Microwave-Assisted Extraction of Cellulose from Aloe Vera Plant Residue and Preparation of Cellulose Nanocrystal–Poly(vinyl alcohol) Hydrogels
Abstract
1. Introduction
2. Results and Discussion
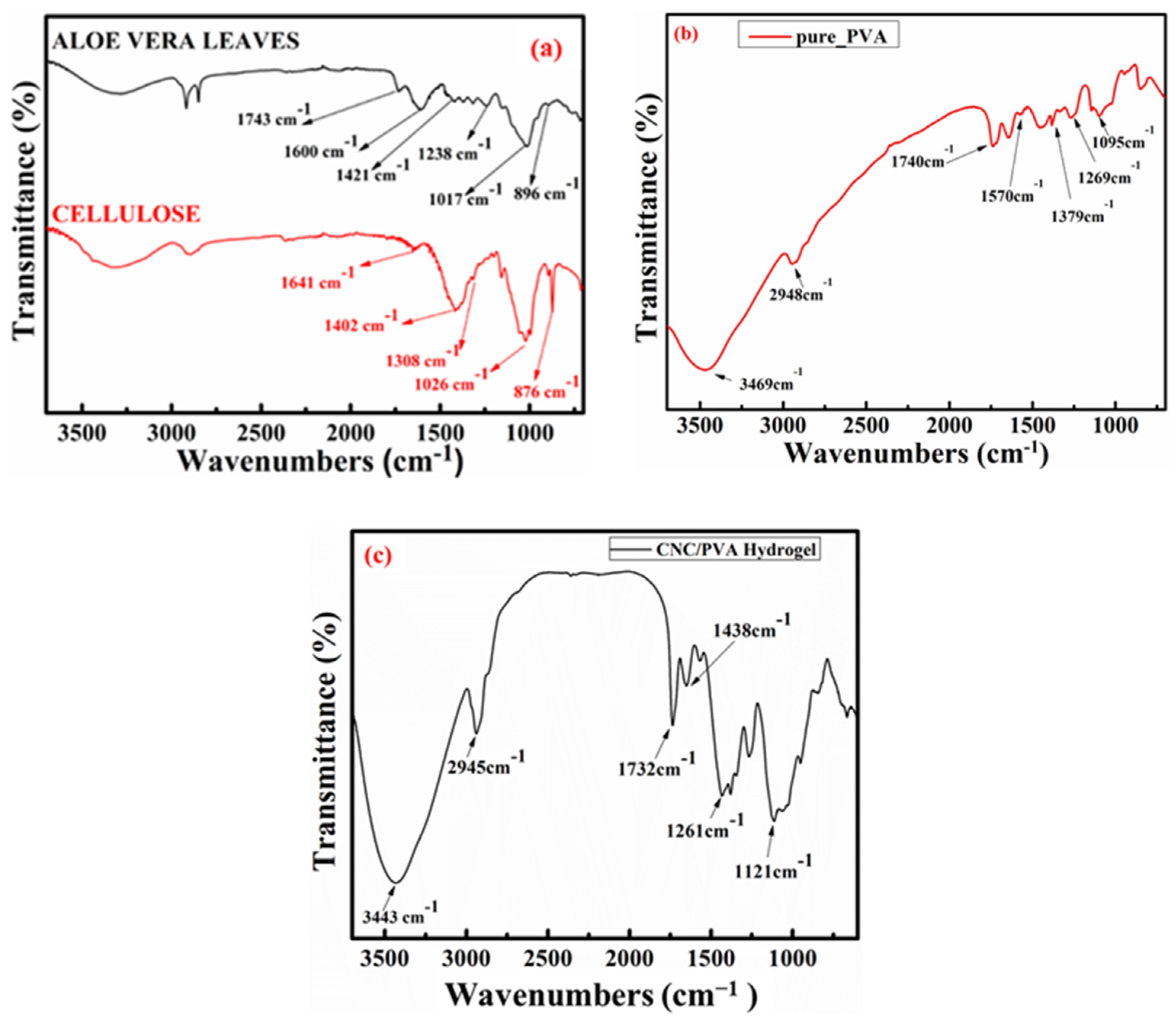
2.1. FT-IR Spectrum
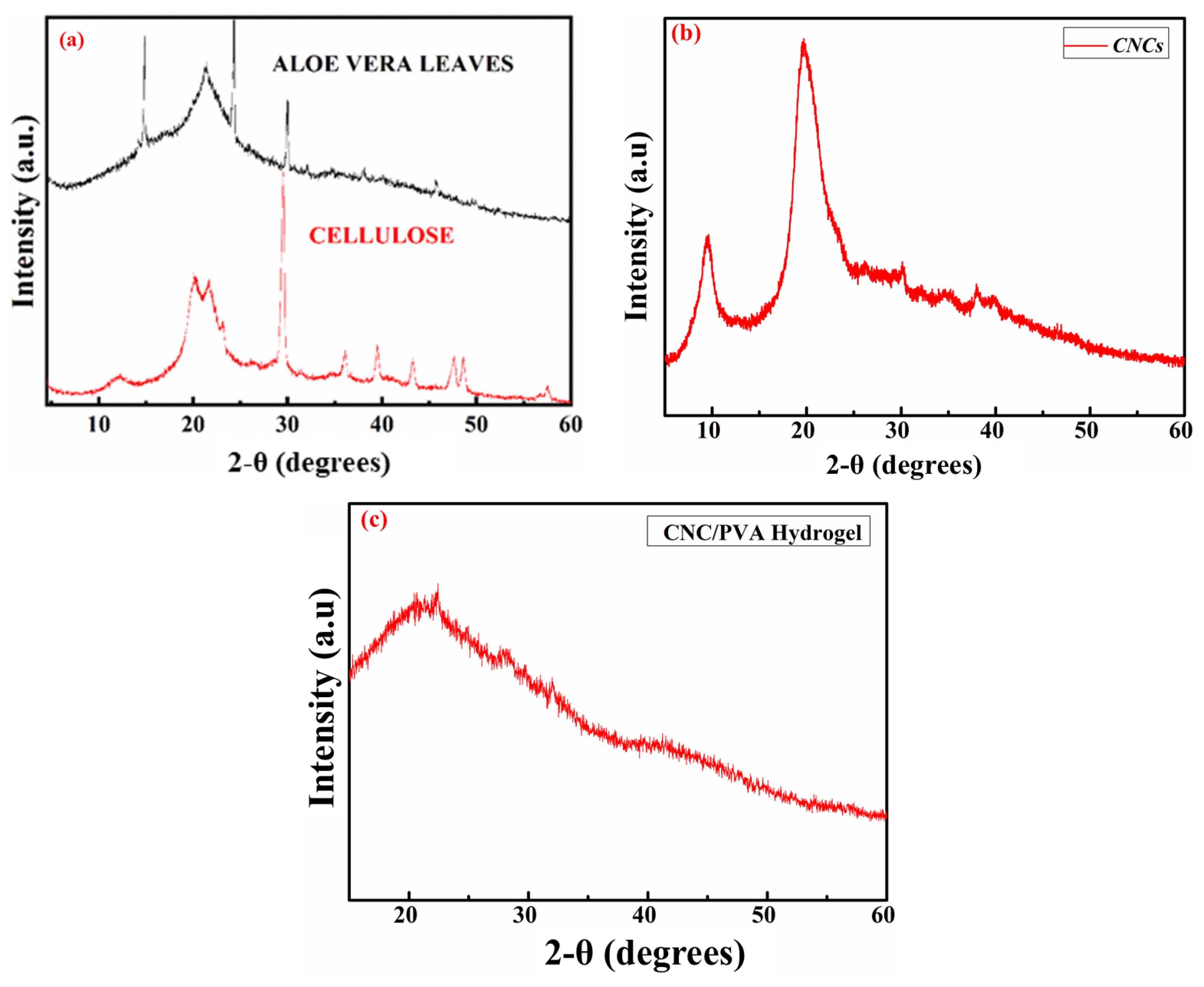
2.2. XRD Analysis
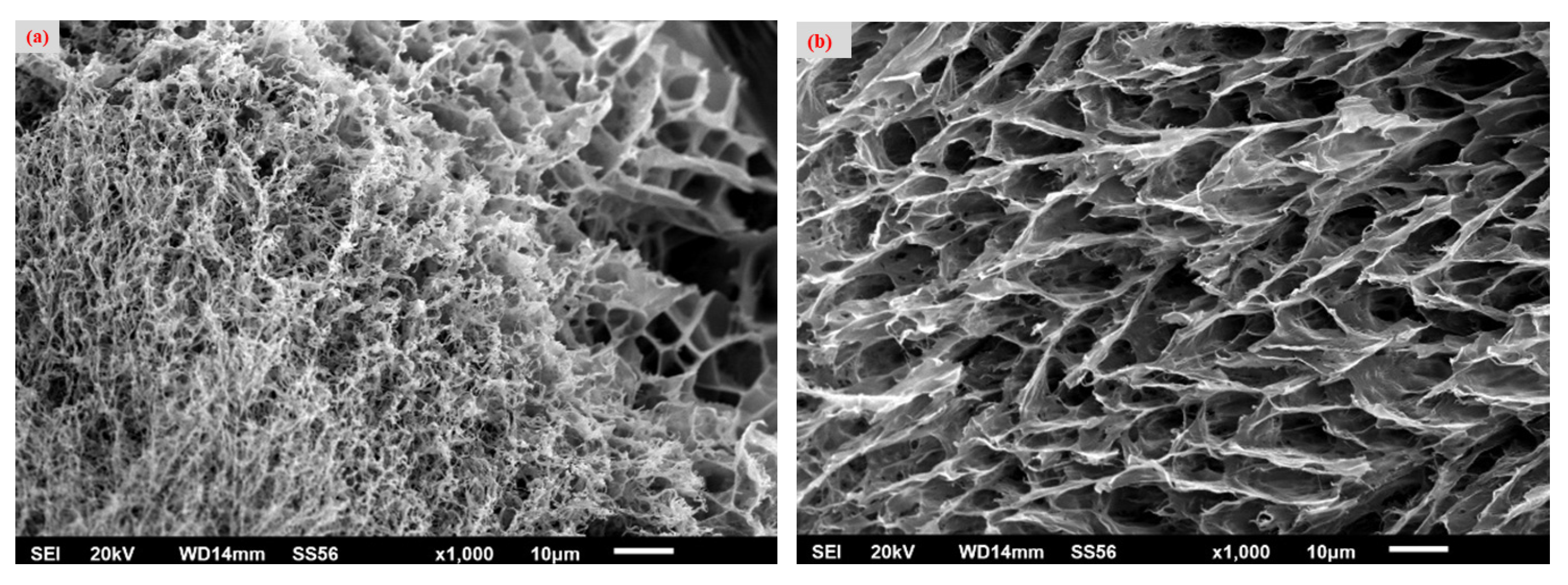
2.3. Scanning Electron Microscopy (SEM)
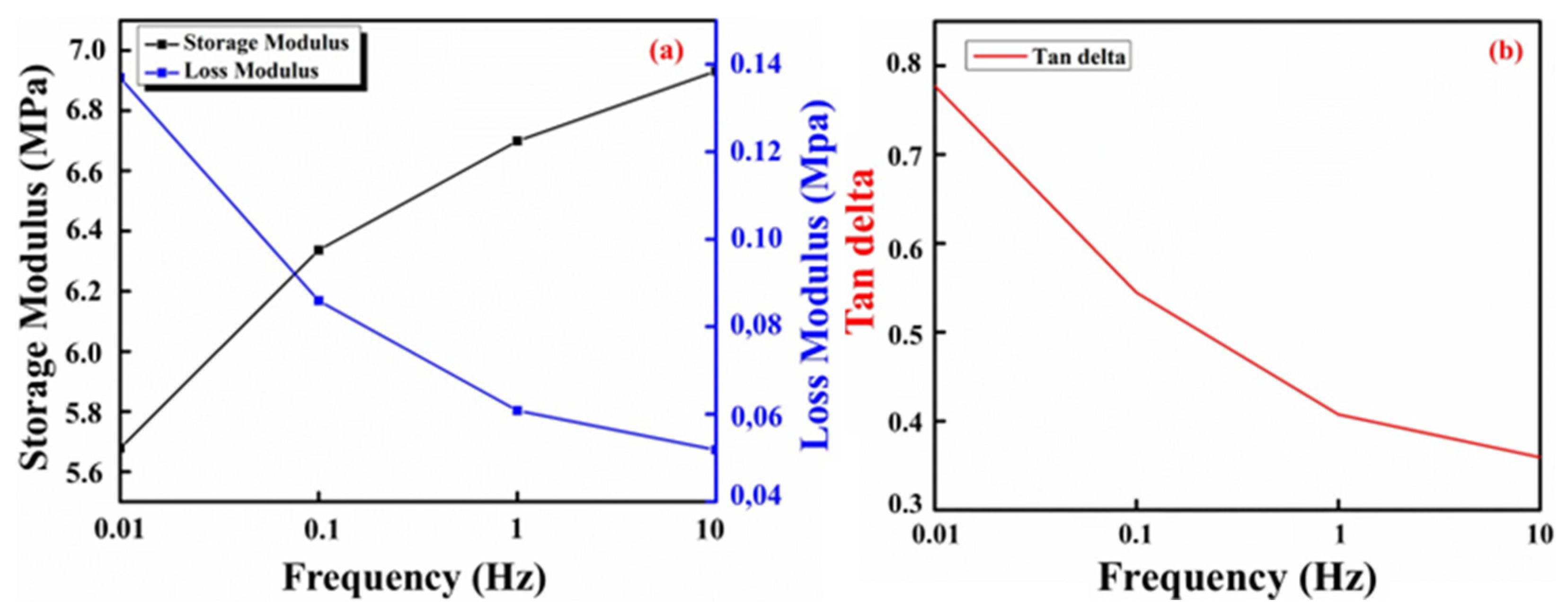
2.4. Dynamic Mechanical Analysis (DMA)
3. Materials and Methods
3.1. Materials
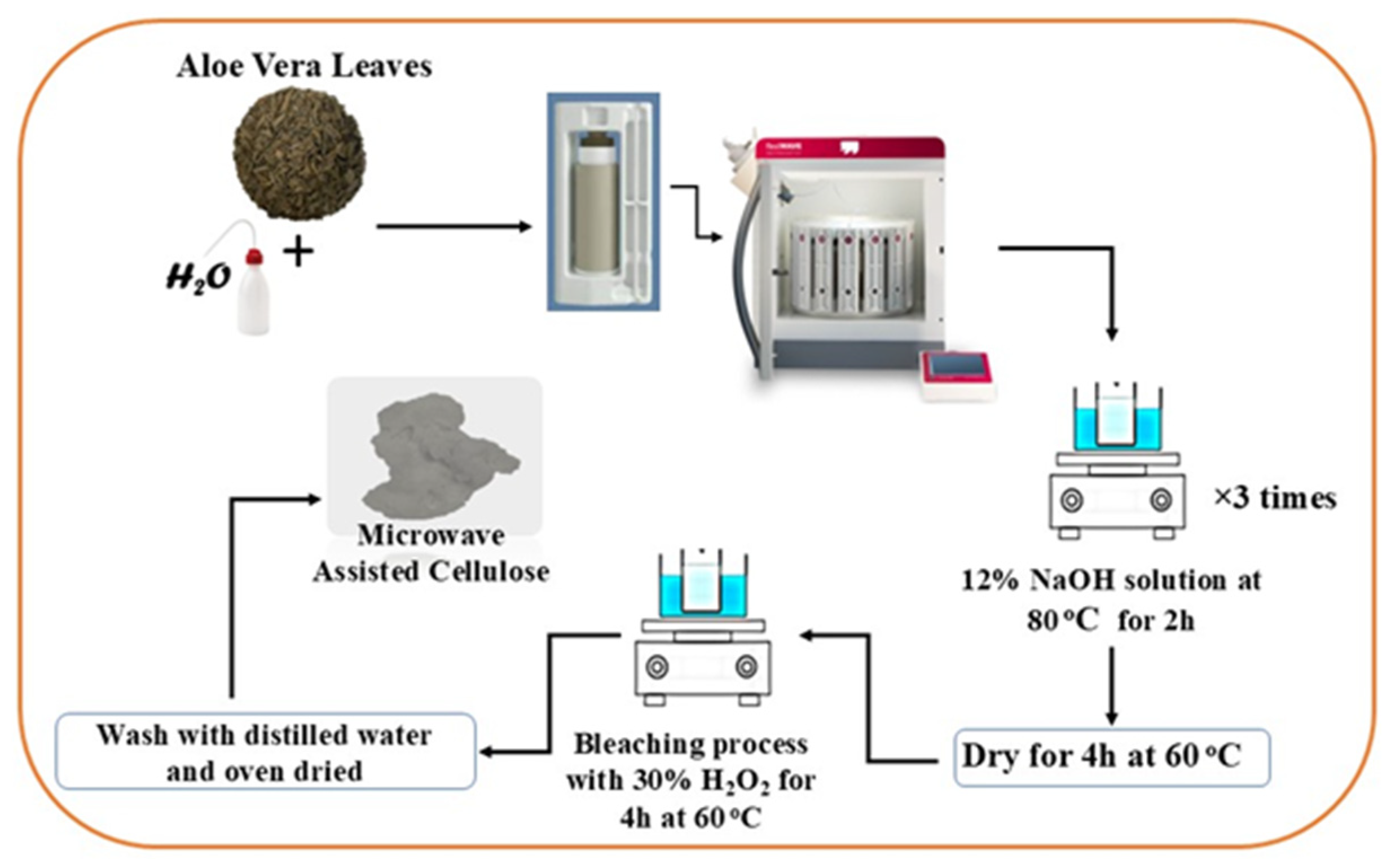
3.2. Cellulose Extraction
3.3. Cellulose Nanocrystal Isolation
3.4. PVA–CNC Hydrogel Preparation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothen, L.A.; Cintil, J.; Thomas, S.; John, M.J.; Anandjiwala, R.; Narine, S.S. Environmental friendly method for the extraction of coir fibre and isolation of nanofibre. Carbohydr. Polym. 2013, 92, 1477–1483. [Google Scholar] [CrossRef]
- Hernandez, C.; Rosa, D. Extraction of cellulose nanowhiskers: Natural fibers source, methodology and application. Matrix 2016, 3, 232–242. [Google Scholar]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, K.J.; Balaji, A.N.; Ramanujam, N.R. Extraction