Analysis of Plasticizer Contamination Throughout Olive Oil Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analytical Method Development
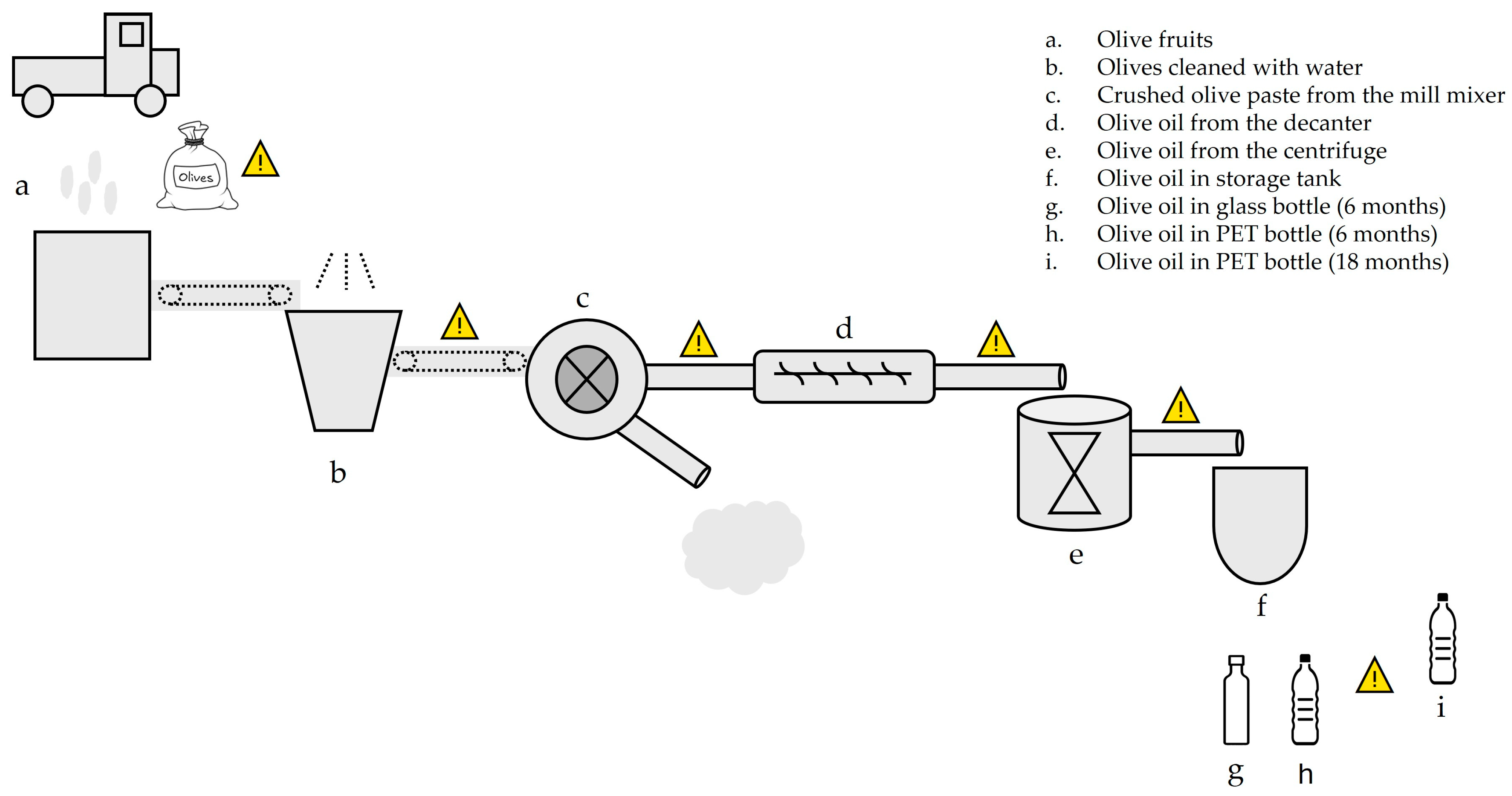
2.2. Olive Oil Production and Plasticizer Contamination
2.3. Off-the-Shelf Olive Oil
3. Experimental Section
3.1. Chemicals
3.2. Sampling
3.3. Sample Analysis and Method Validation
3.4. Chromatographic Conditions for GC-MS/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet: A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Global Consumption of Olive Oil 2022/23. Statista. Available online: https://www.statista.com/statistics/940491/olive-oil-consumption-worldwide/ (accessed on 30 October 2024).
