Removal of Ciprofloxacin and Norfloxacin from Aqueous Solution with Activated Carbon from Cupuaçu (Theobroma grandiflorum) Bark
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of AC
2.1.1. Yield, Moisture, and Ash
2.1.2. Surface Functional Groups, pH at the Zero-Load Point (pHPZC), and Surface pH
2.1.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.1.4. Specific Surface Area
2.1.5. Thermal Analyses
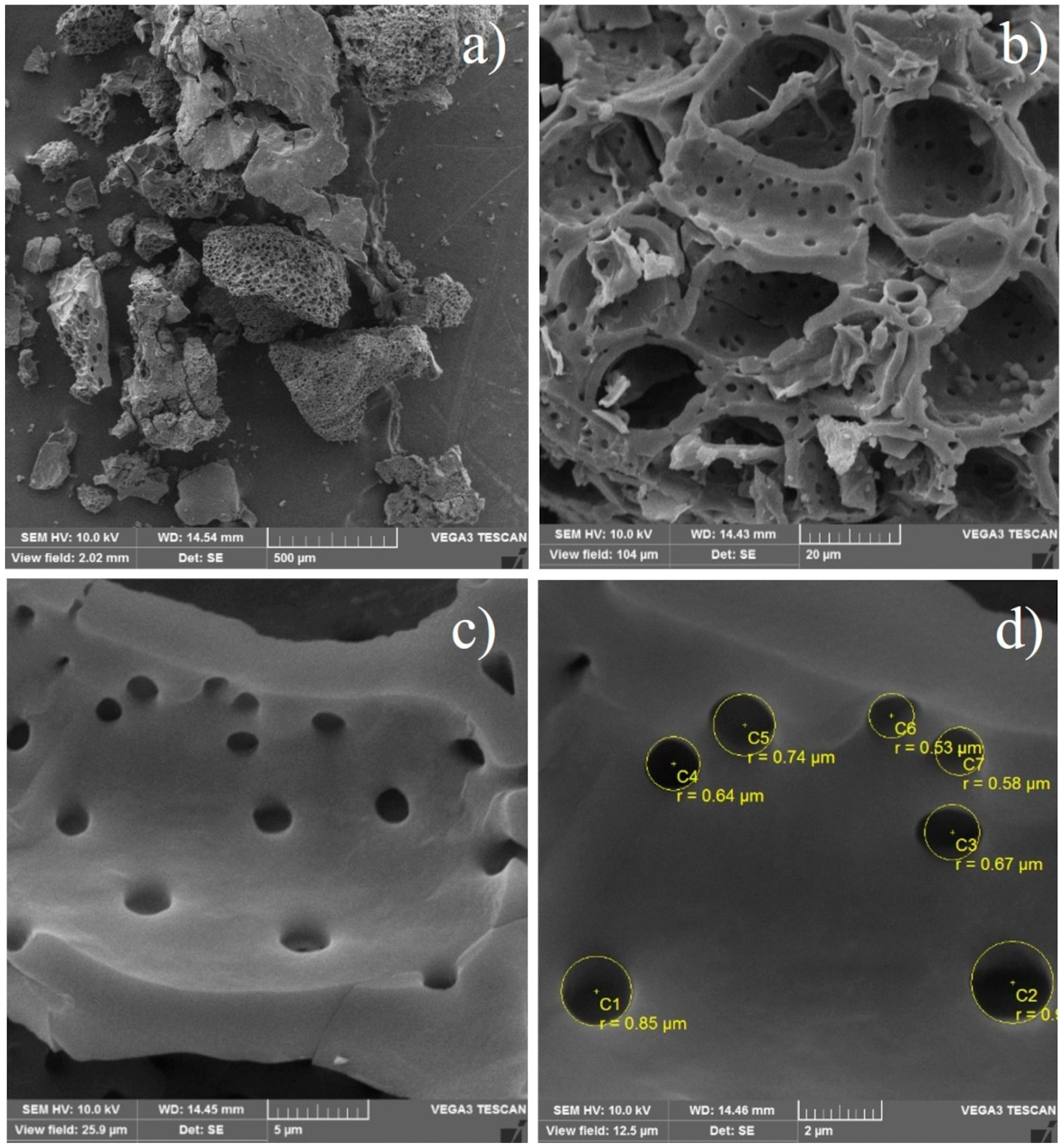
2.1.6. X-Ray Diffraction (XRD) and Morphology
2.2. Preliminary Tests
2.3. Experimental Design
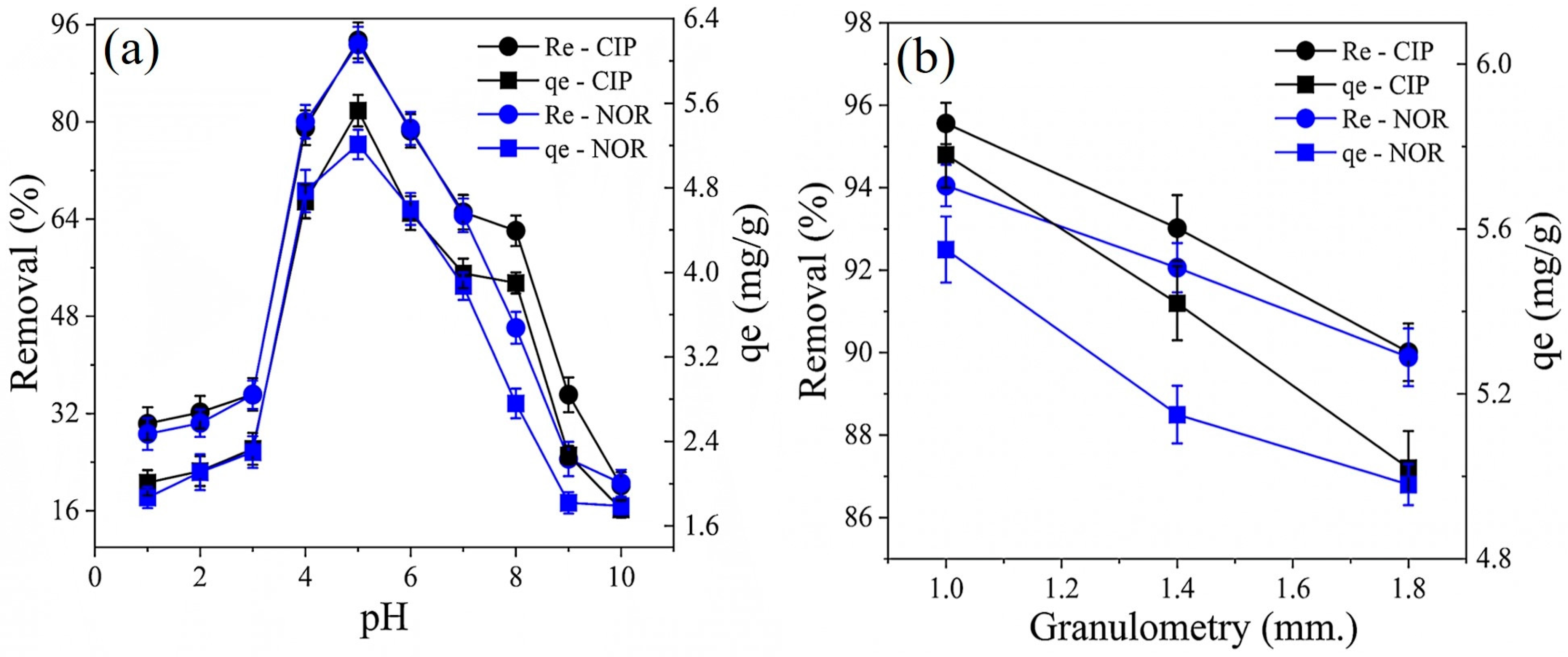
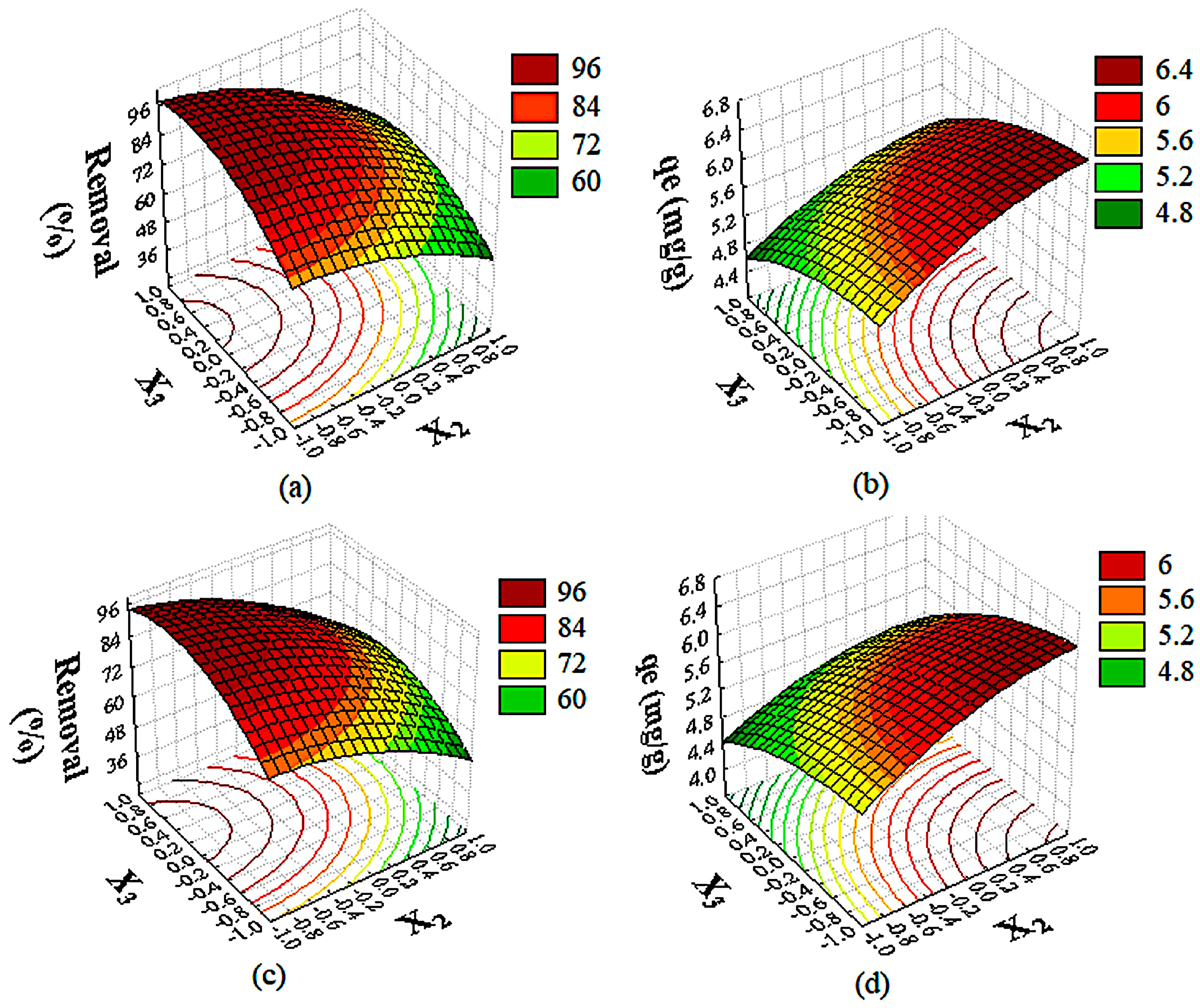
2.3.1. Influence of Operating Conditions on CIP and NOR Removal
2.3.2. Influence of Operating Conditions on CIP and NOR Adsorption Capacity
2.4. Simultaneous Optimization of Responses
2.5. Kinetics and Mass Transfer
2.6. Adsorption Equilibrium
2.7. Adsorption Thermodynamics
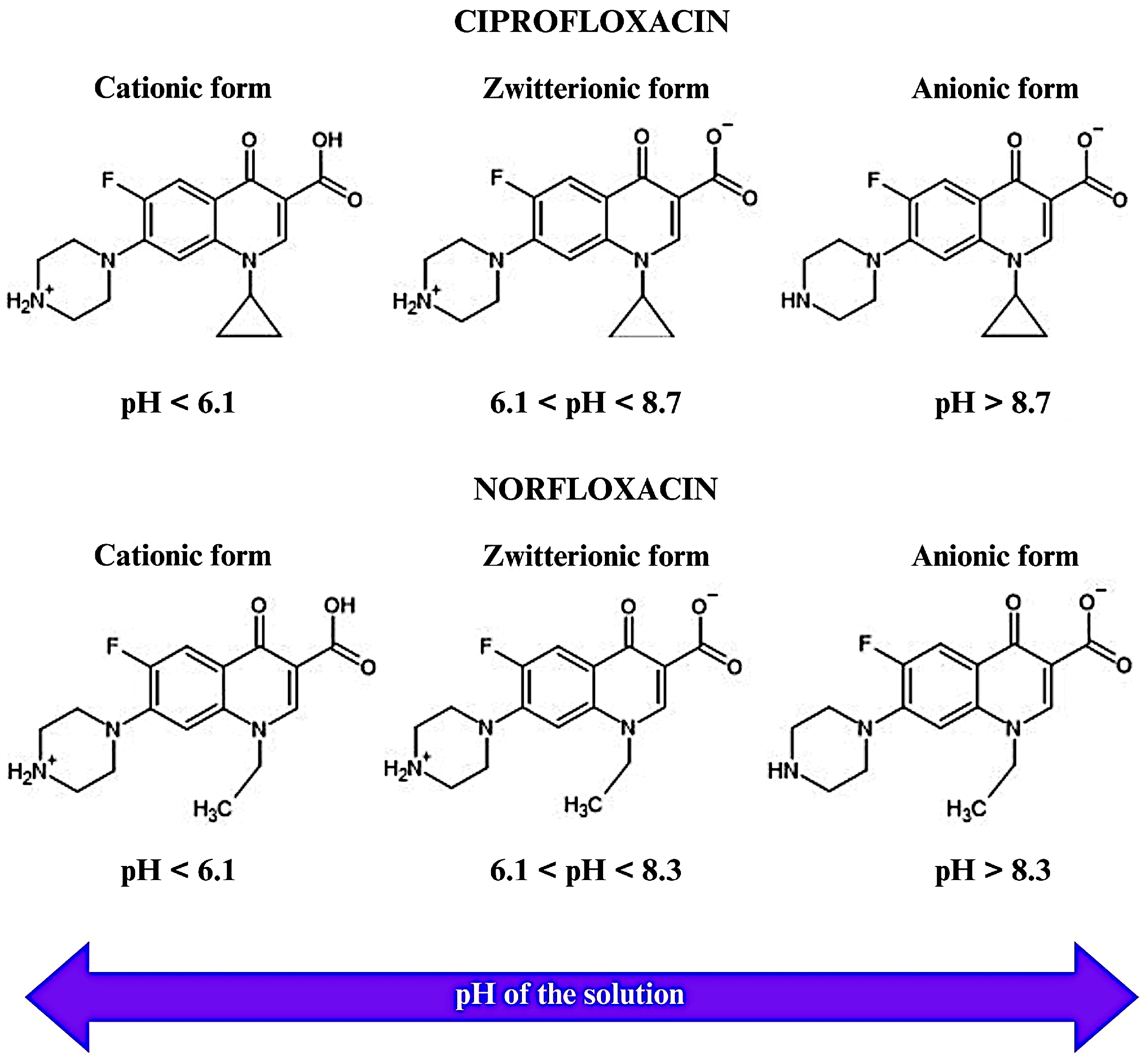
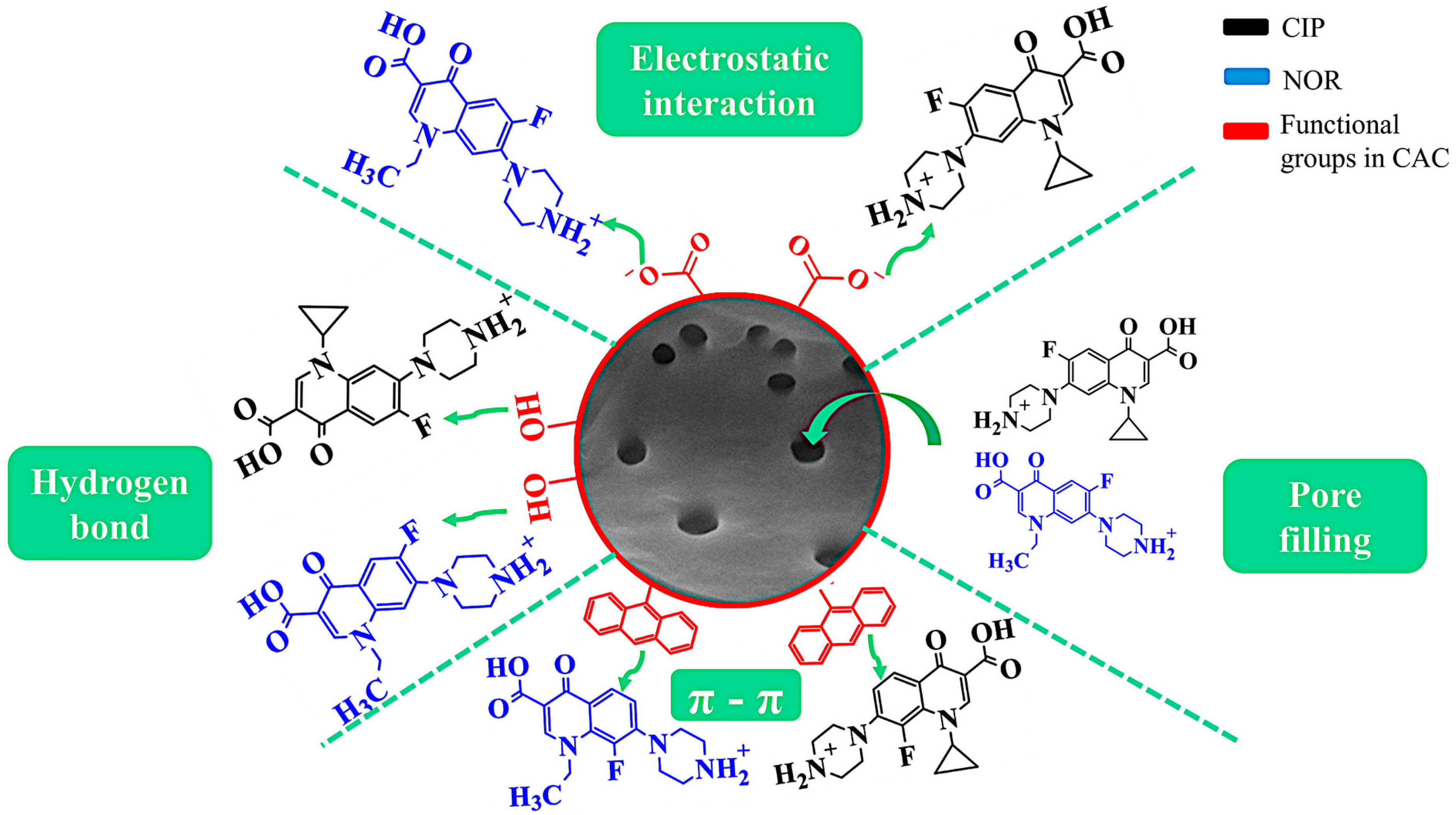
2.8. Adsorption Mechanisms of CIP and NOR on CAC
2.9. Regeneration Study
3. Materials and Methods
3.1. Preparation of AC
3.2. Characterization of AC
3.3. Preparation of Ciprofloxacin and Norfloxacin Solutions
3.4. Batch Adsorption Tests
3.4.1. Preliminary Tests
3.4.2. Experimental Design and Optimization
3.5. Kinetic and Equilibrium Study
3.6. Adsorption Thermodynamics
3.7. Regeneration Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandoval, M.A.; Calzadilla, W.; Vidal, J.; Brillas, E.; Salazar-González, R. Contaminants of Emerging Concern: Occurrence, Analytical Techniques, and Removal with Electrochemical Advanced Oxidation Processes with Special Emphasis in Latin America. Environ. Pollut. 2024, 345, 123397. [Google Scholar] [CrossRef] [PubMed]
