In Vitro Assessment of Cortisol Release Inhibition, Bioaccessibility and Bioavailability of a Chemically Characterized Scutellaria lateriflora L. Hydroethanolic Extract
Abstract
1. Introduction
2. Results
2.1. Bioaccessibility of S. lateriflora Extract after In Vitro Simulated Gastric, Duodenal, and Gastroduodenal Digestion
2.2. Bioavailability of S. lateriflora Extract
2.2.1. Evaluation of Viability of Caco-2 Cells after Treatment with Duodenal Digested S. lateriflora Extract
2.2.2. Evaluation of Transepithelial Electrical Resistance (TEER) and Tight Junction ZO-1 Protein
2.2.3. Lucifer Yellow Permeability Assay
2.2.4. Absorption Experiment on Caco-2 Cells Grown on Transwell Insert
2.2.5. Parallel Artificial Membrane Permeability Assay (PAMPA)
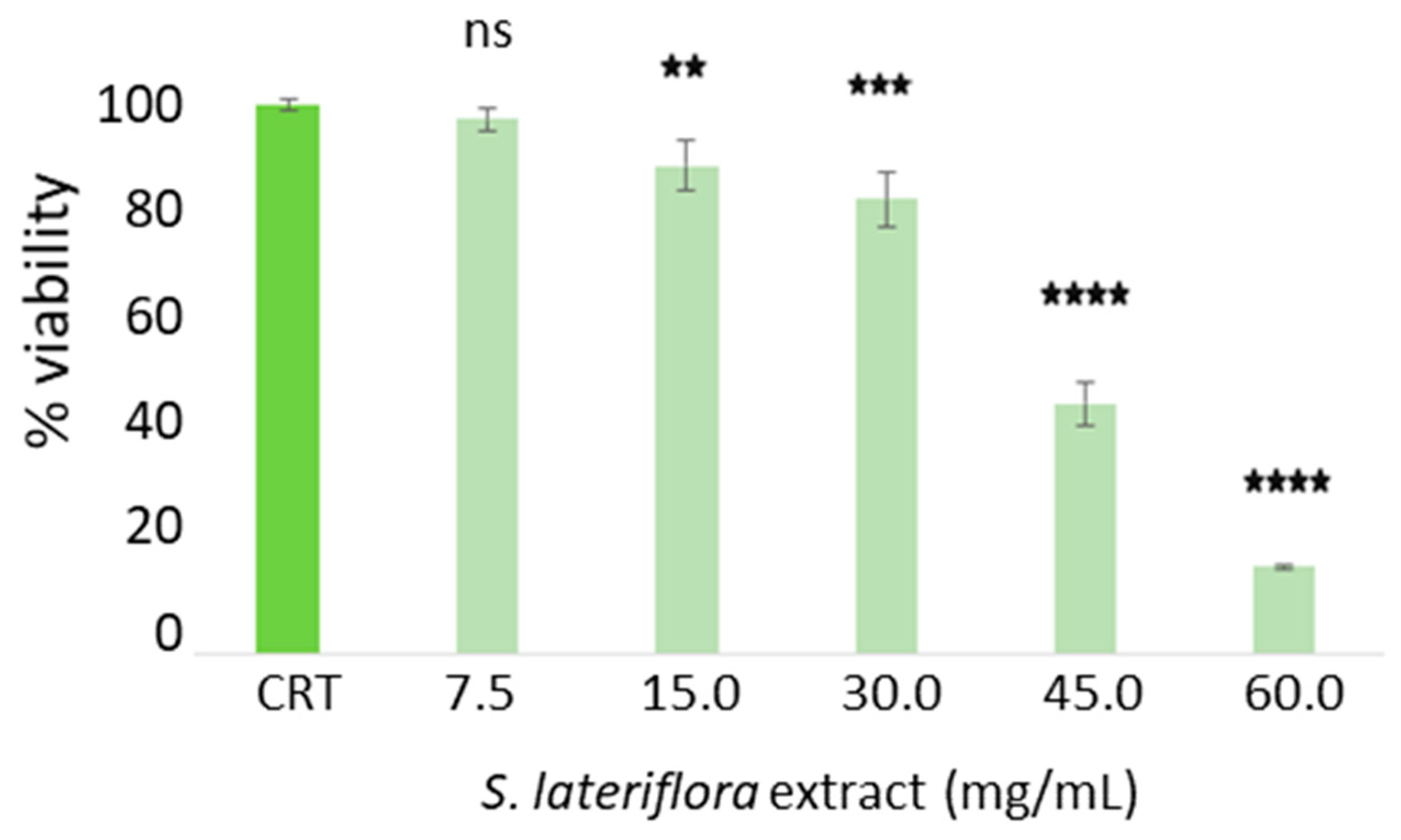
2.3. Determination of Noncytotoxic Concentrations of S. lateriflora Extract in the H295R Cell Model System
2.4. In Vitro Inhibition of Cortisol Release by S. lateriflora Extract
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents and Biological Materials
4.2. In Vitro Bioaccessibility of S. lateriflora Extract
4.2.1. In Vitro Simulated Gastric Digestion
4.2.2. In Vitro Simulated Duodenal Digestion
4.2.3. In Vitro Simulated Gastroduodenal Digestion
4.2.4. RP-UHPLC-PDA-ESI-MS/MS Analysis
4.3. In Vitro Bioavailability
4.3.1. Cell Culture
4.3.2. Cytotoxic Activity of Duodenal Digested S. lateriflora Extract on Caco-2 Cells
4.3.3. PAMPA Assay
4.3.4. Caco-2 Transwell Model System
4.3.5. Immunochemistry
4.4. In Vitro Efficacy of S. lateriflora Extract on the Release of Cortisol
4.4.1. Cell Culture
4.4.2. Cytotoxic Activity of S. lateriflora Extract on H295R Cells
4.4.3. In Vitro Evaluation of the S. lateriflora Extract on Inhibition of CORTISOL Release
4.4.4. Evaluation of Cortisol Levels by ELISA Assay
