Abstract
This study examined the sensitivity of single-walled (5,5) aluminium nitride nanotubes ((5,5) AlNNTs) to carbon monoxide (CO) and carbon dioxide (CO2) gas molecules by performing DFT calculations using a hybrid functional, specifically, B3LYP (Becke’s three-parameter, Lee-Yang-Parr) exchange-correlation functional at a 6–31G* basis set. This research investigates the adsorption behavior of CO2 and CO molecules on pristine and silicon-doped aluminum nitride nanotubes (AlNNTs) and examines their implications for sensor applications. The study assesses each system’s adsorption energy, sensing potential, and recovery time to gain insights into their binding strength and practical viability. For CO2 adsorption on (5,5) AlNNT, significant adsorption energy of −24.36 kcal/mol was observed, indicating a strong binding to the nanotube surface, with a sensing potential of 8.95%. However, the slow recovery time of approximately 4.964 days may limit its real-time application. Si-(5,5) AlNNT exhibited a CO2 adsorption energy of −19.69 kcal/mol, a sensing potential of 5.40%, and a relatively short recovery time of approximately 2.978 min, making it a promising candidate for CO2 sensing. CO adsorption on (5,5) AlNNT showed an adsorption energy of −25.20 kcal/mol, a sensing potential of 9.095%, but a longer recovery time of approximately 20.130 days. Si-(5,5) AlNNT displayed a high CO adsorption energy of −20.78 kcal/mol, a sensing potential of 4.29%, and a recovery time of approximately 18.320 min. These findings provide insights into the adsorption characteristics of carbon molecules on AlNNTs, highlighting their potential for CO2 and CO sensing applications.
1. Introduction
Carbon monoxide (CO) and carbon dioxide (CO2) are two important gases that significantly impact human health and the environment. Carbon monoxide (CO) is a tasteless gas that has no smell [1,2]. It is produced from the incomplete combustion of carbon-containing materials such as fuel, combustible gas, and wood. Major sources include releases from automobile engines, power plants, wildfires, and incinerators [2]. Similarly, photochemical processes in the atmosphere can cause methane and nonmethane hydrocarbons, other unstable natural hydrocarbons in the air, natural atoms in surface waters, and soils to all interact and form CO. CO is a toxic gas that is produced by the burning of fossil fuels. It can cause headaches, nausea, and dizziness, and in high concentrations, it can be fatal [1,2,3]. CO2, on the other hand, is a greenhouse gas that is produced by human activities such as the burning of fossil fuels. It contributes to climate change by trapping heat in the atmosphere [1,2,3,4,5]. According to the World Health Organization (WHO), exposure to high levels of carbon monoxide can lead to unconsciousness and death. In the United States, the Environmental Protection Agency (EPA) estimates that more than 400 Americans die each year from accidental nonfire-related CO poisoning [6,7,8,9,10,11]. CO2 is also a major concern. According to the Intergovernmental Panel on Climate Change (IPCC), human activities are the main driver of climate change, and fossil fuel burning is the largest contributor to CO2 emissions [8,12,13,14,15,16,17,18,19]. The concentration of CO2 in the atmosphere has risen from around 280 parts per million (ppm) in the pre-industrial era to over 400 ppm in recent times [20,21,22,23,24]. This has led to an increase in global temperature and more extreme weather events such as heat waves, droughts, and storms [25,26,27,28,29,30,31,32,33].
In addition to the impact on human health and the environment, CO and CO2 can also have significant economic consequences. For example, the costs of treating illnesses related to air pollution and the costs of adapting to climate change are expected to increase [34,35,36,37,38,39].
AlNNTs have emerged as a unique and highly promising class of nanomaterials that have garnered significant attention in recent years due to their exceptional properties and wide range of potential applications. These nanotubes possess remarkable characteristics, including high thermal conductivity, excellent mechanical strength, and outstanding chemical stability [40]. The high thermal conductivity of AlNNTs makes them particularly suitable for thermal management applications. With their ability to efficiently conduct heat, AlNNTs can be utilized to design and develop thermal management systems. This is particularly important in electronic devices and integrated circuits, where effective heat dissipation is crucial for enhancing performance and reliability [41].
Furthermore, the excellent mechanical strength of AlNNTs opens up opportunities for their use in structural materials and composites. By incorporating AlNNTs into various matrices, such as polymers or metals, the resulting composites can exhibit improved mechanical properties, including enhanced strength and durability. This makes AlNNTs promising for aerospace, automotive, and construction applications, where lightweight yet strong materials are highly sought after [40].
The exceptional chemical stability of AlNNTs allows them to withstand harsh environments, making them suitable for applications in corrosive or chemically aggressive settings. AlNNTs can be employed in sensors for detecting gases, liquids, or even biological analytes. Leveraging their chemical stability, AlNNT-based sensors have the potential to enhance sensitivity, selectivity, and response time, leading to advancements in environmental monitoring, medical diagnostics, and industrial sensing [42,43,44,45]. Energy storage is another area where AlNNTs show great promise. Their unique properties can be leveraged to improve energy storage devices’ performance and efficiency, such as in batteries and supercapacitors. AlNNTs hold the potential to increase energy density, thus improving cycling stability and reducing charging time, which are crucial factors in advancing energy storage technologies [40,42,44].
To synthesize AlNNTs, various methods have been explored. Chemical vapor deposition, chemical etching, and electrochemical techniques are among the commonly employed approaches. The choice of synthesis method plays a critical role in determining the structure, morphology, and properties of the resulting nanotubes. Researchers are actively investigating and optimizing these synthesis techniques to achieve AlNNTs with the desired characteristics for specific applications [40,41,42,43,44,45].
The field of AlNNT research is rapidly expanding, with numerous studies focused on the synthesis, characterization, and potential applications of these materials. Scientists are striving to gain a deeper understanding of the fundamental properties and behaviors of AlNNTs and to explore novel applications across various industries. The continuous efforts in this field hold promise for further advancements and breakthroughs that can unlock the full potential of AlNNTs as a versatile and high-performance nanomaterial [44,45,46,47].
