Antioxidant and Antibacterial Activities of Chinese Native Thyme Essential Oils with Different Chemotypes
Abstract
1. Introduction
2. Results
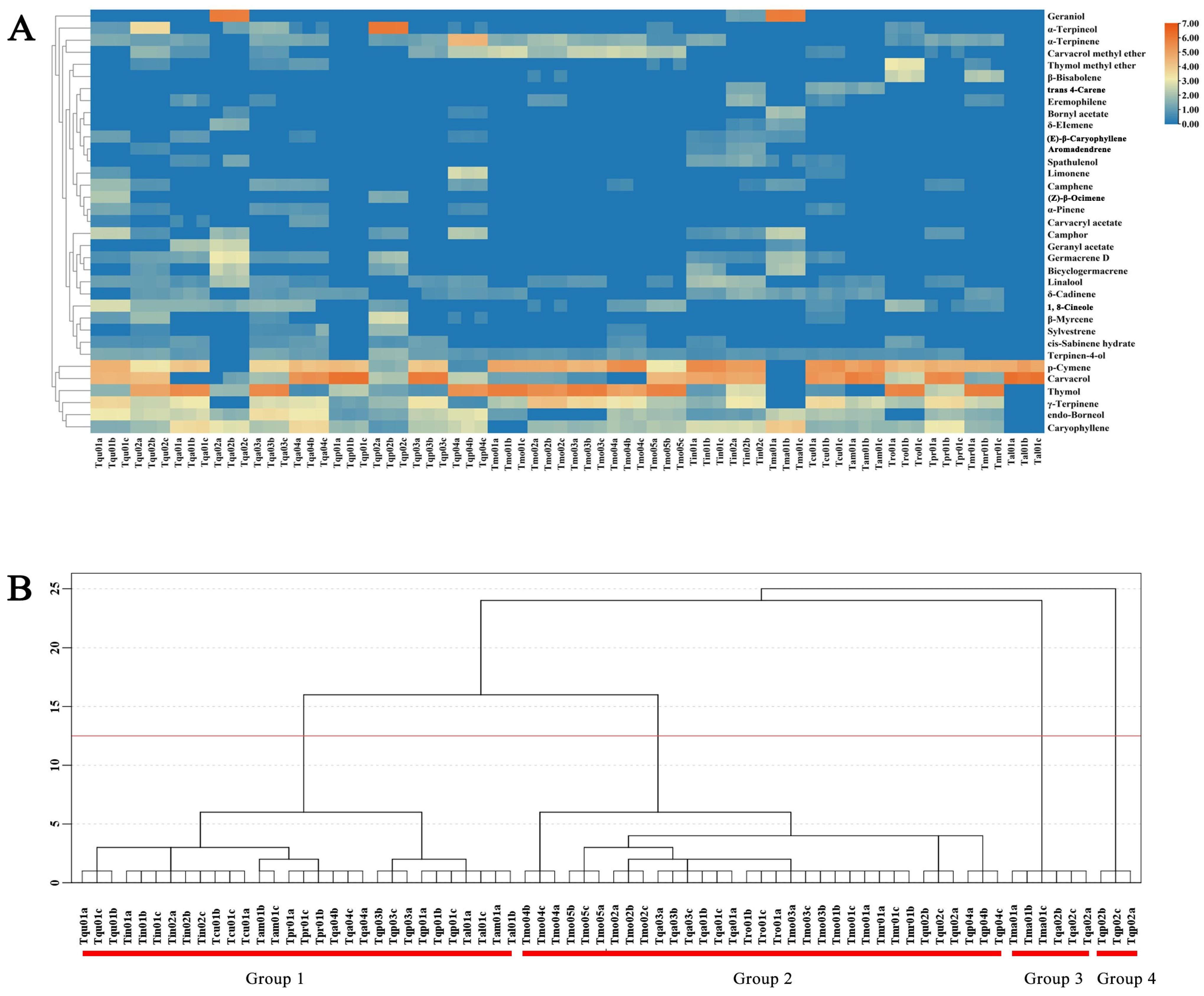
2.1. The Determination of the EO Compositions in Chinese Native Thymes
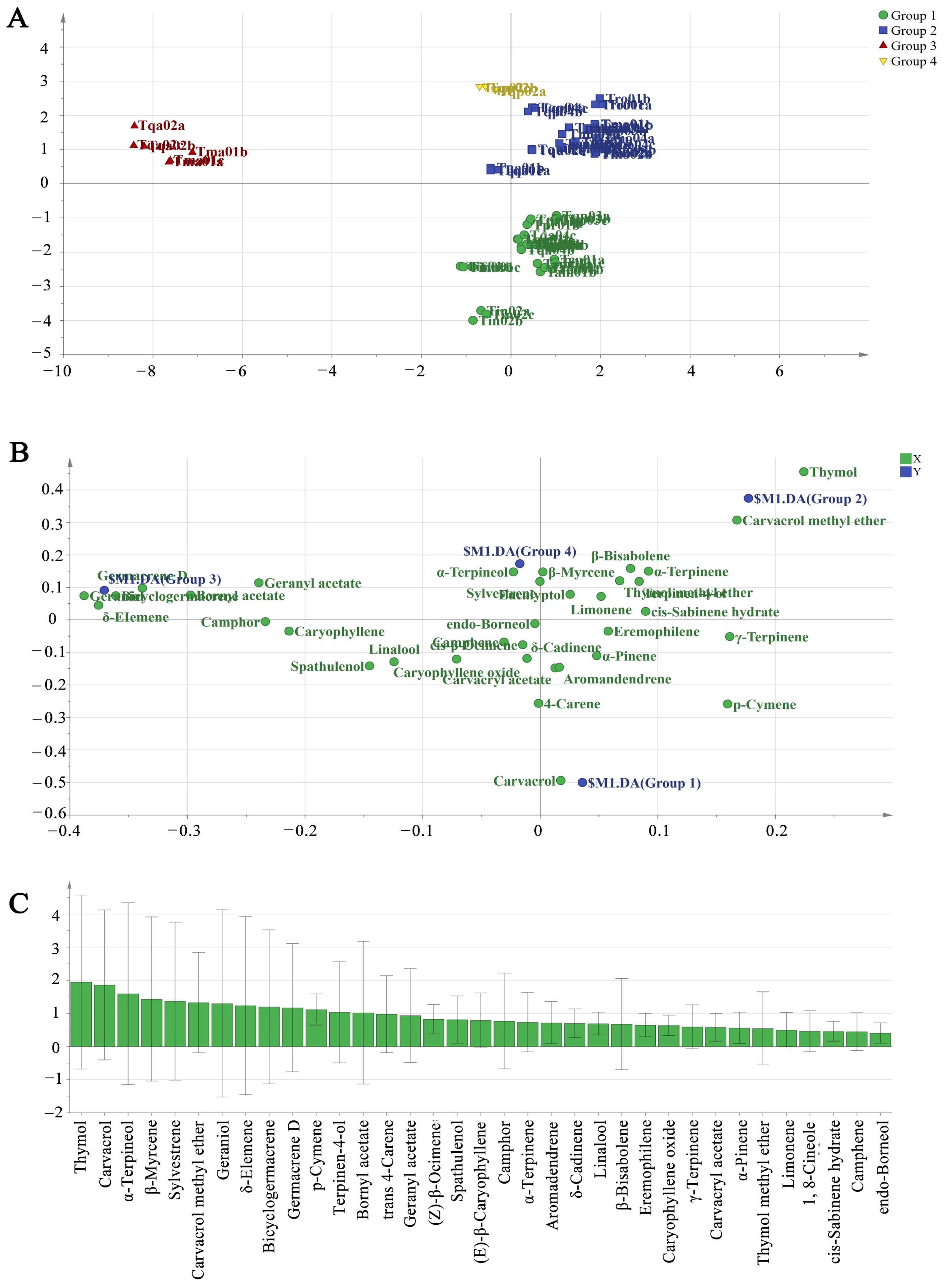
2.2. Chemodiversity Classification of Chinese Native Thyme EO Chemotypes
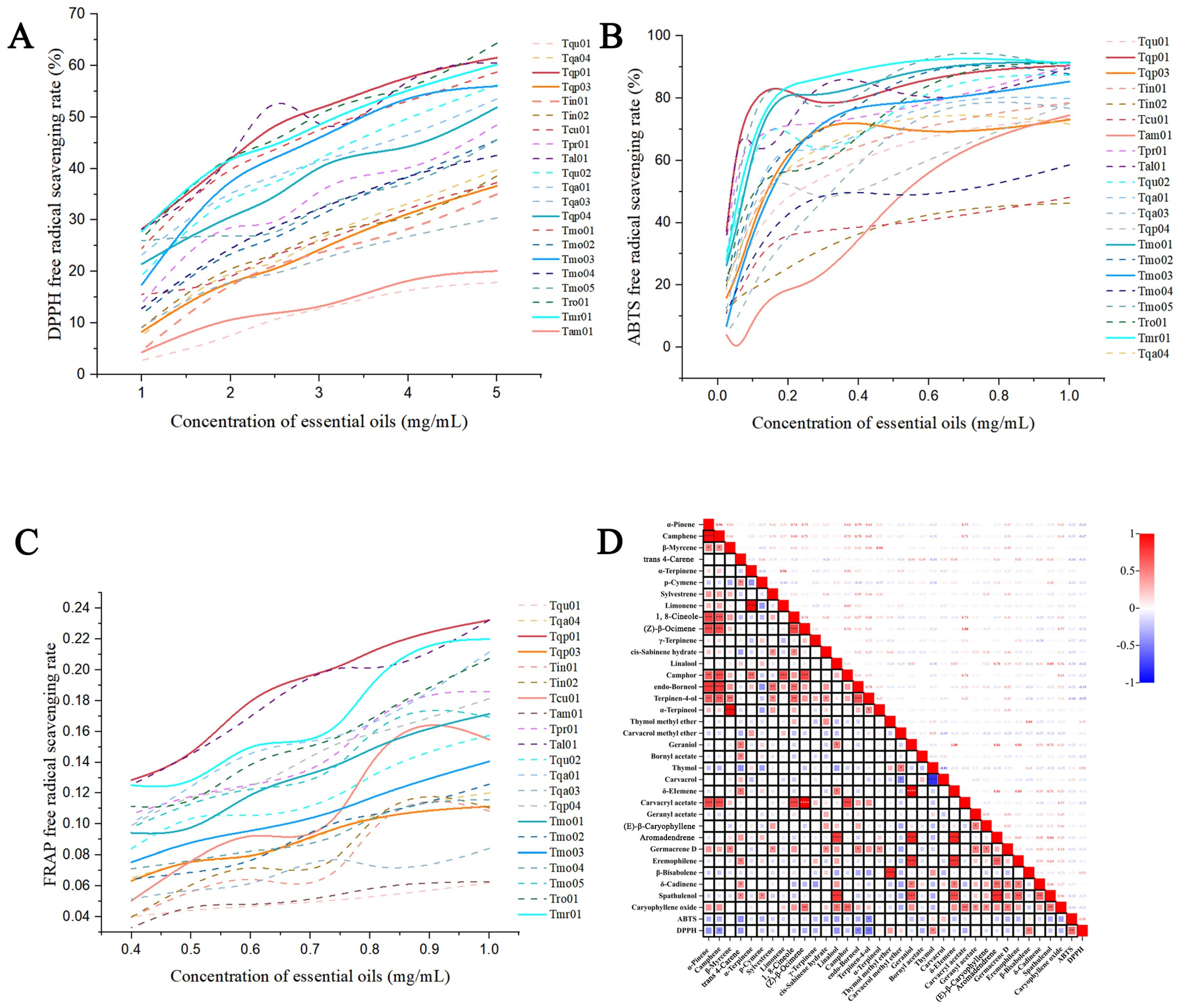
2.3. Antioxidant Activity Analysis of Chinese Native Thyme EOs
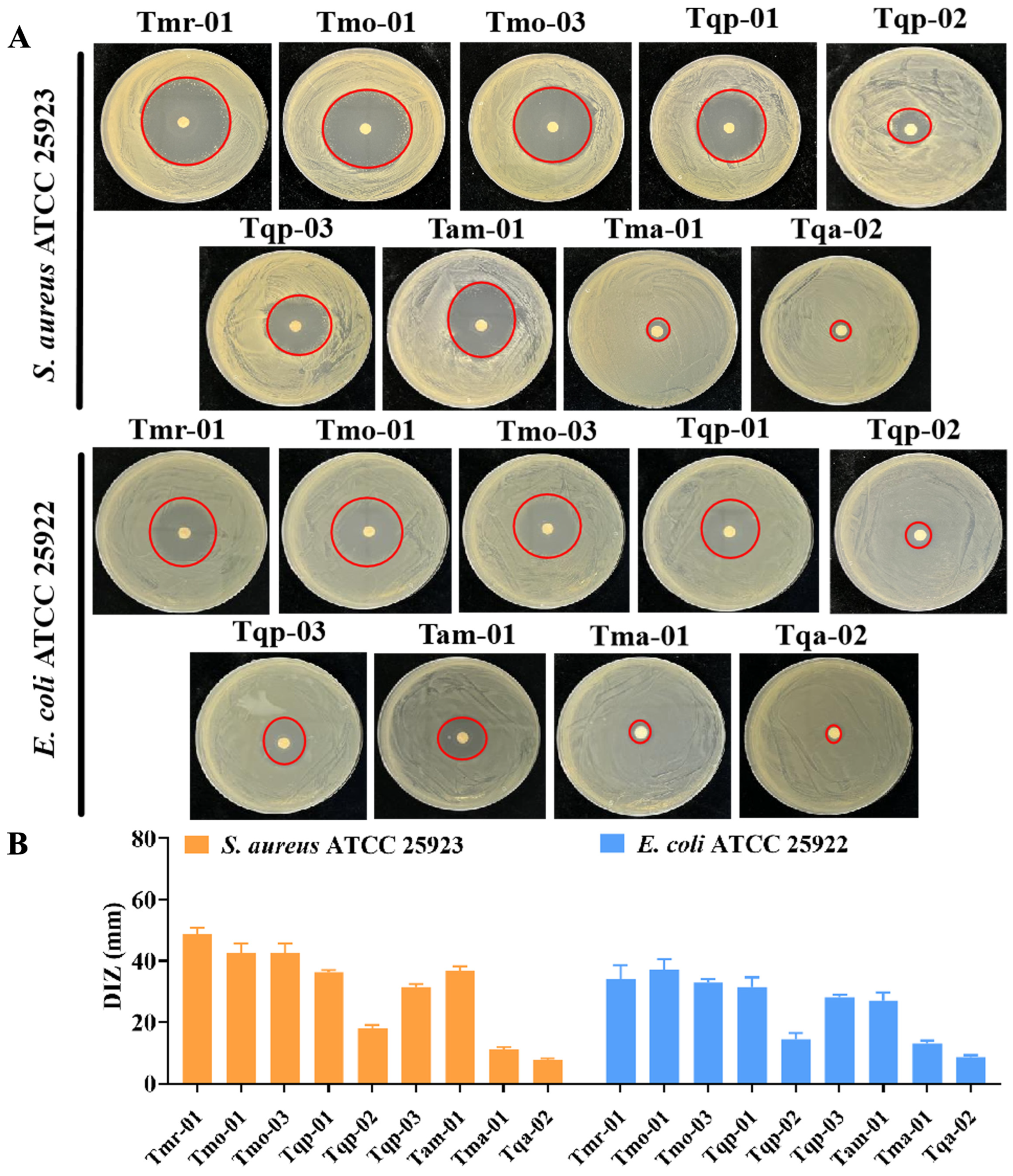
2.4. Antibacterial Activity Analysis of the Chinese Native Thyme EOs
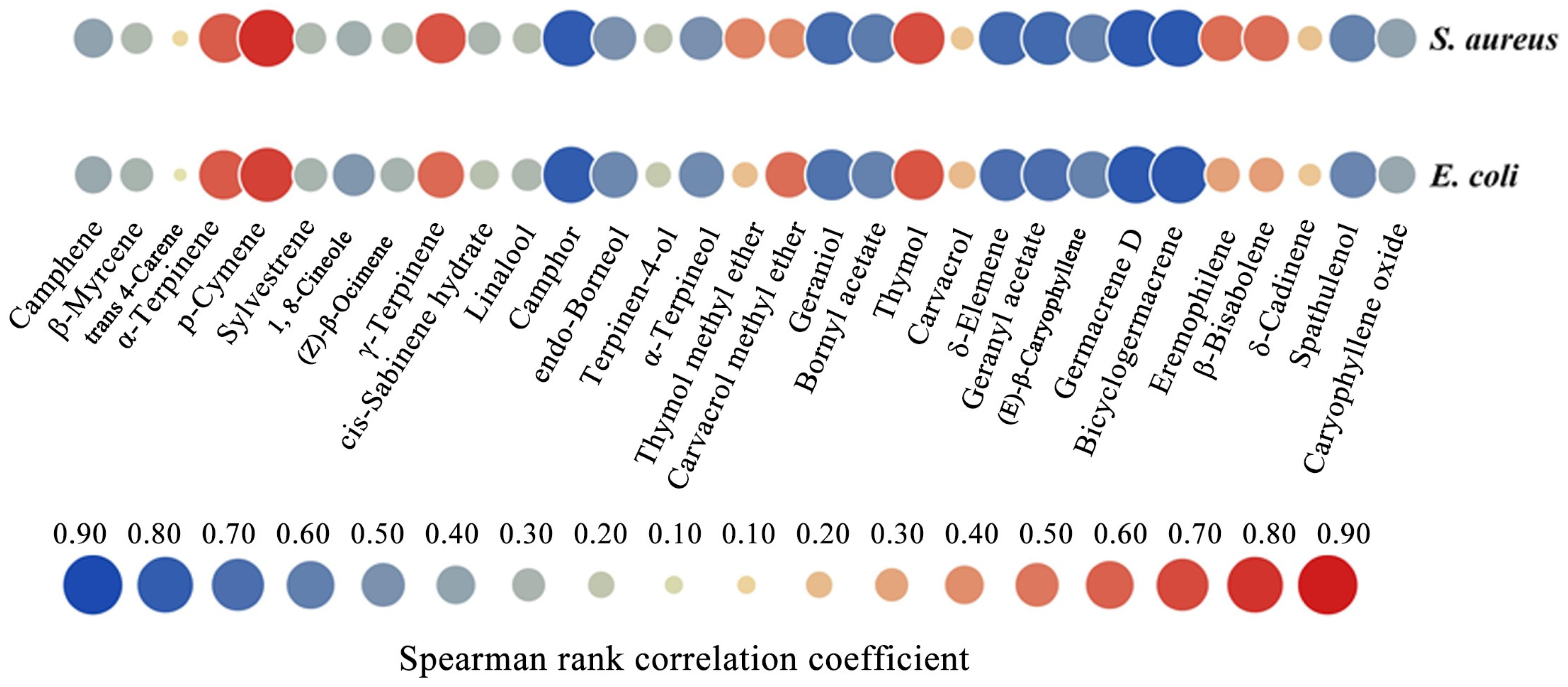
2.5. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Essential Oil (EO) Isolation Using Steam Distillation
4.3. EO Composition Analysis
4.4. Chinese Native Thyme EOs’ Antioxidant Activities
4.4.1. DPPH Free-Radical-Scavenging Activity Assay
4.4.2. ABTS Free-Radical-Scavenging Assay
4.4.3. FRAP Reducibility Determination
4.5. Chinese Native Thyme EOs’ Antibacterial Activities Against S. aureus and E. coli
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehi, B.; Abu-Darwish, M.S.; Tarawneh, A.H.; Cabral, C.; Gadetskaya, A.V.; Salgueiro, L.; Sharifi-Rad, M. Thymus spp. plants-Food applications and phytopharmacy properties. Trends Food Sci. Technol. 2019, 85, 287–306. [Google Scholar] [CrossRef]
- Zaazaa, A.; Mudalal, S.; Alzuheir, I.; Samara, M.; Jalboush, N.; Fayyad, A.; Petracci, M. The impact of thyme and oregano essential oils dietary supplementation on broiler health, growth performance, and prevalence of growth-related breast muscle abnormalities. Animals 2022, 12, 3065. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.M.; Zanuncio, J.C.; Brugger, B.P.; Soares, M.A.; Zanuncio, A.J.V.; Wilcken, C.F.; Sediyama, C.S. Selectivity of the botanical compounds to the pollinators Apis mellifera and Trigona hyalinata (Hymenoptera: Apidae). Sci. Rep. 2020, 10, 4820. [Google Scholar] [CrossRef] [PubMed]
- Ozogul, Y.; Boga, E.K.; Akyol, I.; Durmus, M.; Ucar, Y.; Regenstein, J.M.; Kosker, A.R. Antimicrobial activity of thyme essential oil nanoemulsions on spoilage bacteria of fish and food-borne pathogens. Food Biosci. 2020, 36, 100635. [Google Scholar] [CrossRef]
- Pinto, L.; Cefola, M.; Bonifacio, M.A.; Cometa, S.; Bocchino, C.; Pace, B.; Baruzzi, F. Effect of red thyme oil (Thymus vulgaris L.) vapours on fungal decay, quality parameters and shelf-life of oranges during cold storage. Food Chem. 2021, 336, 127590. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.Y.; Zhang, Y.N.; Bai, H.T.; Sun, G.F.; Zhang, J.Z.; Shi, L. Population diversity analyses provide insights into key horticultural traits of Chinese native thymes. Hortic. Res. 2023, 10, uhac262. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.Y.; Zhang, Y.N.; Zhu, L.; Liu, N.N.; Bai, H.T.; Sun, G.F.; Shi, L. Chromosome-level assembly and analysis of the Thymus genome provide insights into glandular secretory trichome formation and monoterpenoid biosynthesis in thyme. Plant Commun. 2022, 3, 100413. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Variations in chemical composition and bioactive compounds of Thymus kotschyanus Boiss. & Hohen populations originated from different collection sites. J. Essent. Oil Bear. Plants 2018, 21, 1272–1283. [Google Scholar] [CrossRef]
- Rota, M.C.; Herrera, A.; Martinez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Marchese, A.; Izadi, M.; Curti, V.; Daglia, M.; Nabavi, S.F. Plants belonging to the genus Thymus as antibacterial agents: From farm to pharmacy. Food Chem. 2015, 173, 339–347. [Google Scholar] [CrossRef]