- Marsh, K.; Bugusu, B. Food Packaging—Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Mangaraj, S.; Goswami, T.K.; Mahajan, P.V. Applications of Plastic Films for Modified Atmosphere Packaging of Fruits and Vegetables: A Review. Food Eng. Rev. 2009, 1, 133–158. [Google Scholar] [CrossRef]
- Kirwan, M.J.; McDowell, D.; Coles, R. Food Packaging Technology; Blackwell: Oxford, UK, 2003; ISBN 978-1-405-14771-2. [Google Scholar]
- Thompson, R.C.; Moore, C.J.; Saal, F.S.V.; Swan, S.H. Plastics, the Environment and Human Health: Current Consensus and Future Trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and Societal Benefits of Plastics. Philos. Trans. R. Soc. B: Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Staples, C. Phthalate Esters; Springer-Verlag: Berlin, Germany, 2003; ISBN 3540009922. [Google Scholar]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and Exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef]
- Alamri, M.S.; Qasem, A.A.A.; Mohamed, A.A.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food Packaging’s Materials: A Food Safety Perspective. Saudi J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef]
- Craver, C.; Carraher, C. Applied Polymer Science: 21st Century; Elsevier B.V.: Amsterdam, The Netherlands, 2000; ISBN 0080434177. [Google Scholar]
- Hauser, R.; Calafat, A.M.; Hauser, A.R. PHTHALATES AND HUMAN HEALTH. Occup. Environ. Med. 2005, 62, 806–818. [Google Scholar] [CrossRef]
- Hlisníková, H.; Petrovičová, I.; Kolena, B.; Šidlovská, M.; Sirotkin, A. Effects and Mechanisms of Phthalates’ Action on Neurological Processes and Neural Health: A Literature Review. Pharmacol. Rep. 2021, 73, 386–404. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Guo, J.L.; Xue, J.; Bai, C.L.; Guo, Y. Phthalate Metabolites: Characterization, Toxicities, Global Distribution, and Exposure Assessment. Environ. Pollut. 2021, 291, 118106. [Google Scholar] [CrossRef]
- Ventrice, P.; Ventrice, D.; Russo, E.; De Sarro, G. Mini Review Phthalates: European Regulation, Chemistry, Pharmacokinetic and Related Toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, H. Phthalates and Their Impacts on Human Health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef]
- Bølling, A.K.; Sripada, K.; Becher, R.; Bekö, G. Phthalate Exposure and Allergic Diseases: Review of Epidemiological and Experimental Evidence. Environ. Int. 2020, 139, 105706. [Google Scholar] [CrossRef] [PubMed]
- Sree, C.G.; Buddolla, V.; Lakshmi, B.A.; Kim, Y.J. Phthalate Toxicity Mechanisms: An Update. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2023, 263, 109498. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L.; Gutleb, A.C.; Bergman, Å.; Eriksen, G.S.; Murk, A.J.; Ropstad, E.; Saunders, M.; Skaare, J.U. Reproductive and Developmental Toxicity of Phthalates. J. Toxicol. Environ. Health Part B 2009, 12, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Spade, D.J. REPRODUCTIVE TOXICOLOGY: Environmental Exposures, Fetal Testis Development and Function: Phthalates and Beyond. Reproduction 2021, 162, F147–F167. [Google Scholar] [CrossRef]
- Zhang, Y.; Lyu, L.; Tao, Y.; Ju, H.; Chen, J. Health Risks of Phthalates: A Review of Immunotoxicity. Environ. Pollut. 2022, 313, 120173. [Google Scholar] [CrossRef]
- Sedha, S.; Lee, H.; Singh, S.; Kumar, S.; Jain, S.; Ahmad, A.; Bin Jardan, Y.A.; Sonwal, S.; Shukla, S.; Simal-Gandara, J.; et al. Reproductive Toxic Potential of Phthalate Compounds—State of Art Review. Pharmacol. Res. 2021, 167, 105536. [Google Scholar] [CrossRef]
- Document 32004R1935. Regulation (EC) No 1935/2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC; European Parliament and Council of the European Union: Brussels, Belgium, 2004; Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2004R1935:20090807:EN:PDF (accessed on 17 December 2024).
