolipids at present is their high production cost. It is necessary to reduce the cost, increase rhamnolipid production and expand their applications. To enhance the efficiency (e.g., titer, rate, and yield) of rhamnolipid production, a lot of work has been carried out, including strain improvement and fermentation optimization [8,9,10,11]. The amphiphilic structure of rhamnolipids is comparable to that of cell membranes. Rhamnolipids intercalate into cell membranes via interaction with lipopolysaccharide-binding proteins (LBPs), causing toxicity to cells [12,13,14]. Considering the stress and toxicity caused to the producer cells by the high concentration of rhamnolipid accumulation in fermentation broth, it is worth trying to improve the stress tolerance of producers to obtain a higher rhamnolipid titer.
The global transcriptional regulator irrE (or PprI) of Deinococcus radiodurans, a protein of 35-kD, plays a central regulatory role in multiple DNA damage repair and protection pathways in response to radiation stress [15,16]. As a global regulator of extreme stress responses, irrE has been used as a stress-resistant element to build more robust production strains. The heterologous expression of irrE and its mutants in Escherichia coli [17], Saccharomyces cerevisiae [18], Zymomonas mobilis [19], Sphingomonas sp. [20], and Arthrobacter simplex [21] can cause significant changes in the host transcriptome and proteome, and enhance tolerance to various stresses such as acid, salt, organic solvents, inhibitors in lignocellulose hydrolysates, and high temperatures.
Our original intention was to obtain a more robust rhamnolipid-producing strain by heterologous expression of the global transcriptional regulator irrE. Thus, the global transcriptional regulator irrE from Deinococcus species was applied in rhamnolipid producer Pseudomonas aeruginosa YM4. Unexpectedly, a rare mono-rhamnolipids congener could be synthesized in large quantities in one of the irrE-expressing strains. Furthermore, the new products were determined and characterized, as shown in Figure 1. This work emphasizes that the global regulator irrE of D. radiodurans played a complex regulatory role in Pseudomonas aeruginosa YM4, which provides new approaches for improving strains and developing new rhamnolipid products.
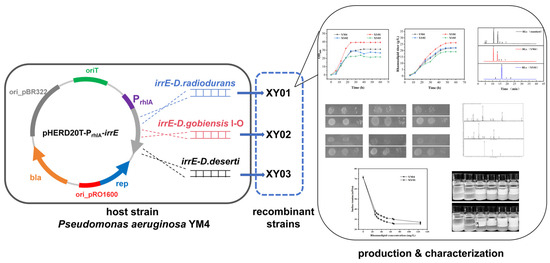
Figure 1.
Experimental design process diagram.
2. Results
2.1. Screening for Suitable Promoters
In the original plasmind pHERD20T, the target gene is under the control of the PBAD promoter. In general, 10 g/L of L-arabinose is needed to induce the expression of the target gene [22]. Considering the cost of the inducer arabinose, it is more beneficial to replace the induced promoter with a constitutive promoter. The acyltransferase RhlA synthesizes 3-(3-hydroxyalkanoyloxy)alcanoic acids (HAAs) by esterification of two 3-hydroxyfatty acids, and this is often seen as the first step in rhamnolipid synthesis in P. aeruginosa [23]. In our work, the original promoter PBAD was replaced with the promoter of rhlA (PrhlA), and PrhlA was used to control the expression of the desired gene. Taking gfp as the reporter gene, the expression cassette PrhlA-gfp was constructed, and the transcription intensity of PrhlA in recombinant strain P. aeruginosa YM4 was identifed by measuring the fluorescence of GFP using a fluorescence spectrophotometer. Recombinant strain P. aeruginosa YM4 containing the original expression cassette PBAD-gfp was used as the control. Figure 2 shows the whole-cell fluorescence of recombinant strain cells containing the PrhlA-gfp cassette and PBAD-gfp. It shows that the gfp gene under the control of PrhlA in recombinant strain YM4 could be well expressed both in lysogeny broth (LB) medium and in the rhamnolipid fermentation medium without the addition of any inducer. No fluorescence was detected in the control group (PBAD-gfp cassette). It confirmed that PrhlA was an efficient constitutive promoter.
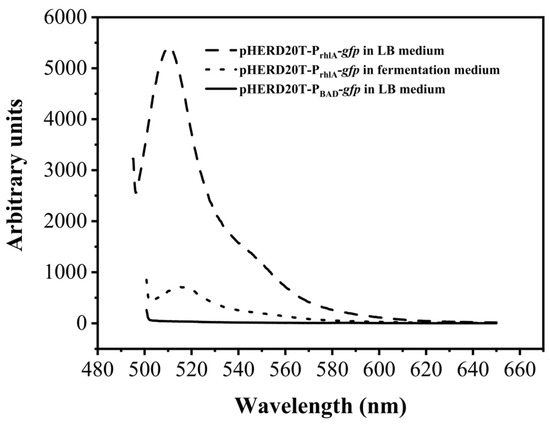
Figure 2.
The fluorescence of recombinant YM4 cells containing the PrhlA-gfp cassette and PBAD-gfp cassette cultivated in LB medium and rhamnolipid fermentation medium without the addition of any inducer.
2.2. Effects of the Global Transcriptional Regulator IrrE on Growth and Rhamnolipds Titer in Batch Fermentation
The global transcriptional regulator irrE from a different source was constitutively expressed in recombinant strain YM4 by constructing the PrhlA-irrE cassette in plasmid pHERD20T; then, the recombinant YM4 cells containing the PrhlA-irrE cassette were named as XY01 (irrE from D. radiodurans), XY02 (irrE from D. gobiensis I-O), and XY03 (irrE from D. deserti). Taking the starting strain YM4 as the control, all strains were cultivated in rhamnolipid fermentation medium. The cell growth was monitored by measuring the optical density at 600 nm (OD600) and the rhamnolipid titer was determined directly using HPLC–ELSD.