- Bigdeloo, M.; Hadian, J.; Nazeri, V. Composition of essential oil compounds from different populations of Thymus caramanicus Jalas. J. Appl. Res. Med. Aromat. Plants 2017, 7, 95–98. [Google Scholar] [CrossRef]
- Kim, M.; Moon, J.C.; Kim, S.; Sowndhararajan, K. Morphological, chemical, and genetic characteristics of Korean native thyme Bak-Ri-Hyang (Thymus quinquecostatus Celak.). Antibiotics 2020, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Conart, C.; Saclier, N.; Foucher, F.; Goubert, C.; Rius-Bony, A.; Paramita, S.N.; Douady, C. Duplication and specialization of NUDX1 in rosaceae led to geraniol production in rose petals. Mol. Biol. Evol. 2022, 39, msac002. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, L.Y.; Zhang, Y.Q.; Huang, Y.X.; Cao, J.K.; Jiang, W.B. Antimicrobial and controlled release properties of nanocomposite film containing thymol and carvacrol loaded UiO-66-NH2 for active food packaging. Food Chem. 2022, 404, 134427. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wang, X.; Zhou, N.; Li, J.; Liu, J.; Yue, J.; Shang, X. Chemical characterization of the polar antibacterial fraction of the ethanol extract from Rosmarinus officinalis. Food Chem. 2021, 344, 128674. [Google Scholar] [CrossRef] [PubMed]
- Almasi, L.; Radi, M.; Amiri, S.; Torri, L. Fully dilutable Thymus vulgaris essential oil:acetic or propionic acid microemulsions are potent fruit disinfecting solutions. Food Chem. 2021, 343, 128411. [Google Scholar] [CrossRef]
- Hao, Y.P.; Kang, J.M.; Yang, R.; Li, H.; Cui, H.X.; Bai, H.T.; Shi, L. Multidimensional exploration of essential oils generated via eight oregano cultivars: Compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022, 374, 131629. [Google Scholar] [CrossRef] [PubMed]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147. [Google Scholar] [CrossRef] [PubMed]
- Zolotilov, V.; Nevkrytaya, N.; Zolotilova, O.; Seitadzhieva, S.; Myagkikh, E.; Pashtetskiy, V.; Karpukhin, M. The essential-oil-bearing rose collection variability study in terms of biochemical parameters. Agronomy 2022, 12, 529. [Google Scholar] [CrossRef]
- Kim, E.S.; Kang, S.Y.; Kim, Y.H.; Lee, Y.E.; Choi, N.Y.; You, Y.O.; Kim, K.J. Chamaecyparis obtusa essential oil inhibits methicillin-resistant Staphylococcus aureus biofilm formation and expression of virulence factors. J. Med. Food 2015, 18, 810–817. [Google Scholar] [CrossRef]
- Beale, D.J.; Morrison, P.D.; Karpe, A.V.; Dunn, M.S. Chemometric analysis of lavender essential oils using targeted and untargeted GC-MS acquired data for the rapid identification and characterization of oil quality. Molecules 2017, 22, 1339. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.B.; Kulawik, P.; Ozogul, F.; Regenstein, J.M.; Ozogul, Y. Biological activity of plant-based carvacrol and thymol and their impact on human health and food quality. Trends Food Sci. Technol. 2021, 116, 733–748. [Google Scholar] [CrossRef]
- Tao, R.; Sedman, J.; Ismail, A. Antimicrobial activity of various essential oils and their application in active packaging of frozen vegetable products. Food Chem. 2021, 360, 129956. [Google Scholar] [CrossRef]
- Hagvall, L.; Bruze, M.; Engfeldt, M.; Isaksson, M.; Lindberg, M.; Ryberg, K.; Christensson, J.B. Contact allergy to oxidized geraniol among Swedish dermatitis patients-A multicentre study by the Swedish Contact Dermatitis Research Group. Contact Dermat. 2018, 79, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Hejazian, L.B.; Amani, R.; Badeli, N.S. Geraniol attenuates oxidative stress, bioaccumulation, serological and histopathological changes during aluminum chloride-hepatopancreatic toxicity in male Wistar rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 20076–20089. [Google Scholar] [CrossRef]
