Recent Advances in the Synthesis of Cyclic Sulfone Compounds with Potential Biological Activity
Abstract
1. Introduction
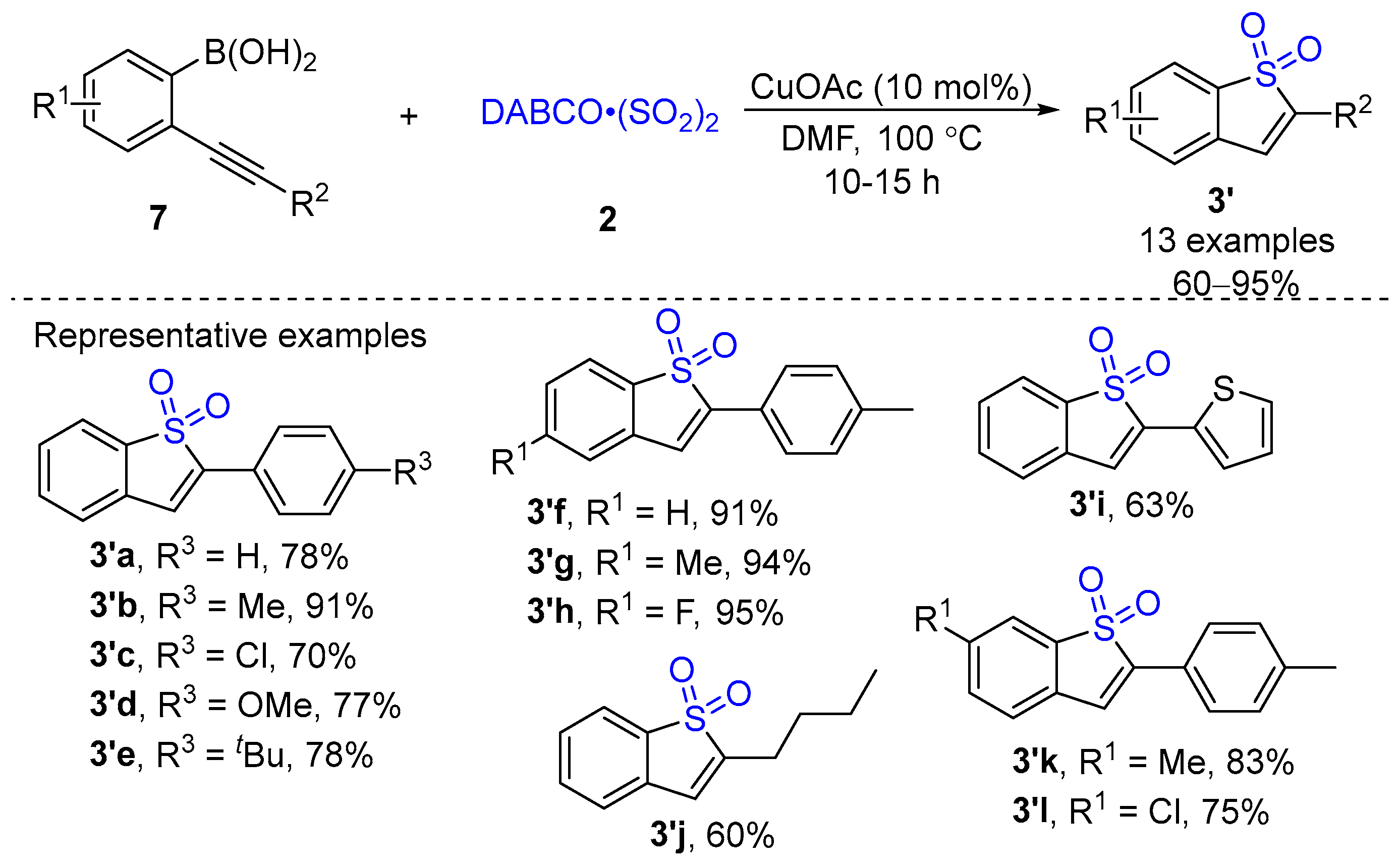
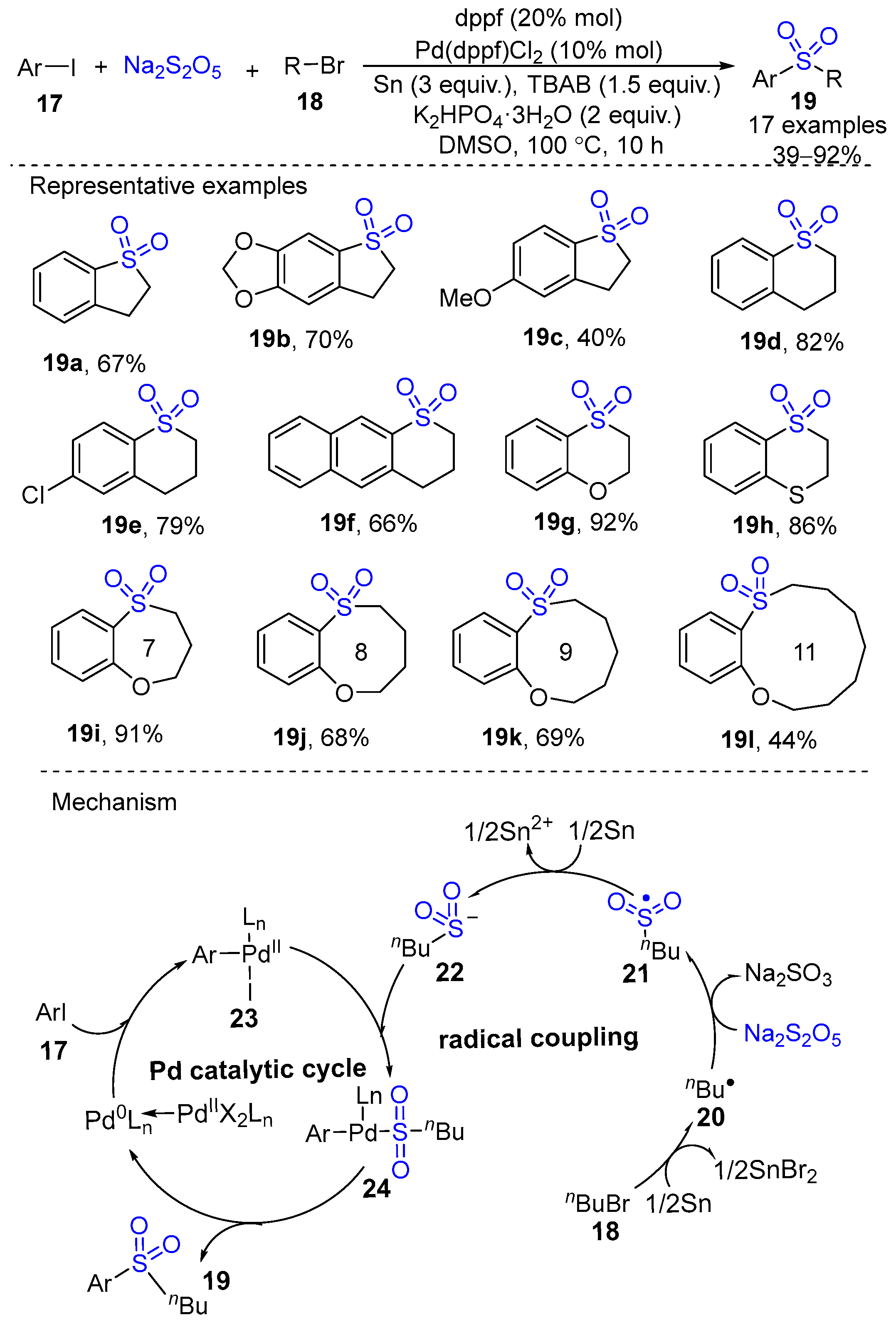
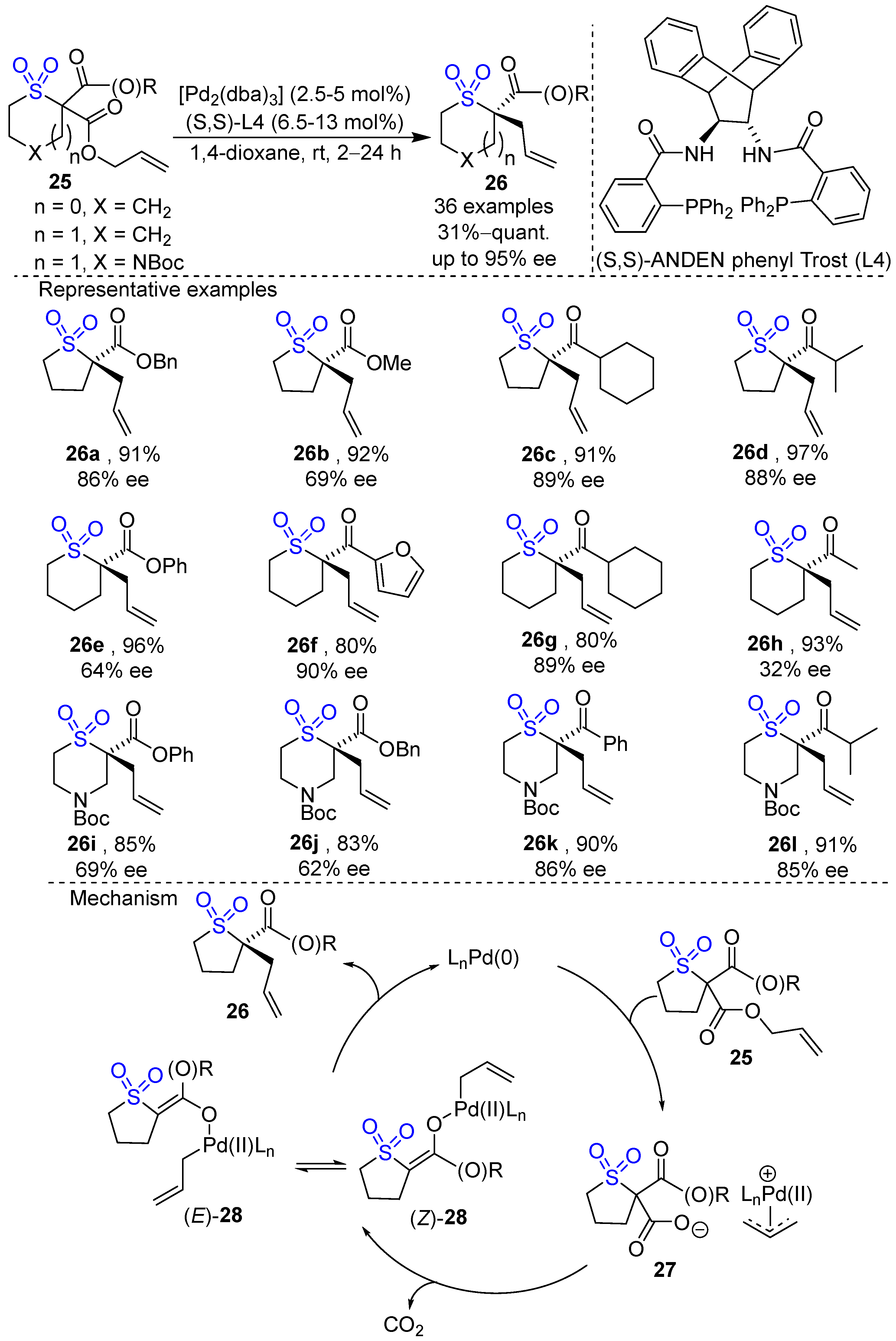
2. Metal-Catalyzed Synthesis of Cyclic Sulfone Compounds
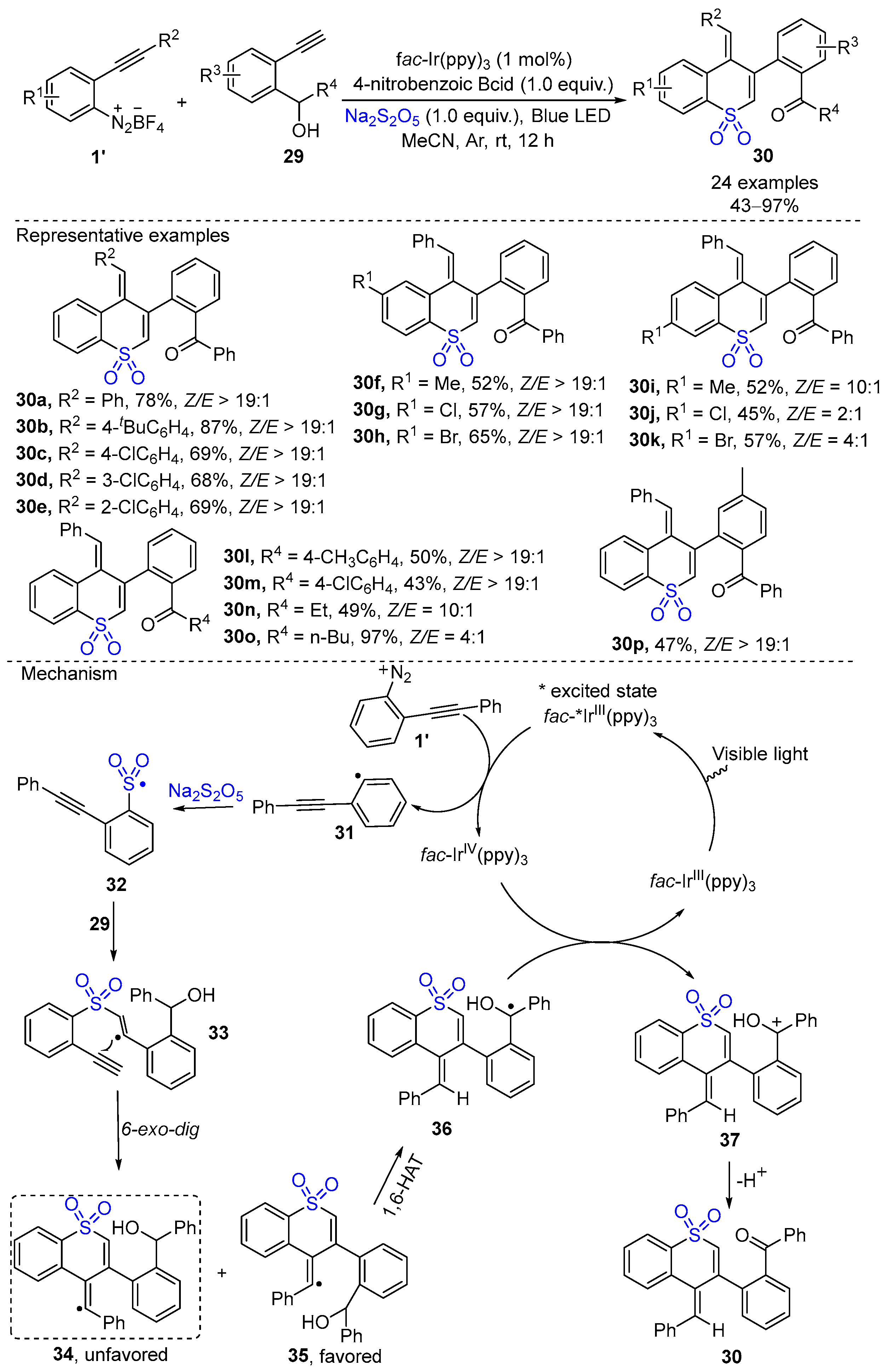
3. Photocatalytic Synthesis of Cyclic Sulfones
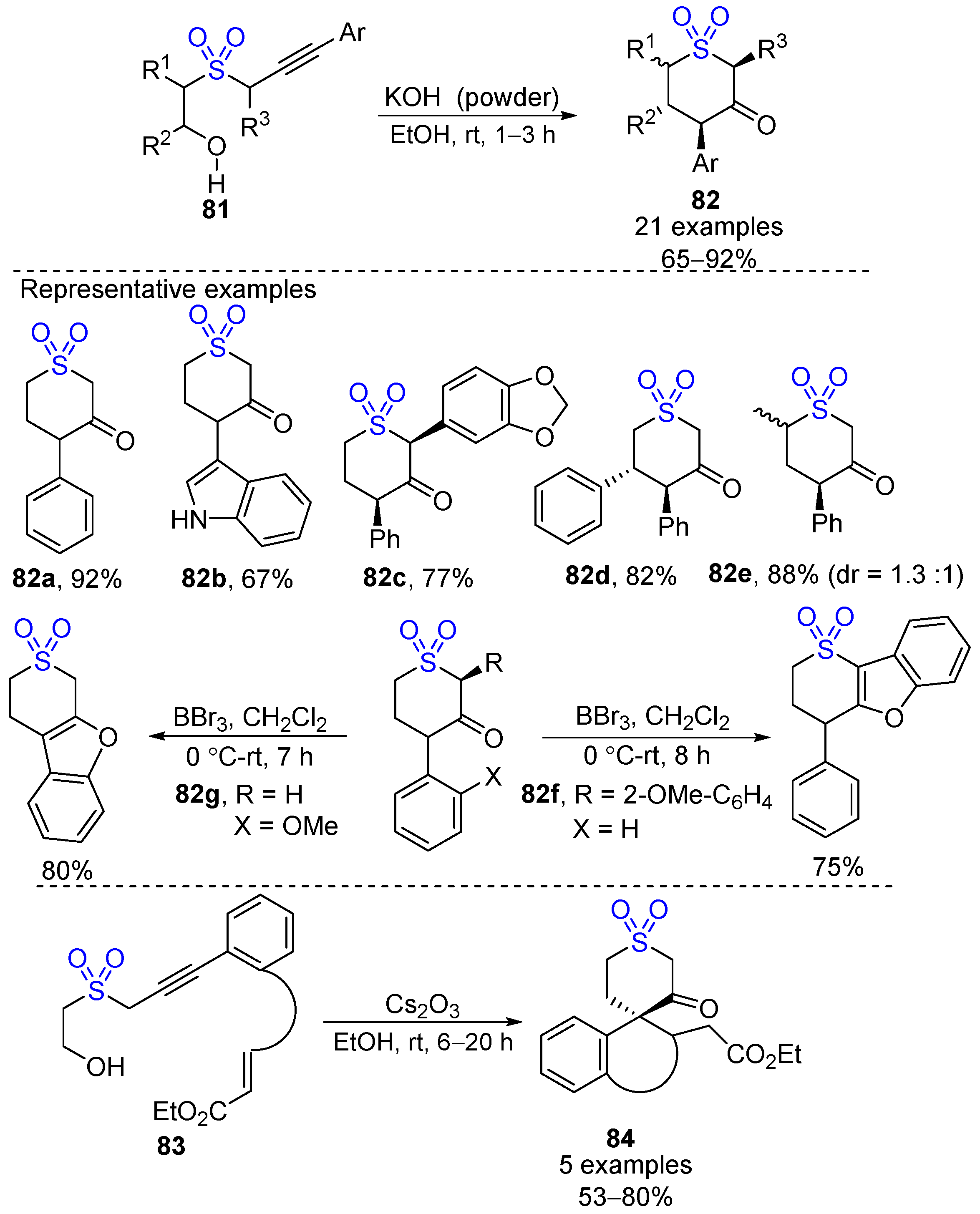
4. Other Methods for Synthesizing Cyclic Sulfones
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
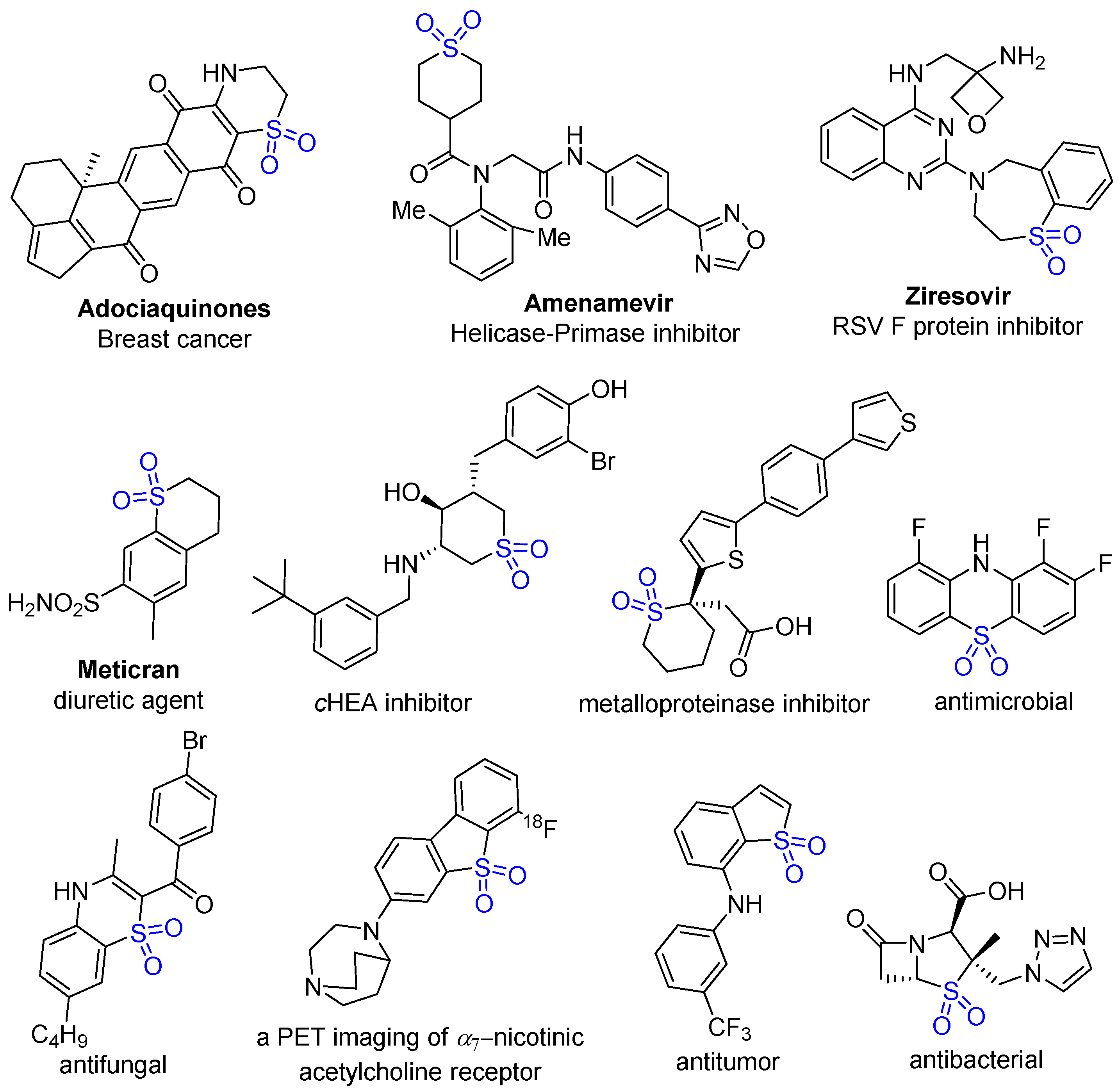
- Alam, M.A.; Shimada, K.; Jahan, A.; Khan, M.W.; Bhuiyan, M.M.H.; Alam, M.S.; Matin, M.M. Synthesis, Reactions and Medicinal Importance of Cyclic Sulfone Derivatives: A Review. Nat. Prod. Chem. Res. 2018, 6, 1000350–1000357. [Google Scholar]
- Regueiro-Ren, A. Chapter One—Cyclic sulfoxides and sulfones in drug design. In Advances in Heterocyclic Chemistry; Academic Press: Cambridge, MA, USA, 2021; Volume 134, pp. 1–30. [Google Scholar]
- Chen, G.; He, R.; Yang, W.; Zhang, B. Synthesis and Optical and Electrochemical Properties of Water-soluble Cationic Fluorophores Based on Bispyridinium and Dibenzothiophene-S,S-Dioxide. New J. Chem. 2017, 41, 1696–1703. [Google Scholar] [CrossRef]
- He, F.; Mai, L.; Longeon, A.; Copp, B.; Loaëc, N.; Bescond, A.; Meijer, L.; Bourguet-Kondracki, M.-L. Novel Adociaquinone Derivatives from the Indonesian Sponge xestospongia sp. Mar. Drugs 2015, 13, 2617–2628. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Liu, H.; Huang, H. Iron-Catalyzed Fluoroalkylative Alkylsulfonylation of Alkenes via Radical-anion Relay. Nat. common. 