- Ashiq, A.; Vithanage, M.; Sarkar, B.; Kumar, M.; Bhatnagar, A.; Khan, E.; Xi, Y.; Ok, Y.S. Carbon-Based Adsorbents for Fluoroquinolone Removal from Water and Wastewater: A Critical Review. Environ. Res. 2021, 197, 111091. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and Hazardous Impact of Pharmaceutical and Personal Care Products and Antibiotics in Environment: A Review on Emerging Contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef] [PubMed]
- Thai, V.-A.; Dang, V.D.; Thuy, N.T.; Pandit, B.; Vo, T.-K.-Q.; Khedulkar, A.P. Fluoroquinolones: Fate, Effects on the Environment and Selected Removal Methods. J. Clean. Prod. 2023, 418, 137762. [Google Scholar] [CrossRef]
- Kujawska, A.; Kiełkowska, U.; Atisha, A.; Yanful, E.; Kujawski, W. Comparative Analysis of Separation Methods Used for the Elimination of Pharmaceuticals and Personal Care Products (PPCPs) from Water—A Critical Review. Sep. Purif. Technol. 2022, 290, 120797. [Google Scholar] [CrossRef]
- Katsigiannis, A.; Noutsopoulos, C.; Mantziaras, J.; Gioldasi, M. Removal of Emerging Pollutants through Granular Activated Carbon. Chem. Eng. J. 2015, 280, 49–57. [Google Scholar] [CrossRef]
- Darweesh, T.M.; Ahmed, M.J. Adsorption of Ciprofloxacin and Norfloxacin from Aqueous Solution onto Granular Activated Carbon in Fixed Bed Column. Ecotoxicol. Environ. Saf. 2017, 138, 139–145. [Google Scholar] [CrossRef]
- Suhas; Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A Review as Natural, Modified and Activated Carbon Adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar] [CrossRef]
- Kumar, P.; Omer, S.N.; Saravanan, P.; Rajeshkannan, R.; Rajasimman, M.; Shanmugam, V.K.; Kamyab, H.; Gupta, V.K.; Vasseghian, Y. Exploring the Role of Activated Charcoal from Lignocellulosic Biomass Wastes for Sustainable Water Treatment. J. Energy Inst. 2024, 114, 101626. [Google Scholar] [CrossRef]