- Mahmoud, N.M.; Elshazly, S.M.; Rezq, S. Geraniol protects against cyclosporine A-induced renal injury in rats: Role of Wnt/beta-catenin and PPAR gamma signaling pathways. Life Sci. 2022, 291, 120259. [Google Scholar] [CrossRef] [PubMed]
- Singulani, J.L.; Pedroso, R.S.; Ribeiro, A.B.; Nicolella, H.D.; Freitas, K.S.; Damasceno, J.L.; Martins, C.H.G. Geraniol and linalool anticandidal activity, genotoxic potential and embryotoxic effect on zebrafish. Future Microbiol. 2018, 13, 1637–1646. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, X.M.; Chen, S.; Deng, L.; Jiang, L.; Yang, S.A.; Dong, Z. Geraniol-mediated suppression of endoplasmic reticulum stress protects against cerebral ischemia-reperfusion injury via the PERK-ATF4-CHOP pathway. Int. J. Mol. Sci. 2023, 24, 544. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Wang, Z.H.; Fu, X.; Lin, Z.; Yu, K.H. Geraniol-mediated osteoarthritis improvement by down-regulating PI3K/Akt/NF-kappa B and MAPK signals: In vivo and in vitro studies. Int. Immunopharmcol. 2020, 86, 106713. [Google Scholar] [CrossRef]
- Ortiz, N.; Jimenez, M.F.; Chaverri, C.; Ciccio, J.F.; Diaz, C. Effect on cell growth, viability and migration of geraniol and geraniol-containing essential oil from Lippia alba (Verbenaceae) on gastric carcinoma cells. J. Essent. Oil Res. 2021, 34, 65–76. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Elmorsy, M.A.; Younis, N.S. Renal ischemia/reperfusion mitigation via geraniol: The role of Nrf-2/HO-1/NQO-1 and TLR2,4/MYD88/NF kappa B pathway. Antioxidants 2022, 11, 1568. [Google Scholar] [CrossRef]
- Ghosh, R.; Metze, D.; Sant, S.; Shaikh, M.; Deshpande, A.; Firake, D.M.; Pandit, S. Chemical ecology of Himalayan eggplant variety’s antixenosis: Identification of geraniol as an oviposition deterrent against the eggplant shoot and fruit borer. New Phytol. 2023, 240, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- El Azab, E.F.; Abdulmalek, S. Amelioration of age-related multiple neuronal impairments and inflammation in high-fat diet-fed rats: The prospective multitargets of geraniol. Oxidative Med. Cell. Longev. 2022, 2022, 4812993. [Google Scholar] [CrossRef]
- Gu, K.X.; Ouyang, P.; Hong, Y.X.; Dai, Y.Y.; Tang, T.; He, C.L.; Yin, L.Z. Geraniol inhibits biofilm formation of methicillin-resistant Staphylococcus aureus and increase the therapeutic effect of vancomycin in vivo. Front. Microbio. 2022, 13, 960728. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Jin, W.; Wang, J.; Sun, Y.; Wu, X.; Liu, L. Antibacterial and antibiofilm activities of peppermint essential oil against Staphylococcus aureus. LWT-Food Sci. Technol. 2019, 101, 639–645. [Google Scholar] [CrossRef]
- Miladinović, D.L.; Dimitrijević, M.V.; Mihajilov-Krstev, T.M.; Marković, M.S.; Ćirić, V.M. The significance of minor components on the antibacterial activity of essential oil via chemometrics. LWT-Food Sci. Technol. 2021, 136, 110305. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Ram, C.; Gairola, S.; Syed, A.; Verma, S.; Mugale, M.N.; Sahu, B.D. Carvacrol preserves antioxidant status and attenuates kidney fibrosis via modulation of TGF-beta 1/Smad signaling and inflammation. Food Funct. 2022, 13, 10587–10600. [Google Scholar] [CrossRef]
- Bendif, H.; Boudjeniba, M.; Miara, M.D.; Biqiku, L.; Bramucci, M.; Lupidi, G.; Maggi, F. Essential oil of Thymus munbyanus subsp coloratus from Algeria: Chemotypification and in vitro biological activities. Chem. Biodivers. 2017, 14, e1600299. [Google Scholar] [CrossRef]
- Akowuah, G.; Ismail, Z.; Norhayati, I.; Sadikun, A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005, 93, 311–317. [Google Scholar] [CrossRef]
