Two Fluorescent Probes for Recognition of Acetylcholinesterase: Design, Synthesis, and Comparative Evaluation
Abstract
1. Introduction
2. Results and Discussion
2.1. Response and Detection of the Probes for AChE In Vitro
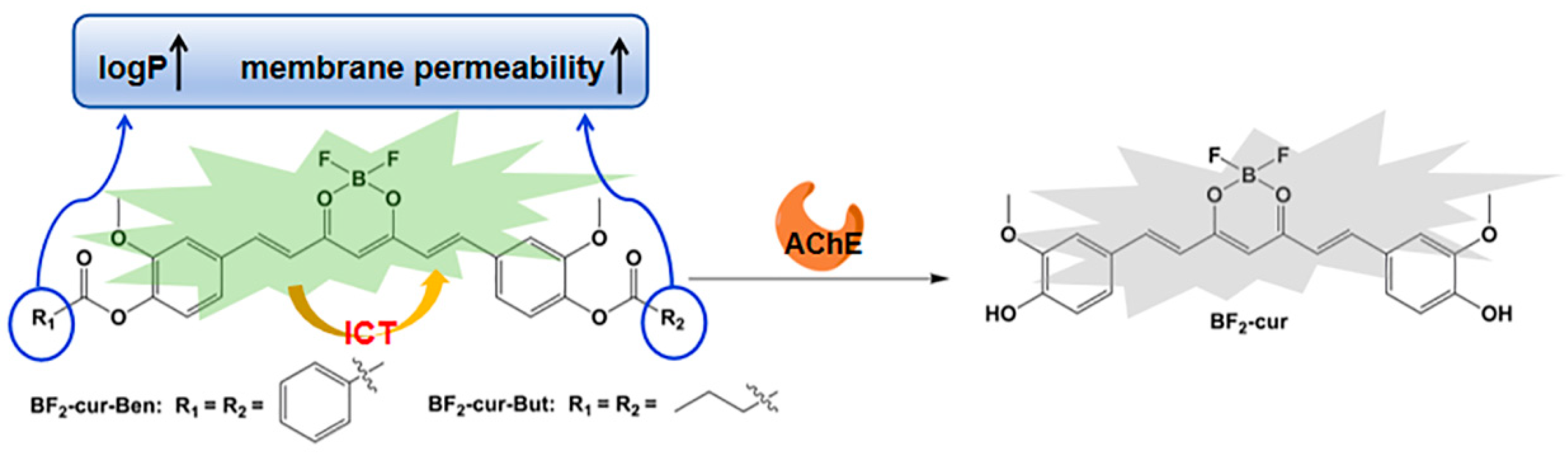
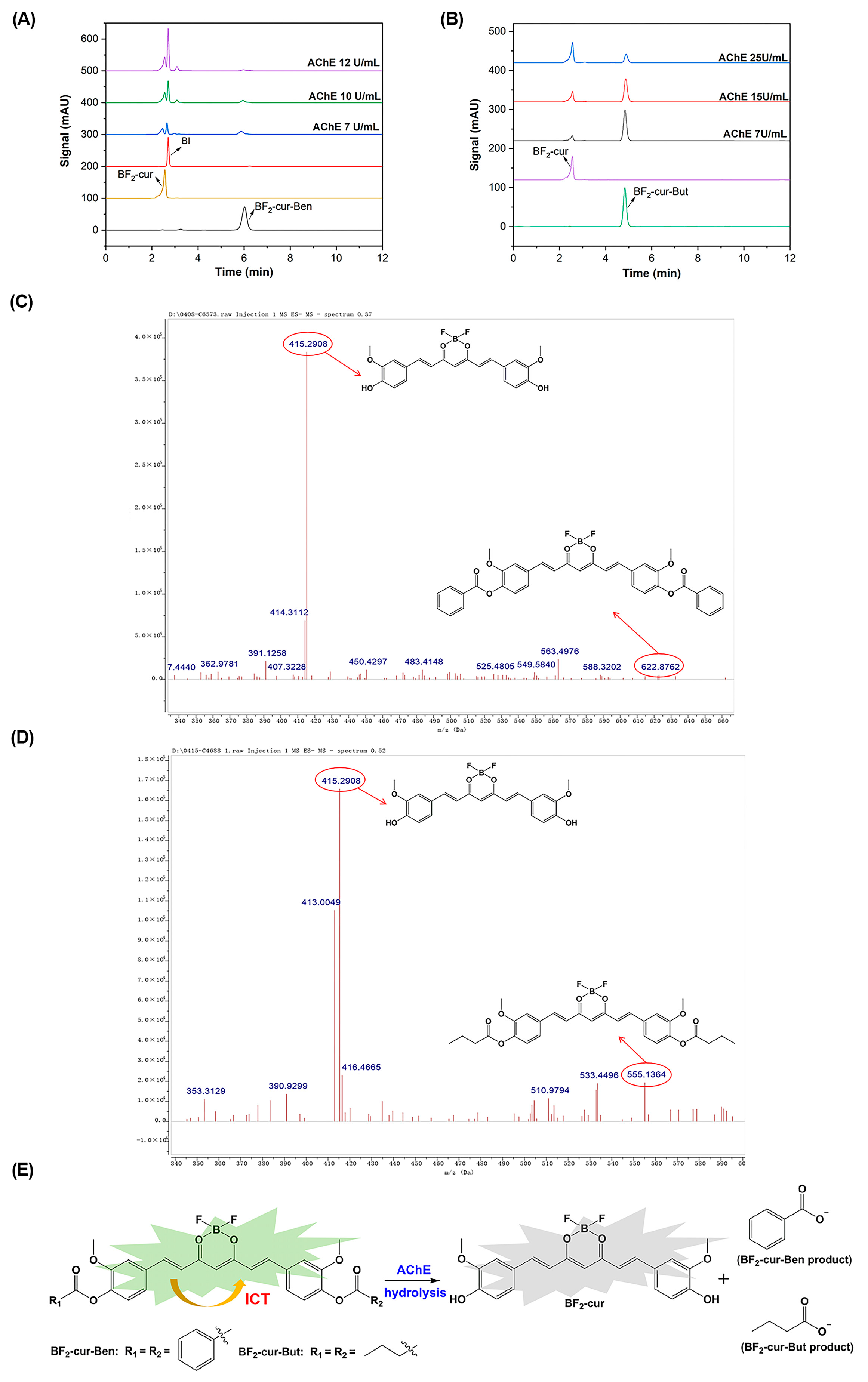
2.2. Recognition and Responsive Mechanisms
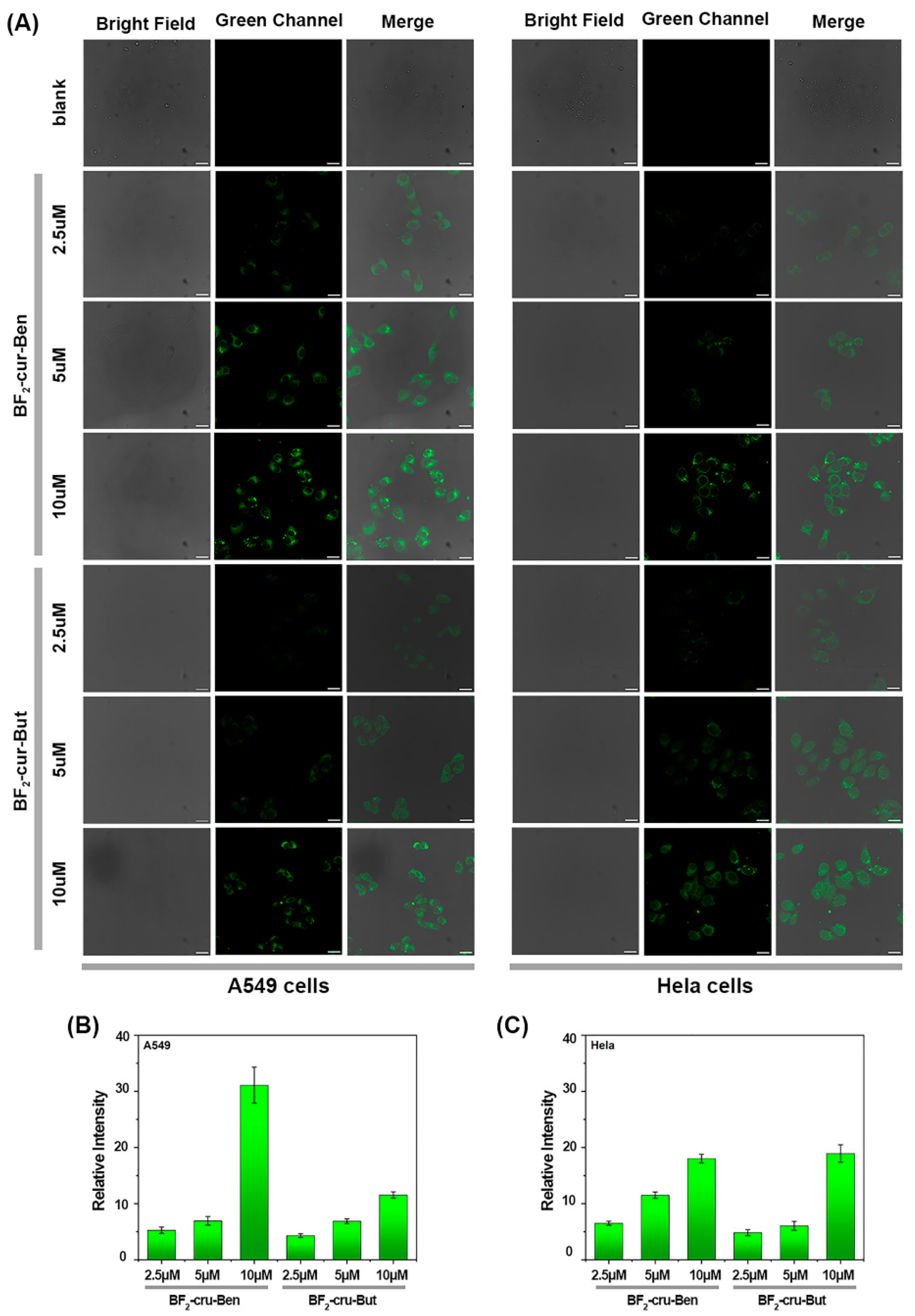
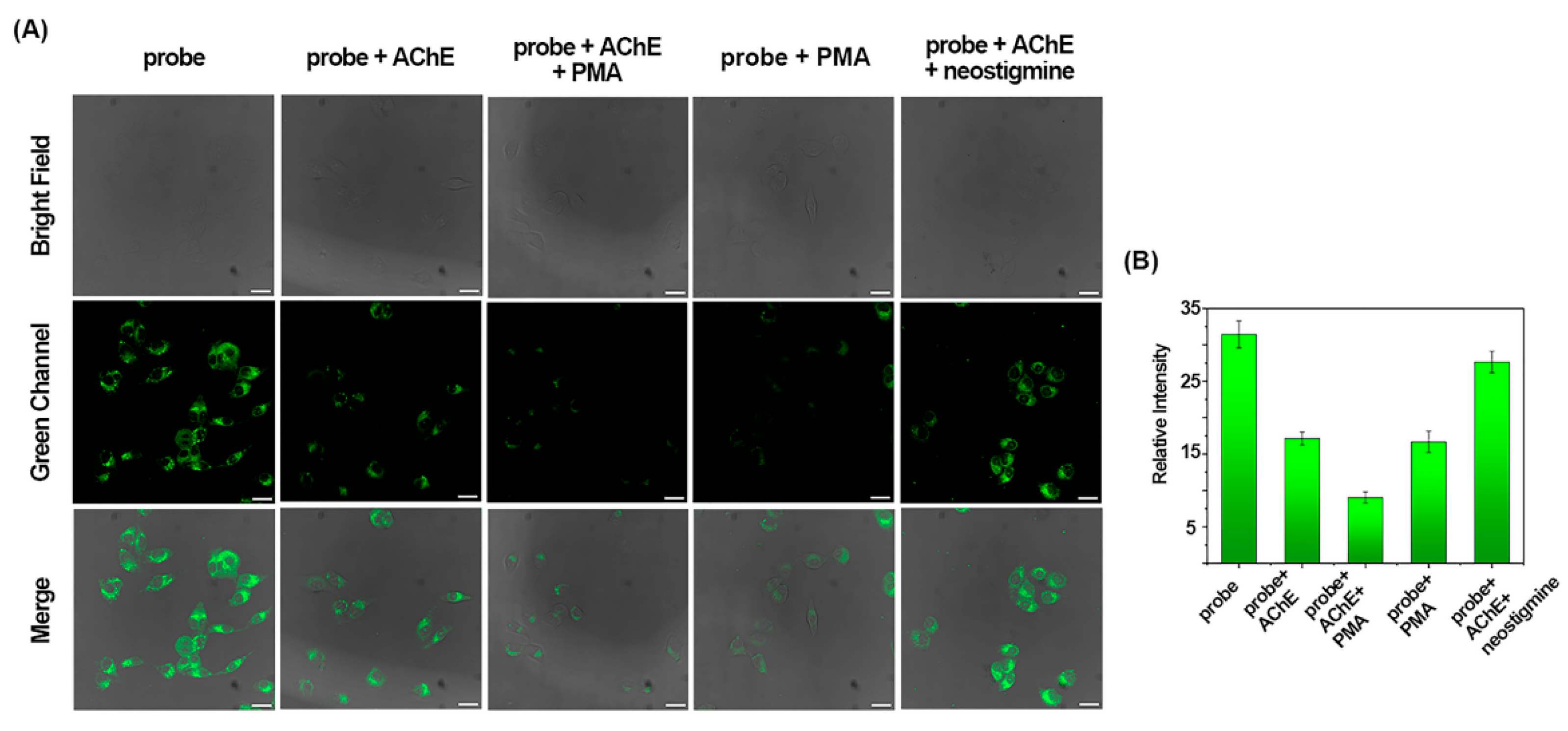
2.3. Detection of AChE with the Probes at the Cellular Level
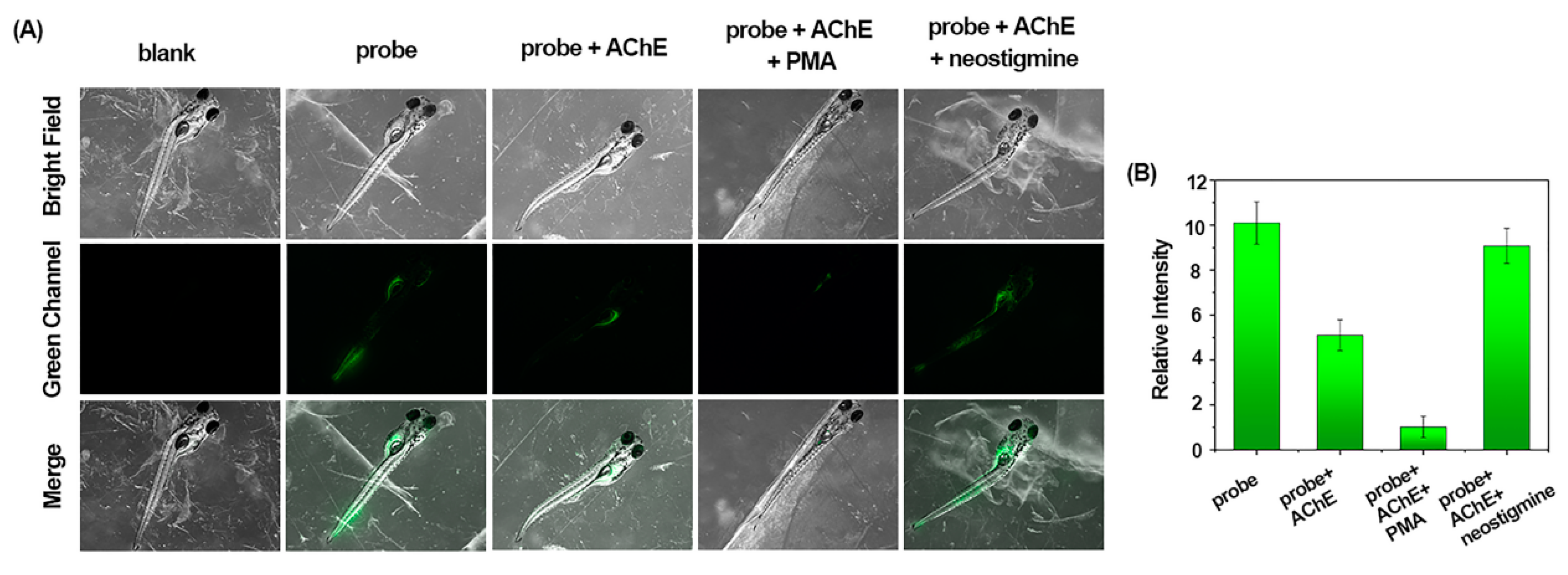
2.4. Detection of AChE with the Probes at the Living Organism Level
2.5. Comparison of Our Probes with Reported Sensors
3. Materials and Methods
3.1. Instruments and Reagents
3.2. Synthesis
3.3. Spectroscopy Experiments
3.4. HPLC and MS Experiments on Samples after Incubation
3.5. Theoretical Calculations and Analysis
3.6. Cytotoxicity and Cell Imaging
3.7. Zebrafish Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sussman, I.S.A.J. Acetylcholinesterase: ‘classical’ and ‘non-classical’ functions and pharmacology. Curr. Opin. Pharmacol. 2005, 5, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Sola, I.; Aso, E.; Frattini, D.; López-González, I.; Espargaró, A.; Sabaté, R.; Di Pietro, O.; Luque, F.J.; Clos, M.V.; Ferrer, I.; et al. Novel Levetiracetam Derivatives That Are Effective against the Alzheimer-like Phenotype in Mice: Synthesis, in Vitro, ex Vivo, and in Vivo Efficacy Studies. J. Med. Chem. 2015, 58, 6018–6032. [Google Scholar] [CrossRef] [PubMed]
- Oukoloff, K.; Chao, S.; CieslikiewiczBouet, M.; Mougeot, R.; Jean, L.; Renard, P.Y. Improved Access to Huprine Derivatives Functionalized at Position 9. Eur. J. Org. Chem. 2016, 2016, 1337–1343. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, X. Acetylcholinesterase and apoptosis A novel perspective for an old enzyme. FEBS J. 2008, 275, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lu, L.; Zhang, X.; Ye, W.; Wu, J.; Xi, Q.; Zhang, X. Hsa-miR-132 regulates apoptosis in non-small cell lung cancer independent of acetylcholinesterase. J. Mol. Neurosci. 