- Document 32011R0010. Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Official Journal of the European Union: Luxembourg, 2011; Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R0010 (accessed on 17 December 2024).
- Silano, V.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mortensen, A.; et al. Update of the Risk Assessment of Di-Butylphthalate (DBP), Butyl-Benzyl-Phthalate (BBP), Bis(2-Ethylhexyl)Phthalate (DEHP), Di-Isononylphthalate (DINP) and Di-Isodecylphthalate (DIDP) for Use in Food Contact Materials. EFSA J. 2019, 17. [Google Scholar] [CrossRef]
- Document 32023R1442; Commission Regulation (EU) 2023/1442 of 11 July 2023 Amending Annex I to Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food, as Regards Changes to Substance Authorisations and Addition of New Substances. Official Journal of the European Union: Luxembourg, 2023.
- Bi, X.; Pan, X.; Yuan, S.; Wang, Q. Plasticizer Contamination in Edible Vegetable Oil in a U.S. Retail Market. J. Agric. Food Chem. 2013, 61, 9502–9509. [Google Scholar] [CrossRef]
- Wang, S.Y.; Wang, M.Q.; Yang, E.Q.; Chen, X.M.; Pan, F.G. Review on Occurrence, Sources of Contamination, and Mitigation Strategies of Phthalates in Vegetable Oils. Eur. J. Lipid Sci. Technol. 2022, 124, 2100086. [Google Scholar] [CrossRef]
- Wang, C.; Huang, P.; Qiu, C.; Li, J.; Hu, S.; Sun, L.; Bai, Y.; Gao, F.; Li, C.; Liu, N.; et al. Occurrence, Migration and Health Risk of Phthalates in Tap Water, Barreled Water and Bottled Water in Tianjin, China. J. Hazard. Mater. 2021, 408, 124891. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, A.; Kirsten, K.L.; Ajmal, Z.; Jiang, X.; Zhao, X. Alternative Plasticizers As Emerging Global Environmental and Health Threat: Another Regrettable Substitution? Environ. Sci. Technol. 2022, 56, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Yang, L.; Liang, X.; Huang, D.; Qiao, X.; Dai, Q.; Chen, D.; Cai, Z. Nonphthalate Plasticizers in House Dust from Multiple Countries: An Increasing Threat to Humans. Environ. Sci. Technol. 2023, 57, 3634–3644. [Google Scholar] [CrossRef]
- Harmon, P.; Otter, R. A Review of Common Non-Ortho-Phthalate Plasticizers for Use in Food Contact Materials. Food Chem. Toxicol. 2022, 164, 112984. [Google Scholar] [CrossRef]
- Jung, J.; Cho, Y.; Lee, Y.; Choi, K. Uses and Occurrences of Five Major Alternative Plasticizers, and Their Exposure and Related Endocrine Outcomes in Humans: A Systematic Review. Crit. Rev. Environ. Sci. Technol. 2024, 54, 1165–1194. [Google Scholar] [CrossRef]
- He, P.; Ling, Y.; Yong, W.; Yao, M.; Zhang, Y.; Feng, X.; Zhang, Y.; Zhang, F. Determination of 22 Alternative Plasticizers in Wrap Film by Solid Phase Extraction and Ultra-High Performance Supercritical Fluid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2022, 1669, 462916. [Google Scholar] [CrossRef]