In recent studies, the adsorption behavior of O2 molecules on silicon-doped graphene (C53H18Si) has been explored. Kuzmin and Shainyan (2020) discovered that the adsorption of O2 on silicon proceeded without any barriers, resulting in the formation of exclusively atop intermediate. The adsorption energy for this process was found to be −2.40 eV. Interestingly, the barrier to dissociation of O2 adsorbed on Si-doped graphene was approximately 16 times lower compared to pristine graphene [48].
In a separate investigation, Zhao et al. (2012) focused on graphene doped with a single Si atom, achieved by replacing one carbon atom with a silicon atom. The adsorption process was studied specifically for the doped site. Notably, the only observed adsorption occurred between the oxygen atom of the H2O molecule and the silicon atom. It should be noted that the H2O molecule did not adsorb onto the silicon atom from its hydrogen atoms or other adjacent positions [49]. Milad et al. (2020) also reported an adsorption energy of −29.92 kcal/mol, which is approximately −1.297 eV, for the interaction between H2O molecules and Si-doped graphene. This finding aligns with the previous study conducted by Zhao et al. (2012) [50]. Overall, the studies on silicon-doped graphene demonstrate the influence of silicon doping on gas adsorption properties. This knowledge can be extended to other silicon-doped materials, including silicon-doped aluminum nitride nanotubes, to design and optimize gas sensors with improved performance for sensing CO and CO2 gases in various environments and applications. The ability to enhance gas sensing properties through silicon doping makes it a promising approach to developing advanced gas sensors with practical applications in environmental monitoring, industrial safety, and more.
Doped AlNNTs represent a novel and highly intriguing class of nanomaterials that have gained considerable attention in recent years. These doped nanotubes exhibit unique properties and offer promising potential applications, particularly in the field of sensing [50,51,52,53,54,55]. Impurity doping of AlNNTs, involving the introduction of elements such as copper, carbon, and titanium, has been found to significantly enhance their sensing capabilities towards specific chemicals, such as formaldehyde (HCOH) and ammonia (NH3) [53,55]. The addition of impurities brings about changes in the electrical conductivity and bandgap of AlNNTs, which can be exploited for sensing purposes. For instance, doping AlNNTs with copper can transform them into p-type semiconductors, thereby improving their sensing properties. This modification leads to increased sensitivity and selectivity, making them more suitable for detecting and sensing specific analytes [52,53,54,55,56].
The introduction of impurities in AlNNTs brings about several benefits for sensing applications. Firstly, it can alter the electronic structure of the nanotubes, influencing their conductivity and bandgap. This modification enables tailored responses to target analytes, enhancing the sensitivity and selectivity of the sensing material. By precisely controlling the type and concentration of the dopants, the sensing performance of AlNNTs can be optimized for specific chemical species [52,55]. Secondly, the introduction of impurities can induce localized defect states within the bandgap of AlNNTs. These localized states serve as active sites for chemical interactions, allowing for enhanced adsorption and reactivity toward target analytes. This feature contributes to the improved sensitivity and selectivity of doped AlNNTs in sensing applications [50,51,52,53,54,55]. The unique combination of impurity doping and the inherent properties of AlNNTs, such as high thermal conductivity and mechanical strength, further enhances their sensing capabilities. The resulting doped AlNNTs offer not only high-performance sensing platforms but also potential advancements in areas such as gas sensing, environmental monitoring, and biomedical applications. The exploration of doped AlNNTs as sensing materials represents a rapidly growing research field, with numerous studies focused on understanding the synthesis, characterization, and sensing performance of these materials. The ability to tailor the properties of AlNNTs through impurity doping opens up exciting possibilities for developing next-generation sensing devices with improved performance and expanded application domains.
This paper aimed to evaluate the performance of pristine AlNNT and Si-doped AlNNT as CO and CO2 gas sensors using DFT calculations. The electrical properties, as well as its interactions of CO and CO2 with armchair AlNNTs (5,5) and Si-doped AlNNTs (5,5), were thoroughly explored.
2. Results and Discussion
2.1. Pristine Aluminum Nitride Nanotube
Based on the results, it was observed that the bond length between Al and N in the (5,5) AlNNT was 1.829 Å, as depicted in Figure 1A, which matches a previous report by Mahdavifar et al. in 2013. Moreover, the band gap (Eg) was measured to be 4.090 eV, indicating that the material holds promise as a semiconductor. Furthermore, Figure 1B revealed that the fermi energy was −0.180 eV. In Table 1, the chemical potential (µ) was determined to be −4.265 eV, which implies that the material was stable. The global hardness (η) was calculated to be 2.045 eV, and the electrophilicity index (ω) was found to be 4.447 eV, which suggested that the material has a high electron affinity.
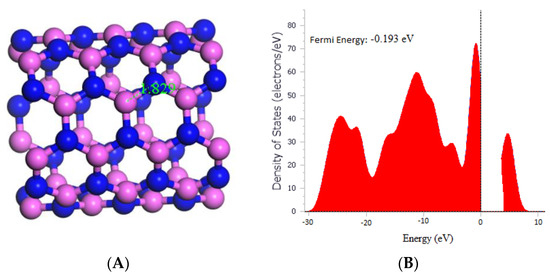
Figure 1.
(A) Geometry optimized structure of the (5,5) AlNNT with the bond length measured in Å, and (B) Density of states for pristine (5,5) AlNNT.

Table 1.
Computed adsorption energy (Eads) measured in kcal/mol, HOMO and LUMO energies in eV, band gap (Eg) in eV, chemical potential (µ) in eV, global hardness (η) in eV, electrophilicity index (ω) in eV, Recovery time (τ) in Seconds and sensing potential (%Eg) in % for the nanotubes and nanotube/gas complexes.