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherman, S.H.; Joshee, N. Current status of research on medicinal plant Scutellaria lateriflora: A review. J. Med. Act. Plants 2022, 11, 22–38. [Google Scholar]
- Upton, R.; Dayu, R.H. Skullcap Scutellaria lateriflora L.: An American nervine. J. Herb. Med. 2012, 2, 76–96. [Google Scholar] [CrossRef]
- Ullah, H.; Minno, A.D.; Filippis, A.D.; Sommella, E.; Buccato, D.G.; Lellis, L.F.D.; El-Seedi, H.R.; Khalifa, S.A.; Piccinocchi, R.; Galdiero, M.; et al. In vitro antimicrobial and antibiofilm properties and bioaccessibility after oral digestion of chemically characterized extracts obtained from Cistus × incanus L., Scutellaria lateriflora L., and their combination. Foods 2023, 12, 1826. [Google Scholar] [CrossRef] [PubMed]
- Udintsev, S.N.; Krylova, S.G.; Konovalova, O.N. Correction by natural adaptogens of hormonal-metabolic status disorders in rats during the development of adaptation syndrome using functional tests with dexamethasone and ACTH. Biull. Eksp. Biol. Med. 1991, 112, 599–601. [Google Scholar] [CrossRef]
- Ryu, J.H.; Tan-Lee, B.S.; Jung, J.W.; Ahn, N.Y.; Lee, S.J.; Yu, G.Y.; Han, S.H.; Lee, J.H.; Lee, G.S.; Cheong, J.H. Anti-stress Effect of Scutellatia baicalensis in SD Rats and ICR Mice. Biomol. Ther. 2004, 12, 34–42. [Google Scholar]
- Lee, S.; Kim, D.H.; Jung, J.W.; Oh, J.H.; Park, H.J.; Park, C.; Huh, Y.; Cheong, J.H.; Oh, T.H.; Ryu, J.H. Schizandra chinensis and Scutellaria baicalensis counter stress behaviors in mice. Phytother. Res. 2007, 21, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Dedovic, K.; Duchesne, A.; Andrews, J.; Engert, V.; Pruessner, J.C. The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. Neuroimage 2009, 47, 864–871. [Google Scholar] [CrossRef]
- Hakamata, Y.; Komi, S.; Moriguchi, Y.; Izawa, S.; Motomura, Y.; Sato, E.; Mizukami, S.; Kim, Y.; Hanakawa, T.; Inoue, Y.; et al. Amygdala-centred functional connectivity affects daily cortisol concentrations: A putative link with anxiety. Sci. Rep. 2017, 7, 8313. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, E.; Choi, M.H. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep. 2015, 48, 209. [Google Scholar] [CrossRef]