- Serafin, J.; Kishibayev, K.; Tokpayev, R.; Khavaza, T.; Atchabarova, A.; Ibraimov, Z.; Nauryzbayev, M.; Nazzal, J.S.; Giraldo, L.; Moreno-Piraján, J.C. Functional Activated Biocarbons Based on Biomass Waste for CO2 Capture and Heavy Metal Sorption. ACS Omega 2023, 8, 48191–48210. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Abreu, V.K.G.; Rodrigues, S. Cupuassu—Theobroma grandiflorum. In Exotic Fruits; Academic Press: London, UK, 2018; pp. 159–162. ISBN 978-0-12-803138-4. [Google Scholar]
- Korzenowski, C.; de Souza, C.B.S.; Aguero, R.R.; dos Santos, M.G.; Santos, B.C.; Voltz, B.H.; Sada, G.D.; Darolt, G.G.; da Silva, L.S.M.; Hillesheim, M.C.; et al. Reaproveitamento de cascas de cupuaçu para a produção de energia através da sua transformação em briquetes. Int. Seven J. Multidiscip. 2023, 2, 189–220. [Google Scholar] [CrossRef]
- de Sousa, H.S.; de Oliveira, J.D. Biomass characterization cupuaçu Bark (Theobroma grandiflorum) and açaí fruit stone (Euterpe oleracea) biomass for the purpose of removing potentially toxic metals from chemical laboratory effluents. J. Interdiscip. Debates 2021, 2, 109–127. [Google Scholar] [CrossRef]
- Marasca, N. Aproveitamento da Casca de Cupuaçu Para Diferentes Aplicações da Biorrefinaria. Master’s Thesis, Universidade Federal do Tocantins, Palmas, Tocantins, Brazil, 2022. [Google Scholar]
- Cruz, O.F.; Campello-Gómez, I.; Casco, M.E.; Serafin, J.; Silvestre-Albero, J.; Martínez-Escandell, M.; Hotza, D.; Rambo, C.R. Enhanced CO2 Capture by Cupuassu Shell-Derived Activated Carbon with High Microporous Volume. Carbon Lett. 2023, 33, 727–735. [Google Scholar] [CrossRef]
- Sousa, M.P.d.S.e.; de Souza, C.D.R.; Nascimento, L.O.N.D.; de Oliveira, M.S. Adsorção do corante alaranjado de metila em carvão ativado obtido da casca do cupuaçu. Res. Soc. Dev. 2023, 12, e17121444394. [Google Scholar] [CrossRef]