2024, 15, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Gao, L.; Wang, L.; Liang, C.; Wang, B.; Liu, Y.; Feng, S.; Zhang, B.; Zhou, M.; Yu, X.; et al. Discovery of Ziresovir as a Potent, Selective, and Orally Bioavailable Respiratory Syncytial Virus Fusion Protein Inhibitor. J. Med. Chem. 2019, 62, 6003–6014. [Google Scholar] [CrossRef]
- Gharpure, S.J.; Fartade, D.J.; Nanda, S.K.; Somani, S. Hydroalkoxylation-Initiated Cascade on Sulfone-Tethered Aryl Alkynols Gives Cyclic and Spiro-Heterocyclic β-Ketosulfones. Org. Lett. 2023, 25, 6155–6160. [Google Scholar] [CrossRef]
- Rueeger, H.; Lueoend, R.; Rogel, O.; Rondeau, J.-M.; Möbitz, H.; Machauer, R.; Jacobson, L.; Staufenbiel, M.; Desrayaud, S.; Neumann, U. Discovery of Cyclic Sulfone Hydroxyethylamines as Potent and Selective β-Site APP-Cleaving Enzyme 1 (BACE1) Inhibitors: Structure-Based Design and in Vivo Reduction of Amyloid β-Peptides. J. Med. Chem. 2012, 55, 3364–3386. [Google Scholar] [CrossRef]
- Bowen, E.; Laidlaw, G.; Atkinson, B.C.; McArdle-Ismaguilov, T.A.; Franckevičius, V. Catalytic Enantioselective Synthesis of α-Difunctionalized Cyclic Sulfones. J. Org. Chem. 2022, 87, 10256–10276. [Google Scholar] [CrossRef]
- Teodoro, R.; Scheunemann, M.; Deuther-Conrad, W.; Wenzel, B.; Fasoli, F.; Gotti, C.; Kranz, M.; Donat, C.; Patt, M.; Hillmer, A.; et al. A Promising PET Tracer for Imaging of α7 Nicotinic Acetylcholine Receptors in the Brain: Design, Synthesis, and in Vivo Evaluation of a Dibenzothiophene-based Radioligand. Molecules 2015, 20, 18387–18421. [Google Scholar] [CrossRef]
- Zhu, C.; Cai, Y.; Jiang, H. Recent Advances for the Synthesis of Chiral Sulfones with the Sulfone Moiety Directly Connected to the Chiral Center. Org. Chem. Front. 2021, 8, 5574–5589. [Google Scholar] [CrossRef]