- Randler, C.; Schaal, S. Morningness–eveningness, habitual sleep-wake variables and cortisol level. Biol. Psychol. 2010, 85, 14–18. [Google Scholar] [CrossRef]
- Hirotsu, C.; Tufik, S.; Andersen, M.L. Interactions between sleep, stress, and metabolism: From physiological to pathological conditions. Sleep Sci. 2015, 8, 143–152. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Tsigos, C.; Bixler, E.O.; Stratakis, C.A.; Zachman, K.; Kales, A.; Vela-Bueno, A.; Chrousos, G.P. Chronic insomnia and activity of the stress system: A preliminary study. J. Psychosom. Res. 1998, 45, 21–31. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Bixler, E.O.; Lin, H.M.; Prolo, P.; Mastorakos, G.; Vela-Bueno, A.; Kales, A.; Chrousos, G.P. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: Clinical implications. J. Clin. Endocrinol. Metab. 2001, 86, 3787–3794. [Google Scholar] [CrossRef]
- Rodenbeck, A.; Hajak, G. Neuroendocrine dysregulation in primary insomnia. Rev. Neurol. 2001, 157, S57–S61. [Google Scholar]
- Dziurkowska, E.; Wesolowski, M. Cortisol as a biomarker of mental disorder severity. J. Clin. Med. 2021, 10, 5204. [Google Scholar] [CrossRef]
- Chiodini, I.; Torlontano, M.; Carnevale, V.; Trischitta, V.; Scillitani, A. Skeletal involvement in adult patients with endogenous hypercortisolism. J. Endocrinol. Investig. 2008, 31, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Diez, J.J.; Iglesias, P. Pharmacological therapy of Cushing’s syndrome: Drugs and indications. Mini Rev. Med. Chem. 2007, 7, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.; Enache, D.; Gianotti, L.; Schatzberg, A.F.; Young, A.H.; Pariante, C.M.; Mondelli, V. Baseline cortisol and the efficacy of antiglucocorticoid treatment in mood disorders: A meta-analysis. Psychoneuroendocrinology 2019, 110, 104420. [Google Scholar] [CrossRef] [PubMed]