of cellulose nanofibers from cocos nucifera var aurantiaca peduncle by ball milling combined with chemical treatment. Carbohydr. Polym. 2019, 212, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Hou, J.; Chang, P.; Huang, J. Structure and Properties of Cellulose Nanocrystals; Wiley Online Library: Hoboken, NJ, USA, 2019; pp. 21–52. [Google Scholar]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Mondragon, G.; Garrigós, M.C.; Eceiza, A.; Jiménez, A. Microwave-assisted extraction of cellulose nanocrystals from almond (Prunus amygdalus) shell waste. Front. Nutr. 2022, 9, 1071754. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef]
- Zhao, Z.; Fang, R.; Rong, Q.; Liu, M. Bioinspired Nanocomposite Hydrogels with Highly Ordered Structures. Adv. Mater. 2017, 29, 1703045. [Google Scholar] [CrossRef]
- Ge, G.; Zhang, Y.; Shao, J.; Wang, W.; Si, W.; Huang, W.; Dong, X. Stretchable, Transparent, and Self-Patterned Hydrogel-Based Pressure Sensor for Human Motions Detection. Adv. Funct. Mater. 2018, 28, 1802576. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly(vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Sumi, K. Thermal decomposition products of poly(vinyl alcohol). J. Polym. Sci. Part A-1 Polym. Chem. 1969, 7, 3151–3158. [Google Scholar] [CrossRef]