- Seeram, N.P.; Henning, S.M.; Niu, Y.; Lee, R.; Scheuller, H.S.; Heber, D. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J. Agric. Food Chem. 2006, 54, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.P.; Li, J.Y.; Shi, L. A carvacrol-rich essential oil extracted from oregano (Origanum vulgare “Hot & Spicy”) exerts potent antibacterial effects against Staphylococcus aureus. Front. Microbiol. 2021, 12, 741861. [Google Scholar] [CrossRef]
| Terpenoid | Empirical Formula | RT | RI | Concentration (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tqu01 | Tqu02 | Tqa01 | Tqa02 | Tqa03 | Tqa04 | Tqp01 | Tqp02 | Tqp03 | Tqp04 | Tin01 | Tin02 | ||||
| α-Pinene | C10H16 | 7.03 | 929 | 1.6 ± 0.0 | 0.5 ± 0.0 | - | - | 0.9 ± 0.1 | 0.8 ± 0.1 | - | - | - | 0.5 ± 0.1 | - | - |
| Camphene | C10H16 | 7.56 | 952 | 2.6 ± 0.0 | 0.7 ± 0.0 | - | - | 1.4 ± 0.1 | 1.2 ± 0.1 | - | - | - | 1.2 ± 0.1 | - | 0.4 ± 0.0 |
| β-Myrcene | C10H16 | 9.16 | 991 | 1.0 ± 0.0 | 2.7 ± 0.0 | - | - | 0.7 ± 0.1 | - | - | 5.8 ± 0.2 | - | 0.4 ± 0.0 | - | - |
| trans 4-Carene | C10H16 | 10.06 | 1009 | - | - | - | - | - | - | - | - | - | - | - | 1.6 ± 0.0 |
| α-Terpinene | C10H16 | 10.06 | 1017 | 1.7 ± 0.0 | 1.1 ± 0.0 | 1.3 ± 0.2 | - | 1.6 ± 0.1 | 1.5 ± 0.1 | - | 1.1 ± 0.1 | 1.4 ± 0.2 | 21.1 ± 0.7 | 1.7 ± 0.1 | - |
| * p-Cymene | C10H14 | 10.38 | 1021 | 23.0 ± 0.1 | 8.7 ± 0.4 | 15.6 ± 0.9 | - | 11.6 ± 0.1 | 17.3 ± 1.5 | 18.0 ± 1.4 | 2.0 ± 0.2 | 15.7 ± 1.0 | 0.6 ± 0.0 | 46.0 ± 1.5 | 33.2 ± 1.1 |
| Sylvestrene | C10H16 | 10.53 | 1027 | - | 0.5 ± 0.0 | - | - | 0.6 ± 0.0 | 1.0 ± 0.9 | - | 2.5 ± 0.1 | - | - | - | - |
| 1,8-Cineole | C10H18O | 10.62 | 1032 | 6.7 ± 0.0 | 2.1 ± 0.0 | 2.1 ± 0.0 | 1.8 ± 0.3 | 2.5 ± 0.1 | 2.3 ± 0.1 | - | 1.2 ± 0.1 | - | 5.3 ± 0.6 | - | - |
| (Z)-β-Ocimene | C10H16 | 11.23 | 1038 | 3.4 ± 0.0 | - | - | - | - | - | - | 1.6 ± 0.1 | - | - | - | - |
| * γ-Terpinene | C10H16 | 11.72 | 1060 | 11.5 ± 0.1 | 4.8 ± 0.1 | 7.5 ± 0.5 | - | 5.8 ± 0.3 | 6.7 ± 0.4 | 1.2 ± 0.1 | 2.5 ± 0.0 | 8.8 ± 0.1 | 0.5 ± 0.0 | 2.0 ± 0.2 | 7.7 ± 0.6 |
| cis-Sabinene hydrate | C10H18O | 12.09 | 1070 | 0.6 ± 0.0 | 0.6 ± 0.0 | 1.0 ± 0.1 | 0.7 ± 0.0 | 1.1 ± 0.1 | 0.7 ± 0.0 | 1.2 ± 0.1 | - | 0.6 ± 0.1 | - | ||
| * Linalool | C10H18O | 13.32 | 1099 | 1.2 ± 0.0 | 1.1 ± 0.1 | 0.7 ± 0.1 | 3.3 ± 0.3 | 0.9 ± 0.0 | 0.5 ± 0.1 | - | - | 0.86 ± 0.1 | 1.0 ± 0.1 | 3.2 ± 0.3 | 2.6 ± 0.1 |
| * Camphor | C10H16O | 15.06 | 1143 | 4.4 ± 0.0 | 0.7 ± 0.0 | 2.3 ± 0.3 | 1.1 ± 0.1 | 3.6 ± 0.3 | 0.7 ± 0.1 | 1.0 ± 0.1 | |||||
| endo-Borneol | C10H18O | 15.80 | 1167 | 7.9 ± 0.1 | 5.1 ± 0.4 | 5.1 ± 0.7 | 2.2 ± 0.4 | 1.0 ± 0.3 | 7.9 ± 0.2 | 0.9 ± 0.1 | 1.5 ± 0.0 | 4.4 ± 0.3 | 6.5 ± 0.6 | 0.9 ± 0.1 | 1.2 ± 0.2 |
| Terpinen-4-ol | C10H18O | 16.31 | 1177 | 1.6 ± 0.0 | 1.5 ± 0.1 | 0.9 ± 0.1 | - | 1.5 ± 0.0 | 1.1 ± 0.0 | - | 2.5 ± 0.1 | 1.2 ± 0.0 | 0.9 ± 0.1 | 0.4 ± 0.0 | 0.8 ± 0.0 |
| * α-Terpineol | C10H18O | 16.77 | 1189 | 1.0 ± 0.0 | 10.5 ± 0.1 | - | 1.2 ± 0.0 | 2.4 ± 0.1 | 0.4 ± 0.0 | - | 65.4 ± 1.0 | - | 0.4 ± 0.0 | - | - |
| Thymol methyl ether | C11H16O | 18.44 | 1235 | - | 0.6 ± 0.0 | - | - | 0.6 ± 0.0 | 1.0 ± 0.0 | - | - | - | - | - | - |
| Carvacrol methyl ether | C11H16O | 18.77 | 1244 | - | 2.3 ± 0.0 | 0.7 ± 0.0 | - | 0.9 ± 0.1 | - | - | - | 1.6 ± 0.2 | 4.0 ± 0.0 | - | - |
| * Geraniol | C10H18O | 19.19 | 1255 | - | - | - | 58.3 ± 2.0 | - | - | - | - | - | - | - | 1.2 ± 0.1 |
| Bornyl acetate | C12H20O2 | 20.18 | 1287 | - | - | - | 0.7 ± 0.0 | - | - | - | - | - | 0.5 ± 0.0 | - | - |
| * Thymol | C10H14O | 20.38 | 1291 | 2.1 ± 0.0 | 28.9 ± 0.5 | 44.5 ± 2.2 | 2.5 ± 0.1 | 48.4 ± 1.4 | 0.5 ± 0.0 | 3.9 ± 0.3 | 1.3 ± 0.1 | 0.7 ± 0.0 | 40.2 ± 1.2 | 1.0 ± 0.0 | 5.9 ± 0.3 |
