Identification and Validation of Magnolol Biosynthesis Genes in Magnolia officinalis
Abstract
1. Introduction
2. Results
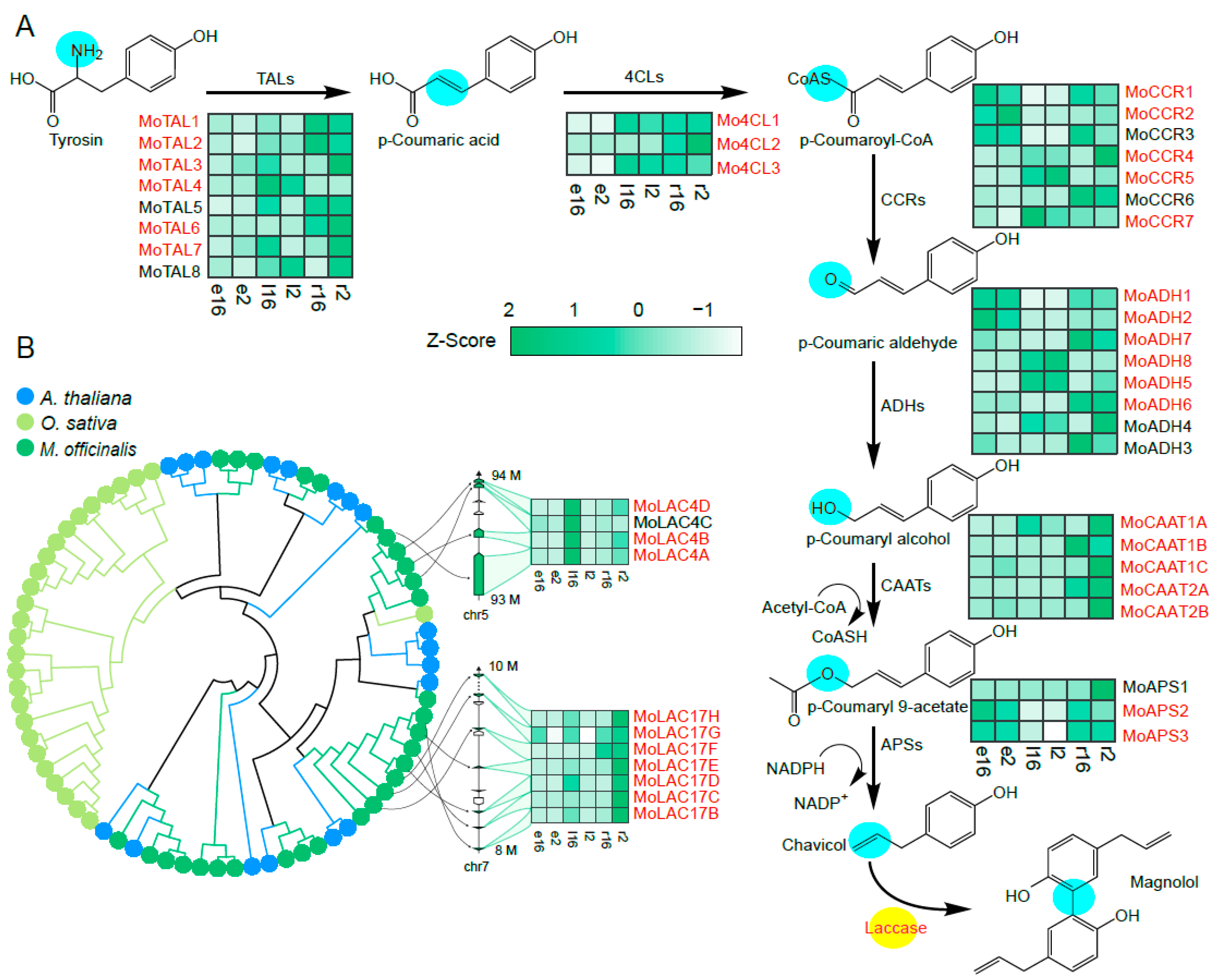
2.1. Biosynthetic Pathway for Magnolol and RNA Sequencing of M. officinalis Tissues
2.2. Laccase-Catalyzed Magnolol Synthesis
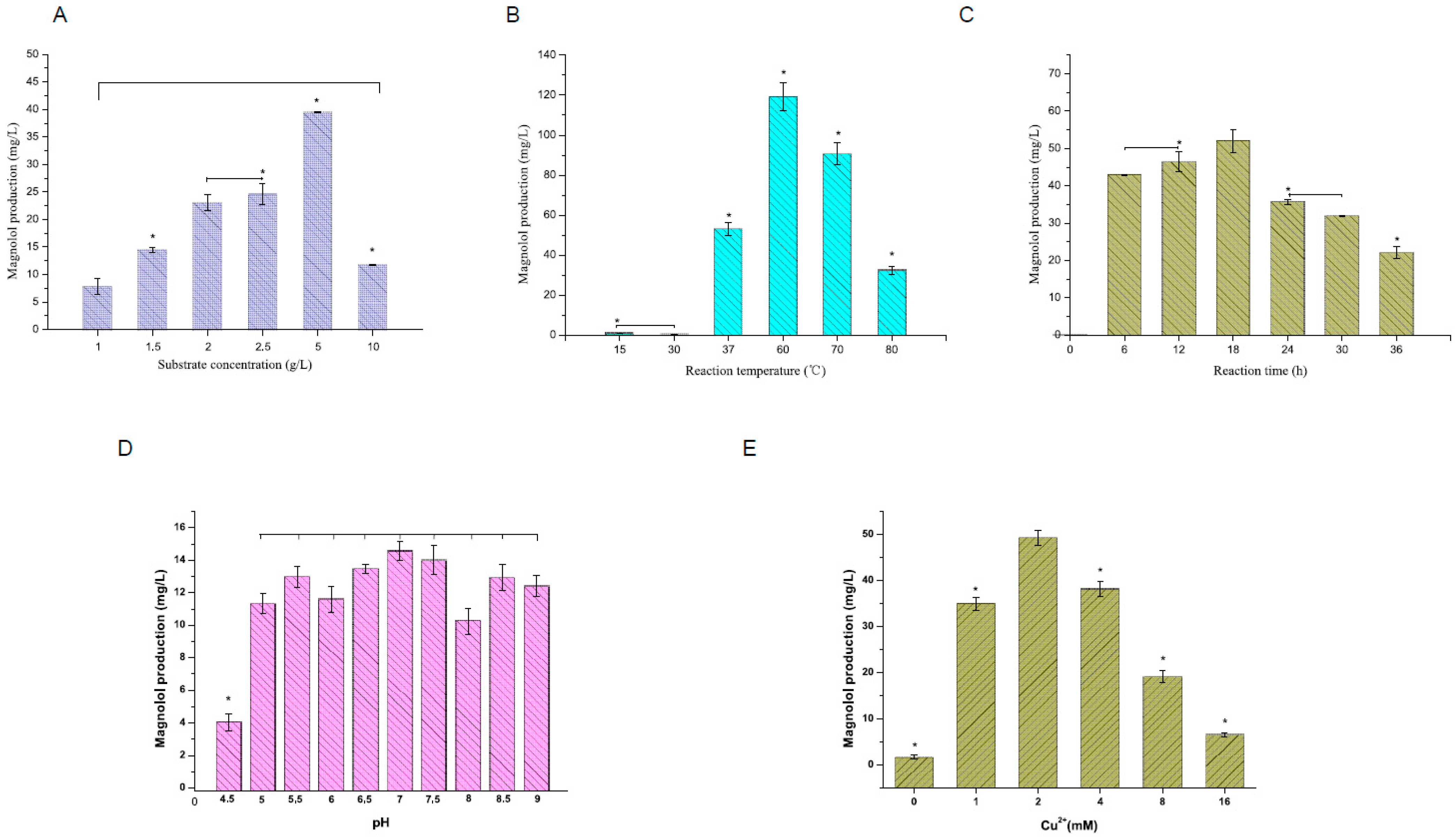
2.3. Optimization of Magnolol Synthesis through the MolAC14 Enzyme
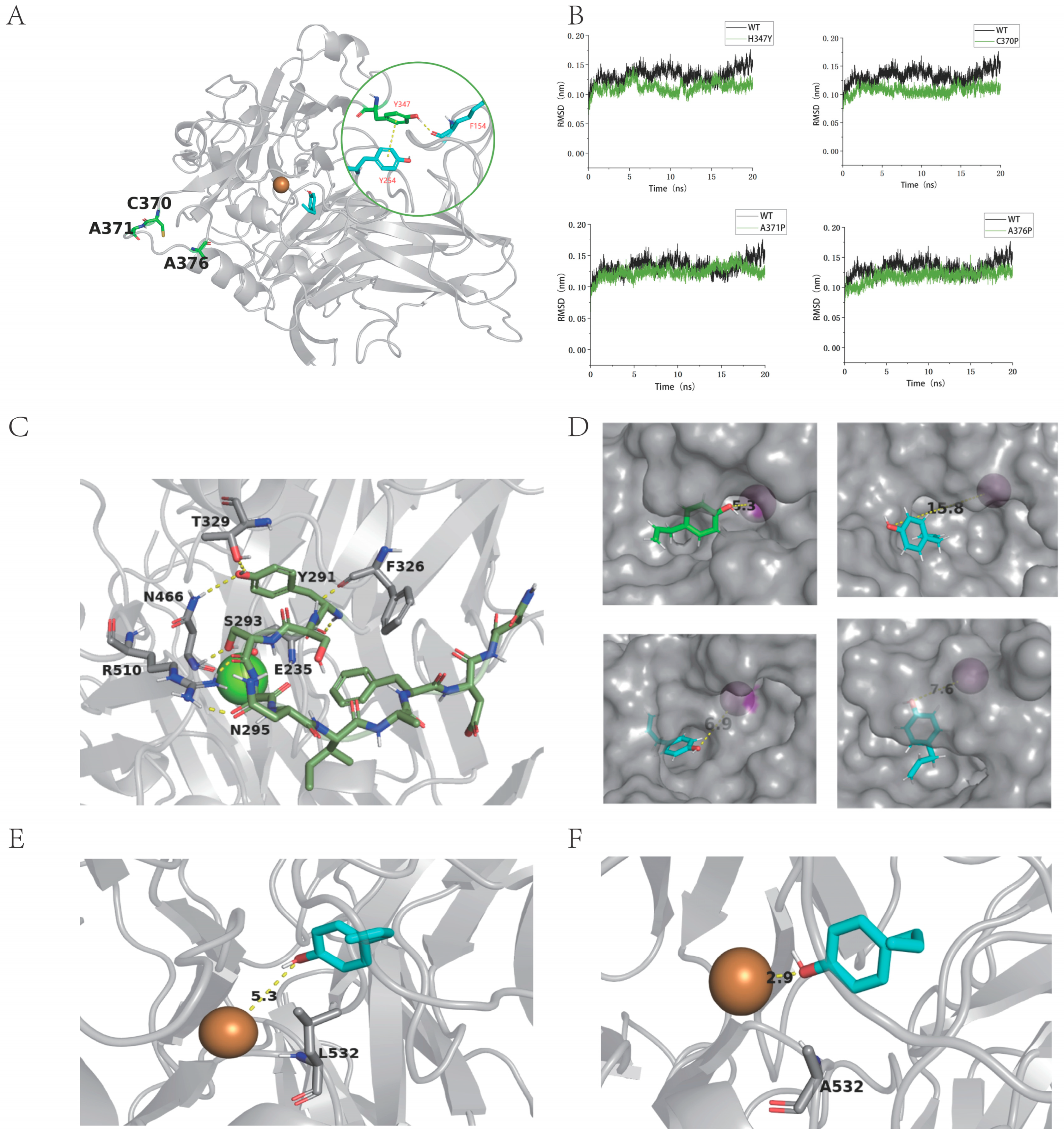
2.4. Modification of MoLAC14 Enzyme
2.5. Mechanism of the Impact on Thermal Stability and Activity by Critical Residues of MoLAC14 Mutants
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Transcriptome Materials
4.3. Transcriptome Analysis
4.4. Gene Cloning, Protein Expression, and Protein Purification
4.5. Structural Simulation and Molecular Modification of MoLAC14
4.6. Enzyme Assay
4.7. Analytical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talebi Bezmin Abadi, A.; Rizvanov, A.A.; Haertlé, T.; Blatt, N.L. World Health Organization report: Current crisis of antibiotic resistance. BioNanoScience 2019, 9, 778–788. [Google Scholar] [CrossRef]
- White, A.; Hughes, J.M. Critical importance of a one health approach to antimicrobial resistance. EcoHealth 2019, 16, 404–409. [Google Scholar] [CrossRef]
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.X. Antibiotics and food safety in aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef]
- Chen, C.; Tan, R.; Qu, W.; Wu, Z.; Wang, Y.; Urade, Y.; Huang, Z. Magnolol, a major bioactive constituent of the bark of Magnolia officinalis, exerts antiepileptic effects via the GABA/benzodiazepine receptor complex in mice. Br. J. Pharmacol. 2011, 164, 1534–1546. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Huang, P.-H.; Lin, F.-Y.; Chen, W.-C.; Chen, Y.-L.; Yin, W.-H.; Man, K.-M.; Liu, P.-L. Magnolol: A multifunctional compound isolated from the Chinese medicinal plant Magnolia officinalis. Euro. J. Integr. Med. 2011, 3, e317–e324. [Google Scholar] [CrossRef]
- Vega-García, A.; Santana-Gómez, C.; Rocha, L.; Magdaleno-Madrigal, V.; Morales-Otal, A.; Buzoianu-Anguiano, V.; Feria-Romero, I.; Orozco-Suarez, S. Magnolia officinalis reduces the long-term effects of the status epilepticus induced by kainic acid in immature rats. Brain Res. Bull. 2019, 149, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.-Y.; Zhang, X.-J.; Han, J.; Li, Y.-Q.; Wang, G.-C. In vitro synergism of magnolol and honokiol in combination with antibacterial agents against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA). BMC Complement. Altern. Med. 2015, 15, 425. [Google Scholar] [CrossRef] [PubMed]
