Abstract
Organic compounds with 1,3-diketone or 3-amino enone functional groups are extremely important as they can be converted into a plethora of carbo- or heterocyclic derivatives or can be used as ligands in the formation of metal complexes. Here, we have achieved the preparation of a series of non-symmetrical β-ketoenamines (O,N,N proligand) of the type (4-MeOC6H4)C(=O)CH=C(R)NH(Q) obtained through the Schiff base condensation of 1,3-diketones (1-anisoylacetone, 1-anisyl-3-(4-cyanophenyl)-1,3-propanedione, and 1-anisyl-3-(4,4,4-trifluorotolyl)-1,3-propanedione) functionalized with electron donor and electron-withdrawing substituents and 8-aminoquinoline (R = CH3, 4-C6H4CN, 4-C6H4CF3; Q = C9H7N). Schiff base ketoimines with a pendant quinolyl moiety were isolated as single regioisomers in yields of 22–56% and characterized with FT-IR, 1H NMR, and UV-visible spectroscopy, as well as single-crystal X-ray crystallography, which allowed for the elucidation of the nature of the isolated regioisomers. The regioselectivity of the condensation of electronically unsymmetrical 1,3-diaryl-1,3-diketones with 8-aminoquinoline was studied by 1H NMR, providing regioisomer ratios of ~3:1 and ~2:1 in the case of CN and CF3 substituents, respectively. The electronic effects correlate well with the difference between the Hammett σ+ coefficients of the two para substituents on the aryl rings.
1. Introduction
Schiff bases, characterized by an imine (>C=N-) group [1,2], form an outstanding class of compounds that play a key role in organic chemistry and in the development of coordination chemistry due to their facile synthesis, structural flexibility, easily tunable electronic properties, versatility, and their ability to form stable complexes under various coordination geometries and oxidation states [3,4,5,6]. ONX-tridentate Schiff base proligands (X = O, N, S), resulting from the monocondensation of 1,3-diketones with primary amines [7,8,9], can exist in solution as a tautomeric mixture of ketoamine (enaminone, -(O=)C(1R)-CH=C(2R)-NH-) and ketoimine (iminone, -(O=)C(1R)-CH2-C(2R)=N-) forms (see Chart 1) [10].
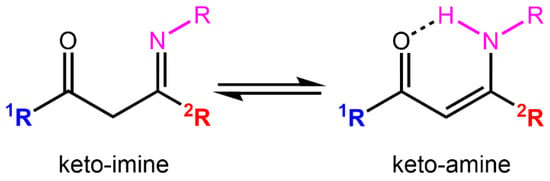
Chart 1.
Tautomeric forms reported for ONN Schiff base derivatives obtained by condensation of β-diketones with primary amines; R1 = electron donor group and R2 = electron acceptor group.
When an unsymmetrical 1,3-diketone (R1 ≠ R2) is reacted with a primary amine, two regioisomers are possible, resulting from amine addition on the R1 or R2 side. The regioselectivity of the reaction has been demonstrated to be determined by a combination of steric and electronic factors; the condensation reaction at the carbonyl group adjacent to the less sterically hindered or more electron-withdrawing substituent is generally favored [11,12,13,14,15]. In their deprotonated forms, non-symmetrical bidentate β-enaminoketonato ligands are among the most ubiquitous chelating systems in coordination chemistry because they are easy to prepare and both their steric and electronic properties can be modified simply [16]. A lot of corresponding metal complexes bearing such pincer-like ligands containing nitrogen donors have, therefore, been prepared and have received increased attention as catalysts for organic synthesis [17,18] and for the preparation of L-lactide ring-opening polymerization initiators [12,13,14,19,20] and olefin polymerization catalysts [15]. β-Enaminoketonato metal complexes acting as metalloligands were also employed to construct new multimetallic architectures with desired properties [21,22,23].
On the other hand, it is worth mentioning that tridentate ONN Schiff base ligands derived from dipolar 1,3-diaryl-1,3-diketones functionalized with terminal electron donor (D) and electron acceptor (A) groups have not been reported. These new molecular systems are of great interest to our research field in pursuing the development of chelating chromophores with modulated push–pull properties for potential applications as functional molecular materials [24,25]. In this contribution, we report the successful synthesis, spectroscopic and structural characterization, and the regioselectivity study of a new class of tridentate ONN Schiff base proligands derived from appropriate 1,3-diaryl-1,3-diketones in which the aryl rings were electronically dissimilar but sterically identical by virtue of para-substitution, and bearing a planar quinolyl pendant donor.
2. Results and Discussion
2.1. Synthesis and Characterization
Compounds SB1–SB3 were synthetized through a Schiff base condensation reaction with 1,3-diketones (β1–β3), which were functionalized with electron donor and electron-withdrawing substituents with 8-aminoquinoline and were isolated as pure samples in yields of 22–56% upon column chromatography on silica gel, followed by fractional crystallization (see Section 3.3) (Scheme 1). The reactions were carried out in refluxing toluene, and the water generated was removed by azeotropic distillation. As expected, the condensation reaction was faster starting from the most electron-rich diketone, β1, being over after 16 h, while 24 h was needed to reach completion with the push–pull-type diketones β2 and β3 (TLC monitoring). The Schiff base derivatives SB1–SB3 are air- and moisture-insensitive, thermally stable, and soluble in common organic solvents such as diethyl ether, DCM, chloroform, THF, and acetone, but they are sparingly soluble in n-hexane, methanol, and ethanol.
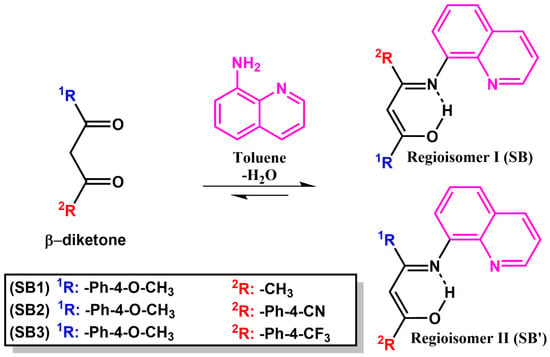
Scheme 1.
Synthetic pathway for obtaining ONN Schiff base molecules SB1–SB3. In SB2 and SB3, both possible regioisomers I and II were detected in the bulk analysis.
The Schiff base derivative SB1 was formed as a single regioisomer, resulting from condensation taking place at the least impeded carbonyl functionality, which is the one nearest to the methyl group of the β-diketonic skeleton of β1. This follows the general trend observed for Schiff condensation of monosubstituted 1-R-1,3-butadione (R = organic or organometallic moieties) with primary amines [8,9,26,27,28]. The synthesis of compounds SB2 and SB3, derived from Schiff condensation between the dipolar 1,3-diketones β2 (MeO/CN) and β3 (MeO/CF3) and 8-aminoquinoline, resulted in the formation of regioisomer mixtures SB2/SB2′ and SB3/SB3′, respectively, due to the addition of 8-aminoquinoline to one or the other carbonyl group of the diketone (Scheme 1).
Analysis of the 1H NMR spectrum of each isomeric mixture, which gave rise to two sets of signals (see Figure 1 and Figures S13 and S14), allowed for the determination of the experimental yield of the condensation reactions and the relative formation ratio of the regioisomers. Comparison of the integration of the signals associated with the methine proton (δ = CH ~6.2 ppm, see Section 3.3 and Table S2) led to formation ratios of ~3:1 and ~2:1 for SB2:SB2′ and SB3/SB3′, respectively. This correlates well with the electronic nature of the substituents. Indeed, according to Hammett’s coefficients (σ), the CN group (0.66), contained in the diketone precursor β2, has a higher electron-withdrawing ability than the CF3 substituent (0.54) borne by the diketone β3 [29]. Such a behavior has previously been observed in the reaction of electronically unsymmetrical 1,3-diaryl-1,3-diketones with hydrazinopyridines to form 3,5-diarylpyrazolylpyridines [11]. The calculated experimental yields of the main reaction products for SB2 and SB3 were 46 and 49%, respectively.