- Prahas, D.; Kartika, Y.; Indraswati, N.; Ismadji, S. Activated Carbon from Jackfruit Peel Waste by H3PO4 Chemical Activation: Pore Structure and Surface Chemistry Characterization. Chem. Eng. J. 2008, 140, 32–42. [Google Scholar] [CrossRef]
- Costa, R.L.T.; Nascimento, R.A.D.; de Araújo, R.C.S.; Vieira, M.G.A.; da Silva, M.G.C.; de Carvalho, S.M.L.; de Faria, L.J.G. Removal of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) from Water with Activated Carbons Synthetized from Waste Murumuru (Astrocaryum murumuru Mart.): Characterization and Adsorption Studies. J. Mol. Liq. 2021, 343, 116980. [Google Scholar] [CrossRef]
- Carmona, I.N.; da Sampaio, J.S.; Andrade, F.W.C.; Júnior, C.D.C.; de Junior, S.M.O.M.; da Moreira, L.S.; Moutinho, V.H.P. Potencial Energético da Biomassa e Carvão Vegetal de Resíduos de Castanha-do-Pará (Bertholletia excelsa Bonpl.). In Proceedings of the Anais Congresso Brasileiro de Ciência e Tecnologia da Madeira, Santa Catarina, Brazil, 4–6 September 2017; Volume 2, pp. 1–9. [Google Scholar]
- da Silva, S.D.P.; Pereira, L.C.; Nobre, J.R.C.; Melo, V.L.M.; Weiler, E.B.; Fantinel, R.A.; dos Santos, F.D.; da Silva, L.B.O.; Marchesan, J.; Loiola, T.M.; et al. Uso do carvão de caroço de açaí (Euterpe oleraceae Mart.) ativado quimicamente como meio filtrante de água. In Produtos Florestais Não Madereiros: Tecnologia, Mercado, Pesquisas e Atualidades; Editora Científica Digital: São Paulo, Brazil, 2021; Volume 1, pp. 388–402. ISBN 9786589826392. [Google Scholar]
- Lam, S.S.; Liew, R.K.; Wong, Y.M.; Yek, P.N.Y.; Ma, N.L.; Lee, C.L.; Chase, H.A. Microwave-Assisted Pyrolysis with Chemical Activation, an Innovative Method to Convert Orange Peel into Activated Carbon with Improved Properties as Dye Adsorbent. J. Clean. Prod. 2017, 162, 1376–1387. [Google Scholar] [CrossRef]
- ASTM D2867-23; Standard Test Methods for Moisture in Activated Carbon. American Society for Testing and Materials: West Conshohocken, PA, USA, 2023.