- Oufensou, S.; Scherm, B.; Pani, G.; Balmas, V.; Fabbri, D.; Dettori, M.A.; Carta, P.; Malbrán, I.; Migheli, Q.; Delogu, G. Honokiol, magnolol and its monoacetyl derivative show strong anti-fungal effect on Fusarium isolates of clinical relevance. PLoS ONE 2019, 14, e0221249. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, Y.; Zeng, Y.; Tian, B.; Qu, X.; Yuan, Q.; Song, Y. Pharmacology, toxicity, bioavailability, and formulation of magnolol: An update. Front. Pharmacol. 2021, 12, 632767. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lu, M.-H.; Guo, D.-S.; Zhai, Y.-Y.; Miao, D.; Yue, J.-Y.; Yuan, C.-H.; Zhao, M.-M.; An, D.-R. Antifungal effect of magnolol and honokiol from Magnolia officinalis on Alternaria alternata causing tobacco brown spot. Molecules 2019, 24, 2140. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Nișca, A.; Mirica, A.; Milan, A.; Boz, I. Wood bark as valuable raw material for compounds with a bioregulator effect in lemon balm (Melissa officinalis L.) plants. Appl. Sci. 2019, 9, 3148. [Google Scholar] [CrossRef]
- Łata, E.; Fulczyk, A.; Ott, P.G.; Kowalska, T.; Sajewicz, M.; Móricz, Á.M. Thin-layer chromatographic quantification of magnolol and honokiol in dietary supplements and selected biological properties of these preparations. J. Chromatogr. A 2020, 1625, 461230. [Google Scholar] [CrossRef] [PubMed]
- Zai-Kang, T.; Yan-Ru, Z.; Jin-ping, S. Variation, heredity and selection of effective ingredients in Magnolis officinalis of different provenances. J. For. Res. 2002, 13, 7–11. [Google Scholar] [CrossRef]
- Thuerig, B.; Ramseyer, J.; Hamburger, M.; Ludwig, M.; Oberhänsli, T.; Potterat, O.; Schärer, H.-J.; Tamm, L. Efficacy of a Magnolia officinalis bark extract against grapevine downy mildew and apple scab under controlled and field conditions. Crop Prot. 2018, 114, 97–105. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, Y.; Chai, C.; Zou, L.-S.; Liu, X.-H.; Wang, S.-N.; Hua, Y.-J. Dynamic changes of eight bioactive constituents in Magnoliae officinalis cortex based on UFLC-QTRAP-MS/MS combined with grey relational analysis. Curr. Pharm. Anal. 2019, 15, 497–504. [Google Scholar] [CrossRef]