2.2. Silicon-Doped (5,5) Aluminum Nitride Nanotube [Si-(5,5) AlNNT]
The data presented in Figure 2A shows that the bond length between Aluminum and Nitrogen in the AlNNT decreased from 1.829 to 1.574 Å after doping with silicon, indicating a significant impact on the electronic structure of the material. The calculated band gap (Eg) obtained from Table 1 also decreased from 4.090 to 3.725 eV after silicon doping. This reduction in Eg suggests the creation of new electronic states in the AlNNT, which could alter its electrical conductivity and optical properties. Hence, the decrease in Eg indicates an increase in the conductivity of AlNNT after doping. The fermi energy of the AlNNT also changed from −0.193 to −0.189 eV after doping, as observed in Figure 2B. Furthermore, the silicon doping also affected the chemical potential (µ), global hardness (η), and electrophilicity index (ω) of the AlNNT, as shown in Table 1. The chemical potential decreased from −4.265 to −4.131 eV after doping, indicating the presence of new chemical states introduced in the AlNNT. The global hardness decreased from 2.045 to 1.863 eV, indicating that the AlNNT could have become less resistant to deformation after doping. In contrast, the electrophilicity index increased from 4.447 to 4.580 eV, suggesting that the AlNNT could have become more susceptible to chemical reactions after doping.
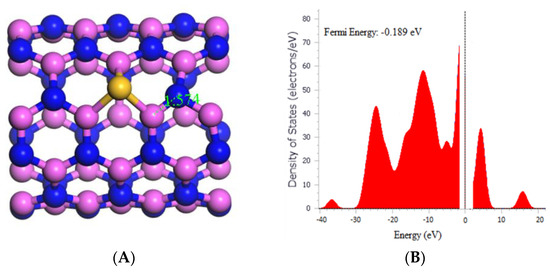
Figure 2.
(A) Geometry optimized structure of Si-(5,5) AlNNT with the bond length measured in Å, and (B) density of states for Si-(5,5) AlNNT.
2.3. CO2 Adsorbed on (5,5) AlNNT [(5,5) AlNNT/CO2]
This study investigated the interaction between CO2 and AlNNT (5,5) through adsorption, which resulted in the significant adsorption energy of −24.36 kcal/mol, indicating a strong binding between the CO2 molecule and (5,5) AlNNT surface, as shown in Table 1. The electronic structure of AlNNT (5,5) was modified by CO2 adsorption, as seen in the change of HOMO and LUMO values and a decrease in the band gap from 4.090 eV to 3.724 eV. The sensing potential of 8.95%, demonstrated in Table 1, suggests that (5,5) AlNNT has the potential to be used as a CO2 sensor; however, the slow recovery time of 428,899.920 s, which is approximately 4.964 days after CO2 adsorption, may limit its practical application. The chemical potential of −4.164 eV, global hardness of 1.862 eV, and electrophilicity index of 4.656 eV suggest that the material is less stable and softer than pristine AlNNT (5,5) due to CO2 adsorption. The molecular orbital analysis showed that the electronic interaction is primarily between the CO2 molecule and (5,5) AlNNT, as the HOMO was only on the (5,5) AlNNT, and the LUMO was on both the (5,5) AlNNT and CO2, as seen in Figure 3D. The charge density diagram in Figure 3C demonstrates that the CO2 molecule interacts with the (5,5) AlNNT through charge transfer, as the charge density is distributed over both the (5,5) AlNNT and CO2. Moreover, there was a decrease in the Fermi energy from −0.180 eV to −0.184 eV, as shown in Figure 3B.
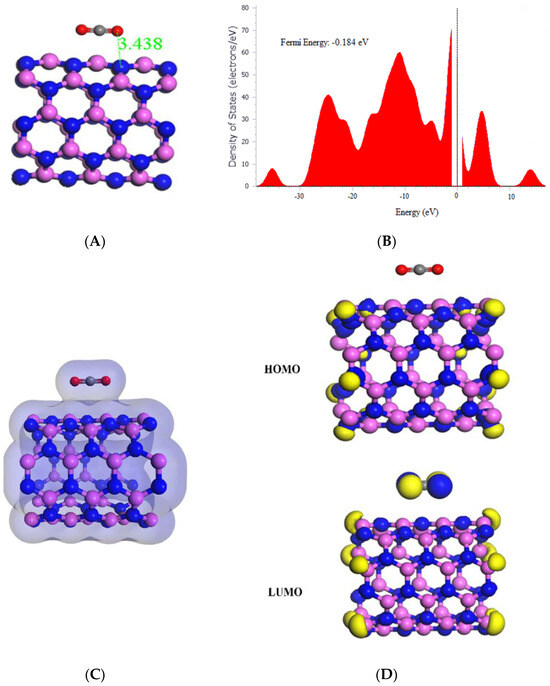
Figure 3.
(A) Geometry optimized structure with the adsorption distance measured in Å, (B) density of states, (C) charge density, and (D) frontier HOMO and LUMO orbitals for the (5,5) AlNNT and its interaction with CO2.
2.4. CO2 Adsorbed on Si-(5,5) AlNNT [Si-(5,5) AlNNT/CO2]
As seen in Table 1, the interaction between CO2 and Si-(5,5) AlNNT was identified as a strong adsorption process with an adsorption energy of −19.69 kcal/mol. The observed decrease in the band gap (Eg) from 3.725 eV to 3.926 eV following CO2 adsorption suggests that the doping process introduced new electronic states in Si-(5,5) AlNNT, as depicted in Figure 4B. This could potentially impact its electrical conductivity and optical properties. The decrease in Eg further implies that Si-(5,5) AlNNT might have higher conductivity after CO2 adsorption. The sensing potential of 5.40% reported in Table 1 suggests that Si-(5,5) AlNNT could be a promising candidate for CO2 sensing applications with a relatively short recovery time of 178.689 s, which is approximately 2.978 min. The results in Table 1 also further reveal that the chemical potential (µ) of −4.117 eV, global hardness (η) of 1.963 eV, and electrophilicity index (ω) of 4.317 eV indicate that Si-(5,5) AlNNT could have become more susceptible to chemical reactions after CO2 adsorption. Figure 4D shows that the HOMO was on both the Si-(5,5) AlNNT and CO2, while the LUMO was present solely on the AlNNT (5,5). Moreover, the charge density diagram in Figure 4C demonstrates that the charge density has covered both the Si-(5,5) AlNNT and CO2, suggesting a strong interaction between them.