- Gautam, N.; Bishnoi, A.K.; Guleria, A.; Jangid, D.K.; Gupta, S.K.; Gautam, D.C. Synthesis, Characterization and in Vitro Antimicrobial Assessment of Some Novel 4H-1, 4-Benzothiazines and their Sulfone Derivatives. Heterocycl. Commun. 2013, 19, 37–42. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, T.; Li, S.; Yang, Y.; Guo, J.; Yu, W.; Kong, L. Antagonizing STAT3 Activation with Benzo[b]thiophene 1,1-Dioxide Based Small Molecules. Eur. J. Med. Chem. 2017, 125, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Efremova, I.E.; Lapshina, L.V.; Baichurin, R.I.; Serebryannikova, A.V.; Savel’ev, I.I. Synthesis of Polycyclic Systems Based on Sulfolenes. Russ. J. Gen. Chem. 2020, 90, 1369–1387. [Google Scholar] [CrossRef]
- Shyshkina, O.O.; Popov, K.S.; Gordivska, O.O.; Tkachuk, T.M.; Kovalenko, N.V.; Volovnenko, T.A.; Volovenko, Y.M. Synthesis and Chemical Properties of Cyclic β-keto Sulfones (review). Chem. Heterocycl. Com. 2011, 47, 923–945. [Google Scholar] [CrossRef]
- Zhang, R.; Ding, H.; Pu, X.; Qian, Z.; Xiao, Y. Recent Advances in the Synthesis of Sulfides, Sulfoxides and Sulfones via C-S Bond Construction from Non-Halide Substrates. Catalysts 2020, 10, 1339. [Google Scholar] [CrossRef]
- Li, C.; Mizuno, N.; Murata, K.; Ishii, K.; Suenobu, T.; Yamaguchi, K.; Suzuki, K. Selectivity Switch in the Aerobic Oxygenation of Sulfides Photocatalysed by Visible-Light-Responsive Decavanadate. Green Chem. 2020, 22, 3896–3905. [Google Scholar] [CrossRef]
- Liu, N.-W.; Liang, S.; Manolikakes, G. Recent Advances in the Synthesis of Sulfones. Synthesis 2016, 48, 1939–1973. [Google Scholar]