2014, 53, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Gong, X.; Xie, J.; Wu, J.; Zhang, X.; Ouyang, Q.; Zhao, X.; Shi, Y.; Zhang, X. AChE deficiency or inhibition decreases apoptosis and p53 expression and protects renal function after ischemia/reperfusion. Apoptosis 2010, 15, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, L.; Liu, S.; Ye, W.; Wu, J.; Zhang, X. Acetylcholinesterase deficiency decreases apoptosis in dopaminergic neurons in the neurotoxin model of Parkinson’s disease. Int. J. Biochem. Cell Biol. 2013, 45, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhu, H.; Zhang, J.; Zhang, X. Calcium signaling-induced Smad3 nuclear accumulation induces acetylcholinesterase transcription in apoptotic HeLa cells. Cell. Mol. Life Sci. 2009, 66, 2181–2193. [Google Scholar] [CrossRef]
- Park, S.E.; Kim, N.D.; Yoo, Y.H. Acetylcholinesterase plays a pivotal role in apoptosome formation. Cancer Res. 2004, 64, 2652–2655. [Google Scholar] [CrossRef]
- Munoz-Delgado, E.; Montenegro, M.F.; Campoy, F.J.; Moral-Naranjo, M.T.; Cabezas-Herrera, J.; Kovacs, G.; Vidal, C.J. Expression of cholinesterases in human kidney and its variation in renal cell carcinoma types. FEBS J. 2010, 277, 4519–4529. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Wang, T.; Hu, X.; Hui, X.; Yan, M.; Gao, Q.; Chen, T.; Li, J.; Yao, M.; et al. Acetylcholinesterase, a key prognostic predictor for hepatocellular carcinoma, suppresses cell growth and induces chemosensitization. Hepatology 2011, 53, 493–503. [Google Scholar] [CrossRef]
- Nieto-Cerón, S.; Vargas-López, H.; Pérez-Albacete, M.; Tovar-Zapata, I.; Martínez-Hernández, P.; Rodríguez-López, J.N.; Cabezas-Herrera, J. Analysis of cholinesterases in human prostate and sperm. Chem. Biol. Interact. 2010, 187, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Ni, Y.; Kokot, S. Unmodified silver nanoparticles for rapid analysis of the organophosphorus pesticide, dipterex, often found in different waters. Sens. Actuators B-Chem. 2014, 193, 205–211. [Google Scholar] [CrossRef]