Figure 3a shows the cell growth of all strains. The growth of XY01 was the best. Compared with strain YM4, the cell density (OD600) of XY01 significantly increased to 39.28, resulting in a 27.4% increase in biomass. However, the growth of XY03 decreased significantly, and the biomass decreased by approximately 23.5%. Figure 3b shows the rhamnolipid production of all strains. We can see that strain XY01 had the highest titer, and the rhamnolipid titer reached 26 g/L at 48 h, which was about 17.9% higher than that of strain YM4. The productivity of strain XY01 was about 541.7 mg/(L·h).
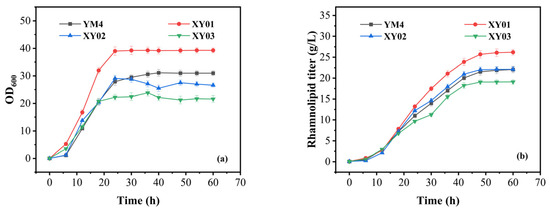
Figure 3.
Fermentation performance of strain YM4 and recombinant YM4 cells containing the PrhlA-irrE cassette in batch fermentation. (a) The growth of strains; (b) the rhamnolipid production of strains. All strains were cultivated in rhamnolipid fermentation medium. The error bars represent standard deviation values of three independent experiments (n = 3).
The parameters μ and qp of strain XY01 and strain YM4 are shown in Figure 4. The maximum specific growth rate (μmax) of XY01 was 0.33 h−1 at 10 h, which was 6.5% higher than that of YM4. It was noteworthy that the maximum specific product synthesis rate (qpmax) of XY01 was 0.21 h−1 at 13 h, which was 31.3% higher than that of YM4.
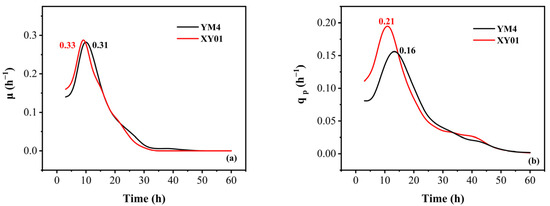
Figure 4.
The variation of kinetic parameters (µ, qp) of strain XY01 and YM4. (a) The specific growth rate (µ, h−1); (b) the specific rhamnolipid production rate (qp, h−1).
These results suggested that the heterologous expression of the global transcriptional regulator irrE from D. radiodurans could improve cell growth, which in turn promoted rhamnolipid production. Thus, strain XY01 was chosen to be further investigated.
2.3. IrrE of D. radiodurans Improved the Robustness of P. aeruginosa
During the batch fermentation in flasks, the rhamnolipids accumulated to high concentration, and the pH of the broth gradually increased from 7.0 to 8.0. This became a stressful environment for producer cells. The tolerance of strain XY01 of rhamnolipids and an alkaline environment were tested by spot assay. As shown in Figure 5, with the increase of rhamnolipid concentration and pH, the growth of original strain YM4 was significantly inhibited. However, the recombinant strain XY01 containing heterologous irrE exhibited better growth performance under the same stress conditions; it grew faster or was numerically more dominant than the control strain YM4. These results indicated that heterologous expression of irrE gene in P. aeruginosa could enhance the robustness of P. aeruginosa and improve its tolerance of stressful environments.
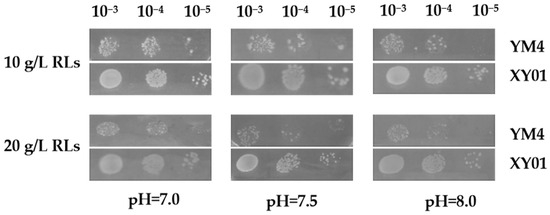
Figure 5.
Response of cells to stress environment. The growth phenotypes of strain XY01 and YM4 were evaluated on LB agar plates supplemented with 10 g/L or 20 g/L rhamnolipids and with pH adjusted to 7.0, 7.5, and 8.0. All plates were photographed after 12 h incubation at 37 °C.
2.4. Characterization of Rhamnolipids
2.4.1. HPLC-ELSD Analysis of Rhamnolipids
In our previous work, strain YM4 was an efficient di-rhamnolipid producer, and di-rhamnolipids accounted for 64.8% and 85.7% of total products with soybean oil and glycerol as carbon sources, respectively [24]. HPLC-ELSD profiles of the rhamnolipid products are shown in Figure 6, and the structural composition of rhamnolipid products is shown in Table 1. A total of nine rhamnolipid congeners were detected. The products of strains YM4, XY02, and XY03 exhibited similar components and contents, with di-rhamnolipids (Rha2–C10–C10) (10.56 min) and mono-rhamnolipids (Rha–C10–C10) (13.28 min) as major components, and together accounting for 97–98% of total products. The rhamnolipids of strain XY01 were significantly different. The congener of peak 6 (16.28 min) was the major component, accounting for 94.1%. However, the congener of peak 6 (16.28 min) also appeared in the rhamnolipids standard and products of YM4 as a rare component, accounting for 0.5–2%. The congeners of peak 8 (11.95 min) and peak 9 (22.33 min) were unique new components of rhamnolipids produced by strain XY01. These congeners were not found in the original products of strain YM4. As the proportion of such congeners was low to less than 3%, we performed no in-depth research on them. This indicated that global regulator irrE from D. radiodurans could regulate the biosynthesis of rhamnolipids and generate novel rhamnolipid products in strain P. aeruginosa YM4. However, IrrE from D. gobiensis I-O and D. deserti could not significantly influence the rhamnolipid composition in recombinant strains. The congener of peak 6 (16.28 min) was further determined by HPLC-MS.