- Garzarella, E.U.; Navajas-Porras, B.; Pérez-Burillo, S.; Ullah, H.; Esposito, C.; Santarcangelo, C.; Hinojosa-Nogueira, D.; Pastoriza, S.; Zaccaria, V.; Xiao, J.; et al. Evaluating the effects of a standardized polyphenol mixture extracted from poplar-type propolis on healthy and diseased human gut microbiota. Biom. Pharmacother. 2022, 148, 112759. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- De Paulo Farias, D.; de Araújo, F.F.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; Catharino, R.R.; Pastore, G.M. Effect of in vitro digestion on the bioaccessibility and bioactivity of phenolic compounds in fractions of Eugenia pyriformis fruit. Food Res. Int. 2021, 150, 110767. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.R.; Zhang, Y.C.; Vodovotz, Y.; Schwartz, S.J.; Failla, M.L. Stability and bioaccessibility of isoflavones from soy bread during in vitro digestion. J. Agric. Food Chem. 2003, 51, 4603–4609. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pi, J.; Zhang, Y.; Ma, X.; Zhang, B.; Wang, S.; Qi, D.; Li, N.; Guo, P.; Liu, Z. A strategy to improve the oral availability of baicalein: The baicalein-theophylline cocrystal. Fitoterapia 2018, 129, 85–93. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Q.; Wang, J.R.; Mei, X. Cocrystals of baicalein with higher solubility and enhanced bioavailability. Cryst. Growth Des. 2017, 17, 1893–1901. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, W.; Ye, J.; Hao, H.; Zhou, J.; Wang, R.; Liu, Y. A novel matrix dispersion based on phospholipid complex for improving oral bioavailability of baicalein: Preparation, in vitro and in vivo evaluations. Drug Deliv. 2017, 24, 720–728. [Google Scholar] [CrossRef]