- Runeberg, J. Phenol dehydrogenations. Acta Chem. Scand. 1958, 12, 188–192. [Google Scholar] [CrossRef][Green Version]
- Clark, A.M.; El-Feraly, A.S.; Li, W.-S. Antimicrobial activity of phenolic constituents of Magnolia grandiflora L. J. Pharm. Sci. 1981, 70, 951–952. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Xie, J.; Li, H.; Zhu, X.; Wang, P.; Su, W. Efficient and selective extraction of magnolol from Magnolia officinalis by mechanochemical extraction technique. Chem. Eng. Process. 2011, 50, 325–330. [Google Scholar] [CrossRef]
- Westfall, P.J.; Pitera, D.J.; Lenihan, J.R.; Eng, D.; Woolard, F.X.; Regentin, R.; Horning, T.; Tsuruta, H.; Melis, D.J.; Owens, A. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc. Natl. Acad. Sci. USA 2012, 109, E111–E118. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, J.; Zhang, G.; Ding, W.; Duan, L.; Yang, J.; Kui, L.; Cheng, X.; Ruan, J.; Fan, W. Engineering yeast for the production of breviscapine by genomic analysis and synthetic biology approaches. Nat. Commun. 2018, 9, 448. [Google Scholar] [CrossRef]
- Luo, X.; Reiter, M.A.; d’Espaux, L.; Wong, J.; Denby, C.M.; Lechner, A.; Zhang, Y.; Grzybowski, A.T.; Harth, S.; Lin, W. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 2019, 567, 123–126. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Z.; Li, X.; Ma, L.; Guan, Q.; Zheng, G.; Liang, H.; Yan, Y.; Shen, X.; Wang, J. Biosynthesis of plant hemostatic dencichine in Escherichia coli. Nat. Commun. 2022, 13, 5492. [Google Scholar] [CrossRef]
- Shi, X.; Yang, L.; Gao, J.; Sheng, Y.; Li, X.; Gu, Y.; Zhuang, G.; Chen, F. Deep sequencing of Magnoliae officinalis reveals upstream genes related to the lignan biosynthetic pathway. J. For. Res. 2017, 28, 671–681. [Google Scholar] [CrossRef]
- Xu, F.; Damhus, T.; Danielsen, S.; Østergaard, L.H. Catalytic applications of laccase. In Modern Biooxidation: Enzymes, Reactions and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 43–75. [Google Scholar]
- Mate, D.M.; Alcalde, M. Laccase: A multi-purpose biocatalyst at the forefront of biotechnology. Microb. Biotechnol. 2017, 10, 1457–1467. [Google Scholar] [CrossRef]