- Qadeer, A.; Anis, M.; Warner, G.R.; Potts, C.; Giovanoulis, G.; Nasr, S.; Archundia, D.; Zhang, Q.; Ajmal, Z.; Tweedale, A.C.; et al. Global Environmental and Toxicological Data of Emerging Plasticizers: Current Knowledge, Regrettable Substitution Dilemma, Green Solution and Future Perspectives. Green. Chem. 2024, 26, 5635–5683. [Google Scholar] [CrossRef]
- Bui, T.T.; Giovanoulis, G.; Cousins, A.P.; Magnér, J.; Cousins, I.T.; de Wit, C.A. Human Exposure, Hazard and Risk of Alternative Plasticizers to Phthalate Esters. Sci. Total Environ. 2016, 541, 451–467. [Google Scholar] [CrossRef]
- Zughaibi, T.A.; Sheikh, I.A.; Beg, M.A. Insights into the Endocrine Disrupting Activity of Emerging Non-Phthalate Alternate Plasticizers against Thyroid Hormone Receptor: A Structural Perspective. Toxics 2022, 10, 263. [Google Scholar] [CrossRef]
- Freitas, F.; Cabrita, M.J.; da Silva, M.G. A Critical Review of Analytical Methods for the Quantification of Phthalates Esters in Two Important European Food Products: Olive Oil and Wine. Molecules 2023, 28, 7628. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Wang, Y.; Ruan, J.; Zhang, J.; Sun, C. Recent Advances in Analysis of Phthalate Esters in Foods. TrAC Trends Anal. Chem. 2015, 72, 10–26. [Google Scholar] [CrossRef]
- Sanchis, Y.; Yusà, V.; Coscollà, C. Analytical Strategies for Organic Food Packaging Contaminants. J. Chromatogr. A 2017, 1490, 22–46. [Google Scholar] [CrossRef] [PubMed]
- Haji Harunarashid, N.Z.I.; Lim, L.H.; Harunsani, M.H. Phthalate Sample Preparation Methods and Analysis in Food and Food Packaging: A Review. Food Anal. Methods 2017, 10, 3790–3814. [Google Scholar] [CrossRef]
- Marega, M.; Grob, K.; Moret, S.; Conte, L. Phthalate Analysis by Gas Chromatography–Mass Spectrometry: Blank Problems Related to the Syringe Needle. J. Chromatogr. A 2013, 1273, 105–110. [Google Scholar] [CrossRef]
- Fankhauser-Noti, A.; Grob, K. Blank Problems in Trace Analysis of Diethylhexyl and Dibutyl Phthalate: Investigation of the Sources, Tips and Tricks. Anal. Chim. Acta 2007, 582, 353–360. [Google Scholar] [CrossRef]
- Vavrouš, A.; Pavloušková, J.; Ševčík, V.; Vrbík, K.; Čabala, R. Solution for Blank and Matrix Difficulties Encountered during Phthalate Analysis of Edible Oils by High Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry. J. Chromatogr. A 2016, 1456, 196–204. [Google Scholar] [CrossRef]
- Guo, Y.; Kannan, K. Challenges Encountered in the Analysis of Phthalate Esters in Foodstuffs and Other Biological Matrices. Anal. Bioanal. Chem. 2012, 404, 2539–2554. [Google Scholar] [CrossRef]
- Kapellakis, I.E.; Tsagarakis, K.P.; Crowther, J.C. Olive Oil History, Production and by-Product Management. Rev. Environ. Sci. Biotechnol. 2008, 7, 1–26. [Google Scholar] [CrossRef]