- Karydis-Messinis, A.; Kyriakaki, C.; Triantafyllou, E.; Tsirka, K.; Gioti, C.; Gkikas, D.; Nesseris, K.; Exarchos, D.A.; Farmaki, S.; Giannakas, A.E.; et al. Development and Physicochemical Characterization of Edible Chitosan–Casein Hydrogel Membranes for Potential Use in Food Packaging. Gels 2024, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green. Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Han, C.; Zhang, X.; Xu, F.; Sun, R.-C. Cellulose Nanocrystals Mechanical Reinforcement in Composite Hydrogels with Multiple Cross-Links: Correlations between Dissipation Properties and Deformation Mechanisms. Macromolecules 2014, 47, 4077–4086. [Google Scholar] [CrossRef]
- You, J.; Cao, J.; Zhao, Y.; Zhang, L.; Zhou, J.; Chen, Y. Improved Mechanical Properties and Sustained Release Behavior of Cationic Cellulose Nanocrystals Reinforeced Cationic Cellulose Injectable Hydrogels. Biomacromolecules 2016, 17, 2839–2848. [Google Scholar] [CrossRef]
- Berglund, L.; Squinca, P.; Baş, Y.; Zattarin, E.; Aili, D.; Rakar, J.; Junker, J.; Starkenberg, A.; Diamanti, M.; Sivlér, P.; et al. Self-Assembly of Nanocellulose Hydrogels Mimicking Bacterial Cellulose for Wound Dressing Applications. Biomacromolecules 2023, 24, 2264–2277. [Google Scholar] [CrossRef]
- Hebeish, A.; Farag, S.; Sharaf, S.; Shaheen, T.I. Development of cellulose nanowhisker-polyacrylamide copolymer as a highly functional precursor in the synthesis of nanometal particles for conductive textiles. Cellulose 2014, 21, 3055–3071. [Google Scholar] [CrossRef]
- Loh, E.Y.X.; Mohamad, N.; Fauzi, M.B.; Ng, M.H.; Ng, S.F.; Mohd Amin, M.C.I. Development of a bacterial cellulose-based hydrogel cell carrier containing keratinocytes and fibroblasts for full-thickness wound healing. Sci. Rep. 2018, 8, 2875. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, Z.; Abd Hamid, S.B. Preparation and Characterization of Nanocrystalline Cellulose using Ultrasonication Combined with a Microwave-assisted Pretreatment Process. BioResources 2016, 11, 3397–3415. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, Y.; Wang, J. Novel Polyvinyl Alcohol (PVA)/Cellulose Nanocrystal (CNC) Supramolecular Composite Hydrogels: Preparation and Application as Soil Conditioners. Nanomaterials 2019, 9, 1397. [Google Scholar] [CrossRef] [PubMed]