| * Carvacrol | C10H14O | 20.70 | 1299 | 20.7 ± 0.1 | 17.6 ± 0.4 | - | 1.3 ± 0.1 | 2.9 ± 0.2 | 41.0 ± 2.7 | 72.4 ± 1.0 | 3.5 ± 0.3 | 59.8 ± 1.7 | 4.3 ± 0.1 | 28.8 ± 1.7 | 26.9 ± 0.8 |
| δ-EIemene | C15H24 | 22.01 | 1338 | - | - | - | 2.0 ± 0.2 | - | - | - | - | - | - | - | 0.5 ± 0.1 |
| Carvacryl acetate | C12H16O2 | 22.95 | 1336 | 0.4 ± 0.0 | - | - | - | - | - | - | - | - | - | - | - |
| Geranyl acetate | C12H20O2 | 23.48 | 1385 | - | - | 3.2 ± 0.1 | 5.0 ± 0.3 | - | - | - | - | - | - | - | - |
| * (E)-β-Caryophyllene | C15H24 | 24.64 | 1419 | 1.7 ± 0.0 | 2.8 ± 0.2 | 10.5 ± 0.8 | 6.7 ± 0.5 | 3.5 ± 0.2 | 11.1 ± 0.3 | 2.5 ± 0.2 | 0.9 ± 0.0 | 3.7 ± 0.1 | 5.4 ± 0.7 | 5.7 ± 0.1 | 5.5 ± 0.5 |
| Aromadendrene | C15H24 | 25.33 | 1440 | - | 0.5 ± 0.0 | - | - | - | - | - | - | - | - | 0.6 ± 0.0 | 1.4 ± 0.1 |
| Germacrene D | C15H24 | 26.57 | 1481 | 0.6 ± 0.0 | 1.2 ± 0.0 | 1.5 ± 0.1 | 7.2 ± 0.4 | 0.8 ± 0.0 | 1.0 ± 0.0 | - | 2.8 ± 0.4 | - | - | 0.8 ± 0.1 | 0.6 ± 0.1 |
| Bicyclogermacrene | C15H24 | 27.04 | 1499 | - | 1.0 ± 0.1 | - | 4.3 ± 0.7 | - | - | - | 1.5 ± 0.2 | - | - | 1.9 ± 0.5 | - |
| Eremophilene | C15H24 | 27.09 | 1499 | - | - | 0.9 ± 0.4 | - | 0.6 ± 0.0 | - | - | - | - | - | - | 2.2 ± 0.3 |
| β-Bisabolene | C15H24 | 27.39 | 1509 | - | - | - | - | - | - | - | - | - | - | - | - |
| δ-Cadinene | C15H26 | 27.94 | 1524 | - | 1.0 ± 0.1 | 1.4 ± 0.2 | - | 0.9 ± 0.0 | 1.1 ± 0.0 | 1.1 ± 0.1 | - | 0.6 ± 0.0 | 0.9 ± 0.1 | 1.1 ± 0.1 | 2.0 ± 0.2 |
| Spathulenol | C15H24O | 29.45 | 1580 | - | - | 0.6 ± 0.0 | 1.5 ± 0.0 | - | - | - | - | - | - | 1.4 ± 0.0 | 1.6 ± 0.3 |
| Caryophyllene oxide | C15H24O | 29.62 | 1581 | 1.1 ± 0.0 | - | 0.9 ± 0.0 | - | - | 0.4 ± 0.0 | - | - | - | 0.4 ± 0.0 | 0.5 ± 0.1 | 0.9 ± 0.0 |
| Total | 94.8 ± 0.0 | 96.5 ± 0.1 | 98.7 ± 0.1 | 98.9 ± 1.0 | 98.8 ± 0.5 | 97.9 ± 0.6 | 100.0 ± 0.0 | 97.8 ± 0.5 | 99.6 ± 0.4 | 97.4 ± 0.2 | 97.4 ± 0.2 | 97.1 ± 0.3 | |||
| EO yields (%) | 0.5 | 0.7 | 1.0 | 0.3 | 0.8 | 0.9 | 0.7 | 1.2 | 0.8 | 0.7 | 0.8 | 0.6 | |||
| Terpenoid | Empirical Formula | RT | RI | Concentration (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tmo01 | Tmo02 | Tmo03 | Tmo04 | Tmo05 | Tma01 | Tcu01 | Tam01 | Tro01 | Tpr01 | Tmr01 | Tal01 | ||||
| α-Pinene | C10H16 | 7.03 | 929 | - | - | - | - | - | - | 0.6 ± 0.2 | - | - | - | - | - |
| Camphene | C10H16 | 7.56 | 952 | - | - | - | 0.5 ± 0.1 | - | 1.1 ± 0.1 | 1.1 ± 0.1 | - | - | 0.6 ± 0.0 | - | - |
| β-Myrcene | C10H16 | 9.16 | 991 | - | - | - | - | - | - | 0.5 ± 0.0 | - | - | - | - | - |
| trans 4-Carene | C10H16 | 10.06 | 1009 | - | - | - | - | - | - | 1.7 ± 0.2 | 1.5 ± 0.3 | - | - | - | - |
| α-Terpinene | C10H16 | 10.06 | 1017 | 1.8 ± 0.2 | 3.1 ± 0.3 | 1.9 ± 0.1 | 2.2 ± 0.1 | 0.6 ± 0.0 | - | - | - | - | 1.5 ± 0.3 | 1.5 ± 0.1 | - |
| * p-Cymene | C10H14 | 10.38 | 1021 | 27.9 ± 0.7 | 26.6 ± 1.0 | 21.0 ± 0.9 | 43.7 ± 2.3 | 7.6 ± 0.3 | - | 38.7 ± 2.5 | 33.7 ± 5.1 | 19.2 ± 1.9 | 22.2 ± 1.5 | 22.8 ± 0.9 | 28.0 ± 4.8 |
| Sylvestrene | C10H16 | 10.53 | 1027 | - | - | - | 0.45 ± 0.01 | - | - | - | - | - | - | - | - |
| 1,8-Cineole | C10H18O | 10.62 | 1032 | - | 0.6 ± 0.0 | - | 1.0 ± 0.0 | 2.2 ± 0.1 | - | 1.2 ± 0.1 | - | 2.6 ± 0.2 | 0.5 ± 0.0 | 0.8 ± 0.1 | - |
| (Z)-β-Ocimene | C10H16 | 11.23 | 1038 | - | - | - | - | - | - | - | - | - | - | - | |
| * γ-Terpinene | C10H16 | 11.72 | 1060 | 3.7 ± 0.3 | 17.6 ± 0.7 | 11.5 ± 0.5 | 8.0 ± 1.2 | 3.5 ± 0.1 | - | 10.8 ± 0.6 | 5.0 ± 0.9 | 3.1 ± 0.3 | 10.5 ± 0.7 | 4.8 ± 0.5 | - |
| cis-Sabinene hydrate | C10H18O | 12.09 | 1070 | - | - | - | 0.7 ± 0.1 | - | - | - | - | 1.2 ± 0.2 | - | - | |
| * Linalool | C10H18O | 13.32 | 1099 | - | 0.7 ± 0.0 | 0.9 ± 0.0 | - | 0.4 ± 0.0 | - | 0.3 ± 0.0 | 0.8 ± 0.0 | - | 0.6 ± 0.1 | - | - |
| * Camphor | C10H16O | 15.06 | 1143 | - | - | 4.4 ± 0.2 | 0.9 ± 0.0 | ||||||||