- Andreani, A.; Burnelli, S.; Granaiola, M.; Guardigli, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Rizzoli, M.; Varoli, L. Chemiluminescent high-throughput microassay applied to imidazo[2,1-b]thiazole derivatives as potential acetylcholinesterase and butyrylcholinesterase inhibitors. Eur. J. Med. Chem. 2008, 43, 657–661. [Google Scholar] [CrossRef]
- Singh, A.P.; Balayan, S.; Hooda, V.; Sarin, R.K.; Chauhan, N. Nano-interface driven electrochemical sensor for pesticides detection based on the acetylcholinesterase enzyme inhibition. Int. J. Biol. Macromol. 2020, 164, 3943–3952. [Google Scholar] [CrossRef]
- Li, Y.P.; Zhao, R.X.; Han, G.Y.; Xiao, Y.M. Novel Acetylcholinesterase Biosensor for Detection of Paraoxon Based on Holey Graphene Oxide Modified Glass Carbon Electrode. Electroanalysis 2018, 30, 2258–2264. [Google Scholar] [CrossRef]
- Lei, C.; Wang, Z.; Nie, Z.; Deng, H.; Hu, H.; Huang, Y.; Yao, S. Resurfaced Fluorescent Protein as a Sensing Platform for Label-Free Detection of Copper(II) Ion and Acetylcholinesterase Activity. Anal. Chem. 2015, 87, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Chai, L.; Tang, C.; Huang, Y.; Chen, J.; Feng, H. A fluorometric assay for acetylcholinesterase activity and inhibitor screening with carbon quantum dots. Sens. Actuators B-Chem. 2016, 222, 879–886. [Google Scholar] [CrossRef]
- Gu, W.; Yan, Y.; Pei, X.; Zhang, C.; Ding, C.; Xian, Y. Fluorescent black phosphorus quantum dots as label-free sensing probes for evaluation of acetylcholinesterase activity. Sens. Actuators B-Chem. 2017, 250, 601–607. [Google Scholar] [CrossRef]
- Li, C.; Wei, C. DNA-functionlized silver nanoclusters as label-free fluorescent probe for the highly sensitive detection of biothiols and acetylcholinesterase activity. Sens. Actuators B-Chem. 2017, 240, 451–458. [Google Scholar] [CrossRef]
- Wang, M.; Li, N.; Wang, S.; Chen, J.; Wang, M.; Liu, L.; Su, X. Constructing self-assembled nanohybrids for the ratiometric fluorescent sensing of acetylcholinesterase activity. Sens. Actuators B-Chem. 2021, 345, 130430. [Google Scholar] [CrossRef]