- Cavaliere, B.; Macchione, B.; Sindona, G.; Tagarelli, A. Tandem Mass Spectrometry in Food Safety Assessment: The Determination of Phthalates in Olive Oil. J. Chromatogr. A 2008, 1205, 137–143. [Google Scholar] [CrossRef]
- Nagorka, R.; Koschorreck, J. Trends for Plasticizers in German Freshwater Environments—Evidence for the Substitution of DEHP with Emerging Phthalate and Non-Phthalate Alternatives. Environ. Pollut. 2020, 262, 114237. [Google Scholar] [CrossRef] [PubMed]
- Nanni, N.; Fiselier, K.; Grob, K.; Di Pasquale, M.; Fabrizi, L.; Aureli, P.; Coni, E. Contamination of Vegetable Oils Marketed in Italy by Phthalic Acid Esters. Food Control 2011, 22, 209–214. [Google Scholar] [CrossRef]
- Pereira, J.; do Céu Selbourne, M.; Poças, F. Determination of Phthalates in Olive Oil from European Market. Food Control 2019, 98, 54–60. [Google Scholar] [CrossRef]
- Arena, A.; Zoccali, M.; Mondello, L.; Tranchida, P.Q. Direct Analysis of Phthalate Esters in Vegetable Oils by Means of Comprehensive Two-Dimensional Gas Chromatography Combined with Triple Quadrupole Mass Spectrometry. Food Chem. 2022, 396, 133721. [Google Scholar] [CrossRef]
- Singh, A.R.; Lawrence, W.H.; Autian, J. Mutagenic and Antifertility Sensitivities of Mice to Di-2-Ethylhexyl Phthalate (DEHP) and Dimethoxyethyl Phthalate (DMEP). Toxicol. Appl. Pharmacol. 1974, 29, 35–46. [Google Scholar] [CrossRef]
- Cao, X.L. Phthalate Esters in Foods: Sources, Occurrence, and Analytical Methods. Compr. Rev. Food Sci. Food Saf. 2010, 9, 21–43. [Google Scholar] [CrossRef]
- Wirnitzer, U.; Rickenbacher, U.; Katerkamp, A.; Schachtrupp, A. Systemic Toxicity of Di-2-Ethylhexyl Terephthalate (DEHT) in Rodents Following Four Weeks of Intravenous Exposure. Toxicol. Lett. 2011, 205, 8–14. [Google Scholar] [CrossRef]
- Hirata-Koizumi, M.; Takahashi, M.; Matsumoto, M.; Kawamura, T.; Ono, A.; Hirose, A. Toxicity Effects of Phthalate Substitute Plasticizers Used in Toys. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku 2012, 31–42. [Google Scholar]
- Kıralan, S.S.; Toptancı, İ.; Öncül Abacıgil, T.; Ramadan, M.F. Phthalates Levels in Olive Oils and Olive Pomace Oils Marketed in Turkey. Food Addit. Contam. Part. A -Chem. 2020, 37, 1332–1338. [Google Scholar] [CrossRef]
- SANTE/11312/2021; Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. EU Reference Laboratories for Residues of Pesticides: Wageningen, The Netherlands, 2021.



| Substance | Regulation (EU) 2023/1442 Amending Annex I to Regulation (EU) 10/2011 | Only to Be Used as: |
|---|---|---|
| Dibutyl Phthalate (DBP) | SML: 0.12 mg/kg Total SML group restriction no. 32: 60 mg/kg Total SML group restriction no. 36: 0.6 mg/kg | (a) Plasticizer in repeated use materials and articles contacting non-fatty foods; (b) Technical support agent in polyolefins in concentrations up to 0.05% (w/w) in the final product. |