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and pharmacokinetics of genistein: Mechanistic studies on its ADME. Anticancer Agents Med. Chem. 2012, 12, 1264–1280. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of neuroprotection by quercetin: Counteracting oxidative stress and more. Oxid. Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef]
- Orhan, I.E.; Nabavi, S.F.; Daglia, M.; Tenore, G.C.; Mansouri, K.; Nabavi, S.M. Naringenin and atherosclerosis: A review of literature. Curr. Pharm. Biotechnol. 2015, 16, 245–251. [Google Scholar] [CrossRef]
- Naeem, A.; Ming, Y.; Pengyi, H.; Jie, K.Y.; Yali, L.; Haiyan, Z.; Shuai, X.; Wenjing, L.; Ling, W.; Xia, Z.M.; et al. The fate of flavonoids after oral administration: A comprehensive overview of its bioavailability. Crit. Rev. Food Sci. Nutr. 2022, 62, 6169–6186. [Google Scholar] [CrossRef]
- Gao, C.; Chen, X.; Zhong, D. Absorption and disposition of scutellarin in rats: A pharmacokinetic explanation for the high exposure of its isomeric metabolite. Drug Metab. Dispos. 2011, 39, 2034–2044. [Google Scholar] [CrossRef]
- Hao, X.; Cheng, G.; Yu, J.E.; He, Y.; An, F.; Sun, J.; Cui, F. Study on the role of hepatic first-pass elimination in the low oral bioavailability of scutellarin in rats. Pharmazie 2005, 60, 477–478. [Google Scholar]
- Domínguez-Avila, J.A.; Wall-Medrano, A.; Velderrain-Rodríguez, G.R.; Chen, C.Y.O.; Salazar-López, N.J.; Robles-Sánchez, M.; González-Aguilar, G.A. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Funct. 2017, 8, 15–38. [Google Scholar] [CrossRef]
- Cassidy, A.; Brown, J.E.; Hawdon, A.; Faughnan, M.S.; King, L.J.; Millward, J.; Zimmer-Nechemias, L.; Wolfe, B.; Setchell, K.D. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J. Nutr. 2006, 136, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, S.; Li, T.; Zhou, R.; Wai, A.T.S.; Yan, R. Oral pharmacokinetics of baicalin, wogonoside, oroxylin A 7-O-β-d-glucuronide and their aglycones from an aqueous extract of Scutellariae