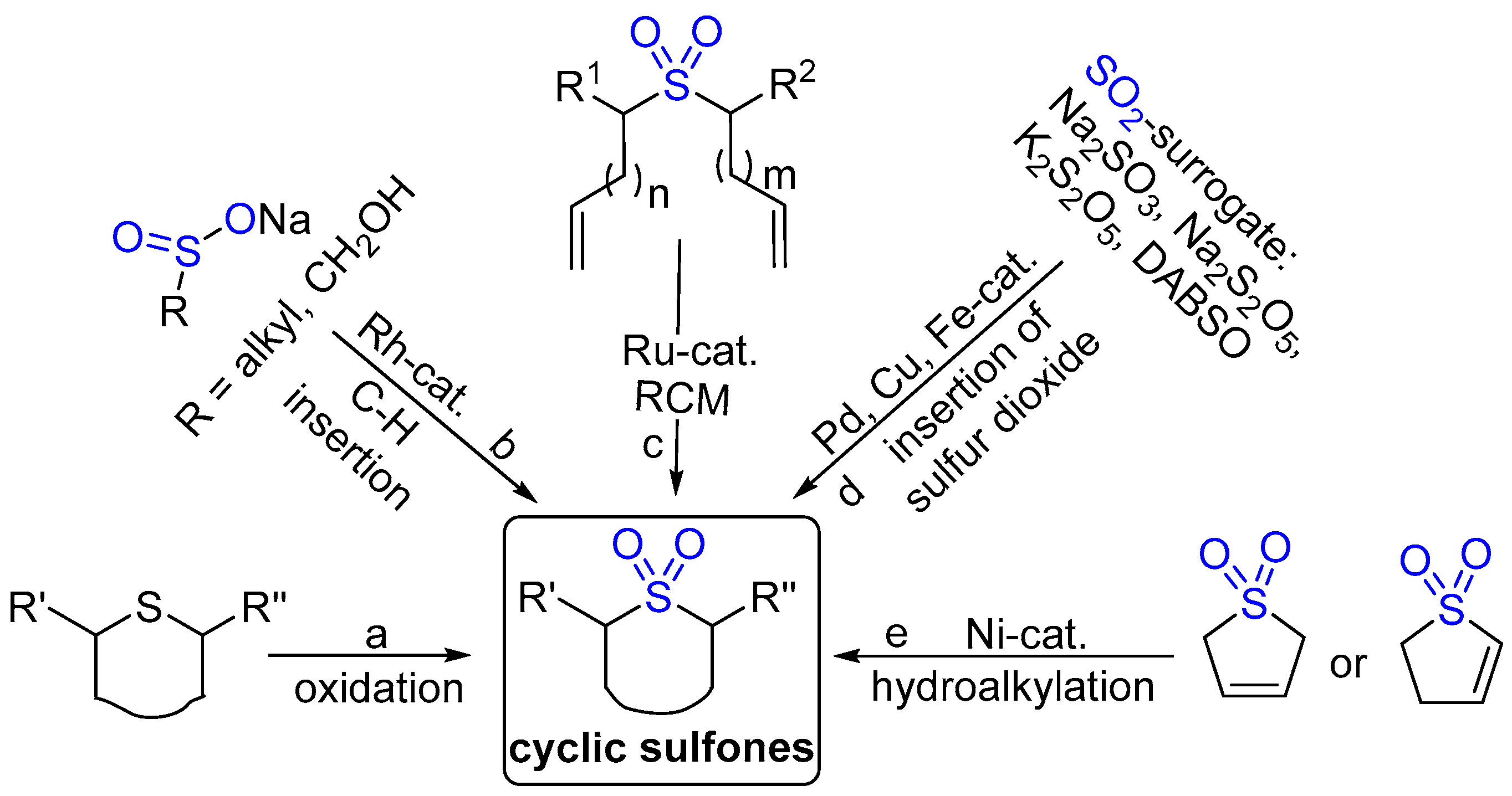
- John, J.P.; Novikov, A.V. Selective Formation of Six-Membered Cyclic Sulfones and Sulfonates by C-H Insertion. Org. Lett. 2007, 9, 61–63. [Google Scholar] [CrossRef]
- Rodríguez, A.; Moran, W.J. Preparation of Alkyl Alkynyl Sulfones and Cyclic Vinyl Sulfones from Alkynyl(aryl)iodonium Salts. J. Org. Chem. 2016, 81, 2543–2548. [Google Scholar] [CrossRef]
- Yao, Q. Synthesis of Cyclic Sulfones by Ring-Closing Metathesis. Org. Lett. 2002, 4, 427–430. [Google Scholar] [CrossRef]
- Lu, X.; Huang, Y.; Yang, Y. Advances in the Synthesis of Sulfur-Containing Cyclic Architectures via Insertion of SO2. Synlett 2024, 36, 15–28. [Google Scholar] [CrossRef]
- Liang, S.; Hofman, K.; Friedrich, M.; Keller, J.; Manolikakes, G. Recent Progress and Emerging Technologies towards a Sustainable Synthesis of Sulfones. ChemSusChem 2021, 14, 4878–4902. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Dhawa, U.; Qian, D.; Sakic, D.; Morel, J.; Hu, X. Regiodivergent and Enantioselective Synthesis of Cyclic Sulfones via Ligand-Controlled Nickel-Catalyzed Hydroalkylation. Angew. Chem. Int. Ed. 2024, 63, e202406767. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-K.; Li, Y.-L.; Zhang, W.; Zhang, S.-S.; Qiu, T.-T.; Ma, X. Facile synthesis of tricyclic isoxazole-fused benzo[b]thiophene1,1-dioxide derivatives via 1,3-dipolar cycloaddition. Tetrahedron Lett. 2020, 6, 151943–151945. [Google Scholar] [CrossRef]
- Chen, X.; Tang, B.; He, C.; Ma, J.; Zhuang, S.; Wu, Y.; Wu, A. Rongalite as a Sulfone Source: A Novel Copper-Catalyzed Sulfur Dioxide Anion Incorporation Process. Chem. Commun. 2020, 56, 13653–13656. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Li, M.-L.; Hu, H.-X.; Teng, F. Recent Advances in Palladium-Catalyzed Sulfonylation via SO2 Insertion. Org. Biomol. Chem. 2024, 22, 5868–5885. [Google Scholar] [CrossRef]