| Benzyl Butyl Phthalate (BBP) | SML: 6.0 mg/kg Total SML group restriction no. 32: 60 mg/kg Total SML group restriction no. 36: 0.6 mg/kg | (a) Plasticizer in repeated use materials and articles; (b) Plasticizer in single-use materials and articles contacting non-fatty foods except for infant formula and follow-on formula; (c) Technical support agent in concentrations up to 0.1% (w/w) in the final product. |
| Di(2-Ethylhexyl) Phthalate (DEHP) | SML: 0.6 mg/kg Total SML group restriction no. 32: 60 mg/kg Total SML group restriction no. 36: 0.6 mg/kg | (a) Plasticizer in repeated use materials and articles contacting non-fatty foods; (b) Technical support agent in concentrations up to 0.1% (w/w) in the final product. |
| Di-isononyl Phthalate and Di-isodecyl Phthalate (DINP and DIDP) | Total SML group restriction no. 26: 1.8 mg/kg (sum of DINP and DIDP) Total SML group restriction no. 32: 60 mg/kg Not to be used in combination with FCM substances DBP, BBP, DEHP, and DIBP. | (a) Plasticizer in repeated use materials and articles; (b) Plasticizer in single-use materials and articles contacting non-fatty foods except for infant formula and follow-on formula; (c) technical support agent in concentrations up to 0.1% (w/w) in the final product. |
| Samples of Production Line | DIBP | DBP | BBP | DEHP | Sum of DINP and DIDP | Sum of 32 Plasticizers | |
|---|---|---|---|---|---|---|---|
| NORTH | a. Olive fruits in plastic bag | 0.011 ± 0.004 | <LOD | <LOD | <LOD | <LOD | 1.987 ± 0.571 |
| b. Olives cleaned with water | 0.008 ± 0.001 | <LOD | <LOD | <LOD | <LOD | 0.971 ± 0.133 | |
| c. Crushed olive paste from the mill mixer | n. a. | ||||||
| d. Olive oil from the decanter | <LOD | <LOQ | <LOQ | <LOD | <LOD | 1.253 ± 0.100 | |
| e. Olive oil from the centrifuge | 0.007 ± 0.002 | <LOQ | <LOQ | <LOD | <LOD | 1.277 ± 0.066 | |
| f. Olive oil in storage tank | <LOD | <LOD | <LOQ | <LOD | 0.103 ± 0.061 | 1.522 ± 0.071 | |
| g. Olive oil in glass bottle (6 months) | <LOD | <LOD | <LOQ | <LOD | 1.278 ± 0.161 | 8.277 ± 0.752 | |
| h. Olive oil in PET bottle (6 months) | 0.019 ± 0.008 | <LOQ | <LOQ | <LOD | 3.527 ± 0.214 | 11.946 ± 1.028 | |
| i. Olive oil in PET bottle (18 months) | 0.028 ± 0.009 | 0.127 ± 0.007 | 0.006 ± 0.001 | 0.454 ± 0.013 | 6.000 ± 0.203 | 14.751 ± 0.506 | |
| CENTRE | a. Olive fruits in plastic bag | n. a. | |||||
| b. Olives cleaned with water | 0.019 ± 0.010 | 0.073 ± 0.004 | <LOD | 0.076 ± 0.007 | 0.146 ± 0.056 | 5.377 ± 0.289 | |
| c. Crushed olive paste from the mill mixer | 0.052 ± 0.010 | 0.085 ± 0.004 | <LOD | <LOD | 0.431 ± 0.145 | 6.372 ± 0.359 | |