- Abbasi Moud, A.; Kamkar, M.; Sanati-Nezhad, A.; Hejazi, S.H.; Sundararaj, U. Viscoelastic properties of poly (vinyl alcohol) hydrogels with cellulose nanocrystals fabricated through sodium chloride addition: Rheological evidence of double network formation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 609, 125577. [Google Scholar] [CrossRef]
- Deleanu, I.; Stoica, A.; Stroescu, M.; Dobre, L.; Dobre, T.; Jinga, S.; Tardei, C. Potassium sorbate release from poly(vinyl alcohol)–bacterial cellulose films. Chem. Pap. 2012, 66, 138–143. [Google Scholar]
- Putri, L.Z.; Ratnawulan. Analysis of Aloe vera Nano Powder (Aloe vera L.) using X-ray Diffraction (XRD). J. Phys. Conf. Ser. 2023, 2582, 012029. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, A.; Ahlawat, V.; Bhattacharya, M.; Goswami, S. Process optimization for the production of cellulose nanocrystals from rice straw derived α-cellulose. Mater. Sci. Energy Technol. 2020, 3, 328–334. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Ko, H.-U.; Kim, H.C.; Kim, J.; Mwongeli, R.; Kim, J. Electroactive Hydrogels Made with Polyvinyl Alcohol/Cellulose Nanocrystals. Materials 2018, 11, 1615. [Google Scholar] [CrossRef] [PubMed]
- Tummala, G.K. Hydrogels of Poly (vinyl alcohol) and Nanocellulose for Ophthalmic Applications: Synthesis, Characterization, Biocompatibility and Drug Delivery Studies; Acta Universitatis Upsaliensis: Uppsala, Sweden, 2018. [Google Scholar]
- Veloso, S.R.S.; Azevedo, A.G.; Teixeira, P.F.; Fernandes, C.B.P. Cellulose Nanocrystal (CNC) Gels: A Review. Gels 2023, 9, 574. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triantafyllou, E.; Karydis-Messinis, A.; Moschovas, D.; Kyriakaki, C.; Vasilopoulos, K.C.; Giannakas, A.E.; Karakassides, M.A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Salmas, C.E. Microwave-Assisted Extraction of Cellulose from Aloe Vera Plant Residue and Preparation of Cellulose Nanocrystal–Poly(vinyl alcohol) Hydrogels. Molecules 2024, 29, 6012. https://doi.org/10.3390/molecules29246012