- Qiu, G.; Lai, L.; Cheng, J.; Wu, J. Recent Advances in the Sulfonylation of Alkenes with the Insertion of Sulfur Dioxide via Radical Reactions. Chem. Commun. 2018, 54, 10405–10414. [Google Scholar] [CrossRef]
- Pincekova, L.; Merot, A.; Schäfer, G.; Willis, M.C. Sandmeyer Chlorosulfonylation of (Hetero)Aromatic Amines Using DABSO as an SO2 Surrogate. Org. Lett. 2024, 26, 5951–5955. [Google Scholar] [CrossRef]
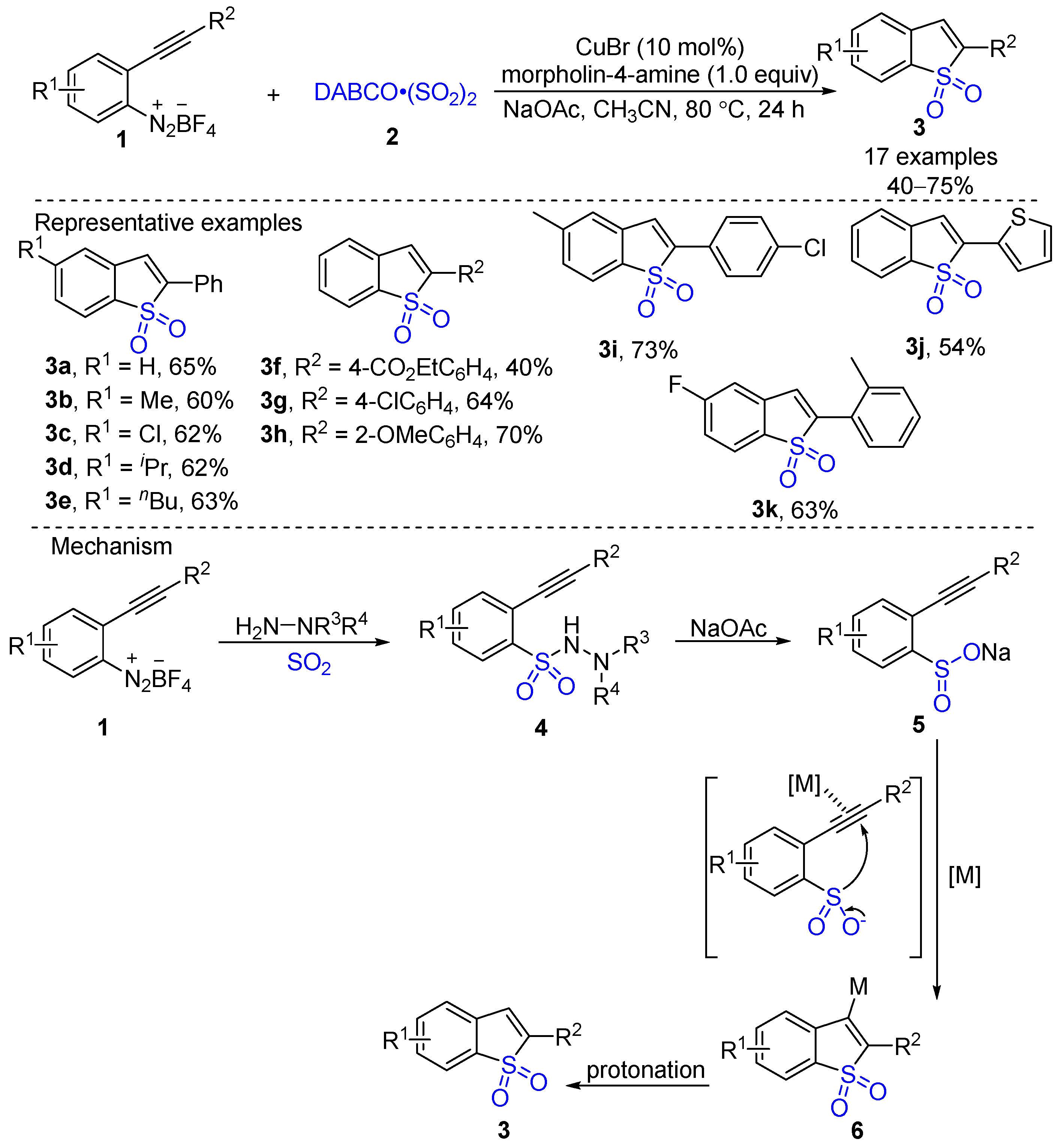
- Luo, Y.; Pan, X.; Chen, C.; Yao, L.; Wu, J. An Unexpected Reaction of 2-Alkynylaryldiazonium Tetrafluoroborate with Sulfur Dioxide. Chem. Commun. 2015, 51, 180–182. [Google Scholar] [CrossRef]
- Mao, R.; Zheng, D.; Xia, H.; Wu, J. Copper(I)-Catalyzed Sulfonylation of (2-Alkynylaryl)Boronic Acids with DABSO. Org. Chem. Front. 2016, 3, 693–696. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Jiang, X. Construction of Functionalized Annulated Sulfone via SO2/I Exchange of Cyclic Diaryliodonium Salts. Org. Lett. 2017, 19, 4916–4919. [Google Scholar] [CrossRef] [PubMed]
- Laha, J.K.; Sharma, S. Palladium-Catalyzed Intramolecular Oxidative Arylations for the Synthesis of Fused Biaryl Sulfones. ACS Omega 2018, 3, 4860–4870. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wang, M.; Jiang, X. Multicomponent Reductive Cross-Coupling of an Inorganic Sulfur Dioxide Surrogate: Straightforward Construction of Diversely Functionalized Sulfones. Angew. Chem. Int. Ed. 2019, 59, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, C.; Wang, X.; Zhou, X.; Xiao, W.; Wu, J. Photoinduced Radical Sulfur DioxideInsertion with Asymmetric Cyclization of Alkenes: Accessing β-Chiral Sulfones bearing S-Stereocentric Cyclic Sulfinamides. Sci. China Chem. 2024. [Google Scholar] [CrossRef]
- Tu, J.-L.; Zhu, Y.; Li, P.; Huang, B. Visible-light Induced direct C(sp3)–H Functionalization: Recent Advances and Future Prospects. Org. Chem. Front. 2024, 11, 5278–5305. [Google Scholar] [CrossRef]
- Song, K.-X.; Qin, X.-Y.; Ma, Z.-X.; Geng, F.-Z.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Photocatalytic Three-Component Annulation Enabling Stereoselective Generation of (Z)-thiochromene 1,1-Dioxides. Org. Chem. Front. 2021, 8, 5681–5686. [Google Scholar] [CrossRef]
- Mandal, T.; Mallick, S.; Islam, M.; Sarkar, S.D. Alcohols as Alkyl Synthons Enabled by Photoredox-Catalyzed Deoxygenative Activation. ACS Catal. 2024, 14, 13451–13496. [Google Scholar] [CrossRef]
- Huang, H.-L.; Li, S.; Lv, Y.-Z.; Shi, Y.-Q.; Pang, T.-T.; Zhang, R.-F.; Huang, W.; Yin, J.; Gao, F. Efficient Functionalization of Organosulfones via Photoredox Catalysis: Direct Incorporation of α-Carbonyl Alkyl Side Chains into α-Allyl-β-Ketosulfones. Molecules 2024, 29, 1971. [Google Scholar] [CrossRef]
- Han, J.-B.; Yang, L.; Chen, X.; Zha, G.-F.; Zhang, C.-P. Perfluoroalkanesulfonylation of Alkynyl(phenyl)iodonium Tosylates by the Weakly Nucleophilic Sodium Perfluoroalkanesulfinates. Adv. Synth. Catal. 2016, 358, 4119–4124. [Google Scholar] [CrossRef]
- Qin, X.-Y.; He, L.; Li, J.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Regioselective Synthesis of Polycyclic Sulfones via Radical-Induced Three-Component Bicyclization Cascades. Chem. Commun. 2019, 55, 3227–3230. [Google Scholar] [CrossRef]