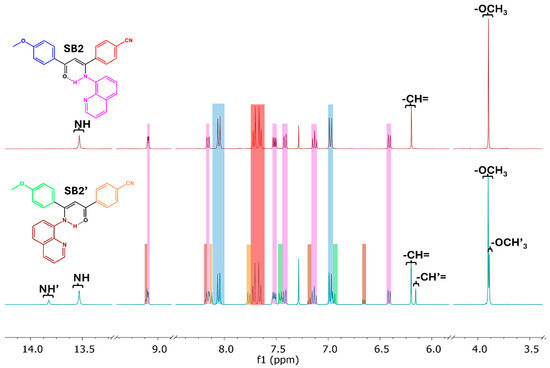
Figure 1.
Comparative 1H NMR spectra of SB2 (top) and regioisomer mixtures SB2/SB2′ (bottom). Spectra acquired in CDCl3 at 400 MHz and 20 °C. The associated resonances are given in the color of the respective fragments in each case.
The regioselectivity in the formation of enaminones is correlated with the electronic nature of the substituents at the para position. This resulted in the formation of a mixture of two regioisomers, with the largest amount of product formed by condensation of the carbonyl closest to the aromatic ring bearing the electron acceptor substituent. Related results have been reported for condensation reactions using 1,3-diaryl-1,3-diketones with terminal electron donor (D) and acceptor (A) substituents as precursors [11,30]. The relative ratio of the products formed increases with an increase in the electron donor/acceptor strength of the terminal substituents.
The formation of the regioisomers is very difficult to follow by thin-layer chromatography (TLC) and to separate by column chromatography. Alternatively, fractional crystallization using the fractions obtained from column chromatography (see Section 3.3) afforded single crystals of one of the two isomers, suitable for X-ray diffraction analysis (see Section 2.2), authenticating the presence of the two regioisomers in the bulk obtained by the Schiff base condensation reaction. Therefore, it was possible to isolate only one of the two isomers, which crystallizes preferentially to the other one, allowing the isolation of presumably the most abundant regioisomer in yields of 26 and 22% for SB2 and SB3, respectively (see Section 3.3). The obtention of the single regioisomer compounds SB1–SB3 was confirmed by FT-IR and 1H NMR spectroscopy. In addition, single-crystal X-ray diffraction analysis was performed to elucidate the molecular structure of the three Schiff base compounds (see Section 2.2).
The solid-state FT-IR spectra (KBr pellets) of the isolated regioisomers SB1–SB3 (Figures S4–S6 and Table S1) exhibit the characteristic absorption bands of Schiff base compounds derived from 1,3-diketones reported in the literature [31]. The spectra show vibrational bands of strong and medium intensity between 1603 and 1545 cm−1, mainly associated with the stretching vibrations of C=O, C=N, and/or C=C. In addition, the spectrum of SB2 displays a low-intensity band attributed to the stretching vibrations of C≡N at 2223 cm−1, while that of SB3 shows a strong intensity absorption band associated to stretching vibration of C-F at 1322 cm−1 [32].
The exploration of the 1H NMR spectra of isolated compound SB1–SB3 (Figure 2 and Figures S10–S12, Table S2) revealed signals associated with the keto-amine tautomer, showing three singlets between 3.87 and 3.88 ppm (3H), 6.03 and 6.21 (1H), and 13.53 and 14.01 (1H) ppm, attributed to the resonances of the methoxy (anisyl), methine (=CH-), and NH amine protons, in agreement with reported observations for a tautomeric enaminone structure [9,27]. The anisyl protons give rise to two doublets at 6.96–6.98 and 8.02–8.06 ppm, integrating both for 2H. On the other hand, in the SB2 spectrum, the benzonitrile protons show up as two doublets at 7.66 and 7.71 ppm (2H:2H), while those of p-C6H4CF3 of SB3 appear at a singlet at 7.68 ppm that integrates for 4H.
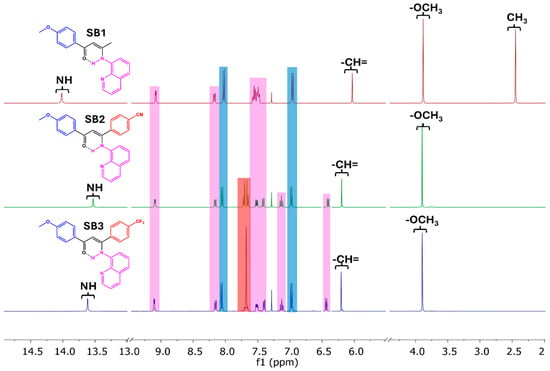
Figure 2.
Comparative 1H NMR spectra of Schiff base compounds SB1–SB3. All spectra were acquired in CDCl3 at 400 MHz and 20 °C. The associated resonances are given in the color of the respective fragments in each case.
The resonances associated with the quinolinic fragment in SB1 are seen as two doublet of doublets at 8.18 and 9.08 ppm, which integrate for 1H, and a multiplet at 7.57–7.47 ppm integrating for 4H. Those observations are in accordance with spectral data previously reported for Schiff base compounds derived from 1,3-diketones and 8-aminoquinoline [26]. On the other hand, the 1H NMR spectra of SB2 and SB3 exhibit the resonances associated with the heterocycle as a doublet at 6.41/6.44 ppm, a triplet at 7.13/7.13 ppm, and four doublets at 7.42/7.40 ppm, 7.52/7.51 ppm, 8.16/8.13 ppm, and 9.09/9.11 ppm, respectively; each of these resonances integrate for 1H. Although these chemical shifts slightly differ from those shown by SB1, they have also been reported for quinoline-derived Schiff base compounds [26]. The presence of an aromatic ring very close to the quinolinic residue in SB2 and SB3 would generate this change in the chemical displacements, with respect to SB1, associated with the magnetic anisotropy of the aromatic fragments. Comparable results were reported by Fritsch et al. [12,13,14,19,20] for the condensation of disubstituted 1,3-diketones with the same primary amine. The selectivity of the reaction was attributed to a steric factor rather than an electronic effect of the terminal substituents, resulting in the condensation of the quinolinic fragment with the carbonyl close to the less bulky group, such as methyl, tert-butyl, or even trifluoromethyl, when the other substituent was a phenyl fragment.
Finally, when comparing the 1H NMR spectra of the crude mixture of regioisomers with those of the isolated pure regioisomers SB2 and SB3 (Figures S11 and S12 vs. Figures S13 and S14, respectively), the presence of signals of lower intensity could be associated in both cases with the minor regioisomer formed. The singlet resonances for N-H show a low-field shift from 13.53 to 13.82 ppm and from 13.58 to 13.79 ppm, the resonance of the methine proton (=CH-) appears more shielded from 6.20 to 6.16 ppm and from 6.21 to 6.19 ppm, and the signal associated with the three protons of the methoxy group remains merely unchanged at 3.88 vs. 3.87 ppm. The spectra also revealed changes in the chemical shifts of the doublets of the aromatic rings. The doublet signals in SB2, at 8.05 and 6.98 ppm (J = 8.9 Hz) and at 7.71 and 7.66 ppm (J = 8.5 Hz), with each pair of doublets associated to an aromatic ring, are now observed at 7.46 and 6.95 ppm (J = 8.7 Hz) and at 8.13 and 7.76 ppm (J = 8.4 Hz), respectively. Similarly, for SB3, the doublets attributed to the protons of one aromatic ring observed at 8.06 and 6.98 ppm (J = 8.8 Hz) shifted to 7.48 and 6.95 ppm (J = 8.8 Hz). On the other hand, the singlet showing up at 7.65 ppm for the trifluoromethylated aromatic ring appears now as two doublets at 8.16 and 7.73 ppm (J = 8.4 Hz). These differences in the chemical shifts of the resonances attributed to the protons of the terminal aromatic rings are related to the change in the position of the quinolinic fragment when condensation occurs in the other carbonyl group, and the disturbance caused by the proximity of the aromatic fragments, as mentioned above.