| endo-Borneol | C10H18O | 15.80 | 1167 | 1.1 ± 0.0 | - | - | 3.2 ± 0.4 | 2.7 ± 0.2 | 4.7 ± 0.5 | 3.4 ± 0.2 | 2.9 ± 0.1 | - | 2.8 ± 0.2 | 1.8 ± 0.2 | - |
| Terpinen-4-ol | C10H18O | 16.31 | 1177 | 0.7 ± 0.1 | 0.9 ± 0.0 | 0.6 ± 0.0 | 1.2 ± 0.1 | 0.8 ± 0.0 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | - | - |
| * α-Terpineol | C10H18O | 16.77 | 1189 | - | - | - | - | 0.6 ± 0.0 | - | - | - | 0.9 ± 0.1 | - | - | - |
| Thymol methyl ether | C11H16O | 18.44 | 1235 | - | - | - | - | 0.6 ± 0.1 | - | - | - | 7.0 ± 0.6 | - | 0.7 ± 0.1 | - |
| Carvacrol methyl ether | C11H16O | 18.77 | 1244 | 5.3 ± 0.4 | 2.5 ± 0.1 | 4.5 ± 0.1 | 4.8 ± 0.4 | 3.3 ± 0.3 | - | 0.6 ± 0.0 | - | - | 0.5 ± 0.0 | - | - |
| * Geraniol | C10H18O | 19.19 | 1255 | - | - | - | - | - | 59.5 ± 2.0 | - | - | - | - | - | - |
| Bornyl acetate | C12H20O2 | 20.18 | 1287 | - | - | - | - | - | - | - | 2.9 ± 0.3 | - | - | - | - |
| * Thymol | C10H14O | 20.38 | 1291 | 56.9 ± 0.1 | 41.9 ± 1.7 | 56.1 ± 1.1 | 30.4 ± 2.0 | 54.1 ± 1.2 | - | 0.8 ± 0.0 | - | 52.3 ± 2.6 | 4.5 ± 0.4 | 58.6 ± 0.8 | - |
| * Carvacrol | C10H14O | 20.70 | 1299 | 1.3 ± 0.1 | 1.4 ± 0.1 | 0.6 ± 0.0 | - | 20.2 ± 0.7 | - | 34.8 ± 3.6 | 51.3 ± 6.8 | 4.6 ± 0.5 | 46.2 ± 2.3 | 1.7 ± 0.2 | 71.7 ± 4.8 |
| δ-EIemene | C15H24 | 22.01 | 1338 | - | - | - | - | - | 1.4 ± 0.1 | - | - | - | - | - | - |
| Carvacryl acetate | C12H16O2 | 22.95 | 1336 | - | - | - | - | - | - | - | - | - | - | - | - |
| Geranyl acetate | C12H20O2 | 23.48 | 1385 | - | - | - | - | - | 0.8 ± 0.1 | - | - | - | - | - | - |
| * (E)-β-Caryophyllene | C15H24 | 24.64 | 1419 | - | 2.7 ± 0.1 | 2.4 ± 0.2 | 3.1 ± 0.4 | 3.4 ± 0.2 | 14.7 ± 1.0 | 2.2 ± 0.2 | 2.4 ± 0.3 | 2.5 ± 0.2 | 7.6 ± 0.3 | 2.3 ± 0.2 | - |
| Aromadendrene | C15H24 | 25.33 | 1440 | - | - | - | - | - | - | - | - | - | - | ||
| Germacrene D | C15H24 | 26.57 | 1481 | - | - | - | - | - | 3.1 ± 0.2 | 0.7 ± 0.2 | - | - | - | - | - |
| Bicyclogermacrene | C15H24 | 27.04 | 1499 | - | - | - | - | - | 3.4 ± 0.4 | - | - | - | - | - | - |
| Eremophilene | C15H24 | 27.09 | 1499 | - | 0.9 ± 0.0 | - | - | - | - | 0.6 ± 0.1 | - | - | - | 0.7 ± 0.1 | - |
| β-Bisabolene | C15H24 | 27.39 | 1509 | - | 0.5 ± 0.0 | - | - | - | - | - | - | 5.0 ± 0.7 | - | 3.5 ± 0.4 | - |
| δ-Cadinene | C15H26 | 27.94 | 1524 | 0.8 ± 0.1 | - | - | - | - | 1.3 ± 0.2 | 0.9 ± 0.2 | 1.1 ± 0.2 | - | - | 1.1 ± 0.1 | - |
| Spathulenol | C15H24O | 29.45 | 1580 | - | - | - | - | - | 0.7 ± 0.0 | 0.5 ± 0.1 | - | - | - | - | - |
| Caryophyllene oxide | C15H24O | 29.62 | 1581 | - | - | - | - | - | 0.7 ± 0.0 | - | - | - | - | - | - |
| Total | 99.6 ± 0.4 | 98.7 ± 0.2 | 99.6 ± 0.3 | 99.0 ± 0.6 | 99.6 ± 0.7 | 99.0 ± 0.3 | 99.0 ± 0.1 | 99.3 ± 0.6 | 99.8 ± 0.3 | 98.9 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | |||
| EO yields (%) | 1.3 | 1.4 | 1.3 | 0.8 | 0.9 | 1.1 | 1.1 | 1.0 | 1.1 | 1.6 | 0.7 | 1.0 | |||
| Sample ID | Radical-Scavenging Activity | |
|---|---|---|
| DPPH/IC50 (mg/mL) | ABTS/IC50 (mg/mL) | |
| Tqu01 | 19.35 | 0.24 |
| Tqu02 | 4.01 | 0.08 |
| Tqa01 | 4.47 | 0.15 |
| Tqa02 | - | - |
| Tqa03 | 13.06 | 0.32 |
| Tqa04 | 7.06 | 0.17 |
| Tqp01 | 2.83 | 0.04 |
| Tqp02 | - | - |
| Tqp03 | 8.12 | 0.17 |
| Tqp04 | 4.92 | 0.22 |
| Tmo01 | 3.36 | 0.07 |
| Tmo02 | 5.99 | 0.11 |
| Tmo03 | 3.58 | 0.16 |
| Tmo04 | 6.59 | 0.48 |
| Tmo05 | 9.32 | 0.05 |
| Tin01 | 8.41 | 0.16 |
| Tin02 | 8.12 | 1.23 |
| Tma01 | - | - |
| Tcu01 | 10.04 | 1.06 |
| Tam01 | 19.95 | 0.54 |
| Tro01 | 2.91 | 0.12 |
| Tpr01 | 5.51 | 0.05 |
| Tmr01 | 3.12 | 0.06 |
| Tal01 | - | - |
| Sample ID | Species | Location | Collection Site | Altitude (m) | Longitude (E) | Latitude (N) | Slope | Soil Texture | Habitat Type |
|---|---|---|---|---|---|---|---|---|---|