- Vassão, D.G.; Gang, D.R.; Koeduka, T.; Jackson, B.; Pichersky, E.; Davin, L.B.; Lewis, N.G. Chavicol formation in sweet basil (Ocimum basilicum): Cleavage of an esterified C9 hydroxyl group with NAD (P) H-dependent reduction. Org. Biomol. Chem. 2006, 4, 2733–2744. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Vassão, D.G.; Moinuddin, S.G.; Bedgar, D.L.; Davin, L.B.; Lewis, N.G. Allyl/propenyl phenol synthases from the creosote bush and engineering production of specialty/commodity chemicals, eugenol/isoeugenol, in Escherichia coli. Arch. Biochem. Biophys. 2014, 541, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sun, X.; Li, Y.; Yan, Y.; Yuan, Q. Metabolic engineering of Escherichia coli for microbial synthesis of monolignols. Metab. Eng. 2017, 39, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Wang, S.; Zhou, W.; Zhuang, Y.; Liu, T. Producing gram-scale unnatural rosavin analogues from glucose by engineered Escherichia coli. ACS Synth. Biol. 2019, 8, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Peng, F.; Zhou, L.; Yin, X.; Chen, J.; Zhong, H.; Hou, F.; Xie, X.; Wang, L.; Shi, X. The chromosome-scale genome of Magnolia officinalis provides insight into the evolutionary position of magnoliids. iScience 2021, 24, 102997. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, S.; Guo, L.; Wang, B.; Jia, Y.; Zhou, J.; Che, Y.; Jia, P.; Lin, J.; Xu, T. Three chromosome-scale Papaver genomes reveal punctuated patchwork evolution of the morphinan and noscapine biosynthesis pathway. Nat. Commun. 2021, 12, 6030. [Google Scholar] [CrossRef] [PubMed]
- Edwards, V.H. The influence of high substrate concentrations on microbial kinetics. Biotechnol. Bioeng. 1970, 12, 679–712. [Google Scholar] [CrossRef]
- Harris, P.R.; Grover, M.A.; Rousseau, R.W.; Bommarius, A.S. Selectivity and kinetic modeling of penicillin G acylase variants for the synthesis of cephalexin under a broad range of substrate concentrations. Biotechnol. Bioeng. 2022, 119, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.R.; Hofsteenge, J. Kinetics of the inhibition of thrombin by hirudin. Biochemistry 1986, 25, 4622–4628. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Verma, P. Laccase: Addressing the ambivalence associated with the calculation of enzyme activity. 3 Biotech 2019, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Xu, D.; Pan, X.; Ma, X. Self-propelled enzymatic nanomotors for enhancing synergetic photodynamic and starvation therapy by self-accelerated cascade reactions. Appl. Mater. Today 2019, 16, 508–517. [Google Scholar] [CrossRef]