2.2. Single-Crystal X-Ray Diffraction Analysis
Diffraction-quality single crystals for X-ray structure investigation of the three Schiff base compounds SB1, SB2, and SB3 were grown by slow evaporation of their respective saturated organic solution (see Section 3.3 for details). Single-crystal X-ray diffraction studies confirmed the proposed structure based on spectroscopic evidence (see above), exhibiting an ONN Schiff base architecture as the result of monocondensation of the 1,3-diketones (β1–β3) and 8-aminoquinoline. The molecular structures of SB1, SB2, and SB3 are displayed in Figure 3 and Chart S1, with selected bond distances and angles gathered in Table 1. Compound SB1 crystallizes in the monoclinic space group P21/n, SB2 crystallizes in the triclinic space group P, and SB3 crystallizes in the monoclinic space group P21/c, with a single molecule in the asymmetric unit in each case. The molecular structure confirms for each compound both the monomeric nature and that they adopt the Z-s-Z conformation, consistent with a keto-enamine tautomeric isomer [10]. Such enaminones may indeed have the following four isomers: E-s-E, E-s-Z, Z-s-E, and Z-s-Z, in which E and Z refer to two possible geometric conformations for the carbon–carbon double bond and s-E and s-Z refer to both isomerisms about the carbon–carbon single bond linking the anisoyl and the enamine fragment [10]. In the observed Z-s-Z conformational form, the mean plane (-O2-C3-C2-C1-N1H1-), the characteristic backbone of β-enaminone conformation, forms a pseudo six-membered aromatic ring. The C1-C2-C3 bond angles for SB1, SB2, and SB3 are 124.35(9)°, 123.63(14)°, and 124.12(19)°, respectively. These values are very close to the idealized value of 120° for atomic systems with sp2 hybridization. Analysis of the O2-C3, C3-C2, C2-C1, and C1-N1 bond distances (Table 1) [33], with slight variations among the three compounds, indicates the presence of a conjugated π-electronic system through a pseudo-heterocycle formed as consequence of the intramolecular hydrogen bond N1-H1···O2 with dN···O = 2.666(12) Å, 2.643(17) Å, and 2.645(2) Å, respectively, closing the planar pseudo six-membered ring through a resonant ···O2=C3-C2=C1-N1-H1 fragment [34]. All those metrical parameters compare favorably to previously reported structural data for variously substituted organic and organometallic enaminone derivatives [9,26,35].
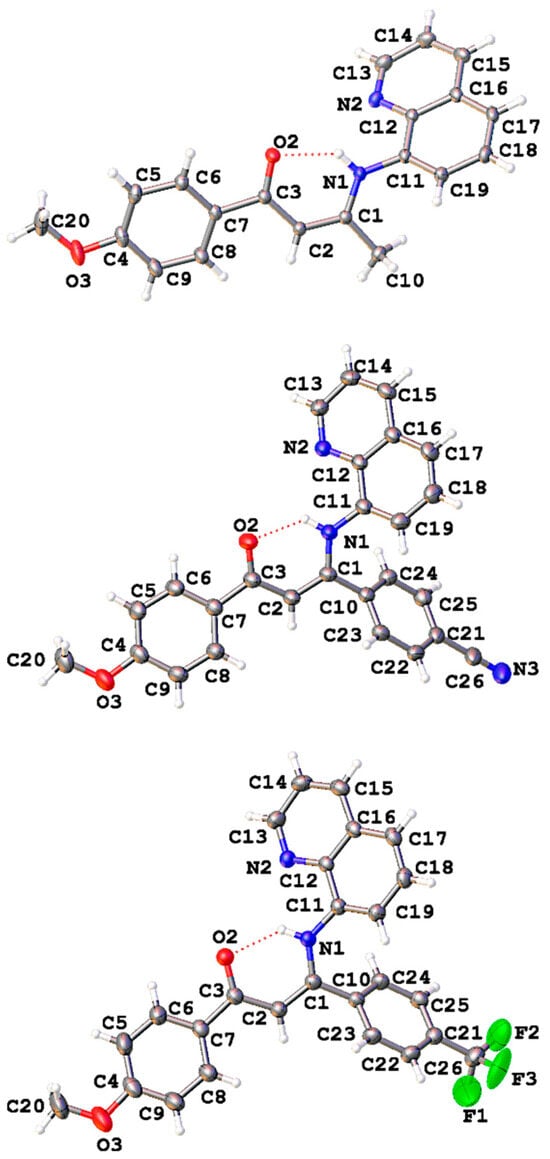
Figure 3.
ORTEP views of Schiff base compounds SB1 (top), SB2 (middle), and SB3 (bottom) with their respective atom numbering scheme. Thermal ellipsoids are drawn with 30% probability, and the intramolecular H-bond is plotted with a dashed line.

Table 1.
Selected bond distances (Å) and angles (°) of SB1–SB3.
In SB1–SB3, the aromatic ring (-C4-C5-C6-C7-C8-C9-) attached to the methoxy electron donor fragment forms dihedral angles with the central plane of 11.46°, 16.40°, and 13.33°, respectively. In addition, the second aromatic ring attached to the electron acceptor group (-C10-C24-C25-C21-C22-C23-), present in SB2 and SB3, deviate by 50.96° and 49.18°, respectively, with respect to the enaminone mean plane. The pseudo heterocycle and the two planes contained in the quinolinic fragment, (-C11-C12-C16-C17-C18-C19-) and (-C12-N13-C14-C15-C16-), form dihedral angles with average values of 55.58(4)°, 42.32(6)°, and 36.01(7)° for SB1, SB2, and SB3, respectively.
The stability of the crystal structure of compounds SB1–SB3 was attributed to intermolecular hydrogen bond interactions and π interactions between the quinolinic fragments. The hydrogen bond distances and symmetry coordinates for intermolecular interactions are detailed in Table 2. For SB1, an antiparallel displaced π–π intermolecular interaction between aromatic ring planes in quinoline moieties was observed. Each aromatic ring in a quinolinic fragment represents a plane, (-C12-N13-C14-C15-C16-) and (-C11-C12-C16-C17-C18-C19-), with each plane interacting with another aromatic ring (-C11-C12-C16-C17-C18-C19-)i of an adjacent quinoline moiety, with centroid–centroid distances of 3.597 and 3.746 Å and shift distances of 1.248 and 1.444 Å, respectively (Figure 4). In this conformation, the presence of weak intermolecular hydrogen interactions C17-H17···O2i of 3.370 Å (donor–acceptor distance), and C19-H19···O2ii of 3.480 Å, was observed.

Table 2.
Hydrogen bond distances and symmetry coordinates for crystal structures of SB1–SB3.
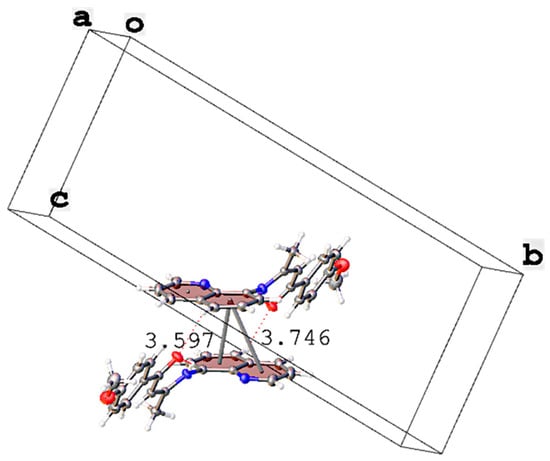
Figure 4.
π–π antiparallel displaced interactions between (-C12-N13-C14-C15-C16-) and (-C11-C12-C16-C17-C18-C19-) with (-C11-C12-C16-C17-C18-C19-)i in the crystal packing of SB1.