| Tqu01 | Thymus quinquecostatus | Hebei Province | Jinhekou Village, Yu County | 1046.00 | 114°55′3″ | 39°57′1″ | Sunny floodplain | Sandy loam | Floodplain in front of a mountain with gravelly sandy land; the main companion plants were Allium polyrhizum, Cymbaria dahurica, and Ephedra intermedia |
| Tqu02 | Thymus quinquecostatus | Inner Mongolia Autonomous Region | Zhenglan Banner | 1415.66 | 116°9′20″ | 42°12′11″ | Sunny slope | Sandy loam | The main companion plants were Soutellari baiclensis, Iris locyzii, Rhaponticum unifloru, and Alliium sp. |
| Tqa01 | Thymus quinquecostatus var. asiaticus | Jilin Province | Songyuan City | 170.00 | 124°1′52″ | 44°36′52″ | Sunny slope | Sandy loam | Sandy land formed by deciduous grass; the main companion plants were Potentilla anserina and Gueldenstaedtia verna subsp. multiflora |
| Tqa02 | Thymus quinquecostatus var. asiaticus | Inner Mongolia Autonomous Region | Zhenglan Banner | 1373.00 | 116°1′43″ | 42°22′49″ | Roadside slope | Sandy loam | Roadside grassland with sandy loam rich in humus |
| Tqa03 | Thymus quinquecostatus var. asiaticus | Inner Mongolia Autonomous Region | Wuhe Erqin Aobao Forest Farm, Zhenglan Banner | 1427.05 | 116°9′44″ | 42°30′19″ | Roadside gentle slope | Dry sandy loam | Dry sandy land formed after grassland degradation |
| Tqa04 | Thymus quinquecostatus var. asiaticus | Shanxi Province | Zuoyun County, Datong City | 1319.00 | 112°43′43″ | 40°6′33″ | Sunny slope | Sandy loam | The main companion plants were Hippophae rhamnoides and Lespedeza davurica |
| Tqp01 | Thymus quinquecostatus var. przewalskii | Hebei Province | Small Wutai Jinhekou Scenic Spot, Yu County | 1117.00 | 114°33′55″ | 39°33′46″ | Half-sunny slope | Sandy loam | Between roadside rock walls or rock crevices; the main companion plants were Selaginella sinensis, Selaginella sanguinolenta, and Spiraea pubescens |
| Tqp02 | Thymus quinquecostatus var. przewalskii | Inner Mongolia Autonomous Region | Duolun Reservoir, Duolun County | 1269.13 | 116°38′44″ | 42°11′44″ | Sunny slope | Sandy loam | The main companion plants were Allium senescens, Patrinia rupestris subsp. scabra, and Spiraea pubescens |
| Tqp03 | Thymus quinquecostatus var. przewalskii | Shanxi Province | Fucheng Town, Linchuan County, Jincheng City | 1093.00 | 113°7′42″ | 36°39′53″ | Sunny steep slope | Calcareous sandy loam | Rocky beach by a cliff |
| Tqp04 | Thymus quinquecostatus var. przewalskii | Shanxi Province | Xiangshan Temple, Hequ County | 926.57 | 111°13′33″ | 39°24′24″ | Sunny slope | Sandy loam | A gravelly yellow sand formed by stratified rock weathering |
| Tmo01 | Thymus mongolicus | Beijing Municipal | Baihua Mountain, Fangshan District | 1891.48 | 115°36′34″ | 39°51′10″ | Roadside sunny slope | Sandy loam | Gravel and stone crevices growth, and a local red ant formation of the associated mound |
| Tmo02 | Thymus mongolicus | Beijing Municipal | Baihua Mountain, Fangshan District | 1935.03 | 115°35′57″ | 39°50′10″ | Half-sunny slope | Sandy loam | Baicao bank southeast side; found in a crack in the rocks by the roadside |
| Tmo03 | Thymus mongolicus | Beijing Municipal | Baihua Mountain, Fangshan District | 1809.23 | 115°35′42″ | 39°49′21″ | Sunny slope | Sandy loam | Roadside rock crevice at the southwest edge of Baicao bank; it is often associated with mounds formed by a local species of red ant |
| Tmo04 | Thymus mongolicus | Beijing Municipal | Dongling Mountain, Mentougou District | 1915.00 | 115°28′29″ | 40°19′31″ | Sunny slope | Sandy loam | The sun was leeward with many rocks, well-drained hillsides, or rock joints; sandy loam rich in humus |