- Nicolás, C.; Martin-Bertelsen, T.; Floudas, D.; Bentzer, J.; Smits, M.; Johansson, T.; Troein, C.; Persson, P.; Tunlid, A. The soil organic matter decomposition mechanisms in ectomycorrhizal fungi are tuned for liberating soil organic nitrogen. ISME J. 2019, 13, 977–988. [Google Scholar] [CrossRef]
- Craig, D.B.; Arriaga, E.A.; Wong, J.C.; Lu, H.; Dovichi, N.J. Studies on single alkaline phosphatase molecules: Reaction rate and activation energy of a reaction catalyzed by a single molecule and the effect of thermal denaturation the death of an enzyme. J. Am. Chem. Soc. 1996, 118, 5245–5253. [Google Scholar] [CrossRef]
- Daniel, R.M.; Dines, M.; Petach, H.H. The denaturation and degradation of stable enzymes at high temperatures. Biochem. J. 1996, 317, 1–11. [Google Scholar] [CrossRef]
- Adachi, T.; Mazurenko, I.; Mano, N.; Kitazumi, Y.; Kataoka, K.; Kano, K.; Sowa, K.; Lojou, E. Kinetic and thermodynamic analysis of Cu2+-dependent reductive inactivation in direct electron transfer-type bioelectrocatalysis by copper efflux oxidase. Electrochim. Acta 2022, 429, 140987. [Google Scholar] [CrossRef]
- Bisswanger, H. Enzyme assays. Perspect. Sci. 2014, 1, 41–55. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Korman, T.P.; Sahachartsiri, B.; Charbonneau, D.M.; Huang, G.L.; Beauregard, M.; Bowie, J.U. Dieselzymes: Development of a stable and methanol tolerant lipase for biodiesel production by directed evolution. Biotechnol. Biofuels 2013, 6, 70. [Google Scholar] [CrossRef]
- Agharahimi, M.R.; LeBel, N.A. Synthesis of (-)-monoterpenylmagnolol and magnolol. J. Org. Chem. 1995, 60, 1856–1863. [Google Scholar] [CrossRef]
- Xu, J.; Xu, H. Magnolol: Chemistry and biology. Ind. Crops Prod. 2023, 205, 117493. [Google Scholar] [CrossRef]
- Wang, J.; Ledesma-Amaro, R.; Wei, Y.; Ji, B.; Ji, X.-J. Metabolic engineering for increased lipid accumulation in Yarrowia lipolytica—A review. Bioresour. Technol. 2020, 313, 123707. [Google Scholar] [CrossRef]