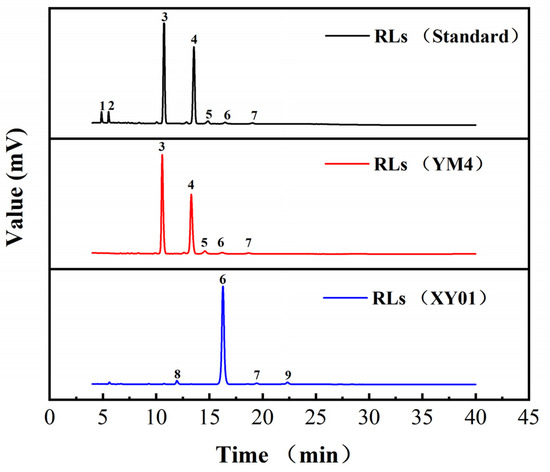
Figure 6.
HPLC-ELSD profiles of rhamnolipids produced from strain YM4 and XY01. Rhamnolipids standard (R90, Lot number: A79125126058) was obtained from AGAE Technologies (Corvallis, OR, USA).

Table 1.
Structural composition of rhamnolipids produced from strains YM4, XY01, XY02, and XY03.
2.4.2. HPLC-MS Analysis of Rhamnolipids
The molecular weight information of the main components that appeared as peaks 3, 4, and 6 was obtained by mass spectrometry analysis. According to the structural characteristics of rhamnolipid congeners and the m/z of each component, the probable structure represented by each peak was deduced. As presented in Figure 7, the congeners of peak 3 and peak 4 were deduced to be Rha2–C10–C10 and Rha–C10–C10, with the major [M + NH4]+ peak at m/z 668 and 522, respectively. The congener of peak 6 was deduced to be Rha–C10–C12, with the [M + NH4]+ peak at m/z 550.
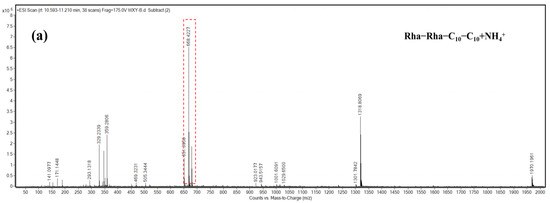
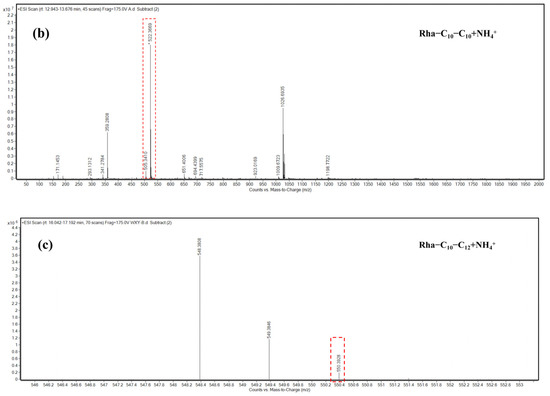
Figure 7.
HPLC-MS characterization of rhamnolipid congeners of peaks 3, 4, and 6. (a) Congener of peak 3 mass spectra of the fragmented pseudomolecular ion at m/z 668 of Rha2–C10–C10 and the daughter ions generated by fragmentation; (b) congener of peak 4 mass spectra of the fragmented m/z 522 pseudomolecular ions of Rha–C10–C10 and the daughter ions generated by fragmentation; (c) congeners of peak 6 mass spectra of the fragmented pseudomolecular ion at m/z 550 of Rha–C10–C12 and the daughter ions generated by fragmentation.
2.4.3. Fourier-Transform Infrared (FTIR) Analysis of Rhamnolipids
The types of functional groups and chemical bonds in the structure of an unknown compound are relatively easy to determine by the FTIR spectra. To further confirm the structure of the new components, the purified products of XY01 were analyzed by infrared spectroscopy. As shown in Figure 8, there were characteristic absorbance bands at 3500 cm−1 (–OH group stretching due to hydrogen bonding), 1738 cm−1 (C=O stretching of the ester linkage), 1721 cm−1 (C=O stretching carboxylate anion), 1319–1052 cm−1 (C–O–C and C–OH stretching in the rhamnose), 2926 cm−1, and 2855 cm−1 (the aliphatic bonds –CH3, –CH2, and –C–H stretching). The absence of bands at 3300–3000 cm−1 demonstrated the absence of H–C=C–H bonds. This indicates that the structure of rhamnolipid XY01 did not contain unsaturated fatty acids.
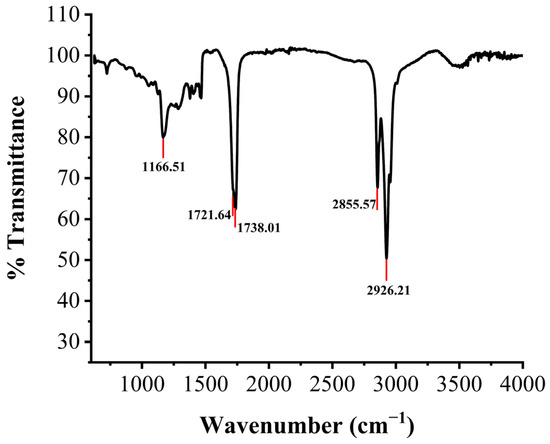
Figure 8.