| Tmo05 | Thymus mongolicus | Ningxia Hui Autonomous Region | Jingyuan County, Guyuan City | 2560.00 | 106°12′41″ | 35°29′43″ | Sunny slope | Sandy loam | Gravel sandy ground formed by weathering sandstone by the highway; the main companion plant was Stachys sieboldii |
| Tin01 | Thymus inaequalis | Heilongjiang Province | Huma County | 440.00 | 124°1′8″ | 52°14′15″ | Dry sunny slope | Sandy loam | The main companion plants were Orostachys cartilaginea, Thymus inaequalis, and Thymus amurensis |
| Tin02 | Thymus inaequalis | Heilongjiang Province | Forest botanical garden | 150.00 | 126°16′ | 45°45′ | Roadside slope | Sandy loam | Roadside slope in the botanical garden area |
| Tma01 | Thymus mandschuricus | Heilongjiang Province | Maoer Mountain, Shangzhi City | 800.00 | 127°32′3″ | 45°20′3″ | Half-sunny slope | Loam | Rocky cracks on top of volcanic rock with medium-acid soil with gravel |
| Tcu01 | Thymus curtus | Heilongjiang Province | Huma County | 440.00 | 124°1′8″ | 52°14′15″ | Dry sunny slope | Sandy loam | The main companion plants were Orostachys cartilaginea, Thymus inaequalis, and Thymus amurensis |
| Tam01 | Thymus amurensis | Heilongjiang Province | Huma County | 440.00 | 124°1′8″ | 52°14′15″ | Dry sunny slope | Sandy loam | The main companion plants were Orostachys cartilaginea, Thymus curtus, and Thymus amurensis |
| Tro01 | Thymus roseus | Xinjiang Uygur Autonomous Region | Sailimu Lake, Yining City | 2038.09 | 81°16′9″ | 44°39′42″ | Slope | Sandy loam | On well-drained sandy loam slopes along the shore of the lake; the main companion plants were Cares sp. and Allium polyrhizum |
| Tpr01 | Thymus proximus | Xinjiang Uygur Autonomous Region | Awuzan Ditch, Yining County | >1637.96 | 81°43′35″ | 44°8′57″ | Sunny slope | Loam | On rock walls or between rock crevices above the snow-ridge spruce line, rich in humus loam; the main companion plants were Eremurus chinensis, Ephedra equisetina, and Hylotelephium ewersii |
| Tmr01 | Thymus marschallianus | Xinjiang Uygur Autonomous Region | Tuolasu Grassland, Yining County | 1792.00 | 81°43′52″ | 44°15′58″ | Grassy slope | Gravelly yellow sand | On an alpine grassland open slope; the main companion plants were Artemisia sp., Stipa sp., Aneurolepidium sp., and Dracocephalum sp. |
| Tal01 | Thymus altaicus | Xinjiang Uygur Autonomous Region | Awuzan Ditch, Yining County | >1637.96 | 81°43′35″ | 44°8′57″ | Sunny slope | Loam | On rock walls or between rock crevices above the lowest snow-ridge spruce line, rich in humus loam; the main companion plants were Eremurus chinensis, Ephedra equisetina, and Hylotelephium ewersii |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Zhang, Y.; Hao, Y.; Miao, J.; Sun, G.; Xiao, J.; Yang, X.; Zhang, J.; Shi, L. Antioxidant and Antibacterial Activities of Chinese Native Thyme Essential Oils with Different Chemotypes. Molecules 2024, 29, 6035. https://doi.org/10.3390/molecules29246035
Sun M, Zhang Y, Hao Y, Miao J, Sun G, Xiao J, Yang X, Zhang J, Shi L. Antioxidant and Antibacterial Activities of Chinese Native Thyme Essential Oils with Different Chemotypes. Molecules. 2024; 29(24):6035. https://doi.org/10.3390/molecules29246035
Chicago/Turabian StyleSun, Meiyu, Yanan Zhang, Yuanpeng Hao, Jiahui Miao, Guofeng Sun, Jianhua Xiao, Xiao Yang, Jinzheng Zhang, and Lei Shi. 2024. "Antioxidant and Antibacterial Activities of Chinese Native Thyme Essential Oils with Different Chemotypes" Molecules 29, no. 24: 6035. https://doi.org/10.3390/molecules29246035
APA StyleSun, M., Zhang, Y., Hao, Y., Miao, J., Sun, G., Xiao, J., Yang, X., Zhang, J., & Shi, L. (2024). Antioxidant and Antibacterial Activities of Chinese Native Thyme Essential Oils with Different Chemotypes. Molecules, 29(24), 6035. https://doi.org/10.3390/molecules29246035