- Çeçen, F. Activated Carbon. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–34. ISBN 978-0-471-23896-6. [Google Scholar]
- Qureshi, K.; Bhatti, I.; Kazi, R.; Ansari, A.K. Physical and Chemical Analysis of Activated Carbon Prepared from Sugarcane Bagasse and Use for Sugar Decolorisation. Int. J. Chem. Biomol. Eng. 2008, 1, 145–149. [Google Scholar]
- Yakout, S.M.; Daifullah, A.A.M.; El-Reefy, S.A.; Ali, H.F. Surface Modification and Characterization of a RS Activated Carbon: Density, Yield, XRD, Ash, and Moisture Content. Desalination Water Treat. 2015, 53, 718–726. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Q.; Zheng, C.; He, C. Sorption of Sulfadiazine, Norfloxacin, Metronidazole, and Tetracycline by Granular Activated Carbon: Kinetics, Mechanisms, and Isotherms. Water Air Soil Pollut. 2017, 228, 129. [Google Scholar] [CrossRef]
- Neme, I.; Gonfa, G.; Masi, C. Preparation and Characterization of Activated Carbon from Castor Seed Hull by Chemical Activation with H3PO4. Results Mater. 2022, 15, 100304. [Google Scholar] [CrossRef]
- de Souza, T.N.V.; Vieira, M.G.A.; da Silva, M.G.C.; Brasil, D.D.S.B.; de Carvalho, S.M.L. H3PO4-Activated Carbons Produced from Açai Stones and Brazil Nut Shells: Removal of Basic Blue 26 Dye from Aqueous Solutions by Adsorption. Environ. Sci. Pollut. Res. 2019, 26, 28533–28547. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castilla, C. Adsorption of Organic Molecules from Aqueous Solutions on Carbon Materials. Carbon 2004, 42, 83–94. [Google Scholar] [CrossRef]
- Alyasiri, H.; Rushdi, S.; Al-Sharify, Z.T. Recent Advances in the Application of Activated Carbon for the Removal of Pharmaceutical Contaminants from Wastewater: A Review. AIP Conf. Proc. 2023, 2787, 040027. [Google Scholar] [CrossRef]
- Zubir, M.H.M.; Zaini, M.A.A. Twigs-Derived Activated Carbons via H3PO4/ZnCl2 Composite Activation for Methylene Blue and Congo Red Dyes Removal. Sci. Rep. 2020, 10, 14050. [Google Scholar] [CrossRef]
- Waghmare, C.; Ghodmare, S.; Ansari, K.; Dehghani, M.H.; Khan, M.A.; Hasan, M.A.; Islan, S.; Khan, N.A.; Zahmatkesh, S. Experimental Investigation of H3PO4 Activated Papaya Peels for Methylene Blue Dye Removal from Aqueous Solution: Evaluation on Optimization, Kinetics, Isotherm, Thermodynamics, and Reusability Studies. J. Environ. Manag. 2023, 345, 118815. [Google Scholar] [CrossRef]
- de Fontoura, C.R.O.; Dutra, L.V.; Guezgüan, S.M.; Nascimento, M.A.; de Oliveira, A.F.; Lopes, R.P. Optimization of One-Pot H3PO4-Activated Hydrochar Synthesis by Doehlert Design: Characterization and Application. J. Anal. Appl. Pyrolysis 2022, 168, 105775. [Google Scholar] [CrossRef]
- Pereira, R.G.; Veloso, C.M.; da Silva, N.M.; de Sousa, L.F.; Bonomo, R.C.F.; de Souza, A.O.; Souza, M.O.D.G.; Fontan, R.D.C.I. Preparation of Activated Carbons from Cocoa Shells and Siriguela Seeds Using H3PO4 and ZnCL2 as Activating Agents for BSA and α-Lactalbumin Adsorption. Fuel Process. Technol. 2014, 126, 476–486. [Google Scholar] [CrossRef]
- El Farissi, H.; Beraich, A.; Lamsayah, M.; Talhaoui, A.; El Bachiri, A. The Efficiency of Carbon Modified by Phosphoric Acid (H3PO4) Used in the Removal of Two Antibiotics Amoxicillin and Metronidazole from Polluted Water: Experimental and Theoretical Investigation. J. Mol. Liq. 2023, 391, 123237. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Martınez-Alonso, A.; Suarez-Garcıa, F.; Tascon, J.M.D. Synthetic Carbons Activated with Phosphoric Acid I. Surface Chemistry and Ion Binding Properties. Carbon 2002, 40, 1493–1505. [Google Scholar] [CrossRef]
- Silva, M.C.; Spessato, L.; Silva, T.L.; Lopes, G.K.P.; Zanella, H.G.; Yokoyama, J.T.C.; Cazetta, A.L.; Almeida, V.C. H3PO4–Activated Carbon Fibers of High Surface Area from Banana Tree Pseudo-Stem Fibers: Adsorption Studies of Methylene Blue Dye in Batch and Fixed Bed Systems. J. Mol. Liq. 2021, 324, 114771. [Google Scholar] [CrossRef]
- Mahmood, T.; Ali, R.; Naeem, A.; Hamayun, M.; Aslam, M. Potential of Used Camellia Sinensis Leaves as Precursor for Activated Carbon Preparation by Chemical Activation with H3PO4; Optimization Using Response Surface Methodology. Process Saf. Environ. Prot. 2017, 109, 548–563. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental Methods in Chemical Engineering: Specific Surface Area and Pore Size Distribution Measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Sych, N.V.; Trofymenko, S.I.; Poddubnaya, O.I.; Tsyba, M.M.; Sapsay, V.I.; Klymchuk, D.O.; Puziy, A.M. Porous Structure and Surface Chemistry of Phosphoric Acid Activated Carbon from Corncob. Appl. Surf. Sci. 2012, 261, 75–82. [Google Scholar] [CrossRef]
- Tareq, R.; Akter, N.; Azam, M.S. Biochars and Biochar Composites. In Biochar from Biomass and Waste; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–209. ISBN 978-0-12-811729-3. [Google Scholar]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Susan, M.A.B.H.; Carabineiro, S.A. Adsorption of Cationic Dyes, Drugs and Metal from Aqueous Solutions Using a Polymer Composite of Magnetic/β-Cyclodextrin/Activated Charcoal/Na Alginate: Isotherm, Kinetics and Regeneration Studies. J. Hazard. Mater. 2021, 409, 124840. [Google Scholar] [CrossRef]