- Chen, J.; Liao, D.; Wang, Y.; Zhou, H.; Li, W.; Yu, C. Real-Time Fluorometric Assay for Acetylcholinesterase Activity and Inhibitor Screening through the Pyrene Probe Monomer–Excimer Transition. Org. Lett. 2013, 15, 2132–2135. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Han, W.; Ding, H.; Xie, D.; Tan, H.; Yang, S.; Wu, Z.; Shen, G.; Yu, R. Modulated Dye Retention for the Signal-On Fluorometric Determination of Acetylcholinesterase Inhibitor. Anal. Chem. 2013, 85, 4968–4973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Fu, C.; Xiao, Y.; Zhang, Q.; Ding, C. Copper(II) complex as a turn on fluorescent sensing platform for acetylcholinesterase activity with high sensitivity. Talanta 2020, 208, 120406. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, F.; Lu, K.; Yang, S.; Tan, B.; Sun, W.; Shangguan, L.; Wang, H.; Liu, Y. Near-infrared fluorescent probe for in vivo monitoring acetylcholinesterase activity. Sens. Actuators B-Chem. 2022, 360, 131647. [Google Scholar] [CrossRef]
- Ma, J.; Si, T.; Yan, C.; Li, Y.; Li, Q.; Lu, X.; Guo, Y. Near-Infrared Fluorescence Probe for Evaluating Acetylcholinesterase Activity in PC12 Cells and In Situ Tracing AChE Distribution in Zebrafish. ACS Sens. 2020, 5, 83–92. [Google Scholar] [CrossRef]
- He, N.; Yu, L.; Xu, M.; Huang, Y.; Wang, X.; Chen, L.; Yue, S. Near-infrared fluorescent probe for evaluating the acetylcholinesterase effect in the aging process and dietary restriction via fluorescence imaging. J. Mat. Chem. B 2021, 9, 2623–2630. [Google Scholar] [CrossRef] [PubMed]
- Fortibui, M.M.; Jang, M.; Lee, S.; Ryoo, I.; Ahn, J.S.; Ko, S.; Kim, J. Near-Infrared Fluorescence Probe for Specific Detection of Acetylcholinesterase and Imaging in Live Cells and Zebrafish. ACS Appl. Bio Mater. 2022, 5, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, P.; Ding, Q.; Wu, C.; Zhang, W.; Tang, B. Observation of Acetylcholinesterase in Stress-Induced Depression Phenotypes by Two-Photon Fluorescence Imaging in the Mouse Brain. J. Am. Chem. Soc. 2019, 141, 2061–2068. [Google Scholar] [CrossRef]
- Masahiro Oe, K.M.A.M. An activator-induced quencher-detachment_x0002_based turn-on probe with a cationic substratemoiety for acetylcholinesterase. Chem. Commun. 2022, 58, 1510–1513. [Google Scholar] [CrossRef]