| d. Olive oil from the decanter | 0.061 ± 0.007 | 0.083 ± 0.012 | <LOD | <LOD | 0.318 ± 0.065 | 7.615 ± 0.086 | |
| e. Olive oil from the centrifuge | 0.079 ± 0.013 | 0.082 ± 0.007 | <LOD | <LOD | 0.625 ± 0.309 | 7.780 ± 0.888 | |
| f. Olive oil in storage tank | 0.093 ± 0.008 | 0.092 ± 0.002 | <LOQ | <LOD | 1.211 ± 0.172 | 9.821 ± 0.331 | |
| g. Olive oil in glass bottle (6 months) | n. a. | ||||||
| h. Olive oil in PET bottle (6 months) | 0.072 ± 0.007 | 0.096 ± 0.009 | <LOQ | <LOD | 3.528 ± 0.323 | 16.224 ± 0.807 | |
| i. Olive oil in PET bottle (18 months) | n. a. | ||||||
| SOUTH | a. Olive fruits in plastic bag | n. a. | |||||
| b. Olives cleaned with water | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| c. Crushed olive paste from the mill mixer | 0.013 ± 0.006 | <LOQ | <LOD | <LOD | <LOQ | 4.385 ± 0.330 | |
| d. Olive oil from the decanter | 0.010 ± 0.008 | <LOQ | <LOD | <LOD | 0.103 ± 0.023 | 6.285 ± 0.271 | |
| e. Olive oil from the centrifuge | 0.010 ± 0.002 | <LOD | <LOD | <LOD | 0.156 ± 0.015 | 6.429 ± 0.212 | |
| f. Olive oil in storage tank | <LOQ | <LOD | <LOD | <LOD | 0.264 ± 0.064 | 6.621 ± 0.200 | |
| g. Olive oil in glass bottle (6 months) | <LOQ | <LOQ | <LOD | 0.037 ± 0.003 | 4.396 ± 0.156 | 5.590 ± 0.215 | |
| h. Olive oil in PET bottle (6 months) | <LOQ | <LOQ | <LOD | 0.078 ± 0.009 | 5.112 ± 0.228 | 14.595 ± 0.262 | |
| i. Olive oil in PET bottle (18 months) | <LOD | 0.038 ± 0.002 | <LOD | 0.095 ± 0.001 | 9.393 ± 0.580 | 24.991 ± 0.786 | |
| DIBP | DBP | BBP | DEHP | Sum of DINP and DIDP | Sum of 32 Plasticizers | ||
|---|---|---|---|---|---|---|---|
| Olive Oil 1 | GLASS | 0.014 ± 0.009 | <LOD | <LOD | <LOD | 1.598 ± 0.145 | 2.688 ± 0.146 |
| PET | <LOQ | <LOD | <LOD | <LOD | 1.807 ± 0.199 | 4.109 ± 0.204 | |
| Olive Oil 2 | GLASS | <LOQ | 0.020 ± 0.005 | <LOD | <LOD | 0.395 ± 0.076 | 1.168 ± 0.116 |
| PET | 0.018 ± 0.005 | <LOQ | <LOD | <LOD | 0.716 ± 0.112 | 1.516 ± 0.117 | |
| Olive Oil 3 | GLASS | <LOD | <LOD | <LOD | <LOD | 3.310 ± 0.053 | 5.763 ± 0.122 |
| PET | <LOQ | <LOD | <LOQ | <LOD | 3.245 ± 0.050 | 5.847 ± 0.131 | |
| Olive Oil 4 | GLASS | <LOD | <LOD | <LOQ | 0.656 ± 0.006 | 1.244 ± 0.086 | 3.299 ± 0.093 |
| Olive Oil 5 | GLASS | 0.02 ± 0.002 | <LOD | <LOD | <LOD | 5.976 ± 0.389 | 7.145 ± 0.391 |
| Olive Oil 6 | CAN | <LOD | <LOD | <LOD | 0.082 ± 0.013 | <LOD | 0.882 ± 0.056 |
| Plasticizers | CAS | Quantifier Transition (eV) | Qualifier Transition (eV) | LOD (mg/kg) | LOQ (mg/kg) |
|---|---|---|---|---|---|
| DMP | 131-11-3 | 163 > 77 (14) | 163 > 92 (28) | 0.002 | 0.007 |
| DMTP | 120-61-6 | 163 > 75 (30) | 163 > 103 (18) | 0.005 | 0.018 |
| DBM | 105-76-0 | 117 > 99 (10) | 117 > 71 (16) | 0.001 | 0.004 |
| DEP | 84-66-2 | 149 > 65 (22) | 149 > 121 (14) | 0.005 | 0.018 |