The SB2 crystal packing analysis reveals an antiparallel displaced overlapping between quinolinic moieties of two neighboring molecules, as described for SB1. In this conformation, the planes (-C11-C12-C16-C17-C18-C19-) and (-C12-N3-C14-C15-C16-) interact with the plane (-C12-N3-C14-C15-C16-)iii of an adjacent molecule with centroid–centroid separations of 3.964 and 3.842 Å, respectively, and shift distances of 1.905 and 1.603 Å, respectively, values that are higher than the ones observed for SB1. The crystal structure of SB2 is stabilized mainly by weak intermolecular hydrogen bond interactions C5-H5···O2iv and C22-H22···O2ii of 3.489 and 3.264 Å, respectively. These interactions occur between an oxygen atom in the β-diketoimine skeleton and aromatic hydrogens contained in phenyl-donor and phenyl-acceptor moieties, respectively. In addition, intermolecular hydrogen bond interactions C18-H18···O3v and C19-H19···O3vi of 3.496 and 3.353 Å between an oxygen atom in the anisyl fragment and aromatic hydrogens of two different quinoline fragments are also observed. As a consequence of these hydrogen interactions, an arrangement of stacked molecules with an alternating disposition of the electron donor and acceptor groups is observed (Figure 5).
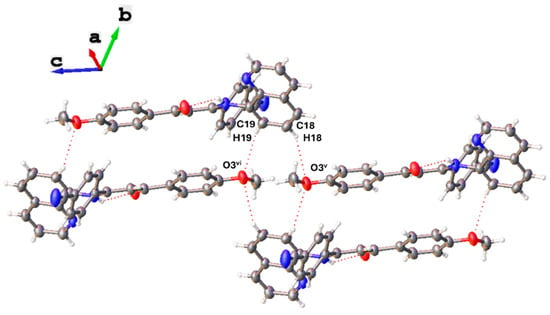
Figure 5.
Intermolecular hydrogen bonds C18-H18···O3v and C19-H19···O3vi in the crystal packing of SB2.
Lastly, the crystal structure of SB3, as described for SB1, is stabilized mainly by π–π intermolecular interactions between the quinolinic aromatic fragments; however, in this case, each aromatic ring plane presents only one π interaction. The interaction observed occurs between the planes (-C12-N3-C14-C15-C16-) and (-C11-C12-C16-C17-C18-C19-)vii of quinoline moieties of adjacent molecules. Compared to what is observed for SB1 and SB2, this interaction shows the shortest centroid–centroid and shift distances of 3.648 Å and 1.076 Å, respectively. Overlapping of the quinoline fragments of neighboring molecules, as observed for SB1 (Figure 6), favors a weak intermolecular interaction C15-H15···O2vii of 3.350 Å between the same molecules. In addition, intermolecular hydrogen bond interactions C6-H6···F3viii and C5-H5···F3Aviii of 3.49 and 3.29 Å are observed. These interactions grow along the c-axis and result in a molecular arrangement with alternating donor and acceptor substituents (Figure 6). The packing is completed with C25-H25···O2ix and C25-H25···N2ix between hydrogen atoms in the aromatic ring bonded to the acceptor moiety and an oxygen atom in the main molecular skeleton (3.354 Å) and a nitrogen atom in the quinolinic aromatic moiety (3.370 Å). Finally, an intermolecular hydrogen bond C24-H24···F1Ax of 3.498 Å is observed (Figure S15).
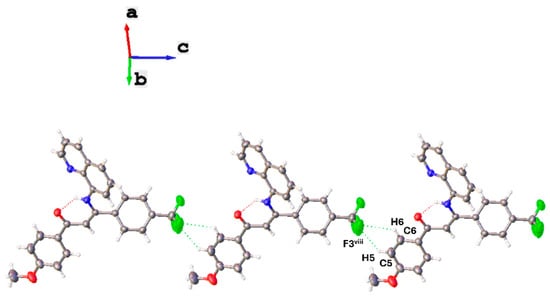
Figure 6.
Intermolecular hydrogen bonds C6-H6···F3 viii and C5-H5···F3A viii in the crystal packing of SB3.
2.3. Electronic Absorption Spectra of SB1–3
The UV-visible absorption spectra of the three Schiff base compounds SB1–SB3 were recorded in both dichloromethane (DCM) and dimethyl sulfoxide (DMSO) solutions at room temperature. The experimental spectra are presented in Figure 7 (see also Figure S16 for comparison with those of 1,3-diketone precursors β1–3), while their absorption maxima and log ε are collected in Table 3. They show three broad absorption bands between 250 and 253 nm, 294 and 304 nm, and 391 and 405 nm in DCM, in accordance with previous literature reports [26,36]. These bands are associated with π-π* charge transfer transitions and ligand-centered n-π* transitions due to the aromatic rings and the imine groups, thus belonging to ligand-to-ligand charge transfer (LLCT) transitions [37]. Switching to a higher polarity solvent (DMSO) induced a modest redshift (2–3 nm of the lowest energy band) in the spectra of compounds SB1–SB3. Thus, some solvatochromic behavior could be ruled out, a property desirable for photophysical applications such as nonlinear optics. However, this electronic absorption band shows a significant bathochromic change of 45–60 nm with respect to the corresponding β-diketone precursors (Figure S16). In this sense, they could be chelators of interest for the formation of coordination compounds with different applications in materials science [38].
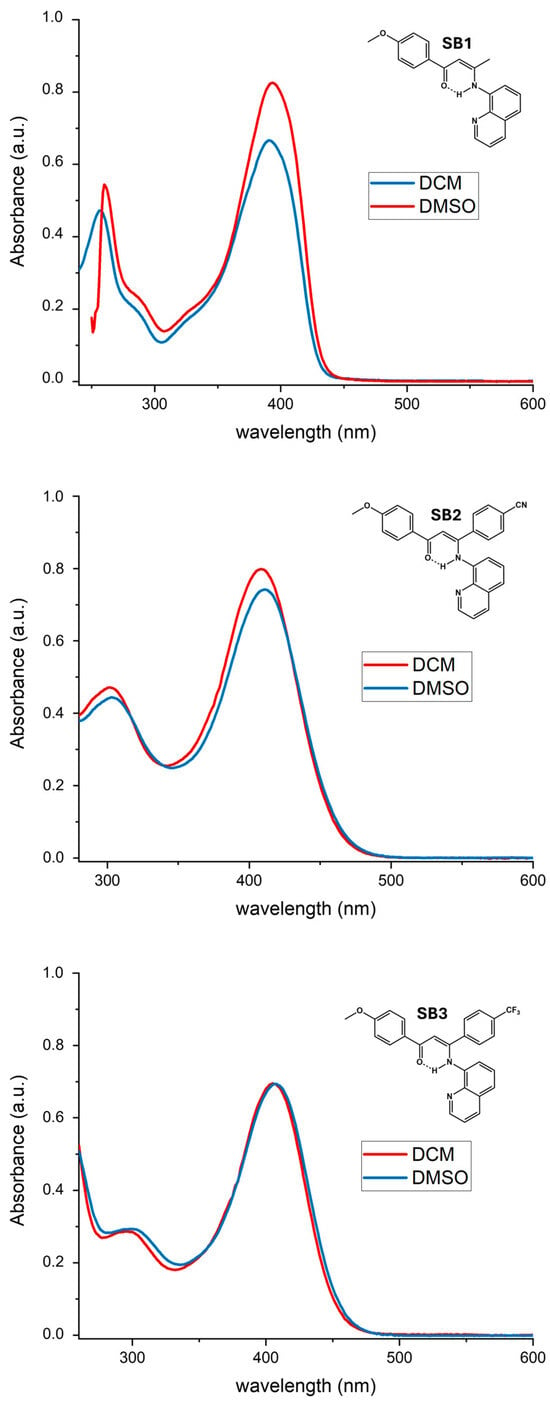
Figure 7.
UV-vis spectra of the Schiff base compounds SB1–SB3 (from top to bottom) recorded in dichloromethane (red line) and DMSO (blue line). Solutions at 20 °C.

Table 3.
UV-vis absorption data for the Schiff base compounds SB1–SB3.