- Chao, S.; Krejci, E.; Bernard, V.; Leroy, J.; Jean, L.; Renard, P.Y. A selective and sensitive near-infrared fluorescent probe for acetylcholinesterase imaging. Chem. Commun. 2016, 52, 11599–11602. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; An, J.M.; Shang, J.; Huh, E.; Qi, S.; Lee, E.; Li, H.; Kim, G.; Ma, H.; Oh, M.S.; et al. A molecular approach to rationally constructing specific fluorogenic substrates for the detection of acetylcholinesterase activity in live cells, mice brains and tissues. Chem. Sci. 2020, 11, 11285–11292. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, P.; Zhao, X.L. Study on Curcumin-induced Apoptosis in Ovarian Cancer Resistant Cell Lines COC1/DDP. J. Sichuan Univ. 2012, 43, 335–339. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhuang, J.; Feng, F.B.; Wei, J.Y.; Sun, Y.; LÜ, Q.L.; Han, H.E.; Sun, C.G. Regulative research of Curcumin mediated angiogenesis mimicry of lung cancer cell by Wnt/β-catenin signaling pathway. Chin. J. Cancer Prev. Treat. 2015, 22, 243–246. [Google Scholar] [CrossRef]
- Qin, J.W.; Chen, L. Effect and Mechanism of Curcumin on Melanoma Growth and Angiogenesis. Chin. J. ETMF 2015, 21, 118–123. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, D.; Zang, W.; Yin, G.; Dai, J.; Sun, Y.U.; Yang, Z.; Hoffman, R.M.; Guo, X. Synergistic Inhibitory Effect of Traditional Chinese Medicine Astragaloside IV and Curcumin on Tumor Growth and Angiogenesis in an Orthotopic Nude-Mouse Model of Human Hepatocellular Carcinoma. Anticancer Res. 2017, 37, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Laali, K.K.; Rathman, B.M.; Bunge, S.D.; Qi, X.; Borosky, G.L. Fluoro-curcuminoids and curcuminoid-BF2 adducts: Synthesis, X-ray structures, bioassay, and computational/docking study. J. Fluor. Chem. 2016, 191, 29–41. [Google Scholar] [CrossRef]
- Laali, K.K.; Greves, W.J.; Correa-Smits, S.J.; Zwarycz, A.T.; Bunge, S.D.; Borosky, G.L.; Manna, A.; Paulus, A.; Chanan-Khan, A. Novel fluorinated curcuminoids and their pyrazole and isoxazole derivatives: Synthesis, structural studies, Computational/Docking and in-vitro bioassay. J. Fluor. Chem. 2018, 206, 82–98. [Google Scholar] [CrossRef]