- Jia, X.; Huang, C.; Zhang, X.; Lian, Z. Metal-free Sulfonylative Annulations of Alkyl Diiodides with Sulfur Dioxide: Synthesis of Cyclic Aliphatic Sulfones. Org. Chem. Front. 2021, 8, 5310–5315. [Google Scholar] [CrossRef]
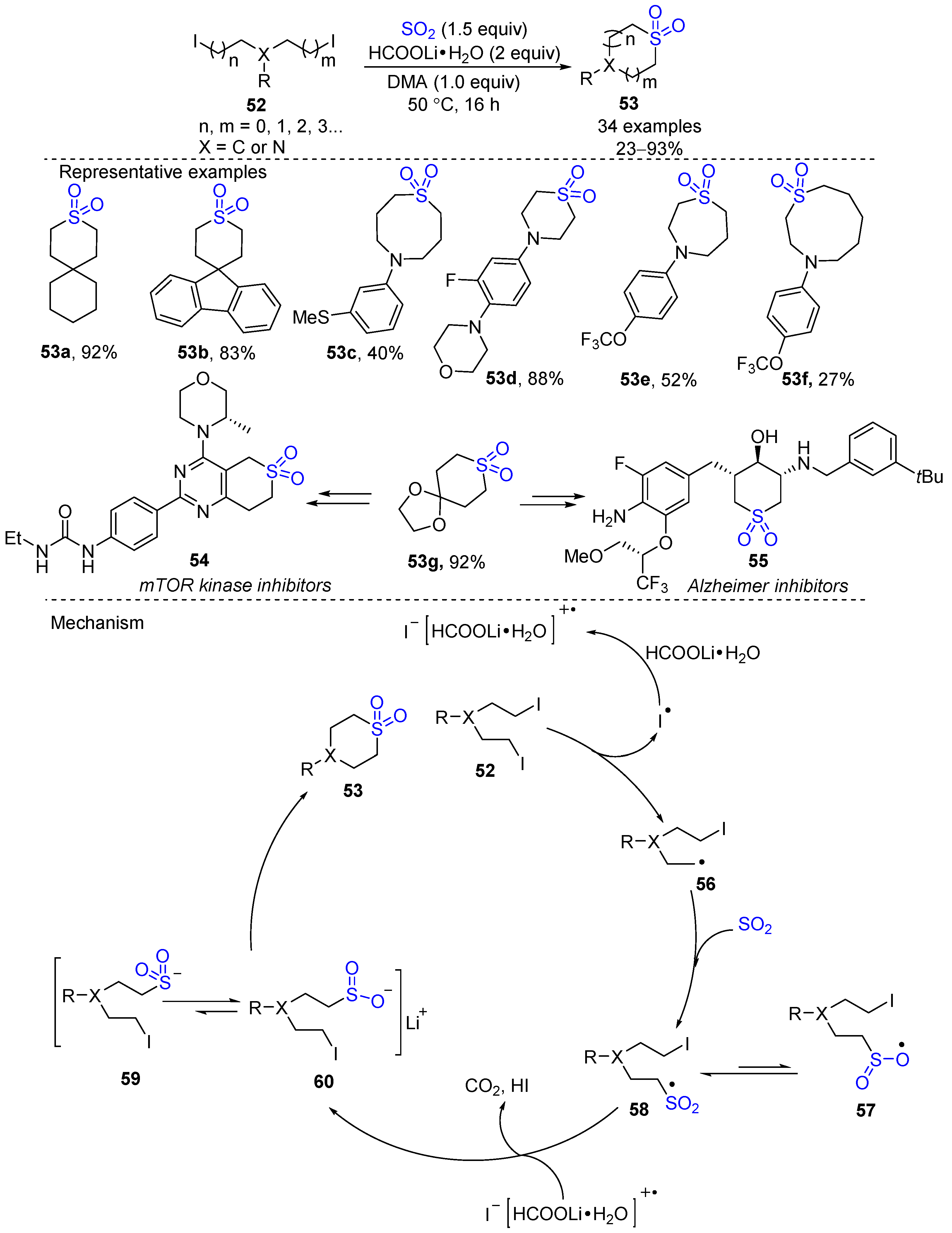
- Hugelshofer, C.L.; Bao, J.; Du, J.; Ashley, E.; Yu, W.; Ji, T.; Hu, B.; Liu, D.; Rondla, R.; Karampuri, S.; et al. Scalable Preparation of 4,4-Disubstituted Six-Membered Cyclic Sulfones. Org. Lett. 2021, 23, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O. Microwave Dielectric Heating in Synthetic Organic Chemistry. Chem. Soc. Rev. 2008, 37, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
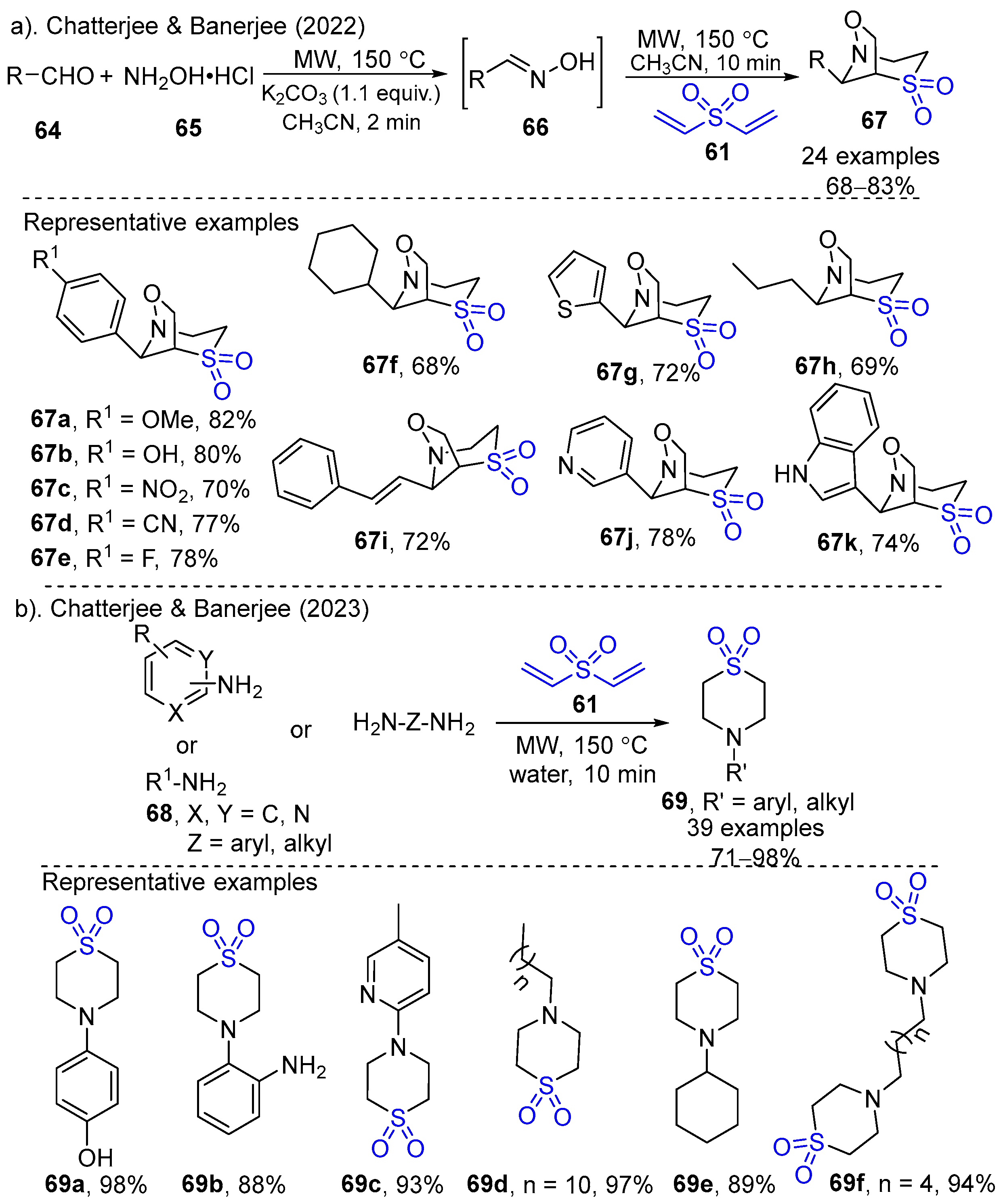
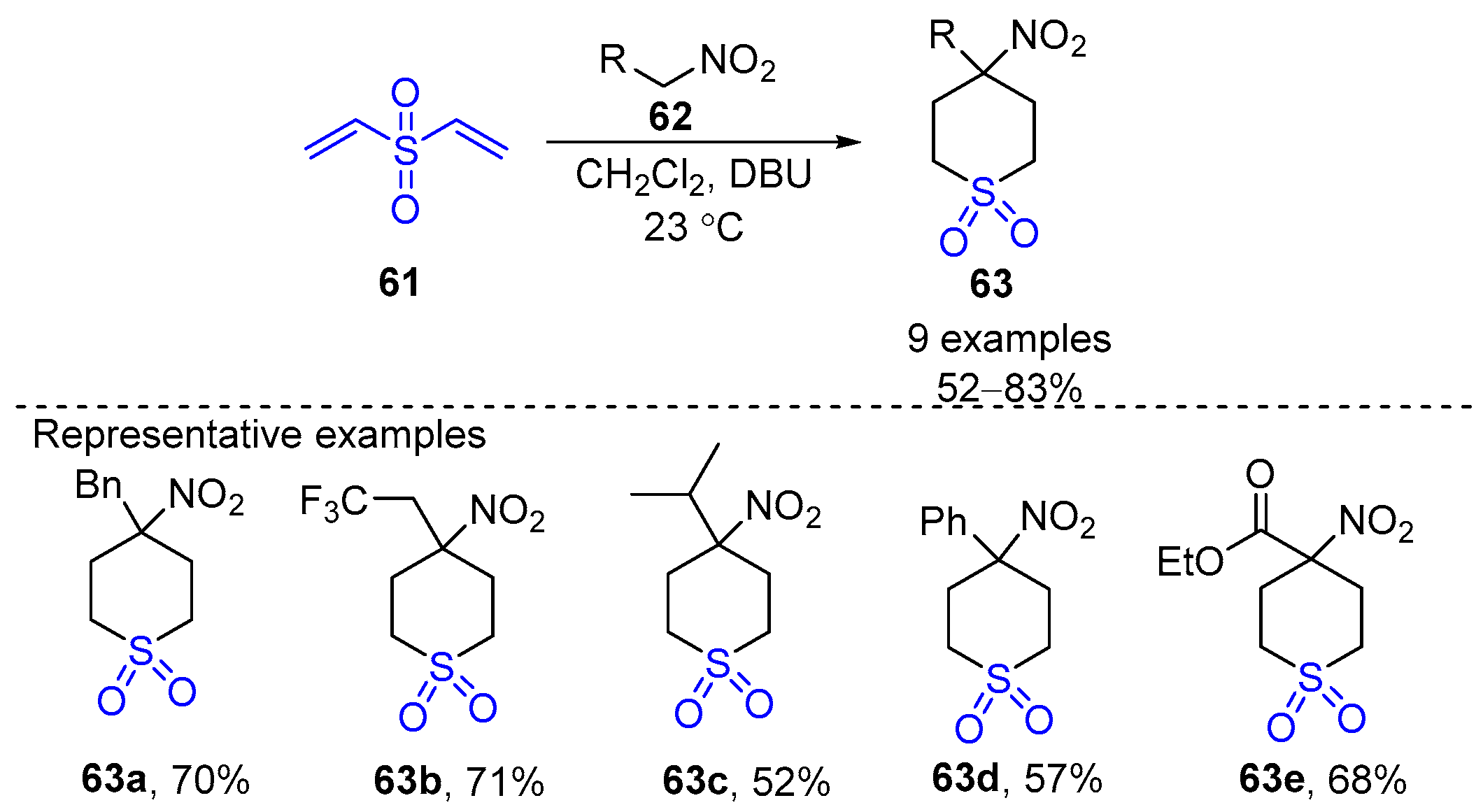
- Panjikar, P.C.; Saha, S.; Chatterjee, A.; Banerjee, M. Microwave Assisted Rapid Synthesis of Bicyclo Aza-Sulfone Derivatives from Aldehydes via Aldoxime Formation Followed by Michael Addition-1,3-Dipolar Cycloaddition with Divinyl Sulfone in one-pot. Tetrahedron Lett. 2022, 111, 154209–154214. [Google Scholar] [CrossRef]
- Saha, S.; Chatterjee, A.; Banerjee, M. Reagentless Chemistry “On-Water”: An Atom-Efficient and “Green” Route to Cyclic and Acyclic β-Amino Sulfones via aza-Michael Addition Using Microwave Irradiation. J. Org. Chem. 2023, 88, 15358–15366. [Google Scholar] [CrossRef]
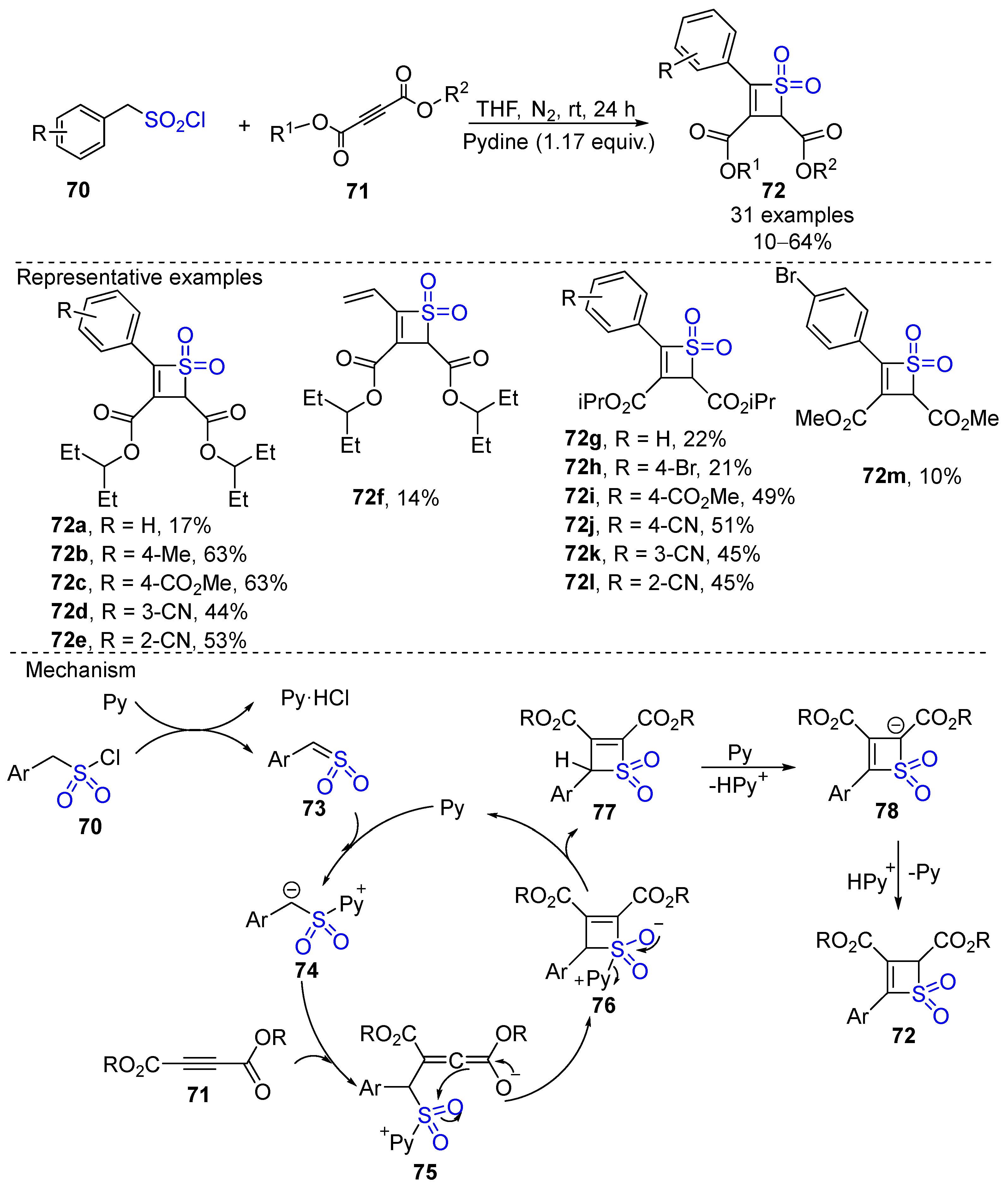
- Sun, S.; Zhang, M.; Xu, J. Direct Synthesis of Highly Strained Bifunctionalized 2H-thiete 1,1-Dioxides. Org. Chem. Front. 2022, 9, 3335–3341. [Google Scholar] [CrossRef]