FTIR spectrum profile of rhamnolipids produced by strain XY01.
2.4.4. The Critical Micelle Concentration (CMC) of Rhamnolipids
CMC is the lowest concentration of surfactant needed to form micelles. In our previous work, the CMC values of rhamnolipids were related to their components, ranged between 57 and 75 mg/L [25]. As demonstrated in Figure 9, both rhamnolipids were able to significantly reduce the surface tension of water; the mono-rhamnolipid products of strain XY01 exhibited a lower CMC value of 62.5 mg/L and a lower surface tension of 25.5 mN/m.
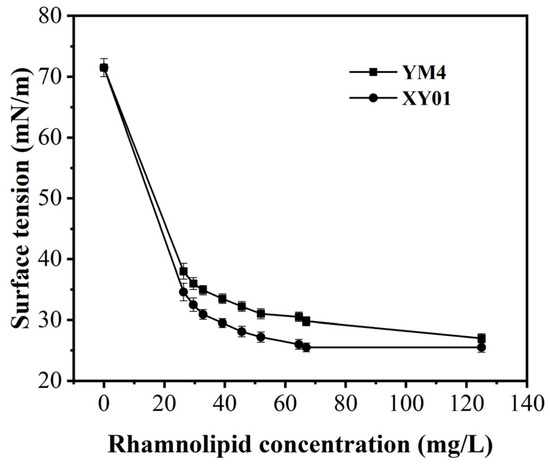
Figure 9.
Surface tension changes and CMC values of rhamnolipids produced by strain XY01 and YM4. The error bars represent standard deviation values of three independent experiments (n = 3).
2.4.5. Emulsifying Activity of Rhamnolipids on Diesel Fuel
It has been shown that the concentration and structural composition of rhamnolipids are important factors in emulsifying activity. Since the rhamnolipid concentrations in the tests were much higher than the CMC values, the emulsifying activity was greatly affected by the structural composition of rhamnolipids rather than rhamnolipid concentrations. The effect of rhamnolipid products on the emulsion layer formed from diesel oil is shown in Figure 10. It shows that the mono-rhamnolipid products (Rha–C10–C12) of strain XY01 exhibited greater emulsifying activity on diesel oil. At the concentration of 1.5 g/L, the EI24 of XY01 rhamnolipids was 72.7%, much higher than that of YM4 rhamnolipids (40.9%).

Figure 10.
The emulsifying activity of rhamnolipids produced by XY01 and YM4 on diesel oil at different concentrations.
3. Discussion
IrrE is a well-conserved global regulator in the Deinococcus species and governs gene transcription in the DNA damage response (DDR), providing extraordinary radiation resistance to the genus Deinococcus. Until now, IrrE from Deinococcus radiodurans and its mutations have been widely used as a tolerance-enhancer for Zymomonas mobilis [19], Saccharomyces cerevisiae [18], and E. coli [26] for ethanol production under various stresses. In this work, the functions of the global regulator irrE from three Deinococcus species were tested, labeled as irrE01 from Deinococcus radiodurans, irrE02 from Deinococcus gobiensis I-O, and irrE03 from Deinococcus deserti. The homologs irrE02 and irrE03 shared 65.2% and 65.1% sequence identity with irrE01, respectively. Studies have shown that irrE02 and irrE03 could restore radiation resistance in the D. radiodurans irrE mutant (∆irrE) and even irrE02 showed a stronger protection phenotype against UV radiation than irrE01 [27,28]. There is no application of irrE02 and irrE03 for enhancing stress tolerance. We tried using irrE from Deinococcus gobiensis I-O and Deinococcus deserti as stress-resistant elements for increasing the efficiency of microbial cell factories for the first time, but unfortunately we did not obtain positive results.
IrrE from D. radiodurans has been used as an heterologous global regulator in the model strain Pseudomonas aeruginosa PAO1 to improve electricity production in microbial fuel cells (MFCs). The results indicated that irrE significantly affected substrate utilization profiling, improving cell growth and cell tolerance to various stresses, and irrE led to many differently expressed genes, which were responsible for phenazines’ core biosynthesis, biofilm formation, QS systems, transcriptional regulation, glucose metabolism, and general stress response [29]. Additionally, heterologous expression of irrE of D. radiodurans had been shown to improve the acid tolerance of pollutant-degrading bacterium Pseudomonas putida S16 and enhance its viability and biodegrading activities in bioremediation of acidic wastes [30]. Similarly, in our work, irrE from D. radiodurans was found to be effective in increasing the rhamnolipid production of Pseudomonas aeruginosa YM4, as well as cell growth and cell tolerance to stress. Therefore, the global regulator irrE can be used as viable tools for improving stress resistance and production efficiency of genus Pseudomonas.
Rhamnolipids are composed of one or two rhamnose molecules linked to one or mostly two 3-hydroxyfatty acids of varying chain lengths. According to the the number of L-rhamnose residues, the rhamnolipids can be classified into mono-rhamnolipids and di-rhamnolipids. At present, rhamnolipids are usually produced by various strains in the form of mixtures of diverse congeners, and the production strains, culture conditions, and carbon sources can affect the components of rhamnolipids [31,32]. Thus, RhlC is the key enzyme for di-rhamnolipid synthesis. Theoretically, P. aeruginosa strains merely synthesizing mono-rhamnolipids are achievable with a natural deficiency of rhlC or by knocking out rhlC. As shown in Table 2, several Pseudomonas strains had been reported to produce mono-rhamnolipids with the predominant congener as Rha–C10–C10. This underlines the truth that P. aeruginosa typically synthesizes various rhamnolipids containing fatty acids with a predominant C10 chain length. P. aeruginosa YM4 was an efficient rhamnolipids producer, with di-rhamnolipids (Rha–Rha–C10–C10) as a predominant component. However, we found that the expression of irrE of D. radiodurans in P. aeruginosa YM4 could make a difference in both the number of L-rhamnose residues and the fatty acids chain lengths of rhamnolipids, and mono-rhamnolipids congener Rha–C10–C12 was almost the sole component, accounting for 94.1% of total products. While we did not expect to synthesize mono-rhamnolipids, the global transcriptional regulator irrE we tried had exactly the same effect. It has been shown that the heterogenous global regulator irrE can affect the expression of thousands of genes in its hosts [20,33,34]. Therefore, the genome-wide transcriptional analysis of irrE-expressing strain XY01 at the stage of rhamnolipids accumulation should be carried out in future work.