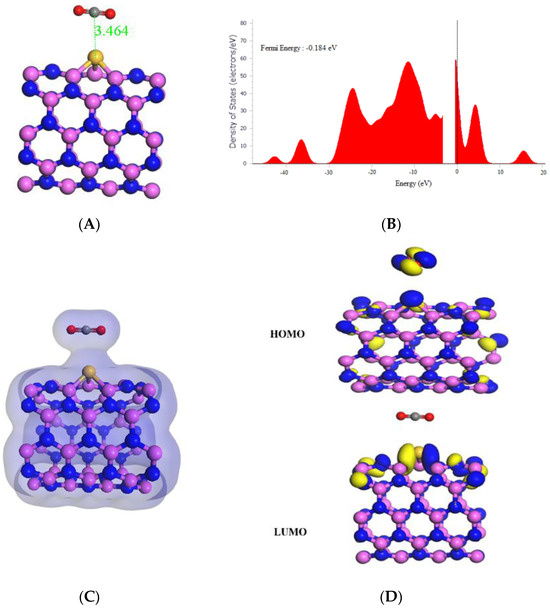
Figure 4.
(A) Geometry optimized structure with the adsorption distance measured in Å, (B) density of states, (C) charge density, and (D) frontier HOMO and LUMO orbitals for the Si-(5,5) AlNNT and its interaction with CO2.
2.5. CO Adsorbed on (5,5) AlNNT [(5,5) AlNNT/CO]
The findings on the interaction of carbon monoxide (CO) with (5,5) AlNNT through adsorption are presented in Table 1. This study revealed a strong binding between the CO molecule and the (5,5) AlNNT surface, with a significant adsorption energy of −25.20 kcal/mol. This interaction caused a significant change in the electronic properties of (5,5) AlNNT, resulting in a reduction in the band gap from 4.090 eV to 3.718 eV. The results indicate that (5,5) AlNNT has the potential to be used as a CO sensor, with a calculated sensing potential of 9.095%. However, the relatively long recovery time of 1,739,274.942 s, which is approximately 20.130 days, indicates that further improvements may be necessary to optimize sensor performance. The charge density diagram in Figure 5C suggests the formation of a hybrid state between the (5,5) AlNNT surface and the CO molecule. Besides, Table 1 shows changes in the chemical potential, global hardness, and electrophilicity index of (5,5) AlNNT after CO adsorption. The chemical potential slightly shifts from −4.265 eV to −4.410 eV, indicating a destabilization of the system upon adsorption. The global hardness decreases from 2.045 eV to 1.859 eV, indicating a decrease in resistance to deformation. Lastly, the electrophilicity index increases from 4.447 eV to 5.230 eV, indicating that the system becomes more electron-seeking. Additionally, the molecular orbital analysis in Figure 5D reveals that the LUMO was localized only on the (5,5) AlNNT, while the HOMO was present on both (5,5) AlNNT and CO.
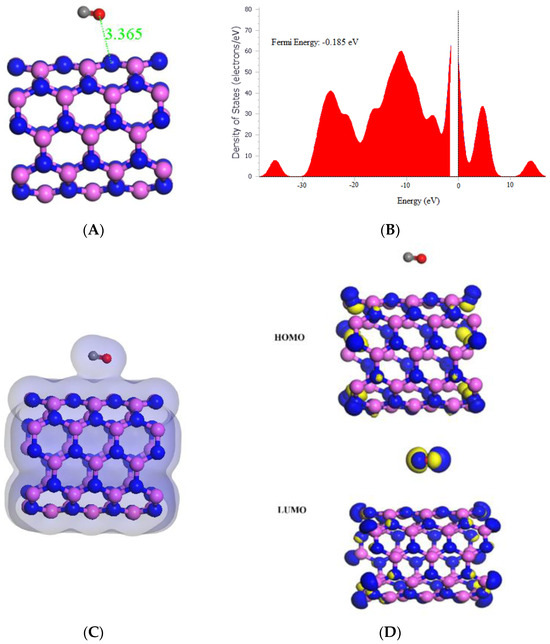
Figure 5.
(A) Geometry optimized structure with the adsorption distance measured in Å, (B) density of states, (C) charge density, and (D) frontier HOMO and LUMO orbitals for the (5,5) AlNNT and its interaction with CO.
2.6. CO Adsorbed on Si-(5,5) AlNNT [Si-(5,5) AlNNT/CO]
As shown in Table 1, the adsorption of CO onto Si-(5,5) AlNNT resulted in a strong and favorable interaction, with a high adsorption energy of −20.78 kcal/mol. The HOMO and LUMO values of the system were −6.033 eV and −2.202 eV, respectively, with a band gap of 3.831 eV. The slight change in Fermi energy from −0.193 eV to −0.183 eV after adsorption was observed in Figure 6B. The Si-(5,5) AlNNT showed a sensing potential of 4.29%, suggesting it could be a potential candidate for CO sensing applications, with a relatively short recovery time of 1099.171 s, which is approximately 18.320 min, which would support its real-time application. After adsorption, the chemical potential (µ) decreased from −4.131 eV to −4.118 eV, indicating new chemical states were introduced in the Si-(5,5) AlNNT. The global hardness (η) decreased from 1.863 eV to 1.916 eV, implying that the Si-AlNNT (5,5) became less resistant to deformation after adsorption. On the other hand, the electrophilicity index (ω) increased from 4.425 eV to 4.580 eV, suggesting that the Si-(5,5) AlNNT may have become more susceptible to chemical reactions after adsorption. The molecular orbital analysis in Figure 6D indicated that the LUMO was localized only on the Si-(5,5) AlNNT, while the HOMO was on both the Si-(5,5) AlNNT and CO. The charge density diagram in Figure 6C suggested that there was a charge transfer between the Si-(5,5) AlNNT and CO, with the charge density distributed on both the nanotube and CO. Thus, this charge transfer may be responsible for the strong adsorption energy observed in the system.