3. Materials and Methods
3.1. Materials and Physical Measurements
Reactions were carried out under a dry nitrogen atmosphere using standard Schlenk techniques. Solvents were dried and distilled under dinitrogen by standard techniques [39]. 4-Methoxyacetophenone, potassium tert-butoxide, ethyl acetate, methyl 4-cyanobenzoate, sodium hydride, methyl 4-(trifluoromethyl)benzoate, p-toluene sulfonic acid, and 8-aminoquinoline were purchased from commercial suppliers and used without further purification. Column chromatography was carried out on silica gel 60 (70–230 mesh ASTM, Merck, Rahway, NJ, USA) as the stationary phase, using the eluents described in each synthetic procedure. Solid-state (KBr disk) FT-IR spectra were recorded on a Nicolet Magna IR-550 FTIR spectrometer in the 4000 to 400 cm−1 range. Absorption bands are expressed with in cm−1 with the following abbreviations to describe the intensity of the bands: vs = very strong, s = strong, m = medium, w = weak, and vw = very weak. Elemental analyses of SB1–SB3 were conducted on a Fisons-EA-1108 CHNS-O Element Analyzer (Thermo Scientific, San Francisco, CA, USA) by the Renewable Resources Laboratory of the Faculty of Chemical Sciences, UdeC (Chile). The 1H NMR spectra were measured at 295 K with a Bruker Ascend-400 spectrometer (Bruker, San Jose, CA, USA). Chemical shifts (δ) are reported in ppm relative to tetramethylsilane (Me4Si). Coupling constants (J) are reported in Hertz (Hz), and the integrations are reported as the number of protons. The following abbreviations are used to describe peak patterns: s = singlet, d = doublet, dd = double doublet, t = triplet, and m = multiplet. UV-vis spectra were registered with a Merck Spectroquant Prove 600 spectrophotometer on compound solutions using quartz cuvettes with a 1.0 cm optical path. Analysis was performed quantitatively using dichloromethane (DCM) and dimethyl sulfoxide (DMSO) solutions at 3.0 × 10−3 mol·L−1.
3.2. Synthesis of β-Diketone (β1–β3)
CH3-O-C4H4-C(O)-CH2-C(O)-CH3 (β1): This compound was synthesized through a previously reported Claisen condensation reaction [40], using, in our case, a new procedure of purification. A Schlenk tube was charged with a magnetic stir bar, 5.00 g (33.29 mmol) of 4-methoxyacetophenone, 5.60 g (49.93 mmol) of potassium tert-butoxide, and 40 mL of THF. The mixture was stirred for 30 min at room temperature, giving a light yellow precipitate. Then, 5.00 mL of ethyl acetate and 20 mL of THF were added, and vigorous stirring was maintained for 16 h. Next, the solid was filtered off and washed with 3 × 50 mL of diethyl ether. The recovered solid was poured into 100 mL of deionized water and 30 mL of HCl 10% v/v and gently stirred. The solid was extracted with 3 × 50 mL of methylene chloride. The organic phases were collected and dried over anhydrous sodium sulphate, and the solvent was evaporated under reduced pressure. The resulting microcrystalline light pink solid was dried under vacuum for 5 h. Yield: 4.59 g (72%). FT-IR (KBr, cm−1): 3430 (w) ν(O-H), 3067, 3008 (vw) ν(=C-H aryl), 2971 (w), 2832 (w) ν(C-H alkyl), 1595 (s) ν(C=O), 1499 (s) ν(C=C aryl), 1261 (m), 1184 (m) ν(-C-O), 830 (m), 783 (m) g (C-H). 1H NMR (400 MHz, CDCl3) δ, keto-enol tautomer: 16.31 (s, 1H), 7.86 (d, J = 8.9 Hz, 2H), 6.94 (d, J = 8.9 Hz, 2H), 6.11 (s, 1H), 3.86 (s, 3H), 2.17 (s, 3H); diketo tautomer: 7.92 (d, J = 8.9 Hz, 2H), 6.94 (d, J = 8.9 Hz, 2H), 4.05 (s, 2H) 3.86 (s, 3H), 2.17 (s, 3H). These data are spectroscopically identical with known material [40].
CH3-O-C4H4-C(O)-CH2-C(O)-C4H4-CN (β2): The following procedure was adapted from a report of Duncan et al. [11]. A two-necked round-bottom flask was charged with a magnetic stir bar, 1.00 g (6.66 mmol) of 4-methoxyacetophenone, 1.39 g (8.66 mmol) of methyl 4-cyanobenzoate, 394 mg (9.99 mmol) of NaH, and 15 mL of THF. The mixture was refluxed for 16 h under a dinitrogen atmosphere. The resulting solution was cooled down to r.t., supplemented with 10 mL of deionized H2O and 5 mL of HCl 10% v/v, and vigorously stirred. The reaction mixture was extracted with DCM (3 × 20 mL), and the organic fractions were collected and dried over anhydrous sodium sulphate. The solvent was removed under reduced pressure, providing a red-orange solid. The crude product was recrystallized from hot ethanol to give a pure yellow solid. Yield: 1.39 g (75%). FT-IR (KBr, cm−1): 3411 (w) ν(O-H), 3071 (vw), 3024 (vw) ν(=C-H aryl), 2928 (w), 2827 (vw) ν(C-H alkyl), 2231 (m) ν(C≡N), 1595 (s) ν(C=O), 1504 (s) ν(C=C), 1265 (m) νasy(-C-O-aryl), 1174(m) νs(-C-O-aryl), 835 (m), 788 (m) g(=C-H). 1H NMR (400 MHz, CDCl3) δ 16.84 (s, 1H), 8.08 (d, J = 8.4 Hz, 2H), 8.02 (d, J = 8.9 Hz, 2H), 7.80 (d, J = 8.4 Hz, 2H), 7.02 (d, J = 8.9 Hz, 2H), 6.82 (s, 1H), 3.93 (s, 3H).
CH3-O-C4H4-C(O)-CH2-C(O)-C4H4-CF3 (β3): This compound was synthesized using an analogous procedure to that previously described for β2, using 1.00 g (6.66 mmol) of 4-methoxyacetophenone, 1.39 g (1.39 mL, 8.66 mmol) of methyl 4-(trifluoromethyl)benzoate, and 394 mg (9.99 mmol) of NaH. Yield: 1.58 g (74%) of pale yellow solid. FT-IR (KBr, cm−1): 3428(w) ν(O-H), 3091 (vw), 3010 (vw) ν(=C-H aryl), 2919 (w), 2844 (w) ν(C-H alkyl), 1604 (s) ν(C=O), 1499 (s) ν(C=C aryl), 1327 (s) ν(C-F), 1251 (m) νasym(-C-O-aryl), 1166 (s) νs(-C-O-aryl), 848 (m), 798 (m) g(=C-H). 1H NMR (400 MHz, CDCl3) δ 16.90 (s, OH, 1H), 8.09 (d, J = 8.1 Hz, 2H), 8.02 (d, J = 9.1 Hz, 2H), 7.76 (d, J = 8.2 Hz, 2H), 7.02 (d, J = 8.7 Hz, 2H), 6.83 (s, 1H), 3.92 (s, 3H). These spectroscopic data agree with the published ones [41].