Table 2.
Mono-rhamnolipid-producing strains and characteristics.
4. Conclusions
Expression of irrE of D. radiodurans in P. aeruginosa YM4 increased its rhamnolipid production, as well as cell growth and cell tolerance to stress. Unexpectedly, mono-rhamnolipids congener Rha–C10–C12, accounting for about 94%, could be efficiently produced in the irrE-expression strain; the maximum titer reached 26 g/L using soybean oil and nitrate. This is the first report on altering the abundance and composition of rhamnolipids by the heterogenous expression of global regulator irrE in P. aeruginosa. This provides a new perspective for constructing efficient rhamnolipid-producing bacteria using synthetic biology techniques.
5. Materials and Methods
5.1. Strain, Medium, and Culture Conditions
Strain Pseudomonas aeruginosa YM4 (CCTCC M2017494) [24] was an efficient rhamnolipids producer and used as the starting strain for molecular manipulation. Table 3 provides a list of the strains and plasmids used in this work. All strains, except for those used in rhamnolipid production, were cultivated in LB medium containing peptone (10 g/L), yeast extract (5 g/L), and NaCl (10 g/L). Carbenicillin of 350 mg/L in LB medium was used for recombinant P. aeruginosa strains and ampicillin of 100 mg/L for E. coli strains.

Table 3.
Strains and plasmids used in this study.
The rhamnolipid fermentation medium consisted of soybean oil (50 g/L), NaNO3 (8 g/L), Na2HPO4 (7.888 g/L), KH2PO4 (1.972 g/L), CaCl2 (0.1 g/L), MgSO4 (0.2 g/L), NaCl (1 g/L), KCl (1 g/L), yeast powder (1 g/L), and a trace elements solution (2 mL/L) [24]. Strains were cultivated in 250 mL baffled flasks filled with 50 mL of medium and incubated at 37 °C with 200 rpm orbital shaking.
5.2. Construction of Reporter Plasmid
The broad host-range vector pHERD20T (5087 bp) was used as the backbone plasmid. However, the arabinose-inducible promoter PBAD (located at 2303–3472 bp) present in the plasmid was replaced with the promoter of acyltransferase rhlA (PrhlA) in P. aeruginosa PAO1 (located at 3,893,009–3,893,431 bp of NC_002516.2, GenBank). To confirm the working effect of promoter PrhlA, we constructed expression cassette PrhlA-gfp in pHERD20T and the green fluorescent protein (GFP) was used as a reporter. The primers used in this study are listed in Table 4. Overlap PCR was employed to construct the PrhlA-gfp cassette. Firstly, the PrhlA gene fragment was amplified from the genome of P. aeruginosa PAO1 using the primer pairs PrhlA-F/PrhlA-R·gfp, and the gfp gene was amplified from the genome of B. subtilis 168 (Pveg-gfp) using the primer pairs gfp-F/gfp-R. Subsequently, fragments PrhlA and gfp were fused together using the primers PrhlA-F/gfp-R to generate the PrhlA-gfp cassette. The resulting product was purified and sequenced. Next, the PrhlA-gfp cassette was joined to the linearized vector pHERD20T using TGGCGATAGCCCGGG and AAGCTTAGCTTGGCA as homology arms in a one-step cloning process. The arabinose operon region and the multi-cloning sites (located at 2303–3472 bp) were removed from the vector, resulting in the reporter plasmid pHERD20T-PrhlA-gfp. Sequencing was performed in the extracted reporter plasmid. The reporter plasmid was first transformed into E. coli TOP10 for amplification and then into strain YM4 for expression using chemical transformation methods as described in the literature [22].

Table 4.
Primers used in this study.
5.3. Promoter Strength Measurements
The strength of the promoter was measured by the whole-cell fluorescence of recombinant strain YM4 (PrhlA-gfp) cultivated in LB medium and rhamnolipid fermentation medium using a fluorescence spectrophotometer (Hitachi Fluorescence Spectrophotometer F-7000, Hitachi, Naka, Japan). After culturing for 12 h, 1 mL of fermentation broth was centrifuged at 12,000 rpm for 10 min. The cells were collected and washed three times. The washed cells were resuspended in deionized water to the same optical density (OD600) 0.07. The excitation and emission wavelengths were 485 and 525 nanometers, respectively.
5.4. Construction of PrhlA-irrE Recombinant Plasmid
The GenBank accession numbers of irrE01, irrE02, and irrE03 are WP_010886813.1 (D. radiodurans), WP_014686212.1 (D. gobiensis I-O), and 3DTI_A (D. deserti), respectively; and all the three irrE genes were synthesized by GENEWIZ and sub-cloned into the pUC18 vector. The method described in 5.2 was also applicable to construct a PrhlA-irrE cassette of different origin and insert into the pHERD20T vector. The three recombinant expression plasmids pHERD20T-PrhlA-irrE were transformed into strain P. aeruginosa YM4, and finally the strain YM4 derivatives were named as strain XY01 (harboring irrE01), XY02 (harboring irrE02), and XY03 (harboring irrE03), respectively.