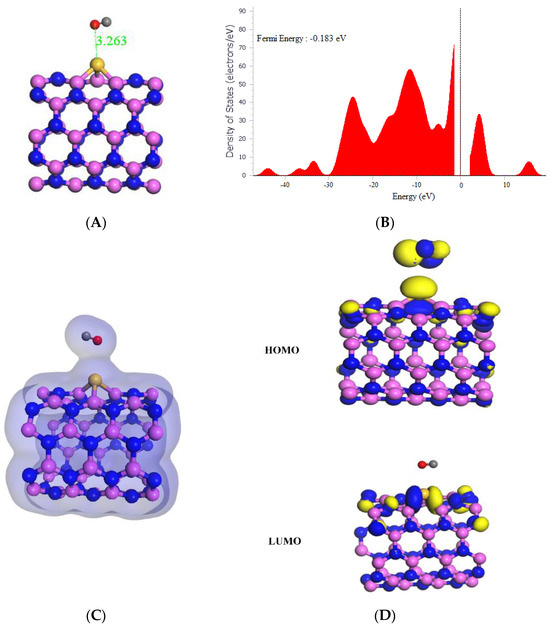
Figure 6.
(A) Geometry optimized structure with the adsorption distance measured in Å, (B) density of states, (C) charge density, and (D) frontier HOMO and LUMO orbitals for the Si-(5,5) AlNNT and its interaction with CO.
2.7. Potential Challenges of Implementing AlNNT-Based Sensors
AlNNT-based sensors hold significant promise for real-world applications due to their distinctive properties; however, their practical implementation presents notable challenges and constraints. To begin with, the synthesis and production of AlNNTs can be intricate and costly, raising concerns about purity and scalability [44,57]. Moreover, these nanotubes demonstrate sensitivity to environmental factors like temperature and humidity, potentially impacting their performance and long-term reliability [40]. Consequently, it is imperative to focus on optimizing production parameters and mastering control over nanotube growth.
Lastly, when comparing the (5,5) nanotube configuration to zig-zag and chiral configurations, the (5,5) configuration exhibited the greatest stability following molecular adsorption [58]. Given our study context, we may anticipate distinct behaviors for MWNTs, zig-zag, and chiral AlNNT configurations when exposed to CO and CO2 molecules. For the other configurations, we recommend both computational and experimental assessments in future research endeavors.
3. Materials and Methods
Computational Details
The Nanotube builder software [54] and Avogadro software [56,59,60] were utilized to create models of the armchair AlNNT (5,5), Si-doped AlNNT (5,5), Carbon monoxide (CO), and Carbon dioxide (CO2) gas molecules for the study, where AlNNT (5,5) and Si-doped AlNNT (5,5) are the pristine aluminum nitride nanotube and silicon replacing a nitrogen atom in the aluminum nitride nanotube, respectively. Subsequently, the molecular geometries were optimized using DFT and Quantum Espresso software [61]. The quantum chemical periodic approach was used throughout the calculations. We employed a single starting geometry per system for the adsorption studies. Equation (1) presents the adsorption model in the following form:
Eads = Eadsorbate_adsorbent − (Eadsorbate + Eadsorbent)
In this equation, Eads represents the adsorption energy, Eadsorbate_adsorbent denotes the cluster energy, Eadsorbate represents the ground state energy of the relaxed adsorbate molecule, and Eadsorbent refers to the energy of the relaxed adsorbent molecule being investigated.
The recovery time (RT) plays a crucial role in the development of sensors, as it is influenced by the interaction strength, which in turn determines the difficulty of the adsorption process. A more negative value of Eads results in a higher recovery time (RT).
where = recovery time; ν0 = attempt frequency; k = Boltzmann’s constant (∼2.0 × 10−3 kcal/mol. K); and T = temperature. According to experimental research studies, different photonic frequencies (υ0), thermal energy, and irradiation can be employed for the desorption of molecules [62,63]. In this study, υ0 = 1012 s−1 and T = 300 K will be the values for the attempt frequency and temperature, respectively, throughout the research. The choice of υ0 = 1012 s−1 and T = 300 K for attempt frequency and temperature in this study is justified by their consistent usage in the work of Wang et al. (2022) [61].
The calculation of the band gap (Eg) between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) will be performed using the following Equation (3):
where the energy of the LUMO and the HOMO are denoted by ELUMO and EHOMO, respectively.
To evaluate the sensitivity of the nanomaterials, the variation in the energy gap (Eg) will be determined using the following equation, where Eg1 represents the energy gap of the aluminum nitride nanotube, and Eg2 represents the energy gap of the nanotube-adsorbate complex:
The descriptors of chemical potential (µ), global hardness (η), and electrophilicity index (ω) offer valuable insights into the chemical reactivity and stability of interactions. They provide a means to understand the energy stabilization that occurs when a molecular system undergoes charge transfer from its surrounding environment. The electrophilicity index (ω) is closely related to both the chemical potential (µ) and global hardness (η), and their relationship can be expressed as follows:
ω = µ2/2η
The global hardness parameter provides an indication of the molecular system’s ability to resist changes in electron distribution. It is calculated by taking half of the difference between the energies of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). The chemical potential (µ) is determined by averaging the energies of the HOMO and LUMO. These calculations were performed using the computational resources available at the South African Centre of High-Performance Computing (CHPC) cluster.