3.3. Synthesis of Schiff Bases (SB1–SB3)
CH3-O-C6H4-C(O)-CH=C(CH3)-NH-(8-C9H7N) (SB1): This compound was synthetized through a Schiff base condensation reaction reported by Fritsch et al. [26]. A two-necked round-bottom flask was charged with 10 mL of toluene, 600 mg (3.12 mmol) of β1, and p-toluene sulfonic acid in a catalytic amount (~5.0 mg), and the mixture was stirred for 20 min under a dinitrogen atmosphere. Then, 359 mg (2.40 mmol) of 8-aminoquinoline was added, and the reaction was kept under reflux for 16 h using a system equipped with a Dean–Stark apparatus. The progress of the reaction was followed by silica-coated TLC plates using a mixture of hexane/ethyl acetate (2:1). The reaction mixture was allowed to cool down to room temperature, and the solvent was removed under vacuum. The solid residue was purified by column chromatography using a hexane/ethyl acetate (4:1, v:v) mixture as the eluent. The intense yellow band was collected, and the solvent was evaporated under reduced pressure to give a bright yellow solid. Yield: 435 mg (56%). Suitable single crystals for X-ray diffraction crystallography were grown by slow evaporation of a hexane/ethyl acetate (4:1) solution. Analysis calculated for C20H19N2O2 (319.38 g mol−1): C, 75.21; H, 6.00; N, 8.77. Found: C, 75.01; H, 6.24; N, 8.51. FT-IR (KBr, cm−1): 3437 (w) ν(N-H), 3066 (vw) ν(=C-H aryl), 2934 (w) νasym(C-H), 2846 (vw) νs(-C-H), 1590 (s) ν(C=O), 1555 (s) ν(C=C aryl) and/or ν(C=N), 1225 (M) δ(C-O-aryl), 1164 (m) ν(C-O-aryl)., 1280 (m) ν(C-H), 1106 (m), 827 g(=C-H). 1H NMR (400 MHz, CDCl3) δ 14.01 (s, 1H), 9.08 (dd, J = 4.1, 1.7 Hz, 1H), 8.18 (dd, J = 8.3, 1.7 Hz, 1H), 8.02(d, J = 8.8, 2H), 7.52 (m, 4H), 6.96 (d, J = 8.9, 2H), 6.03 (s, 1H), 3.88 (s, 3H), 2.45 (s, 3H).
CH3-O-C6H4-C(O)-CH=C(C6H4-CN)-NH-(8-C9H7N) (SB2): This compound was synthesized using a similar procedure to that previously described for SB1, in this case using 630 mg (1.79 mmol) of β2, 284 mg (1.97 mmol) of 8-aminoquinoline, and a reaction time of 24 h. The obtained solid material was purified by column chromatography using a mixture of hexane/ethyl acetate (6:1) as the eluent. An intense yellow band was collected in several fractions (20 mL). The different solutions were allowed to evaporate at room temperature for 3 to 4 days to afford yellow needle-shaped crystals. The crystals were combined and washed with small amounts of diethyl ether followed by hexane. A crystal from this crop was selected for X-ray diffraction determination. Yield: 189 mg (26%). Analysis calculated for C27H20N3O2 (418.47 g mol−1): C, 77.49; H, 4.82; N, 10.04. Found: C, 76.97; H, 4.67; N, 10.31. FT-IR (KBr, cm−1): 3440 (w) ν(N-H), 3057 (vw) ν(=C-H aryl), 2927 (w) νasy(C-H), 2848 (w) νs(C-H), 2227 (m) ν(C≡N), 1603 (s) ν(C=O), (w), 1545 (s) ν(C=C aryl) and/or ν(C=N), 1177 νasy(-C-O-aryl), 1105 νs(-C-O-aryl), 800 γ (=C-H). 1H NMR (400 MHz, CDCl3) δ 13.53 (s, 1H), 9.09 (dd, J = 4.2,1.7 Hz, 1H), 8.16 (dd, J = 8.3, 1.7 Hz, 1H), 8.05 (d, J = 8.9 Hz, 2H), 7.71 (d, J = 8.6 Hz, 2H), 7.66 (d, J = 8.4 Hz, 2H), 7.52 (dd, J = 8.3, 4.2 Hz, 1H), 7.42 (dd, J = 8.3, 1.1 Hz, 1H), 7.13 (t, J = 7.9 Hz, 1H), 6.98 (d, J = 8.9 Hz, 2H), 6.41 (dd, J = 7.8, 1.2 Hz, 1H), 6.20 (s, 1H), 3.88 (s, 3H).
CH3-O-C6H4-C(O)-CH=C(C6H4-CF3)-NH-(8-C9H7N) (SB3): This compound was synthesized using a similar procedure to that previously described for SB1, using in this case 340 mg (1.05 mmol) of β3, 166.5 mg (1.15 mmol) of 8-aminoquinoline and 24 h of reaction. The solid residue was purified by column chromatography using a mixture of hexane/THF (4:1) as the eluent. An intense yellow band was collected in several fractions (20 mL). The different fractions were allowed to evaporate at room temperature for 3 to 4 days to obtain yellow square-sheet-like crystals. The crystals were combined and washed with small amounts of cold diethyl ether followed by hexane. A crystal from this crop was selected for X-ray crystallography. Yield: 103 mg (22%). Analysis calculated for C26H20N2O2F3 (449.44 g mol−1): C, 69.48; H, 4.49; N, 6.23. Found: C, 69.22; H, 4.12; N, 6.51. FT-IR (KBr, cm−1): 3431 (w) ν(N-H), 3077 (vw) ν(=C-H aryl), 2948 (m) νasy(-C-H), 2834 (w) νs(C-H), 1563 (s) ν(C=O), (w), 1545 (s) ν(C=C aryl) and/or ν(C=N), 1322 (s) ν(C-F), 1225 νasy(-C-O-aryl), 1173 νs(-C-O-aryl), 847 g(=C-H), 726 (w). 1H NMR (400 MHz, CDCl3) δ 13.58 (s, 1H), 9.11 (dd, J = 4.3, 1.7 Hz, 1H), 8.13 (dd, J = 8.3 Hz, 1.7 1H), 8.06 (d, J = 8.8 Hz, 2H), 7.68 (s, 4H), 7.51 (dd, J = 8.4, 4.1 Hz, 1H), 7.40 (dd, J = 8.2, 1.2 Hz, 1H), 7.13 (t, J = 8.0 Hz, 1H), 6.98 (d, J = 8.9 Hz, 2H), 6.44 (dd, J = 7.7, 1.3 Hz, 1H), 6.21 (s, 1H), 3.87 (s, 3H).
3.4. Single-Crystal X-Ray Structure Determinations
Molecular structures were determined using the single-crystal X-ray diffraction technique. Intensity data were collected in a BRUKER D8 VENTURE dual-source Cu/Mo system using Mo-Kα radiation (λ = 0.71073 Å) at 296 K. Intensity data were processed with the APEX4 package and were corrected for absorptions using SADABS [42]. Structures were solved and refined with OLEX2 1.5 [43] by least squares with ShelXL [44] and ShelXT [45] using the intrinsic phasing method. The crystallographic and refinement data for compounds SB1–SB3 are given in Table 4. All non-hydrogen atoms were refined with anisotropic thermal parameters. The hydrogen atoms were positioned and refined with riding coordinates, except for the methyl terminal groups which were treated as a rotating group. The disorder of the -CF3 fragments in SB3 was fixed with rigid body restraints (RIGU) with a standard deviation (sigma) of 0.004 for 1–2 and 1–3 distances. Additionally, to restrain the distances equivalently, the same distance (SADI) with a sigma of 0.02 was applied for C-F distances. The crystallographic cif files have been deposited within the Cambridge Structural Database with the identifiers CCDC 2390921-2390923, containing the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, accessed on 2 December 2024; by emailing data_request@ccdc.cam.ac.uk; or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge, CB2 1EZ, UK.

Table 4.
Crystal data collection and structure refinement for compounds SB1–SB3.