- Laali, K.K.; Greves, W.J.; Zwarycz, A.T.; Correa, S.S.; Troendle, F.J.; Borosky, G.L.; Akhtar, S.; Manna, A.; Paulus, A.; Chanan-Khan, A.; et al. Synthesis, Computational Docking Study, and Biological Evaluation of a Library of Heterocyclic Curcuminoids with Remarkable Antitumor Activity. Chem. Med. Chem. 2018, 13, 1895–1908. [Google Scholar] [CrossRef]
- Abonia, R.; Laali, K.K.; Somu, D.R.; Bunge, S.D.; Wang, E.C. A Flexible Strategy for Modular Synthesis of Curcuminoid-BF2/Curcuminoid Pairs and Their Comparative Antiproliferative Activity in Human Cancer Cell Lines. Chem. Med. Chem. 2020, 15, 354–362. [Google Scholar] [CrossRef]
- Laali, K.K.; Zwarycz, A.T.; Bunge, S.D.; Borosky, G.L.; Nukaya, M.; Kennedy, G.D. Deuterated Curcuminoids: Synthesis, Structures, Computational/Docking and Comparative Cell Viability Assays against Colorectal Cancer. Chem. Med. Chem. 2019, 14, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, Y.; Wang, Q.; Li, Z.; Zhang, C. Detection of acetylcholinesterase and butyrylcholinesterase in vitro and in vivo using a new fluorescent probe. Chem. Commun. 2024, 60, 2082–2085. [Google Scholar] [CrossRef] [PubMed]
- Sherin, D.R.; Thomas, S.G.; Rajasekharan, K.N. Mechanochemical synthesis of 2,2-difluoro-4,6-bis(β-styryl)-1,3,2-dioxaborines and their use in cyanide ion sensing. Heterocycl. Commun. 2015, 21, 381–385. [Google Scholar] [CrossRef]
- Margar, S.N.; Rhyman, L.; Ramasam, P.; Sekar, N. Fluorescent difluoroboron-curcumin analogs: An investigation of the electronic structures and photophysical properties. Spectrochim. Acta A 2016, 152, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Canard, G.; Ponce-Vargas, M.; Jacquemin, D.; Guennic, B.L.; Felouat, A.; Rivoal, M.; Zaborova, E.; D’Aleo, A.; Fages, F. Influence of the electron donor groups on the optical and electrochemical properties of borondifluoride complexes of curcuminoid derivatives: A joint theoretical and experimental study. RSC Adv. 2017, 7, 10132. [Google Scholar] [CrossRef]