4. Conclusions
This computational research focused on investigating the interactions between carbon dioxide (CO2) and carbon monoxide (CO) gas molecules with pristine (5,5) AlNNT and silicon-doped (5,5) AlNNT. The adsorption of CO2 on (5,5) AlNNT showed a strong binding with significant adsorption energy, indicating its potential as a CO2 sensor. However, the slow recovery time after CO2 adsorption may limit its practical application. The electronic structure of (5,5) AlNNT was modified by CO2 adsorption, leading to a decrease in the band gap and changes in the HOMO and LUMO values. Similarly, the adsorption of CO on (5,5) AlNNT exhibited a strong binding, suggesting its potential as a CO sensor. However, the relatively long recovery time indicates the need for further optimization. The interaction between CO2 and Si-(5,5) AlNNT demonstrated a strong adsorption process, with changes in the band gap and the introduction of new electronic states. Si-(5,5) AlNNT showed promising potential as a CO2 sensor with a relatively short recovery time. Additionally, the interactions between CO and Si-(5,5) AlNNT resulted in strong and favorable adsorption, with changes in the chemical potential, global hardness, and electrophilicity index. These findings suggest that Si-(5,5) AlNNT could be a potential candidate for CO sensing applications. Molecular orbital analysis and charge density diagrams provided insights into the electronic interaction and charge transfer between the nanotubes and the molecules. Overall, this research sheds light on the potential applications of pristine and silicon-doped aluminum nitride nanotubes in gas sensing and provides valuable insights into their electronic properties and interactions with CO2 and CO molecules.
Author Contributions
Conceptualization, A.Y.; methodology, N.S., K.K.-D. and V.A.A.; software, N.S. and A.Y.; validation, B.M., K.K.-D., V.A.A. and A.Y.; formal analysis, N.S. and A.Y.; investigation, N.S.; resources, A.Y. and V.A.A.; data curation, K.K.-D. and A.Y.; writing—original draft preparation, N.S.; writing—review and editing, A.Y., V.A.A., K.K.-D. and B.M.; visualization, N.S. and B.M.; supervision, A.Y.; project administration, A.Y.; funding acquisition, V.A.A. and A.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Building the next generation of African resesercher (BANGA-AFRICA project) University of Ghana, Legon with sponsorship from Carnegie Corporation of New York, USA. And The APC was funded by BANGA-AFRICA Project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data for this work in contained in the article.
Acknowledgments
A.Y., V.A.A. and K.D.-D. are grateful to the BANGA-AFRICA project with sponsorship from Carnegie Corporation of New York. The authors thank the CHPC, South Africa (Anton Lopis), for computational time on the Lengau cluster.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Green, E.; Short, S.; Shuker, L.; Harrison, P. Carbon Monoxide Exposure in the Home Environment and the Evaluation of Risks to Health—A UK Perspective. Indoor Built Environ. 1999, 8, 168–175. [Google Scholar] [CrossRef]
- Kinoshita, H.; Türkan, H.; Vucinic, S.; Naqvi, S.; Bedair, R.; Rezaee, R.; Tsatsakis, A. Carbon monoxide poisoning. Toxicol. Rep. 2020, 7, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Mangia, C.; D’andrea, A. Climate Change, Carbon Dioxide Emissions, and Medical Imaging Contribution. J. Clin. Med. 2023, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, R.; Mochizuki, H.; Moriguchi, Y.; Nakano, T.; Motohashi, M.; Sakai, Y.; Inaba, A. Estimation of CO2 Emissions of Internal Combustion Engine Vehicle and Battery Electric Vehicle Using LCA. Sustainability 2019, 11, 2690. [Google Scholar] [CrossRef]
- Bierwirth, P. Long-Term Carbon Dioxide Toxicity and Climate Change: A Major Unapprehended Risk for Human Health; Working Paper. 6 April 2023. [Google Scholar] [CrossRef]
- Johansen, N. Carbon Monoxide Detection and Alarm Requirements: Literature Review. 6 April 2023. [Google Scholar]
- Hnatov, M.V. Non-Fire Carbon Monoxide Deaths Associated with the Use of Consumer Products: 2007 Annual Estimates, January 2011. 6 April 2023. [Google Scholar]
- Francisco, P.W.; Pigg, S.; Cautley, D.; Rose, W.B.; Jacobs, D.E.; Cali, S. Carbon monoxide measurements in homes. Sci. Technol. Built Environ. 2018, 24, 118–123. [Google Scholar] [CrossRef]
- Fisher, D.S.; Bowskill, S.; Saliba, L.; Flanagan, R.J. Unintentional domestic non-fire related carbon monoxide poisoning: Data from media reports, UK/Republic of Ireland 1986–2011. Clin. Toxicol. 2013, 51, 409–416. [Google Scholar] [CrossRef]
- Long, J.; Sun, Y.; Zhao, J.; Liu, J.; Peng, X. Temporal trends of carbon monoxide poisoning mortality at the global, regional and national levels: A cross-sectional study from the Global Burden of Disease study, 1990 and 2017. BMJ Open 2021, 11, e053240. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.I.; Al-Zahrani, A.E.; Halwani, M.A. Carbon monoxide related deaths in Jeddah, Saudi Arabia: A forensic carboxyhemoglobin autopsy-based study. Forensic Sci. Int. Rep. 2021, 4, 100232. [Google Scholar] [CrossRef]
- Saleem, M.W.; Tahir, M.H.; Ashfaq, M.W. Fossil Fuel Based Carbon Footprint of Pakistan and Its Role towards Fossil Fuel Based Carbon Footprint of Pakistan and Its Role towards Sustainable Development. 6 April 2023. [Google Scholar]