Radix in the rat. J. Chromatogr. B 2016, 1026, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Chen, H.; Zhang, M.; Yang, N.; Yang, H.; Xu, C.; Li, J.; Ning, C.; Song, Z.; Zhou, S.; et al. Pharmacokinetics, tissue distribution and excretion study of Oroxylin A, Oroxylin A 7-O-glucuronide and Oroxylin A sodium sulfonate in rats after administration of Oroxylin A. Fitoterapia 2020, 142, 104480. [Google Scholar] [CrossRef] [PubMed]
- Sajeev, A.; Hegde, M.; Girisa, S.; Devanarayanan, T.N.; Alqahtani, M.S.; Abbas, M.; Sil, S.K.; Sethi, G.; Chen, J.T.; Kunnumakkara, A.B. Oroxylin A: A promising flavonoid for prevention and treatment of chronic diseases. Biomolecules 2022, 12, 1185. [Google Scholar] [CrossRef]
- Lu, L.; Guo, Q.; Zhao, L. Overview of oroxylin A: A promising flavonoid compound. Phytother. Res. 2016, 30, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Fan, H.; Gao, S.Y.; Jin, Y.F.; Jiang, B.; Shen, J. Antidepressant-like activity of oroxylin A in mice models of depression: A behavioral and neurobiological characterization. Front. Pharmacol. 2022, 13, 921553. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.L.; Kathol, R.G.; Noyes, R., Jr. Reduction in urinary free cortisol during benzodiazepine treatment of panic disorder. Psychoneuroendocrinology 1990, 15, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Sengos, G.; Wedekind, D.; Huether, G.; Pilz, J.; Broocks, A.; Hajak, G.; Rüther, E. Urinary excretion of cortisol, norepinephrine, testosterone, and melatonin in panic disorder. Pharmacopsychiatry 1997, 30, 113–117. [Google Scholar] [CrossRef]
- Vedhara, K.; Miles, J.; Bennett, P.; Plummer, S.; Tallon, D.; Brooks, E.; Gale, L.; Munnoch, K.; Schreiber-Kounine, C.; Fowler, C.; et al. An investigation into the relationship between salivary cortisol, stress, anxiety and depression. Biol. Psychol. 2003, 62, 89–96. [Google Scholar] [CrossRef]