5.5. Extraction of Rhamnolipids and Determination of Strain Growth Rate
For seed culture, the strain YM4 and its derivants were inoculated into LB medium. After overnight incubation, 1 mL of the inoculum was transferred to each flask containing rhamnolipid fermentation medium. Strains were cultivated in 250 mL baffled flasks filled with 50 mL of medium and incubated at 37 °C with 200 rpm orbital shaking. The fermentation was carried out for 60 h. During fermentation, 500 μL culture fluid was taken for analysis of cell and rhamnolipid concentrations. The culture broth was mixed vigorously with n-hexane 1:1 (v/v) and centrifuged (12,000 rpm, 4 °C, 10 min) for separation of cells, and aqueous and n-hexane phases. The cell pellets were washed in deionized water (12,000 rpm, 4 °C, 10 min) and resuspended in deionized water for detection of the cell content at 600 nm. The aqueous phase was used for detecting the rhamnolipid content.
After fermentation, cell-free supernatants were collected for rhamnolipid extraction. The rhamnolipid products were precipitated and extracted using the method described in the literature [24].
The specific cell growth rate (μ, h−1) and specific rhamnolipid production rate (qp, h−1) of strain XY01 and strain YM4 were calculated using Equations (1) and (2).
5.6. Effect of IrrE on the Stress Tolerance of P. aeruginosa Cells
To explore whether the global regulator irrE of D. radiodurans could enhance the tolerance of P. aeruginosa cells to stress environments, spot tests were conducted. The seed cultures of strain XY01 and YM4 in LB medium were diluted to 3.0 at OD600, and 5 µL aliquots of a tenfold dilution series were spotted onto LB agar plates supplemented with 10 g/L or 20 g/L rhamnolipid and the pH adjusted to 7.0, 7.5, and 8.0 with 2M NaOH solution. The plates were incubated at 37 °C for 12 h. The experiments were repeated twice.
5.7. Quantitative Analysis of Rhamnolipids
Rhamnolipids were detected directly by HPLC using an evaporative light scattering detector (ELSD). As described in the literature [24], rhamnolipid samples were prepared; the equipment used was a 1260 Infinity HPLC-ELSD (Thermo Scientific Ultimate 3000 HPLC, Palo Alto, CA, USA)−(Alltech ELSD2000ES, Chicago, IL, USA) equipped with a C18 column (4.6 × 150 mm, 5 µm; Sepax Technologies, Inc., Suzhou, China). The ELSD was set up with a drift temperature of 103 °C and a nebulizer flow rate of 2.8 L/min. A linear gradient from 30% to 100% of acetonitrile in 40 min was used with mobile phase A (acetonitrile) and mobile phase B (water with 0.05% formic acid) at the flow rate of 1 mL/min. Rhamnolipids standard (R90, Lot number: A79125126058) was obtained from AGAE Technologies (Corvallis, OR, USA); and standard curves comprising 0.1 to 1 mg/mL of rhamnolipids were used for quantification. The area normalization method was used to determine relative content of rhamnolipids congeners.
5.8. Characterization of Rhamnolipids
5.8.1. HPLC-MS Analysis of Rhamnolipids
The sample preparation, C18 column, and the parameters of the mobile phase were the same as those in 5.7. The mass spectrometry analysis was performed using an Agilent 6530 Q-TOF (Agilent Technologies 6530 Accurate-Mass Q-TOF LC/MS, Palo Alto, CA, USA) instrument with electrospray ionization (ESI) in positive ion mode. The capillary voltage was set to 4 kV. The gas temperature was 350 °C. The drying gas flow rate was 5 L/min. The Oct 1 RF Vpp was set to 750 V. The mass spectrum scanning range (m/z) was 50–2000.
5.8.2. Fourier-Transform Infrared (FTIR) Analysis of Rhamnolipids
The spectral analysis of the purified XY01 rhamnolipids was performed using FTIR spectroscopy, determining the significant chemical bonds present in the fingerprint area. An infrared spectrophotometer (Thermo Scientific Nicolet iS5, Waltham, MA, USA) operating in the attenuated total reflection (ATR) mode was used to collect the spectra with a resolution of 4 cm−1 from 400 to 4000 wavenumbers (cm−1).
5.8.3. Determination of the Critical Micelle Concentration (CMC)
The CMC values were determined by measuring the changes of surface tension with different concentrations of rhamnolipids. The surface tension of rhamnolipid solutions with varying concentrations (0–200 mg/L) was measured at room temperature using an automated tensiometer (BZY-3B, Shanghai Automation Instrumentation Sales Center, Shanghai, China).
5.8.4. Determination of the Emulsification Index (EI24)
The emulsifying activity of rhamnolipids produced by strain XY01 and strain YM4 were comparatively evaluated using the emulsification index (EI24). Diesel oil was used as the organic phase in this test. Briefly, 2 mL of diesel oil and 2 mL of rhamnolipid sample were vortexed at high speed (~2800 rpm) for 2 min in 5 mL stoppered glass tubes. These mixtures were then left to stand at room temperature for 24 h. The EI24 was calculated by dividing the measured height of the emulsion layer (mm) by the total height of the mixture (mm) and multiplying by 100.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29091992/s1, Data S1: Supplementary data.
Author Contributions
X.W.: conceptualization, investigation, writing—original draft; D.L.: investigation, formal analysis; S.Y. and Z.Y.: formal analysis; S.L.: conceptualization, supervision, writing—reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (grant no. 2022YFC2105200).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We are grateful to the Jiangsu Synergetic Innovation Center for Advanced Bio—Manufacture for providing analytical equipment for our work.