- Madzak, C. Yarrowia lipolytica strains and their biotechnological applications: How natural biodiversity and metabolic engineering could contribute to cell factories improvement. J. Fungi 2021, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Liu, X.; Chen, X.; Cai, Y.; Zhuang, Y.; Liu, T.; Zhu, X.; Wang, H.; Liu, Y.; Jiang, H. Raising the production of phloretin by alleviation of by-product of chalcone synthase in the engineered yeast. Sci. China Life Sci. 2020, 63, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Chen, Z.; Ren, Y.; Sun, Y.; Wang, Y. Whole-cell biocatalyst for rubusoside production in Saccharomyces cerevisiae. J. Agric. Food Chem. 2021, 69, 13155–13163. [Google Scholar] [CrossRef]
- Wu, M.; Gong, D.-C.; Yang, Q.; Zhang, M.-Q.; Mei, Y.-Z.; Dai, C.-C. Activation of naringenin and kaempferol through pathway refactoring in the endophyte Phomopsis liquidambaris. ACS Synth. Biol. 2021, 10, 2030–2039. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Chen, B.; Liu, W.; Guo, Z.; Liu, X.; Zhu, X.; Liu, J.; Zhang, J.; Li, J. Metabolic engineering of Yarrowia lipolytica for scutellarin production. Syn. Syst. Biotechnol. 2022, 7, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, V.; Monteiro, L.; Pedada, D.; Stohr, A.; Blenner, M. High-Efficiency Multiplexed Cytosine Base Editors for Natural Product Synthesis in Yarrowia lipolytica. ACS Synth. Biol. 2023, 12, 3082–3091. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.G.; Yook, S.; Oh, H.; Rao, C.V.; Jin, Y.-S. Toward rapid and efficient utilization of nonconventional substrates by nonconventional yeast strains. Curr. Opin. Biotech. 2024, 85, 103059. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Q.; Xue, Z.-Z.; Yang, B. Chemical components of Magnoliae officinalis Cortex of different origins and with different tree ages before and after being processed with ginger juice: A qualitative and quantitative analysis. China J. Chin. Mater. Med. 2023, 48, 2435–2454. [Google Scholar]
- Sarrica, A.; Kirika, N.; Romeo, M.; Salmona, M.; Diomede, L. Safety and toxicology of magnolol and honokiol. Planta Med. 2018, 84, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Tong, Z.; Zhu, Y.; Si, J.; Pan, X. A study on the relationship between tree age and bark quality in Magnolia officinalis. J. Chin. Med. Mater. 1999, 22, 379–381. [Google Scholar]