- Zimakowska-Laskowska, M.; Laskowski, P. Emission from Internal Combustion Engines and Battery Electric Vehicles: Case Study for Poland. Atmosphere 2022, 13, 401. [Google Scholar] [CrossRef]
- Lamb, W.F.; Wiedmann, T.; Pongratz, J.; Andrew, R.; Crippa, M.; Olivier, J.G.J.; Wiedenhofer, D.; Mattioli, G.; Al Khourdajie, A.; House, J.; et al. A review of trends and drivers of greenhouse gas emissions by sector from 1990 to 2018. Environ. Res. Lett. 2021, 16, 073005. [Google Scholar] [CrossRef]
- Yue, X.-L.; Gao, Q.-X. Contributions of natural systems and human activity to greenhouse gas emissions. Adv. Clim. Chang. Res. 2018, 9, 243–252. [Google Scholar] [CrossRef]
- Marland, G.; Rotty, R.M. Carbon dioxide emissions from fossil fuels: A procedure for estimation and results for 1950-1982. Tellus B Chem. Phys. Meteorol. 1984, 36, 232. [Google Scholar] [CrossRef]
- Kweku, D.W.; Bismark, O.; Maxwell, A.; Desmond, K.A.; Danso, K.B.; Oti-Mensah, E.A.; Quachie, A.T.; Adormaa, B.B. Greenhouse Effect: Greenhouse Gases and Their Impact on Global Warming. J. Sci. Res. Rep. 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Venterea, R.T. Climate Change 2007: Mitigation of Climate Change. J. Environ. Qual. 2009, 38, 837. [Google Scholar] [CrossRef]
- Gaffney, J.S.; Marley, N.A. The impacts of combustion emissions on air quality and climate—From coal to biofuels and beyond. Atmos. Environ. 2009, 43, 23–36. [Google Scholar] [CrossRef]
- Florides, G.A.; Christodoulides, P. Global warming and carbon dioxide through sciences. Environ. Int. 2009, 35, 390–401. [Google Scholar] [CrossRef]
- Ahn, J.; Brook, E.J.; Mitchell, L.; Rosen, J.; McConnell, J.R.; Taylor, K.; Etheridge, D.; Rubino, M. Atmospheric CO2over the last 1000 years: A high-resolution record from the West Antarctic Ice Sheet (WAIS) Divide ice core. Glob. Biogeochem. Cycles 2012, 26. [Google Scholar] [CrossRef]
- Le Quéré, C.; Raupach, M.R.; Canadell, J.G.; Marland, G.; Bopp, L.; Ciais, P.; Conway, T.J.; Doney, S.C.; Feely, R.A.; Foster, P.; et al. Trends in the sources and sinks of carbon dioxide. Nat. Geosci. 2009, 2, 831–836. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. Global warming: Causes and impacts on agroecosystems productivity and food security with emphasis on cassava comparative advantage in the tropics/subtropics. Photosynthetica 2014, 52, 161–178. [Google Scholar] [CrossRef]
- Sparavigna, A.C. Carbon Dioxide Concentration and Emissions in Atmosphere: Trends and Recurrence Plots. Int. J. Sci. 2014, 3, 8–15. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.; Van Der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Vidal, J.-P.; Soubeyroux, J.-M. Impact of climate change on drought and soil moisture in France. In Proceedings of the SEC2008—International Symposium—Drought and Constructions, Paris, France, 1–3 September 2008; pp. 25–31. [Google Scholar]
- North, G.R. Global Climate Change. In A World after Climate Change and Culture-Shift; Springer: Dordrecht, The Netherlands, 2014; pp. 25–42. [Google Scholar] [CrossRef]
- Marx, W.; Haunschild, R.; Bornmann, L. Heat waves: A hot topic in climate change research. Theor. Appl. Clim. 2021, 146, 781–800. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W. Extreme Weather Events and their Consequences. Pap. Glob. Chang. IGBP 2016, 23, 59–69. [Google Scholar] [CrossRef]
- Ebi, K.L.; Vanos, J.; Baldwin, J.W.; Bell, J.E.; Hondula, D.M.; Errett, N.A.; Hayes, K.; Reid, C.E.; Saha, S.; Spector, J.; et al. Extreme Weather and Climate Change: Population Health and Health System Implications. Annu. Rev. Public Health 2020, 42, 293–315. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, A.; Trenberth, K.E. Climate Change and Drought: A Perspective on Drought Indices. Curr. Clim. Chang. Rep. 2018, 4, 145–163. [Google Scholar] [CrossRef]
- Matawal, D.S.; Maton, D.J. Climate Change and Global Warming: Signs, Impact and Solutions. Int. J. Environ. Sci. Dev. 2013, 4, 62–66. [Google Scholar] [CrossRef]
- Martinez, P.; Bandala, E.R. Heat Waves: A Growing Climate Change-Related Risk. 6 April 2023; pp. 1–3. [Google Scholar]
- Xia, Y.; Guan, D.; Jiang, X.; Peng, L.; Schroeder, H.; Zhang, Q. Assessment of socioeconomic costs to China’s air pollution. Atmos. Environ. 2016, 139, 147–156. [Google Scholar] [CrossRef]
- Ciscar, J.-C.; Iglesias, A.; Feyen, L.; Szabó, L.; Van Regemorter, D.; Amelung, B.; Nicholls, R.; Watkiss, P.; Christensen, O.B.; Dankers, R.; et al. Physical and economic consequences of climate change in Europe. Proc. Natl. Acad. Sci. USA 2011, 108, 2678–2683. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Li, C. Theoretical Model and Actual Characteristics of Air Pollution Affecting Health Cost: A Review. Int. J. Environ. Res. Public Health 2022, 19, 3532. [Google Scholar] [CrossRef]
- Sonwani, S.S.S.; Maurya, V.M.V. Impact of air pollution on the environment and economy. In Air Pollution: Sources, Impacts and Controls; CAB International: Wallingford, UK, 2019; pp. 113–134. [Google Scholar] [CrossRef]
- Meisner, C.; Gjorgjev, D.; Tozija, F. Estimating health impacts and economic costs of air pollution in the Republic of Macedonia. South East Eur. J. Public Health 2015, 10, 1–8. [Google Scholar] [CrossRef]