| DiPrP | 605-45-8 | 149 > 65 (24) | 149 > 121 (16) | 0.001 | 0.004 |
| DAP | 131-17-9 | 149 > 65 (22) | 149 > 121 (14) | 0.013 | 0.043 |
| DPrp | 131-16-8 | 149 > 65 (24) | 149 > 121 (14) | 0.005 | 0.018 |
| DES | 110-40-7 | 171 > 55 (23) | 171 > 97 (12) | 0.031 | 0.103 |
| DIBP | 84-69-5 | 149 > 65 (24) | 149 > 121 (16) | 0.002 | 0.007 |
| DBP | 84-74-2 | 149 > 65 (24) | 149 > 121 (16) | 0.005 | 0.018 |
| DMEP | 117-82-8 | 149 > 65 (24) | 149 > 121 (16) | 0.103 | 0.343 |
| BMPP | 84-63-9 | 149 > 65 (24) | 251 > 149 (15) | 0.005 | 0.018 |
| DIPP | 605-50-5 | 149 > 65 (24) | 237 > 149 (12) | 0.005 | 0.018 |
| DEEP | 605-54-9 | 149 > 65 (22) | 149 > 121 (14) | 0.005 | 0.018 |
| DPP | 131-18-0 | 149 > 65 (24) | 149 > 121 (16) | 0.001 | 0.004 |
| BPA | 80-05-7 | 231 > 91 (28) | 119 > 91 (14) | 0.031 | 0.103 |
| ATBC | 77-90-7 | 129 > 69 (18) | 185 > 69 (24) | 0.001 | 0.004 |
| BBP | 85-68-7 | 149 > 65 (24) | 238 > 149 (18) | 0.001 | 0.004 |
| DHXP | 84-75-3 | 149 > 65 (24) | 251 > 149 (14) | 0.001 | 0.004 |
| DEHA | 103-23-1 | 129 > 55 (16) | 129 > 111 (17) | 0.005 | 0.018 |
| DBEP | 117-83-9 | 149 > 65 (22) | 149 > 121 (14) | 0.031 | 0.103 |
| DCHP | 84-61-7 | 149 > 65 (24) | 167 > 149 (10) | 0.005 | 0.018 |
| DPhP | 84-62-8 | 225 > 77 (22) | 225 > 51 (50) | 0.001 | 0.004 |
| DEHP | 117-81-7 | 149 > 65 (20) | 279 > 149 (18) | 0.005 | 0.018 |
| DHP | 3648-21-3 | 149 > 65 (24) | 265 > 149 (15) | 0.009 | 0.030 |
| DOP | 117-84-0 | 149 > 65 (24) | 149 > 121 (16) | 0.001 | 0.004 |
| DEHT | 6422-86-2 | 149 > 65 (19) | 167 > 79 (14) | 0.005 | 0.018 |
| DEHS | 122-62-3 | 185 > 69 (16) | 203 > 121 (14) | 0.005 | 0.018 |
| DNP | 84-76-4 | 149 > 65 (24) | 149 > 121 (16) | 0.005 | 0.018 |
| DINP | 28553-12-0 | 293 > 149 (5) | 293 > 71 (5) | 0.031 | 0.103 |
| DIDP | 26761-40-0 | 307 > 149 (5) | 307 > 71 (5) | 0.103 | 0.343 |
| TOMT | 3319-31-1 | 305 > 193 (20) | 193 > 81 (26) | 0.005 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, F.; Brinco, J.; Cabrita, M.J.; Gomes da Silva, M. Analysis of Plasticizer Contamination Throughout Olive Oil Production. Molecules 2024, 29, 6013. https://doi.org/10.3390/molecules29246013
Freitas F, Brinco J, Cabrita MJ, Gomes da Silva M. Analysis of Plasticizer Contamination Throughout Olive Oil Production. Molecules. 2024; 29(24):6013. https://doi.org/10.3390/molecules29246013
Chicago/Turabian StyleFreitas, Flávia, João Brinco, Maria João Cabrita, and Marco Gomes da Silva. 2024. "Analysis of Plasticizer Contamination Throughout Olive Oil Production" Molecules 29, no. 24: 6013. https://doi.org/10.3390/molecules29246013
APA StyleFreitas, F., Brinco, J., Cabrita, M. J., & Gomes da Silva, M. (2024). Analysis of Plasticizer Contamination Throughout Olive Oil Production. Molecules, 29(24), 6013. https://doi.org/10.3390/molecules29246013