4. Conclusions
In summary, we have reported the synthesis of a series of non-symmetrical β-1-anisyl-ketoen-3-amines bearing a pendant quinolyl donor through Schiff base condensation of readily available 1,3-diketones containing varying degrees of electron-donating and electron-withdrawing substituents and 8-aminoquinoline. The three new enaminones were characterized spectroscopically and crystallographically, including an examination of the role of sterics and electronics in the observed regioisomer ratios. The regioselectivity of the condensation of electronically unsymmetrical 1,3-diaryl-1,3-diketones with 8-aminoquinoline was studied by 1H NMR, providing regioisomer ratios of ~3:1 and ~2:1 in the case of CN and CF3 substituents, respectively. The electronic effects correlate well with the difference between the Hammett σ+ coefficients of the two para substituents on the aryl rings. The regioisomers were separated upon column chromatography and fractional crystallization, and the structure of the isolated ones was established with single-crystal X-ray diffraction analysis, showing that the Schiff condensation reactions took place at the carbonyl group adjacent to the electron-deficient substituent. ONN-tridentate Schiff base ligands are known to act as anionic pincer-type ligands to efficiently chelate metal ions to generate versatile, conformationally rigid metalloligands, thus allowing the construction of new multimetallic architectures with desired properties and applications, such as second-order nonlinear optics, sensors, molecular magnets, catalysts, and many others [3,22,46]. We believe the push–pull enaminones reported here also have promise for coordinating metal centers to build new molecular materials, and such experiments are in progress.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29245863/s1, Chart S1: Molecular structure with atomic numbering of SB1–SB3; Figures S1–S3: Solid-state FT-IR spectra of 1,3-diketone precursors; Figures S4–S6: Solid-state FT-IR spectra of compounds SB1–SB3; Figures S7–S9: 1H NMR spectra of 1,3-diketone precursors; Figures S10–S12: 1H NMR spectra of compounds SB1–SB3; Figure S13: 1H NMR spectra of regioisomeric mixture of compounds SB2 and SB2′; Figure S14: 1H NMR spectra of regioisomeric mixture of compounds SB3 and SB3′; Figure S15: Intermolecular hydrogen bonds in SB3; Figure S16: Overplot UV-visible spectra of 1,3-diketone precursors and their tridentate ONN SB1–SB3. Table S1: Most important FT-IR absorptions of 1,3-diketone precursors and SB1–SB3; Table S2: 1H NMR chemical shifts of 1,3-diketone precursors and SB1–SB3.
Author Contributions
Conceptualization, N.N.; methodology, P.C.-T. and N.N.; software, P.C.-T., D.V., and N.N.; validation, J.-R.H. and N.N.; formal analysis, J.-R.H. and N.N.; investigation, P.C.-T. and D.V.; resources, N.N.; data curation, J.-R.H. and N.N.; writing—original draft preparation, P.C.-T., D.V., and J.-R.H.; writing—review and editing, P.C.-T., J.-R.H., and N.N.; supervision, N.N.; project administration,. N.N.; funding acquisition, N.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), Chile, under grant no. 1201680 (IR = N.N.), and FONDEQUIP MEDIANO EQM200138—ANID (Chile) for single-crystal X-ray diffraction equipment.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
Financial support from the Agencia Nacional de Investigación y Desarrollo (ANID), Chile, through the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), Chile, under grant no. 1201680 (IR = N.N.) and FONDEQUIP MEDIANO EQM200138—ANID (Chile) for single-crystal X-ray diffraction equipment, the CNRS and the Université de Rennes are is gratefully acknowledged. This research was performed as part of the Chilean–French International Research Project “IRP-CoopIC” 2022–2026. P.C.-T. also thanks the ANID (Chile) Beca Doctorado Nacional 2020-21202204 for supporting a graduate scholarship (PhD Program UdeC—Chile).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Qin, W.; Long, S.; Panunzio, M.; Biondi, S. Schiff Bases: A Short Survey on an Evergreen Chemistry Tool. Molecules 2013, 18, 12264–12289. [Google Scholar] [CrossRef] [PubMed]
- Fabbrizzi, L. Beauty in Chemistry: Making Artistic Molecules with Schiff Bases. J. Org. Chem. 2020, 85, 12212–12226. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Manzur, C.; Novoa, N.; Celedón, S.; Carrillo, D.; Hamon, J.R. Multidentate Unsymmetrically-Substituted Schiff Bases and Their Metal Complexes: Synthesis, Functional Materials Properties, and Applications to Catalysis. Coord. Chem. Rev. 2018, 357, 144–172. [Google Scholar] [CrossRef]
- Vigato, P.A.; Tamburini, S. The Challenge of Cyclic and Acyclic Schiff Bases and Related Derivatives. Coord. Chem. Rev. 2004, 248, 1717–2128. [Google Scholar] [CrossRef]
- Bargujar, S.; Ratnani, S.; Jain, R. Recent Advances in Microwave Assisted Synthesis of Schiff Base Metal Complexes. Inorg. Chem. Commun. 2024, 162, 112250. [Google Scholar] [CrossRef]
- Kargar, H.; Fallah-Mehrjardi, M.; Munawar, K.S. Metal Complexes Incorporating Tridentate ONO Pyridyl Hydrazone Schiff Base Ligands: Crystal Structure, Characterization and Applications. Coord. Chem. Rev. 2024, 501, 215587. [Google Scholar] [CrossRef]
- Costes, J.P. Synthetic Possibilities of the 2,4-Pentanedione-1,2-Diaminoethane System: An Overall View. Polyhedron 1987, 6, 2169–2175. [Google Scholar] [CrossRef]
- Fuentealba, M.; Trujillo, A.; Hamon, J.R.; Carrillo, D.; Manzur, C. Synthesis, Characterization and Crystal Structure of the Tridentate Metalloligand Formed from Mono-Condensation of Ferrocenoylacetone and 1,2-Phenylenediamine. J. Mol. Struct. 2008, 881, 76–82. [Google Scholar] [CrossRef]
- Novoa, N.; Roisnel, T.; Hamon, P.; Kahlal, S.; Manzur, C.; Ngo, H.M.; Ledoux-Rak, I.; Saillard, J.-Y.; Carrillo, D.; Hamon, J.-R. Four-Coordinate Nickel(II) and Copper(II) Complex Based ONO Tridentate Schiff Base Ligands: Synthesis, Molecular Structure, Electrochemical, Linear and Nonlinear Properties, and Computational Study. Dalton Trans. 2015, 44, 18019–18037. [Google Scholar] [CrossRef]
- Greenhill, J.V. Enaminones. Chem. Soc. Rev. 1977, 6, 277–294. [Google Scholar] [CrossRef]
- Duncan, N.C.; Garner, C.M.; Nguyen, T.; Hung, F.; Klausmeyer, K. Electronic Effects in the Reaction of 1,3-Diaryl-1,3-Diketones with Hydrazinopyridines. Tetrahedron Lett. 2008, 49, 5766–5769. [Google Scholar] [CrossRef]
- Rezayee, N.M.; Gerling, K.A.; Rheingold, A.L.; Fritsch, J.M. Synthesis and Structures of Tridentate Ketoiminate Zinc Complexes Bearing Trifluoromethyl Substituents That Act as L-Lactide Ring Opening Polymerization Initiators. Dalton Trans. 2013, 42, 5573–5586. [Google Scholar] [CrossRef] [PubMed]
- Gerling, K.A.; Rezayee, N.M.; Rheingold, A.L.; Green, D.B.; Fritsch, J.M. Synthesis and Structures of Bis-Ligated Zinc Complexes Supported by Tridentate Ketoimines That Initiate L-Lactide Polymerization. Dalton Trans. 2014, 43, 16498–16508. [Google Scholar] [CrossRef] [PubMed]
- Slattery, R.M.; Stahl, A.E.; Brereton, K.R.; Rheingold, A.L.; Green, D.B.; Fritsch, J.M. Ring Opening Polymerization and Copolymerization of L-Lactide and ε-Caprolactone by Bis-Ligated Magnesium Complexes. J. Polym. Sci. A Polym. Chem. 2019, 57, 48–59. [Google Scholar] [CrossRef]