- Vreeburg, S.A.; Zitman, F.G.; van Pelt, J.; DeRijk, R.H.; Verhagen, J.C.; van Dyck, R.; Hoogendijk, W.J.; Smit, J.H.; Penninx, B.W. Salivary cortisol levels in persons with and without different anxiety disorders. Psychosom. Med. 2010, 72, 340–347. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
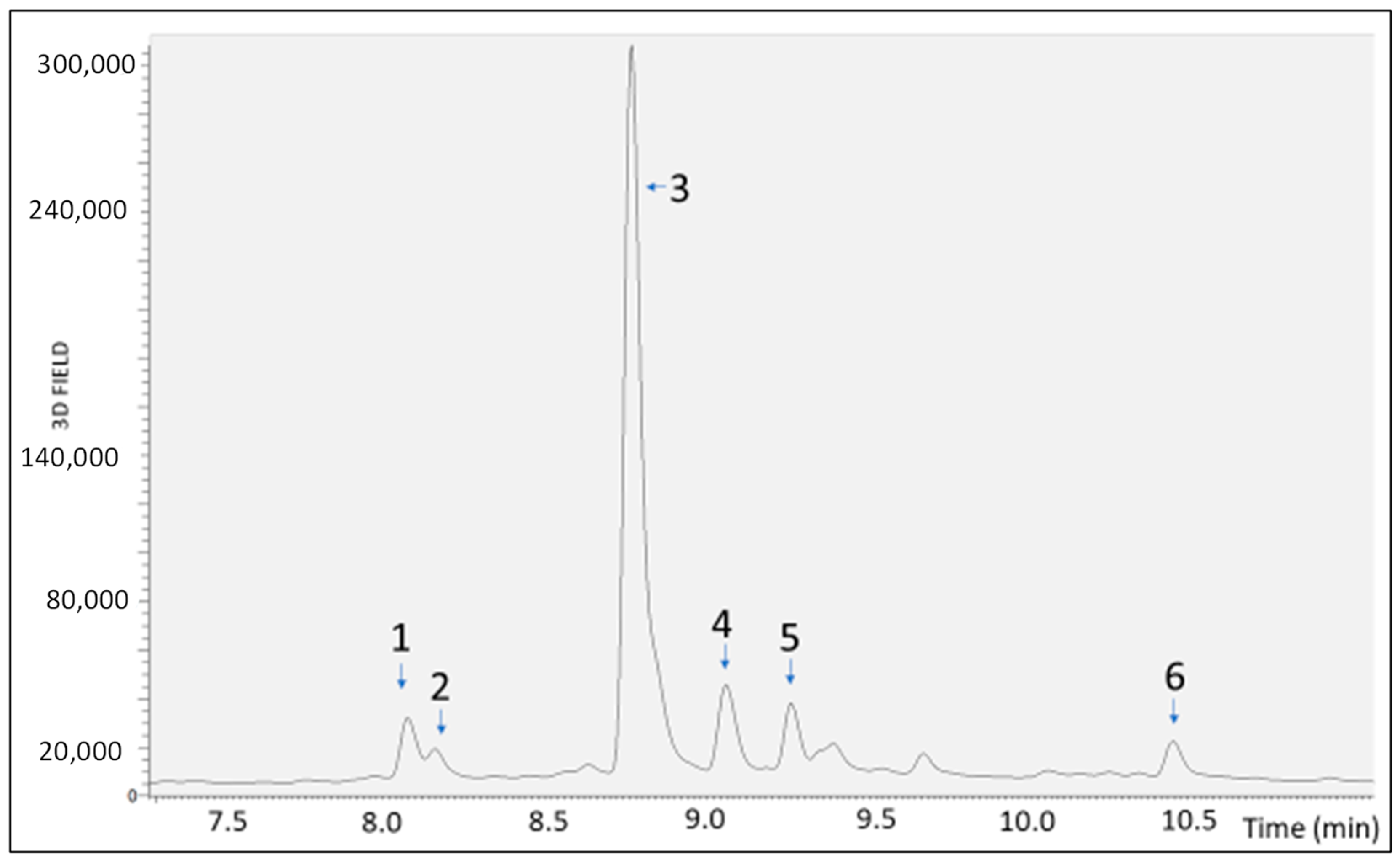
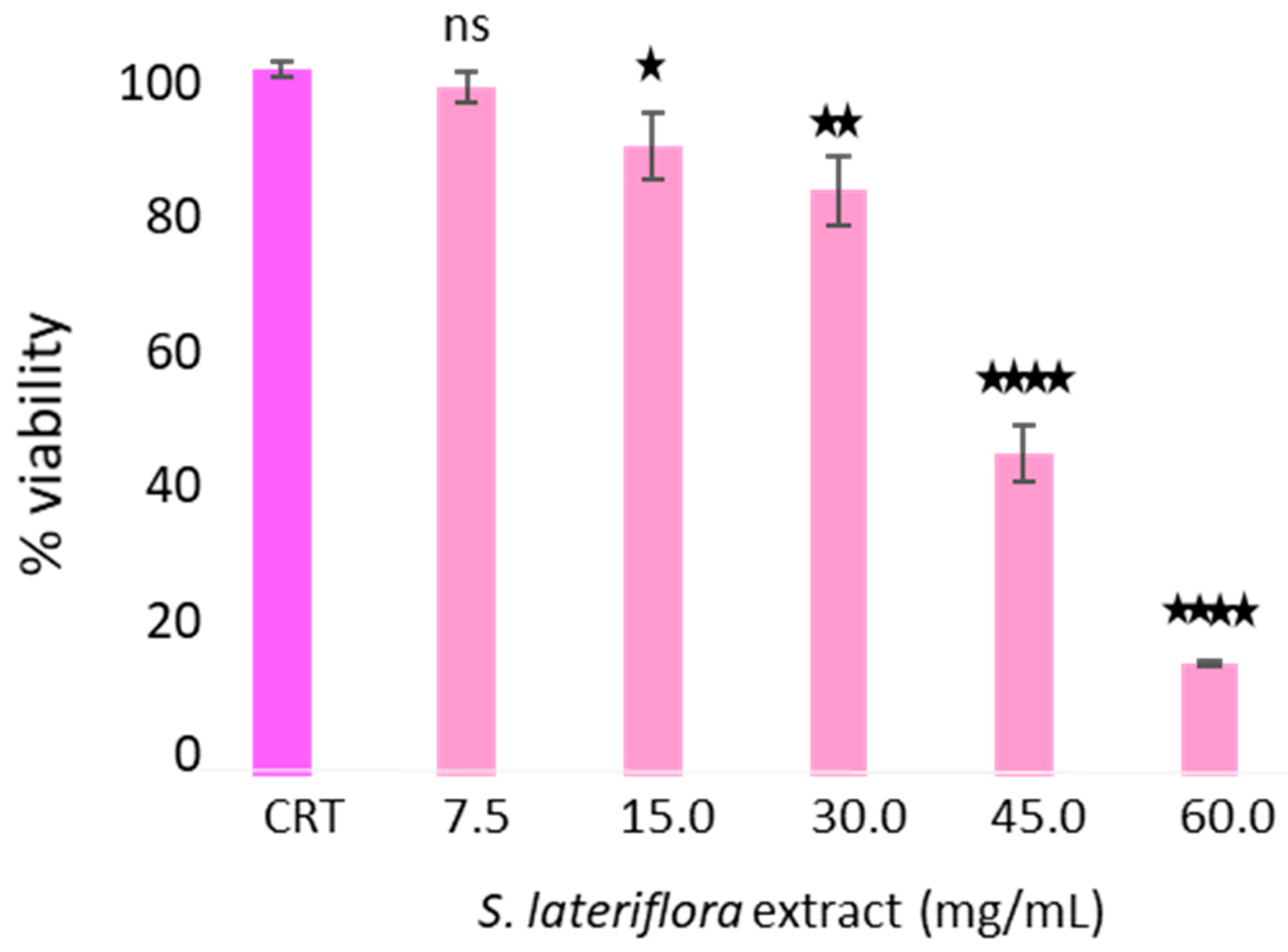
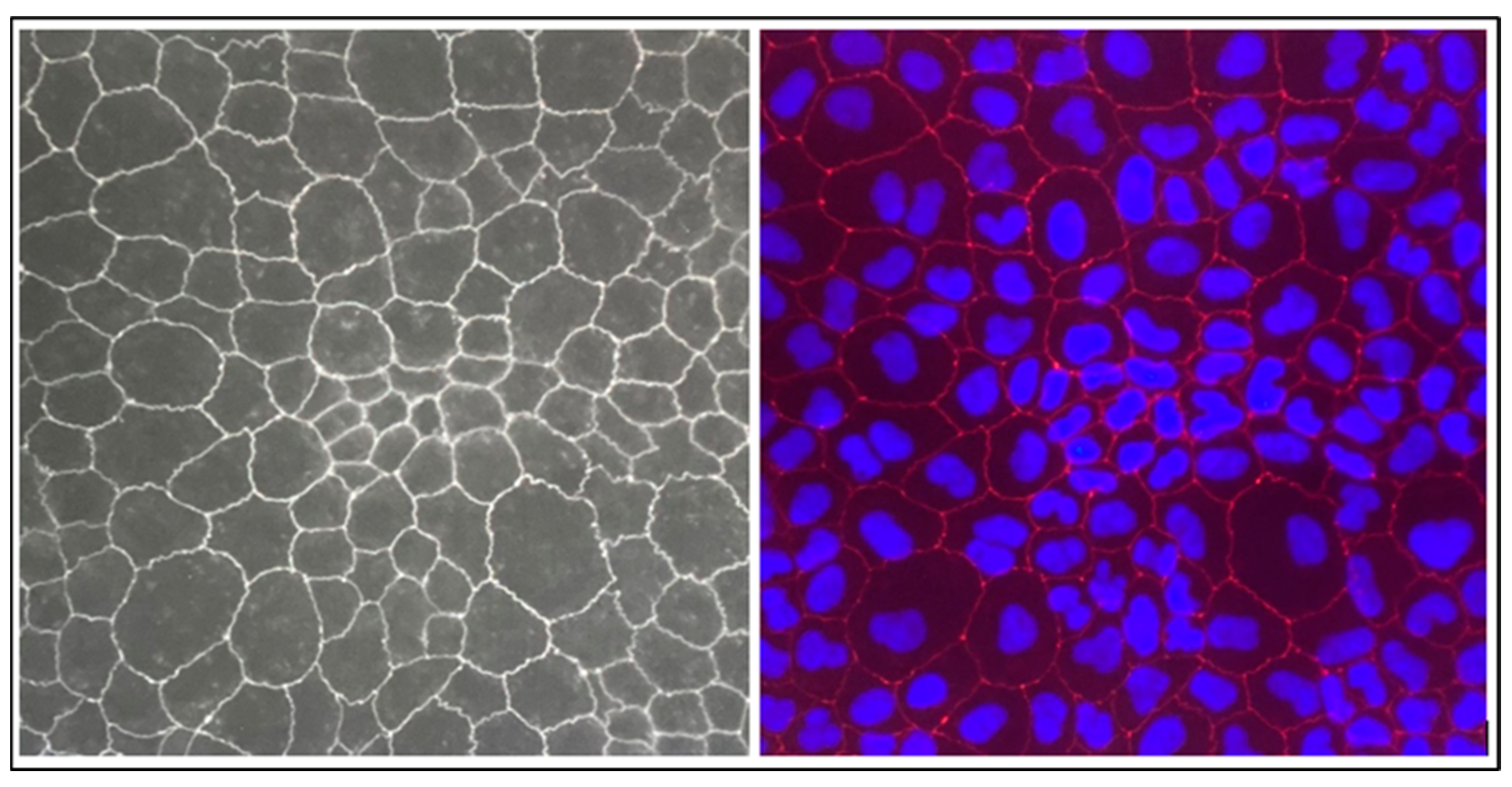
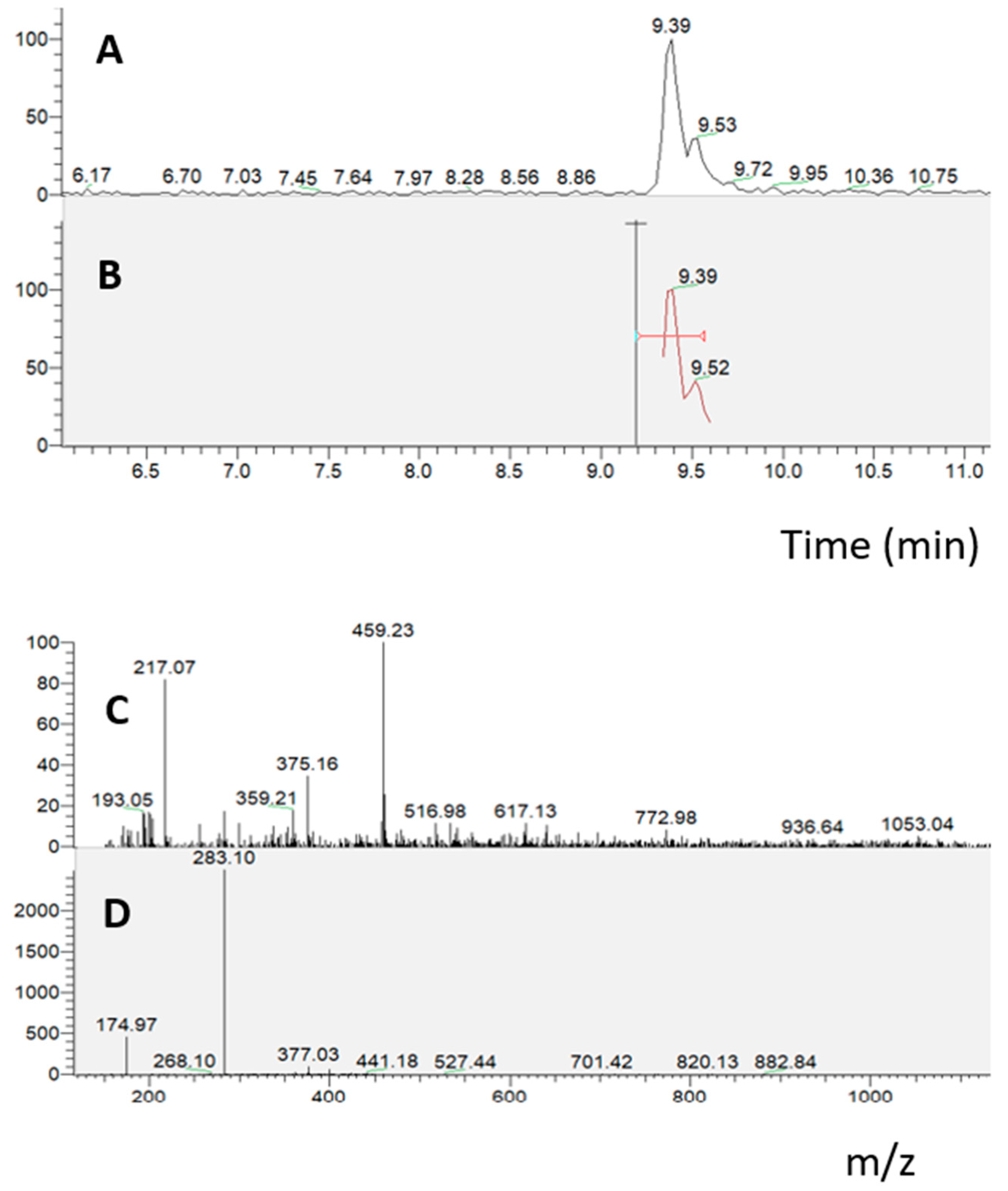


| Sample | RT [min] | Mean Area | Area Reduction Percentage (%) |
|---|---|---|---|
| UND | 8.08 | 5.62 × 104 | |
| 8.16 | 1.55 × 104 | ||
| 8.77 | 1.17 × 106 | ||
| 9.06 | 1.28 × 105 | ||
| 9.26 | 7.50 × 104 | ||
| 10.44 | 4.70 × 104 | ||
| GD | 8.08 | 3.64 × 104 | 35.3 |
| 8.17 | 9.70 × 103 | 37.2 | |
| 8.77 | 8.67 × 105 | 25.6 | |
| 9.06 | 8.74 × 104 | 31.6 | |
| 9.26 | 5.92 × 104 | 21.3 | |
| 10.44 | 2.09 × 104 | 55.7 | |
| DD | 8.08 | 3.63 × 104 | 35.6 |
| 8.17 | 8.58 × 103 | 44.5 | |
| 8.77 | 8.64 × 105 | 25.9 | |
| 9.05 | 8.92 × 104 | 30.2 | |
| 9.25 | 5.97 × 104 | 20.7 | |
| 10.44 | 1.59 × 104 | 66.3 | |
| GDD | 8.07 | 2.63 × 105 | 53.2 |
| 8.16 | 5.93 × 103 | 61.6 | |
| 8.76 | 7.63 × 105 | 34.7 | |
| 9.05 | 7.82 × 104 | 38.9 | |
| 9.25 | 4.93 × 104 | 34.3 | |
| 10.44 | 1.27 × 104 | 73.2 |
| Sample | TEER Ω (Sample Value) | TEER Ω Corrected |
|---|---|---|
| S1 | 1625 | 1510 |
| S2 | 1720 | 1605 |
| S3 | 1780 | 1665 |
| SD1 | 1785 | 1670 |
| SD2 | 1760 | 1645 |
| SD3 | 1768 | 1653 |
| BLANK | 115 | 0 |
| Concentration | OD 1 | OD 2 | OD 3 | Mean Abs |
|---|---|---|---|---|
| Caffein 500 μM | 0.839 | 0.938 | 0.961 | 0.913 |
| Caffein 250 μM | 0.528 | 0.504 | 0.526 | 0.519 |
| Caffein 125 μM | 0.277 | 0.264 | 0.291 | 0.277 |
| Caffein 62.5 μM | 0.152 | 0.145 | 0.153 | 0.150 |
| Caffein 31.25 μM | 0.081 | 0.088 | 0.105 | 0.091 |
| Caffein 15.62 μM | 0.059 | 0.054 | 0.055 | 0.056 |
| Caffein 7.81 μM | 0.035 | 0.040 | 0.045 | 0.040 |