- Szlávik, J.; Füle, M. Economic consequences of climate change. AIP Conf. Proc. 2009, 1157, 73–82. [Google Scholar] [CrossRef]
- Noei, M.; Soleymanabadi, H.; Peyghan, A.A. Aluminum nitride nanotubes. Chem. Pap. 2017, 71, 881–893. [Google Scholar] [CrossRef]
- Hao, S.; Zhang, L.; Wang, X.; Zhao, G.; Hou, P.; Xu, X. Design of Multilayered Porous Aluminum Nitride for Supercapacitor Applications. Energy Fuels 2021, 35, 12628–12636. [Google Scholar] [CrossRef]
- Beheshtian, J.; Baei, M.T.; Bagheri, Z.; Peyghan, A.A. AlN nanotube as a potential electronic sensor for nitrogen dioxide. Microelectron. J. 2012, 43, 452–455. [Google Scholar] [CrossRef]
- Zheng, N.; Zhang, C.; Fan, R.; Sun, Z. Melamine foam-based shape-stable phase change composites enhanced by aluminum nitride for thermal management of lithium-ion batteries. J. Energy Storage 2022, 52, 105052. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Z.; Wang, X.; Lu, Y.; Chen, X.; Xu, H.; Chen, Y. Synthesis and Characterization of Faceted Hexagonal Aluminum Nitride Nanotubes. J. Am. Chem. Soc. 2003, 125, 10176–10177. [Google Scholar] [CrossRef]
- Yin, L.-W.; Bando, Y.; Zhu, Y.-C.; Li, M.-S.; Tang, C.C.; Golberg, D. Single-Crystalline AlN Nanotubes with Carbon-Layer Coatings on the Outer and Inner Surfaces via a Multiwalled-Carbon-Nanotube-Template-Induced Route. Adv. Mater. 2005, 17, 213–217. [Google Scholar] [CrossRef]
- Hesabi, M.; Hesabi, M. The interaction between carbon nanotube and skin anti-cancer drugs: A DFT and NBO approach. J. Nanostructure Chem. 2013, 3, 22. [Google Scholar] [CrossRef]
- Peyghan, A.A.; Omidvar, A.; Hadipour, N.L.; Bagheri, Z.; Kamfiroozi, M. Can aluminum nitride nanotubes detect the toxic NH3 molecules? Phys. E Low-Dimens. Syst. Nanostruct. 2012, 44, 1357–1360. [Google Scholar] [CrossRef]
- Kuzmin, A.V.; Shainyan, B.A. Single Si-Doped Graphene as a Catalyst in Oxygen Reduction Reactions: An In Silico Study. ACS Omega 2020, 5, 15268–15279. [Google Scholar] [CrossRef]
- Zhao, J.-X.; Chen, Y.; Fu, H.-G. Si-embedded graphene: An efficient and metal-free catalyst for CO oxidation by N2O or O2. Theor. Chem. Accounts 2012, 131, 1–11. [Google Scholar] [CrossRef]
- Ghanbari, M.; Afshari, S.; Amri, S.A.N. New capability of graphene as hydrogen storage by Si and/or Ge doping: Density functional theory. Int. J. Hydrogen Energy 2020, 45, 23048–23055. [Google Scholar] [CrossRef]
- Shahabi, M.; Raissi, H. Investigation of the molecular structure, electronic properties, AIM, NBO, NMR and NQR parameters for the interaction of Sc, Ga and Mg-doped (6,0) aluminum nitride nanotubes with COCl2 gas by DFT study. J. Incl. Phenom. Macrocycl. Chem. 2015, 84, 99–114. [Google Scholar] [CrossRef]
- Ahmadi, A.; Hadipour, N.L.; Kamfiroozi, M.; Bagheri, Z. Theoretical study of aluminum nitride nanotubes for chemical sensing of formaldehyde. Sens. Actuators B Chem. 2012, 161, 1025–1029. [Google Scholar] [CrossRef]
- Mahdavifar, Z.; Abbasi, N. The influence of Cu-doping on aluminum nitride, silicon carbide and boron nitride nanotubes’ ability to detect carbon dioxide; DFT study. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 56, 268–276. [Google Scholar] [CrossRef]
- Mirzaei, M.; Seif, A.; Hadipour, N.L. The C-doped zigzag AlN nanotube: A computational NMR study. Chem. Phys. Lett. 2008, 461, 246–248. [Google Scholar] [CrossRef]
- Soltani, A.; Raz, S.G.; Rezaei, V.J.; Khalaji, A.D.; Savar, M. Ab initio investigation of Al- and Ga-doped single-walled boron nitride nanotubes as ammonia sensor. Appl. Surf. Sci. 2012, 263, 619–625. [Google Scholar] [CrossRef]
- Cao, Y.; Farahmand, M.; Ahmadi, R.; Heravi, M.R.P.; Ahmadi, S.; Mahmoud, M.Z. Unraveling the effect of Ti doping on the sensing properties of AlN nanotubes toward acrylonitrile gas. Inorg. Chem. Commun. 2022, 137, 109161. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Menazea, A.A.; Awwad, N.S.; Ibrahium, H.A.; Ebaid, G.; Ali, H.E. Selective detection of sulfur trioxide in the presence of environmental gases by AlN nanotube. J. Sulfur Chem. 2022, 43, 290–303. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Fang, R.; Jing, L.; Wu, P.; Tian, S. Adsorption and Sensing of CO2, CH4 and N2O Molecules by Ti-Doped HfSe2 Monolayer Based on the First-Principle. Chemosensors 2022, 10, 414. [Google Scholar] [CrossRef]
- Balasubramanian, C.; Bellucci, S.; Castrucci, P.; De Crescenzi, M.; Bhoraskar, S. Scanning tunneling microscopy observation of coiled aluminum nitride nanotubes. Chem. Phys. Lett. 2004, 383, 188–191. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Beni, A.S.; Eskandari, R.; Karami, M.; Khorram, M. Interaction of propylthiouracil, an anti-thyroid drug with boron nitride nanotube: A DFT study. Adsorption 2020, 26, 1385–1396. [Google Scholar] [CrossRef]
- Sakharova, N.A.; Antunes, J.M.; Pereira, A.F.G.; Fernandes, J.V. Developments in the evaluation of elastic properties of carbon nanotubes and their heterojunctions by numerical simulation. AIMS Mater. Sci. 2017, 4, 706–737. [Google Scholar] [CrossRef]
- Rayan, B.; Rayan, A. Avogadro Program for Chemistry Education: To What Extent can Molecular Visualization and Three-dimensional Simulations Enhance Meaningful Chemistry Learning? World J. Chem. Educ. 2017, 5, 136–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).