- Li, X.F.; Dai, K.; Ye, W.P.; Pan, L.; Li, Y.S. New Titanium Complexes with Two β-Enaminoketonato Chelate Ligands: Syntheses, Structures, and Olefin Polymerization Activities. Organometallics 2004, 23, 1223–1230. [Google Scholar] [CrossRef]
- Bourget-Merle, L.; Lappert, M.F.; Severn, J.R. The Chemistry of β-Diketiminatometal Complexes. Chem. Rev. 2002, 102, 3031–3065. [Google Scholar] [CrossRef]
- Muthu Tamizh, M.; Cooper, B.F.T.; MacDonald, C.L.B.; Karvembu, R. Palladium(II) Complexes with Salicylideneimine Based Tridentate Ligand and Triphenylphosphine: Synthesis, Structure and Catalytic Activity in Suzuki–Miyaura Cross Coupling Reactions. Inorganica Chim. Acta 2013, 394, 391–400. [Google Scholar] [CrossRef]
- Muthu Tamizh, M.; Mereiter, K.; Kirchner, K.; Karvembu, R. Ruthenium(II) Carbonyl Complexes Containing ‘Pincer like’ ONS Donor Schiff Base and Triphenylphosphine as Catalyst for Selective Oxidation of Alcohols at Room Temperature. J. Organomet. Chem. 2012, 700, 194–201. [Google Scholar] [CrossRef]
- Schmitz, L.A.; McCollum, A.M.; Rheingold, A.L.; Green, D.B.; Fritsch, J.M. Synthesis and Structures of Aluminum Ion-Pair Complexes That Act as L- and Racemic-Lactide Ring Opening Polymerization Initiators. Polyhedron 2018, 147, 94–105. [Google Scholar] [CrossRef]
- McCollum, A.M.; Longo, A.M.; Stahl, A.E.; Butler, A.S.; Rheingold, A.L.; Cundari, T.R.; Green, D.B.; Brereton, K.R.; Fritsch, J.M. Synthesis, Spectroscopy, and Crystallography of Mononuclear, Five-Coordinate Aluminum Complexes That Act as Cyclic Ester Polymerization Initiators. Polyhedron 2021, 204, 115233. [Google Scholar] [CrossRef]
- Ghorai, P.; Brandão, P.; Benmansour, S.; García, C.J.G.; Saha, A. Azido and Thiocyanato Bridged Dinuclear Ni(II) Complexes Involving 8-Aminoquinoline Based Schiff Base as Blocking Ligands: Crystal Structures, Ferromagnetic Properties and Magneto-Structural Correlations. Polyhedron 2020, 188, 114708. [Google Scholar] [CrossRef]
- Thakurta, S.; Maiti, M.; Butcher, R.J.; Gómez-García, C.J.; Tsaturyan, A.A. A Trinuclear Nickel(II) Schiff Base Complex with Phenoxido- and Acetato-Bridges: Combined Experimental and Theoretical Magneto-Structural Correlation. Dalton Trans. 2021, 50, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Novoa, N.; Roisnel, T.; Dorcet, V.; Cador, O.; Manzur, C.; Carrillo, D.; Hamon, J.R. Efficient Preparation of Multimetallic ONO-Based Schiff Base Complexes of Nickel(II) and Copper(II). New J. Chem. 2016, 40, 5920–5929. [Google Scholar] [CrossRef]
- Novoa, N.; Manzur, C.; Roisnel, T.; Dorcet, V.; Cabon, N.; Robin-Le Guen, F.; Ledoux-Rak, I.; Kahlal, S.; Saillard, J.Y.; Carrillo, D.; et al. Redox-Switching of Ternary Ni(II) and Cu(II) Complexes: Synthesis, Experimental and Theoretical Studies along with Second-Order Nonlinear Optical Properties. New J. Chem. 2019, 43, 10468–10481. [Google Scholar] [CrossRef]
- González, D.M.; Hernández, L.A.; Oyarce, J.; Alfaro, A.; Novoa, N.; Cisterna, J.; Brito, I.; Carrillo, D.; Manzur, C. A new and efficient high-performance electrochemical glucose sensor based on a metallopolymer derived from a cobaltate(III) Schiff base complex. Synth. Met. 2021, 271, 116633. [Google Scholar] [CrossRef]
- Roberts, C.C.; Fritsch, J.M. Synthesis and Crystal Structures of Magnesium Complexes with NNO Schiff Base Ligands Bearing Quinolyl Pendant Donors. Polyhedron 2010, 29, 1271–1278. [Google Scholar] [CrossRef]
- Cisterna, J.; Artigas, V.; Fuentealba, M.; Hamon, P.; Manzur, C.; Dorcet, V.; Hamon, J.R.; Carrillo, D. Nickel(II) and Copper(II) Complexes of New Unsymmetrically-Substituted Tetradentate Schiff Base Ligands: Spectral, Structural, Electrochemical and Computational Studies. Inorganica Chim. Acta 2017, 462, 266–280. [Google Scholar] [CrossRef]
- Celedón, S.; Hamon, P.; Artigas, V.; Fuentealba, M.; Kahlal, S.; Carrillo, D.; Saillard, J.Y.; Hamon, J.R.; Manzur, C. Ferrocene Functionalized Enantiomerically Pure Schiff Bases and Their Zn(II) and Pd(II) Complexes: A Spectroscopic, Crystallographic, Electrochemical and Computational Investigation. New J. Chem. 2022, 46, 3948–3960. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Barbera, J.; Gimenez, R.; Serrano, J.L.; Alcala, R.; Villacampa, B.; Villalba, J.; Ledoux, I.; Zyss, J. Beta-Diketone, Pyrazole and Isoxazole Derivatives with Polar Groups: Liquid Crystalline and Non-Linear Optical Properties. Liq. Cryst. 1997, 22, 265–273. [Google Scholar] [CrossRef]
- Danilova, J.S.; Avdoshenko, S.M.; Karushev, M.P.; Timonov, A.M.; Dmitrieva, E. Infrared Spectroscopic Study of Nickel Complexes with Salen-Type Ligands and Their Polymers. J. Mol. Struct. 2021, 1241, 130668. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Elsevier: Amsterdam, The Netherlands, 1991; ISBN 978-0-12-451160-6. [Google Scholar]
- Gilli, P.; Bertolasi, V.; Ferretti, V.; Gilli, G. Evidence for Intramolecular N-H···O Resonance-Assisted Hydrogen Bonding in β-Enaminones and Related Heterodienes. A Combined Crystal-Structural, IR and NMR Spectroscopic, and Quantum-Mechanical Investigation. J. Am. Chem. Soc. 2000, 122, 10405–10417. [Google Scholar] [CrossRef]
- Taylor, R.; Wood, P.A. A Million Crystal Structures: The Whole Is Greater than the Sum of Its Parts. Chem. Rev. 2019, 119, 9427–9477. [Google Scholar] [CrossRef]
- Celedon, S.; Fuentealba, M.; Roisnel, T.; Hamon, J.R.; Carrillo, D.; Manzur, C. Stepwise Construction of a 4-Hydroxyphenyl Functionalized O,N,N-Tridentate Ferrocene-Containing Enaminone: Spectral, Analytical and Structural Studies. Inorganica Chim. Acta 2012, 390, 184–189. [Google Scholar] [CrossRef]
- González, D.; Arrué, R.; Matamala-Cea, E.; Arancibia, R.; Hamon, P.; Cador, O.; Roisnel, T.; Hamon, J.R.; Novoa, N. Homoleptic CoII, NiII, CuII, and ZnII Complexes Based on 8-Hydroxylquinoline Schiff Base Derivative: A Combined Synthetic, Spectral, Structural, and Magnetic Study. Eur. J. Inorg. Chem. 2018, 2018, 4720–4730. [Google Scholar] [CrossRef]
- Kalsi, P.S. Spectroscopy of Organic Compounds; New Age International: Mumbai, India, 2007; ISBN 8122415431. [Google Scholar]
- Bureš, F. Fundamental Aspects of Property Tuning in Push–Pull Molecules. RSC Adv. 2014, 4, 58826–58851. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Cha, C.L.L. Purification of Laboratory Chemicals, 5th ed.; Butterworth-Heinemann Ltd.: London, UK, 2003; ISBN 9780750675710. [Google Scholar]
- Popic, V.V.; Korneev, S.M.; Nikolaev, V.A.; Korobitsyna, I.K. An Improved Synthesis of 2-Diazo-1,3-Diketones. Synthesis 1991, 1991, 195–198. [Google Scholar] [CrossRef]
- Rao, H.S.P.; Muthanna, N. Variations in the Blaise Reaction: Conceptually New Synthesis of 3-Amino Enones and 1,3-Diketones. Eur. J. Org. Chem. 2015, 2015, 1525–1532. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-Ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. IUCr Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. IUCr SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Novoa, N.; Manzur, C.; Roisnel, T.; Kahlal, S.; Saillard, J.Y.; Carrillo, D.; Hamon, J.R. Nickel(II)-Based Building Blocks with Schiff Base Derivatives: Experimental Insights and DFT Calculations. Molecules 2021, 26, 5316. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).