- Azar, F.-Z.; El Kasmi, A.; Cruz Junior, O.F.; Lillo-Ródenas, M.Á.; Román-Martínez, M.D.C. Direct Cost-Efficient Hydrothermal Conversion of Amazonian Lignocellulosic Biomass Residue. Biomass Conv. Bioref. 2023, 14, 18041–18049. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Vo, T.-D.-H.; Nguyen, T.-B.; Dat, N.D.; Huu, B.T.; Nguyen, X.-C.; Tran, T.; Le, T.-N.-C.; Duong, T.-G.-H.; Bui, M.-H.; et al. Adsorption of Norfloxacin from Aqueous Solution on Biochar Derived from Spent Coffee Ground: Master Variables and Response Surface Method Optimized Adsorption Process. Chemosphere 2022, 288, 132577. [Google Scholar] [CrossRef]
- Dąbrowska, W.; Gargol, M.; Gil-Kowalczyk, M.; Nowicki, P. The Influence of Oxidation and Nitrogenation on the Physicochemical Properties and Sorption Capacity of Activated Biocarbons Prepared from the Elderberry Inflorescence. Molecules 2023, 28, 5508. [Google Scholar] [CrossRef]
- Liou, T.-H. Development of Mesoporous Structure and High Adsorption Capacity of Biomass-Based Activated Carbon by Phosphoric Acid and Zinc Chloride Activation. Chem. Eng. J. 2010, 158, 129–142. [Google Scholar] [CrossRef]
- Higai, D.; Huang, Z.; Qian, E.W. Preparation and Surface Characteristics of Phosphoric Acid-activated Carbon from Coconut Shell in Air. Environ. Prog. Sustain. Energy 2021, 40, e13509. [Google Scholar] [CrossRef]
- Lazzarini, A.; Piovano, A.; Pellegrini, R.; Agostini, G.; Rudić, S.; Lamberti, C.; Groppo, E. Graphitization of Activated Carbons: A Molecular-Level Investigation by INS, DRIFT, XRD and Raman Techniques. Phys. Procedia 2016, 85, 20–26. [Google Scholar] [CrossRef]
- Hu, L.; Cheng, W.; Zhang, W.; Wu, F.; Peng, S.; Li, J. Monolithic Bamboo-Based Activated Carbons for Dynamic Adsorption of Toluene. J. Porous Mater. 2017, 24, 541–549. [Google Scholar] [CrossRef]
- Mopoung, S.; Dejang, N. Activated Carbon Preparation from Eucalyptus Wood Chips Using Continuous Carbonization–Steam Activation Process in a Batch Intermittent Rotary Kiln. Sci. Rep. 2021, 11, 13948. [Google Scholar] [CrossRef]
- Guilarduci, V.V.d.S.; de Mesquita, J.P.; Martelli, P.B.; Gorgulho, H.d.F. Adsorção de fenol sobre carvão ativado em meio alcalino. Quím. Nova 2006, 29, 1226–1232. [Google Scholar] [CrossRef]
- Martínez, R.J.; Godíne, L.A.; Robles, I. Waste Resources Utilization for Biosorbent Preparation, Sorption Studies, and Electrocatalytic Applications. In Valorization of Wastes for Sustainable Development; Elsevier: Amsterdam, Netherlands, 2023; pp. 395–418. ISBN 978-0-323-95417-4. [Google Scholar]
- Belhachemi, M. Adsorption of Organic Compounds on Activated Carbons. In Sorbents Materials for Controlling Environmental Pollution; Elsevier: Amsterdam, Netherlands, 2021; pp. 355–385. [Google Scholar]
- Othmani, A.; John, J.; Rajendran, H.; Mansouri, A.; Sillanpää, M.; Velayudhaperumal Chellam, P. Biochar and Activated Carbon Derivatives of Lignocellulosic Fibers towards Adsorptive Removal of Pollutants from Aqueous Systems: Critical Study and Future Insight. Sep. Purif. Technol. 2021, 274, 119062. [Google Scholar] [CrossRef]
- Pathak, S.; Sakhiya, A.K.; Anand, A.; Pant, K.K.; Kaushal, P. A State-of-the-Art Review of Various Adsorption Media Employed for the Removal of Toxic Polycyclic Aromatic Hydrocarbons (PAHs): An Approach towards a Cleaner Environment. J. Water Process Eng. 2022, 47, 102674. [Google Scholar] [CrossRef]
- de Oliveira Brito, S.M.; Andrade, H.M.C.; Soares, L.F.; de Azevedo, R.P. Brazil Nut Shells as a New Biosorbent to Remove Methylene Blue and Indigo Carmine from Aqueous Solutions. J. Hazard. Mater. 2010, 174, 84–92. [Google Scholar] [CrossRef]
- Ferreira, R.M.; de Oliveira, N.M.; Lima, L.L.S.; Campista, A.L.D.M.; Stapelfeldt, D.M.A. Adsorption of Indigo Carmine on Pistia Stratiotes Dry Biomass Chemically Modified. Environ. Sci. Pollut. Res. 2019, 26, 28614–28621. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Q. Exploring the Adsorption Potential of Na2SiO3-Activated Porous Carbon Materials from Waste Bamboo Biomass for Ciprofloxacin Rapid Removal in Wastewater. Environ. Technol. Innov. 2023, 32, 103318. [Google Scholar] [CrossRef]
- de Franco, M.A.E.; de Carvalho, C.B.; Bonetto, M.M.; Soares, R.D.P.; Féris, L.A. Removal of Amoxicillin from Water by Adsorption onto Activated Carbon in Batch Process and Fixed Bed Column: Kinetics, Isotherms, Experimental Design and Breakthrough Curves Modelling. J. Clean. Prod. 2017, 161, 947–956. [Google Scholar] [CrossRef]
- Luo, B.; Huang, G.; Yao, Y.; An, C.; Zhang, P.; Zhao, K. Investigation into the Influencing Factors and Adsorption Characteristics in the Removal of Sulfonamide Antibiotics by Carbonaceous Materials. J. Clean. Prod. 2021, 319, 128692. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Sadegh, N. Effective Removal of Amoxicillin, Cephalexin, Tetracycline and Penicillin G from Aqueous Solutions Using Activated Carbon Nanoparticles Prepared from Vine Wood. J. Water Process Eng. 2014, 1, 64–73. [Google Scholar] [CrossRef]
- Antonelli, R.; Malpass, G.R.P.; da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Ciprofloxacin onto Thermally Modified Bentonite Clay: Experimental Design, Characterization, and Adsorbent Regeneration. J. Environ. Chem. Eng. 2020, 8, 104553. [Google Scholar] [CrossRef]