Triantafyllou E, Karydis-Messinis A, Moschovas D, Kyriakaki C, Vasilopoulos KC, Giannakas AE, Karakassides MA, Avgeropoulos A, Zafeiropoulos NE, Salmas CE. Microwave-Assisted Extraction of Cellulose from Aloe Vera Plant Residue and Preparation of Cellulose Nanocrystal–Poly(vinyl alcohol) Hydrogels. Molecules. 2024; 29(24):6012. https://doi.org/10.3390/molecules29246012
Chicago/Turabian StyleTriantafyllou, Eleni, Andreas Karydis-Messinis, Dimitrios Moschovas, Christina Kyriakaki, Konstantinos C. Vasilopoulos, Aris E. Giannakas, Michael A. Karakassides, Apostolos Avgeropoulos, Nikolaos E. Zafeiropoulos, and Constantinos E. Salmas. 2024. "Microwave-Assisted Extraction of Cellulose from Aloe Vera Plant Residue and Preparation of Cellulose Nanocrystal–Poly(vinyl alcohol) Hydrogels" Molecules 29, no. 24: 6012. https://doi.org/10.3390/molecules29246012
APA StyleTriantafyllou, E., Karydis-Messinis, A., Moschovas, D., Kyriakaki, C., Vasilopoulos, K. C., Giannakas, A. E., Karakassides, M. A., Avgeropoulos, A., Zafeiropoulos, N. E., & Salmas, C. E. (2024). Microwave-Assisted Extraction of Cellulose from Aloe Vera Plant Residue and Preparation of Cellulose Nanocrystal–Poly(vinyl alcohol) Hydrogels. Molecules, 29(24), 6012. https://doi.org/10.3390/molecules29246012