- Luo, H.; Wu, H.; Yu, X.; Zhang, X.; Lu, Y.; Fan, J.; Tang, L.; Wang, Z. A review of the phytochemistry and pharmacological activities of Magnoliae officinalis cortex. J. Ethnopharmacol. 2019, 236, 412–442. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, J.; Jeong, S.-I.; Jahng, K.Y.; Yu, K.-Y. Antimicrobial effects and resistant regulation of magnolol and honokiol on methicillin-resistant Staphylococcus aureus. BioMed Res. Int. 2015, 2015, 283630. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Hu, B.; Hu, J.; Xu, H.; He, H.; Han, C.; Kang, B.; Bai, L.; Zhang, R. Transcriptome reveals genes involving in black skin color formation of ducks. Genes Genom. 2021, 43, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Saddam, M.; Ahsan Habib, M.; Abrar Fahim, M.; Mimi, A.; Islam, S.; Mostofa Uddin Helal, M. Engineered BCL6 BTB Domain of the Bcl-2 Protein Family shows Dynamic Structural Behavior: Insights from Molecular Dynamics Simulations. bioRxiv 2023. [Google Scholar] [CrossRef]
- Pederson, J.P.; McDaniel, J.G. DFT-based QM/MM with particle-mesh Ewald for direct, long-range electrostatic embedding. J. Chem. Phys. 2022, 156, 174105. [Google Scholar] [CrossRef]


| Strains | Vmax | Km (mol/L) | Kcat (1/S) | Km/Kcat |
|---|---|---|---|---|
| L532A | 8.76 × 10−3 | 8.76 × 10−9 | 15.33 | 5.71 × 10−10 |
| MoLAC14 | 3.36 × 10−3 | 3.36 × 10−10 | 5.89 | 5.71 × 10−11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, Z.; Zong, H.; Liu, S.; Du, Q.; Wu, H.; Li, Z.; Wang, X.; Huang, L.; Lai, C.; et al. Identification and Validation of Magnolol Biosynthesis Genes in Magnolia officinalis. Molecules 2024, 29, 587. https://doi.org/10.3390/molecules29030587
Yang Y, Li Z, Zong H, Liu S, Du Q, Wu H, Li Z, Wang X, Huang L, Lai C, et al. Identification and Validation of Magnolol Biosynthesis Genes in Magnolia officinalis. Molecules. 2024; 29(3):587. https://doi.org/10.3390/molecules29030587
Chicago/Turabian StyleYang, Yue, Zihe Li, Hang Zong, Shimeng Liu, Qiuhui Du, Hao Wu, Zhenzhu Li, Xiao Wang, Lihui Huang, Changlong Lai, and et al. 2024. "Identification and Validation of Magnolol Biosynthesis Genes in Magnolia officinalis" Molecules 29, no. 3: 587. https://doi.org/10.3390/molecules29030587
APA StyleYang, Y., Li, Z., Zong, H., Liu, S., Du, Q., Wu, H., Li, Z., Wang, X., Huang, L., Lai, C., Zhang, M., Wang, W., & Chen, X. (2024). Identification and Validation of Magnolol Biosynthesis Genes in Magnolia officinalis. Molecules, 29(3), 587. https://doi.org/10.3390/molecules29030587