- Zhao, L.; He, X.; Huang, Y.; Li, J.; Li, Y.; Tao, S.; Sun, Y.; Wang, X.; Ma, P.; Song, D. A novel ESIPT-ICT-based near-infrared fluorescent probe with large stokesshift for the highly sensitive, specific, and non-invasive in vivo detection of cysteine. Sens. Actuators B-Chem. 2019, 296, 126571. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Q.; Wang, Z.; Liu, J.; Guo, Y.; Pan, C.; Li, X.; Che, J.; Shi, Z.; Zhang, S. Log P analyzation-based discovery of GSH activated biotin-tagged fluorescence probe for selective colorectal cancer imaging. Eur. J. Med. Chem. 2022, 239, 114555. [Google Scholar] [CrossRef]
- Zhang, X.J.; Yang, L.; Zhao, Q.; Caen, J.P.; He, H.Y.; Jin, Q.H.; Guo, L.H.; Alemany, M.; Zhang, L.Y.; Shi, Y.F. Induction of acetylcholinesterase expression during apoptosis in various cell types. Cell Death Differ. 2002, 9, 790–800. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, X.; Zhang, B.; Wu, J.; Zhang, X. Synaptic acetylcholinesterase targeted by microRNA-212 functions as a tumor suppressor in non-small cell lung cancer. Int. J. Biochem. Cell Biol. 2013, 45, 2530–2540. [Google Scholar] [CrossRef]
- Yuan, J.J.; Shen, Z.X.; Mou, N.; Cao, Y.Q. Use of Acetylcholinesterase, Butyrylcholinesterase or Their Mutants in the Preparation or Screening of Drugs for the Treatment of Tumors. China Patent CN 107029218 A, 11 August 2017. [Google Scholar]
- Kim, E.; Felouat, A.; Zaborova, E.; Ribierre, J.; Wu, J.W.; Senatore, S.; Matthews, C.; Lenne, P.; Baffert, C.; Karapetyan, A.; et al. Borondifluoride complexes of hemicurcuminoids as bio-inspired push-pull dyes for bioimaging. Org. Biomol. Chem. 2016, 14, 1311–1324. [Google Scholar] [CrossRef]
- Xie, R.J.; Liu, X.G.; Xu, G.S.; Liu, Z.X. Synthesis of difluoroboron curcumin allylic ether and its application as fluorescent sensor for palladium(0). J. Zhejiang Univ. 2014, 41, 314–317. [Google Scholar] [CrossRef]



| Luminophore | Linearity Range (U/mL) | LOD (U/mL) | Application | Ref. |
|---|---|---|---|---|
| Fluorescent protein | 2.5 × 10−5–2 × 10−3 | 1.5 × 10−5 | food | [17] |
| Carbon quantum dots | 1.42 × 10−2–1.218 × 10−1 | 4.25 × 10−3 | human serum, semen | [18] |
| Black phosphorus quantum dots | 2 × 10−4–5 × 10−3 | 4 × 10−5 | / | [19] |
| Silver nanoclusters | 0–4 × 10−3 | 7.1 × 10−5 | / | [20] |