- Cheikh, S.; Imessaoudene, A.; Bollinger, J.-C.; Manseri, A.; Bouzaza, A.; Hadadi, A.; Hamri, N.; Amrane, A.; Mouni, L. Adsorption Behavior and Mechanisms of the Emerging Antibiotic Pollutant Norfloxacin on Eco-Friendly and Low-Cost Hydroxyapatite: Integrated Experimental and Response Surface Methodology Optimized Adsorption Process. J. Mol. Liq. 2023, 392, 123424. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Z.; Li, J.; Du, H.; Li, Z. Efficient with Low-Cost Removal and Adsorption Mechanisms of Norfloxacin, Ciprofloxacin and Ofloxacin on Modified Thermal Kaolin: Experimental and Theoretical Studies. J. Hazard. Mater. 2022, 430, 128500. [Google Scholar] [CrossRef]
- Avcı, A.; İnci, İ.; Baylan, N. Adsorption of Ciprofloxacin Hydrochloride on Multiwall Carbon Nanotube. J. Mol. Struct. 2020, 1206, 127711. [Google Scholar] [CrossRef]
- Zhang, B.; Han, X.; Gu, P.; Fang, S.; Bai, J. Response Surface Methodology Approach for Optimization of Ciprofloxacin Adsorption Using Activated Carbon Derived from the Residue of Desilicated Rice Husk. J. Mol. Liq. 2017, 238, 316–325. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Lazić, Ž.R. Design of Experiments in Chemical Engineering: A Practical Guide, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; ISBN 978-3-527-31142-2. [Google Scholar]
- Rocha, L.S.; Pereira, D.; Sousa, É.; Otero, M.; Esteves, V.I.; Calisto, V. Recent Advances on the Development and Application of Magnetic Activated Carbon and Char for the Removal of Pharmaceutical Compounds from Waters: A Review. Sci. Total Environ. 2020, 718, 137272. [Google Scholar] [CrossRef]
- Saucier, C.; Adebayo, M.A.; Lima, E.C.; Prola, L.D.T.; Thue, P.S.; Umpierres, C.S.; Puchana-Rosero, M.J.; Machado, F.M. Comparison of a Homemade Bacuri Shell Activated Carbon With Carbon Nanotubes for Food Dye Removal. CLEAN Soil Air Water 2015, 43, 1389–1400. [Google Scholar] [CrossRef]
- Zhu, X.; Tsang, D.C.W.; Chen, F.; Li, S.; Yang, X. Ciprofloxacin Adsorption on Graphene and Granular Activated Carbon: Kinetics, Isotherms, and Effects of Solution Chemistry. Environ. Technol. 2015, 36, 3094–3102. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Laverde, M.; Silva-Agredo, J.; Torres-Palma, R.A. Removal of Norfloxacin in Deionized, Municipal Water and Urine Using Rice (Oryza sativa) and Coffee (Coffea arabica) Husk Wastes as Natural Adsorbents. J. Environ. Manag. 2018, 213, 98–108. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Zhang, C.; Ren, L. Sorption of Norfloxacin by Lotus Stalk-Based Activated Carbon and Iron-Doped Activated Alumina: Mechanisms, Isotherms and Kinetics. Chem. Eng. J. 2011, 171, 431–438. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Mangwandi, C. Mechanisms of Alizarin Red S and Methylene Blue Biosorption onto Olive Stone By-Product: Isotherm Study in Single and Binary Systems. J. Environ. Manag. 2015, 164, 86–93. [Google Scholar] [CrossRef]
- Hameed, B.H.; Tan, I.A.W.; Ahmad, A.L. Adsorption Isotherm, Kinetic Modeling and Mechanism of 2,4,6-Trichlorophenol on Coconut Husk-Based Activated Carbon. Chem. Eng. J. 2008, 144, 235–244. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Kinetic Models: Physical Meanings, Applications, and Solving Methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A General Treatment and Classification of the Solute Adsorption Isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Huang, X.; Zhang, Q.; Wang, T.; Guo, X. Guideline for Modeling Solid-Liquid Adsorption: Kinetics, Isotherm, Fixed Bed, and Thermodynamics. Chemosphere 2024, 349, 140736. [Google Scholar] [CrossRef]
- Ouyang, J.; Chen, J.; Chen, W.; Zhou, L.; Cai, D.; Ren, C. H3PO4 Activated Biochars Derived from Different Agricultural Biomasses for the Removal of Ciprofloxacin from Aqueous Solution. Particuology 2023, 75, 217–227. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Patra, C.; Narayanasamy, S.; Subbiah, S. Adsorptive Removal of Ciprofloxacin and Amoxicillin from Single and Binary Aqueous Systems Using Acid-Activated Carbon from Prosopis juliflora. Environ. Res. 2020, 188, 109825. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, M.; Zhou, R.; Li, J.; Zhao, W.; Zhou, J. Adsorption Characteristics and Mechanism of Norfloxacin in Water by γ-Fe2O3@BC. Water Sci. Technol. 2020, 82, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N. Improper Estimation of Thermodynamic Parameters in Adsorption Studies with Distribution Coefficient KD (qe/Ce) or Freundlich Constant (KF): Considerations from the Derivation of Dimensionless Thermodynamic Equilibrium Constant and Suggestions. Adsorpt. Sci. Technol. 2022, 2022, 5553212. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Dhiman, N. Analysis of Non Competitive and Competitive Adsorption Behaviour of Ciprofloxacin Hydrochloride and Ofloxacin Hydrochloride from Aqueous Solution Using Oryza Sativa Husk Ash (Single and Binary Adsorption of Antibiotics). Clean. Mater. 2022, 5, 100108. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Meili, L.; Bonilla-Petriciolet, A.; Kurniawan, T.A.; Imanova, G.; Demir, E.; Ali, I. Environmental Remediation of the Norfloxacin in Water by Adsorption: Advances, Current Status and Prospects. Adv. Colloid Interface Sci. 2024, 324, 103096. [Google Scholar] [CrossRef]
- Li, X.; Wang, W.; Dou, J.; Gao, J.; Chen, S.; Quan, X.; Zhao, H. Dynamic Adsorption of Ciprofloxacin on Carbon Nanofibers: Quantitative Measurement by in Situ Fluorescence. J. Water Process Eng. 2016, 9, e14–e20. [Google Scholar] [CrossRef]