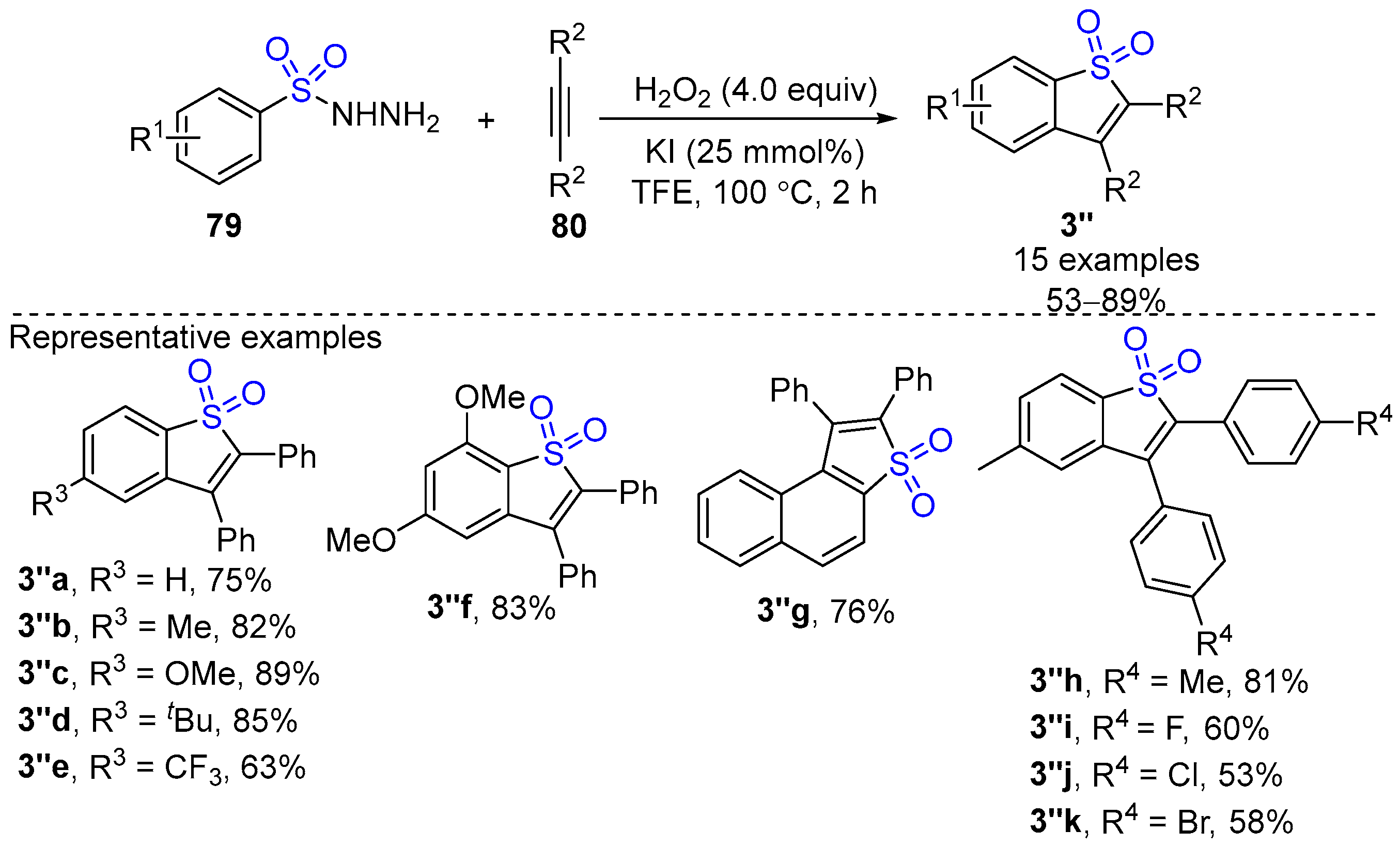
- Ma, Y.; Wang, K.; Zhang, D.; Sun, P. Solvent Controlled Transformation between Sulfonyl Hydrazides and Alkynes: Divergent Synthesis of Benzo[b]thiophene-1,1-Dioxides and (E) -β-iodo Vinylsulfones. Adv. Synth. Catal. 2018, 361, 597–602. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, J.; Wang, T.; Yang, X. Base-Promoted S-Arylation of Sulfenamides for the Synthesis of Sulfilimines. Org. Lett. 2023, 25, 4335–4339. [Google Scholar] [CrossRef]
- Chen, S.; Yang, L.; Shang, Y.; Mao, J.; Walsh, P.J. Base-Promoted Tandem Synthesis of 2-Azaaryl Tetrahydroquinolines. Org. Lett. 2021, 23, 1594–1599. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-L.; Shi, Y.-Q.; Ning, J.-X.; Li, S.; Song, D.-T.; Gao, F.; Ma, N. Recent Advances in the Synthesis of Cyclic Sulfone Compounds with Potential Biological Activity. Molecules 2024, 29, 5868. https://doi.org/10.3390/molecules29245868
Huang H-L, Shi Y-Q, Ning J-X, Li S, Song D-T, Gao F, Ma N. Recent Advances in the Synthesis of Cyclic Sulfone Compounds with Potential Biological Activity. Molecules. 2024; 29(24):5868. https://doi.org/10.3390/molecules29245868
Chicago/Turabian StyleHuang, Hong-Li, Ya-Qian Shi, Jia-Xin Ning, Shan Li, Dian-Tao Song, Fei Gao, and Na Ma. 2024. "Recent Advances in the Synthesis of Cyclic Sulfone Compounds with Potential Biological Activity" Molecules 29, no. 24: 5868. https://doi.org/10.3390/molecules29245868
APA StyleHuang, H.-L., Shi, Y.-Q., Ning, J.-X., Li, S., Song, D.-T., Gao, F., & Ma, N. (2024). Recent Advances in the Synthesis of Cyclic Sulfone Compounds with Potential Biological Activity. Molecules, 29(24), 5868. https://doi.org/10.3390/molecules29245868