| Ag+-modified Au nanoclusters | 3 × 10−5–4 × 10−3 | 2.1 × 10−5 | human serum | [21] |
| Pyrene (polymer PVS assist) | / | 1.5 × 10−2 | screening enzyme inhibitors | [22] |
| Benzothiazoles (Cu2+ and ATCh assist) | 2.5 × 10−5–4 × 10−3 | 1.8 × 10−5 | HEK293 cells | [24] |
| Hemicyanine (with ATCh as substrate) | 0–1 × 10−2 | 2 × 10−4 | HeLa cells, MCF-7 cells, mice | [25] |
| Hemicyanine | 0–8.0 | 0.1173 | PC12 cells, zebrafish | [26] |
| Boron dipyrrole fluoride | 0–20 | 0.21 | PC12 cells, brains of mice, mice | [27] |
| Tricyanofurans (TCF) | 0–50 | 0.17 | PC12 cells, zebrafish | [28] |
| Hemicyanine | 0–20 | 0.36 | PC12 cells, brains of mice | [29] |
| Hemicyanine | / | / | neuromuscular junctions | [31] |
| Phenoxazin | 0–20 | 1.7 × 10−2 | U87MG cells, brains of mice, mice | [32] |
| Curcumin difluoroboride | 0.5–7 | 3.1 × 10−2 | A549 cells, HeLa cells | This work |
| 0.5–25 | 8.7 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Yi, Q.; Qing, B.; Lan, W.; Jiang, F.; Lai, Z.; Huang, J.; Liu, Q.; Jiang, J.; Wang, M.; et al. Two Fluorescent Probes for Recognition of Acetylcholinesterase: Design, Synthesis, and Comparative Evaluation. Molecules 2024, 29, 1961. https://doi.org/10.3390/molecules29091961
Lin X, Yi Q, Qing B, Lan W, Jiang F, Lai Z, Huang J, Liu Q, Jiang J, Wang M, et al. Two Fluorescent Probes for Recognition of Acetylcholinesterase: Design, Synthesis, and Comparative Evaluation. Molecules. 2024; 29(9):1961. https://doi.org/10.3390/molecules29091961
Chicago/Turabian StyleLin, Xia, Qingyuan Yi, Binyang Qing, Weisen Lan, Fangcheng Jiang, Zefeng Lai, Jijun Huang, Qing Liu, Jimin Jiang, Mian Wang, and et al. 2024. "Two Fluorescent Probes for Recognition of Acetylcholinesterase: Design, Synthesis, and Comparative Evaluation" Molecules 29, no. 9: 1961. https://doi.org/10.3390/molecules29091961
APA StyleLin, X., Yi, Q., Qing, B., Lan, W., Jiang, F., Lai, Z., Huang, J., Liu, Q., Jiang, J., Wang, M., Zou, L., Huang, X., & Wang, J. (2024). Two Fluorescent Probes for Recognition of Acetylcholinesterase: Design, Synthesis, and Comparative Evaluation. Molecules, 29(9), 1961. https://doi.org/10.3390/molecules29091961