| Furosemide 500 μM | 0.010 | 0.010 | 0.010 | 0.010 |
| Furosemide 250 μM | 0.024 | 0.030 | 0.022 | 0.025 |
| Furosemide 125 μM | 0.018 | 0.019 | 0.019 | 0.019 |
| Furosemide 125 μM | 0.031 | 0.021 | 0.029 | 0.027 |
| Furosemide 62.5 μM | 0.021 | 0.021 | 0.020 | 0.021 |
| Furosemide 31.25 μM | 0.033 | 0.018 | 0.020 | 0.024 |
| Blank | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buccato, D.G.; Ullah, H.; De Lellis, L.F.; Piccinocchi, R.; Baldi, A.; Xiao, X.; Arciola, C.R.; Di Minno, A.; Daglia, M. In Vitro Assessment of Cortisol Release Inhibition, Bioaccessibility and Bioavailability of a Chemically Characterized Scutellaria lateriflora L. Hydroethanolic Extract. Molecules 2024, 29, 586. https://doi.org/10.3390/molecules29030586
Buccato DG, Ullah H, De Lellis LF, Piccinocchi R, Baldi A, Xiao X, Arciola CR, Di Minno A, Daglia M. In Vitro Assessment of Cortisol Release Inhibition, Bioaccessibility and Bioavailability of a Chemically Characterized Scutellaria lateriflora L. Hydroethanolic Extract. Molecules. 2024; 29(3):586. https://doi.org/10.3390/molecules29030586
Chicago/Turabian StyleBuccato, Daniele Giuseppe, Hammad Ullah, Lorenza Francesca De Lellis, Roberto Piccinocchi, Alessandra Baldi, Xiang Xiao, Carla Renata Arciola, Alessandro Di Minno, and Maria Daglia. 2024. "In Vitro Assessment of Cortisol Release Inhibition, Bioaccessibility and Bioavailability of a Chemically Characterized Scutellaria lateriflora L. Hydroethanolic Extract" Molecules 29, no. 3: 586. https://doi.org/10.3390/molecules29030586
APA StyleBuccato, D. G., Ullah, H., De Lellis, L. F., Piccinocchi, R., Baldi, A., Xiao, X., Arciola, C. R., Di Minno, A., & Daglia, M. (2024). In Vitro Assessment of Cortisol Release Inhibition, Bioaccessibility and Bioavailability of a Chemically Characterized Scutellaria lateriflora L. Hydroethanolic Extract. Molecules, 29(3), 586. https://doi.org/10.3390/molecules29030586