Conflicts of Interest
Author Shenghui Yue and Zhangzhong Yuan were employed by the company Sinopec Shengli Oilfield, Co., Ltd. The other authors declare no conflicts of interest.
References
- Nguyen, T.T.; Sabatini, D.A. Characterization and emulsification properties of rhamnolipid and sophorolipid biosurfactants and their applications. Int. J. Mol. Sci. 2011, 12, 1232–1244. [Google Scholar] [CrossRef]
- Sinumvayo, J.P.; Ishimwe, N. Agriculture and food applications of rhamnolipids and its production by Pseudomonas aeruginosa. J. Chem. Eng. Process Technol. 2015, 6, 223. [Google Scholar] [CrossRef]
- Piljac, A.; Stipčević, T.; Piljac-Žegarac, J.; Piljac, G. Successful treatment of chronic decubitus ulcer with 0.1% dirhamnolipid ointment. J. Cutan. Med. Surg. 2008, 12, 142–146. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, J.; Verma, N. Optimization of rhamnolipid production from Pseudomonas aeruginosa PBS towards application for microbial enhanced oil recovery. 3 Biotech 2018, 8, 20. [Google Scholar] [CrossRef]
- Palos Pacheco, R.; Kegel, L.L.; Pemberton, J.E. Interfacial and Solution Aggregation Behavior of a Series of Bioinspired Rhamnolipid Congeners Rha–C14–C x (x = 6, 8, 10, 12, 14). J. Phys. Chem. B 2021, 125, 13585–13596. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lai, L.; Lu, Q.; Mei, P.; Wang, Y.; Cheng, L.; Liu, Y. Comparative studies on the surface/interface properties and aggregation behavior of mono-rhamnolipid and di-rhamnolipid. Colloids Surf. B Biointerfaces 2019, 181, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Costa, S.G.; Contiero, J. Structure and applications of a rhamnolipid surfactant produced in soybean oil waste. Appl. Biochem. Biotechnol. 2010, 160, 2066–2074. [Google Scholar] [CrossRef]
- Cabrera-Valladares, N.; Richardson, A.-P.; Olvera, C.; Treviño, L.G.; Déziel, E.; Lépine, F.; Soberón-Chávez, G. Monorhamnolipids and 3-(3-hydroxyalkanoyloxy) alkanoic acids (HAAs) production using Escherichia coli as a heterologous host. Appl. Microbiol. Biotechnol. 2006, 73, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wittgens, A.; Tiso, T.; Arndt, T.T.; Wenk, P.; Hemmerich, J.; Müller, C.; Wichmann, R.; Küpper, B.; Zwick, M.; Wilhelm, S. Growth independent rhamnolipid production from glucose using the non-pathogenic Pseudomonas putida KT2440. Microb. Cell Factories 2011, 10, 80. [Google Scholar] [CrossRef]
- Invally, K.; Sancheti, A.; Ju, L.-K. A new approach for downstream purification of rhamnolipid biosurfactants. Food Bioprod. Process. 2019, 114, 122–131. [Google Scholar] [CrossRef]
- Bazsefidpar, S.; Mokhtarani, B.; Panahi, R.; Hajfarajollah, H. Overproduction of rhamnolipid by fed-batch cultivation of Pseudomonas aeruginosa in a lab-scale fermenter under tight DO control. Biodegradation 2019, 30, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.; Tiso, T.; Blank, L.M.; Winter, R. Interaction of rhamnolipids with model biomembranes of varying complexity. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183431. [Google Scholar] [CrossRef]
- Angelova, B.; Schmauder, H.-P. Lipophilic compounds in biotechnology—Interactions with cells and technological problems. J. Biotechnol. 1999, 67, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Andrä, J.; Rademann, J.; Howe, J.; Koch, M.H.; Heine, H.; Zähringer, U.; Brandenburg, K. Endotoxin-like properties of a rhamnolipid exotoxin from Burkholderia (Pseudomonas) plantarii: Immune cell stimulation and biophysical characterization. Biol. Chem. 2006, 387, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Gao, G.; Xu, G.; Fan, L.; Yin, L.; Shen, B.; Hua, Y. Deinococcus radiodurans PprI switches on DNA damage response and cellular survival networks after radiation damage. Mol. Cell. Proteom. 2009, 8, 481–494. [Google Scholar] [CrossRef]
- Hua, Y.; Narumi, I.; Gao, G.; Tian, B.; Satoh, K.; Kitayama, S.; Shen, B. PprI: A general switch responsible for extreme radioresistance of Deinococcus radiodurans. Biochem. Biophys. Res. Commun. 2003, 306, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, J.; Zhu, X.; Song, M.; Zhang, T.; Xin, F.; Dong, W.; Ma, J.; Jiang, M. Expression of global regulator IrrE for improved succinate production under high salt stress by Escherichia coli. Bioresour. Technol. 2018, 254, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Zhang, Y.; Suo, Y.; Liao, Z.; Ma, Y.; Fu, H.; Wang, J. The global regulator IrrE from Deinococcus radiodurans enhances the furfural tolerance of Saccharomyces cerevisiae. Biochem. Eng. J. 2018, 136, 69–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, R.; Zhao, Z.; Zhou, Z.; Lu, W.; Zhang, W.; Chen, M. IrrE, an exogenous gene from Deinococcus radiodurans, improves the growth of and ethanol production by a Zymomonas mobilis strain under ethanol and acid stresses. J. Microbiol. Biotechnol. 2010, 20, 1156–1162. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, M.; Xu, Z.; Xu, H.; Li, S. Construction of a robust Sphingomonas sp. strain for welan gum production via the expression of global transcriptional regulator IrrE. Front. Bioeng. Biotechnol. 2020, 8, 674. [Google Scholar] [CrossRef]
- Song, B.; Zhou, Q.; Xue, H.-J.; Liu, J.-J.; Zheng, Y.-Y.; Shen, Y.-B.; Wang, M.; Luo, J.-M. IrrE improves organic solvent tolerance and Δ1-dehydrogenation productivity of Arthrobacter simplex. J. Agric. Food Chem. 2018, 66, 5210–5220. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Zhang, X.; Cao, S.; Zhou, X.; Yu, Z.; Qian, X.; Zhou, J.; Dong, W.; Jiang, M. Transcription-associated fluorescence-activated droplet sorting for Di-rhamnolipid hyperproducers. ACS Synth. Biol. 2022, 11, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Wittgens, A.; Rosenau, F. On the road towards tailor-made rhamnolipids: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 8175–8185. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Lin, J.; Wang, W.; Li, S. High-yield di-rhamnolipid production by Pseudomonas aeruginosa YM4 and its potential application in MEOR. Molecules 2019, 24, 1433. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tao, W.; Yu, D.; Li, S. Emulsifying Properties of Rhamnolipids and Their In Vitro Antifungal Activity against Plant Pathogenic Fungi. Molecules 2022, 27, 7746. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, J.; Yang, R.; Li, J.; Lin, M.; Lin, Z. Laboratory-evolved mutants of an exogenous global regulator, IrrE from Deinococcus radiodurans, enhance stress tolerances of Escherichia coli. PLoS ONE 2011, 6, e16228. [Google Scholar] [CrossRef][Green Version]
- Vujičić-Žagar, A.; Dulermo, R.; Le Gorrec, M.; Vannier, F.; Servant, P.; Sommer, S.; de Groot, A.; Serre, L. Crystal structure of the IrrE protein, a central regulator of DNA damage repair in Deinococcaceae. J. Mol. Biol. 2009, 386, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, Z.; Zhang, W.; Chen, Z.; Song, Y.; Lu, W.; Lin, M.; Chen, M. The site-directed A184S mutation in the HTH domain of the global regulator IrrE enhances Deinococcus radiodurans R1 tolerance to UV radiation and MMC shock. J. Microbiol. Biotechnol. 2015, 25, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, T.; Li, X.; Yang, Y.; Zhou, M.; Li, M.; Yan, Z. Enhancement of bioelectricity generation via heterologous expression of IrrE in Pseudomonas aeruginosa-inoculated MFCs. Biosens. Bioelectron. 2018, 117, 23–31. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Zanaroli, G.; Wang, Z.; Xu, P.; Tang, H. Enhancing bioremediation potential of Pseudomonas putida by developing its acid stress tolerance with glutamate decarboxylase dependent system and global regulator of extreme radiation resistance. Front. Microbiol. 2019, 10, 2033. [Google Scholar] [CrossRef]
- Zhao, F.; Han, S.; Zhang, Y. Comparative studies on the structural composition, surface/interface activity and application potential of rhamnolipids produced by Pseudomonas aeruginosa using hydrophobic or hydrophilic substrates. Bioresour. Technol. 2020, 295, 122269. [Google Scholar] [CrossRef] [PubMed]
- Sodagari, M.; Invally, K.; Ju, L.-K. Maximize rhamnolipid production with low foaming and high yield. Enzym. Microb. Technol. 2018, 110, 79–86. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, Z.; Zhang, W.; Lin, M.; Chen, M.; Wei, G. Global transcriptional analysis of Escherichia coli expressing IrrE, a regulator from Deinococcus radiodurans, in response to NaCl shock. Mol. BioSystems 2015, 11, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, W.; Chen, M.; Pan, J.; Lu, W.; Ping, S.; Yan, Y.; Hou, X.; Yuan, M.; Zhan, Y. Genome-wide transcriptome and proteome analysis of Escherichia coli expressing IrrE, a global regulator of Deinococcus radiodurans. Mol. Biosyst. 2011, 7, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Tiso, T.; Zauter, R.; Tulke, H.; Leuchtle, B.; Li, W.-J.; Behrens, B.; Wittgens, A.; Rosenau, F.; Hayen, H.; Blank, L.M. Designer rhamnolipids by reduction of congener diversity: Production and characterization. Microb. Cell Factories 2017, 16, 225. [Google Scholar] [CrossRef]
- Zhao, F.; Yuan, M.; Lei, L.; Li, C.; Xu, X. Enhanced production of mono-rhamnolipid in Pseudomonas aeruginosa and application potential in agriculture and petroleum industry. Bioresour. Technol. 2021, 323, 124605. [Google Scholar] [CrossRef] [PubMed]
- Shatila, F.; Diallo, M.M.; Şahar, U.; Ozdemir, G.; Yalçın, H.T. The effect of carbon, nitrogen and iron ions on mono-rhamnolipid production and rhamnolipid synthesis gene expression by Pseudomonas aeruginosa ATCC 15442. Arch. Microbiol. 2020, 202, 1407–1417. [Google Scholar] [CrossRef]
- Sood, U.; Singh, D.N.; Hira, P.; Lee, J.-K.; Kalia, V.C.; Lal, R.; Shakarad, M. Rapid and solitary production of mono-rhamnolipid biosurfactant and biofilm inhibiting pyocyanin by a taxonomic outlier Pseudomonas aeruginosa strain CR1. J. Biotechnol. 2020, 307, 98–106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).