- Bednárek, J.; Matějová, L.; Koutník, I.; Vráblová, M.; Cruz, G.J.F.; Strašák, T.; Šiler, P.; Hrbáč, J. Revelation of High-Adsorption-Performance Activated Carbon for Removal of Fluoroquinolone Antibiotics from Water. Biomass Conv. Bioref. 2024, 14, 2585–2599. [Google Scholar] [CrossRef]
- Wang, M.; Li, G.; Huang, L.; Xue, J.; Liu, Q.; Bao, N.; Huang, J. Study of Ciprofloxacin Adsorption and Regeneration of Activated Carbon Prepared from Enteromorpha Prolifera Impregnated with H3PO4 and Sodium Benzenesulfonate. Ecotoxicol. Environ. Saf. 2017, 139, 36–42. [Google Scholar] [CrossRef]
- García-Reyes, C.B.; Salazar-Rábago, J.J.; Sánchez-Polo, M.; Loredo-Cancino, M.; Leyva-Ramos, R. Ciprofloxacin, Ranitidine, and Chlorphenamine Removal from Aqueous Solution by Adsorption. Mechanistic and Regeneration Analysis. Environ. Technol. Innov. 2021, 24, 102060. [Google Scholar] [CrossRef]
- ASTM D2866-11; Standard Test Method for Total Ash Content of Activated Carbon. American Society for Testing and Materials: West Conshohocken, PA, USA, 2018.
- ASTM 3838-05; Standard Test Method for pH of Activated Carbon. American Society for Testing and Materials: West Conshohocken, PA, USA, 2017.
- Boehm, H.P. Some Aspects of the Surface Chemistry of Carbon Blacks and Other Carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Boehm, H.P. Surface Oxides on Carbon and Their Analysis: A Critical Assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- ASTM D2862-16; Standard Test Method for Particle Size Distribution of Granular Activated Carbon. American Society for Testing and Materials: West Conshohocken, PA, USA, 2022.
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 978-1-119-11347-8. [Google Scholar]
- Maia, G.S.; de Andrade, J.R.; da Silva, M.G.; Vieira, M.G.A. Adsorption of Diclofenac Sodium onto Commercial Organoclay: Kinetic, Equilibrium and Thermodynamic Study. Powder Technol. 2019, 345, 140–150. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A Critical Review of the Estimation of the Thermodynamic Parameters on Adsorption Equilibria. Wrong Use of Equilibrium Constant in the Van’t Hoof Equation for Calculation of Thermodynamic Parameters of Adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- DasSharma, D.; Samanta, S.; Dharun Nithish Kumar, S.; Halder, G. A Mechanistic Insight into Enrofloxacin Sorptive Affinity of Chemically Activated Carbon Engineered from Green Coconut Shell. J. Environ. Chem. Eng. 2020, 8, 104140. [Google Scholar] [CrossRef]
- Mbarki, F.; Selmi, T.; Kesraoui, A.; Seffen, M. Low-Cost Activated Carbon Preparation from Corn Stigmata Fibers Chemically Activated Using H3PO4, ZnCl2 and KOH: Study of Methylene Blue Adsorption, Stochastic Isotherm and Fractal Kinetic. Ind. Crops Prod. 2022, 178, 114546. [Google Scholar] [CrossRef]
- Harabi, S.; Guiza, S.; Álvarez-Montero, A.; Gómez-Avilés, A.; Belver, C.; Rodríguez, J.J.; Bedia, J. Adsorption of 2,4-Dichlorophenoxyacetic Acid on Activated Carbons from Macadamia Nut Shells. Environ. Res. 2024, 247, 118281. [Google Scholar] [CrossRef]
- Avcı, A.; İnci, İ.; Baylan, N. A Comparative Adsorption Study with Various Adsorbents for the Removal of Ciprofloxacin Hydrochloride from Water. Water Air Soil Pollut. 2019, 230, 250. [Google Scholar] [CrossRef]
- Gupta, A.; Garg, A. Adsorption and Oxidation of Ciprofloxacin in a Fixed Bed Column Using Activated Sludge Derived Activated Carbon. J. Environ. Manag. 2019, 250, 109474. [Google Scholar] [CrossRef]
- Liao, X.; Chen, C.; Liang, Z.; Zhao, Z.; Cui, F. Selective Adsorption of Antibiotics on Manganese Oxide-Loaded Biochar and Mechanism Based on Quantitative Structure–Property Relationship Model. Bioresour. Technol. 2023, 367, 128262. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; He, Q.; Sheng, L.; Xi, Y.; Zhang, L.; Liu, H.; Cheng, H. Toward Broader Applications of Iron Ore Waste in Pollution Control: Adsorption of Norfloxacin. J. Hazard. Mater. 2021, 418, 126273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Chen, Y.; Li, Z.-J.; Li, X.; Fan, G. Bioactive Properties of the Aromatic Molecules of Spearmint (Mentha spicata L.) Essential Oil: A Review. Food Funct. 2022, 13, 3110–3132. [Google Scholar] [CrossRef] [PubMed]
- PubChem National Library of Medicine: National Center for Biotechnology Information (NCBI). DataBase Pubchem DataBase. Ciprofloxacin and Norfloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 21 October 2024).
- Lagergren, S. Zur Theorie Der Sogenannten Adsorption Gelöster Stoffe Kungliga Svenska Vetenskapsakademien. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of Dye from Aqueous Solution by Peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Aharoni, C.; Tompkins, F.C. Kinetics of Adsorption and Desorption and the Elovich Equation. In Advances in Catalysis; Eley, D.D., Pines, H., Weisz, P.B., Eds.; Academic Press: Cambridge, MA, USA, 1970; Volume 21, pp. 1–49. [Google Scholar]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S., Jr. The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. II. Kinetics1. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Puranik, P.R.; Modak, J.M.; Paknikar, K.M. A Comparative Study of the Mass Transfer Kinetics of Metal Biosorption by Microbial Biomass. Hydrometallurgy 1999, 52, 189–197. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 1100–1107. [Google Scholar]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]

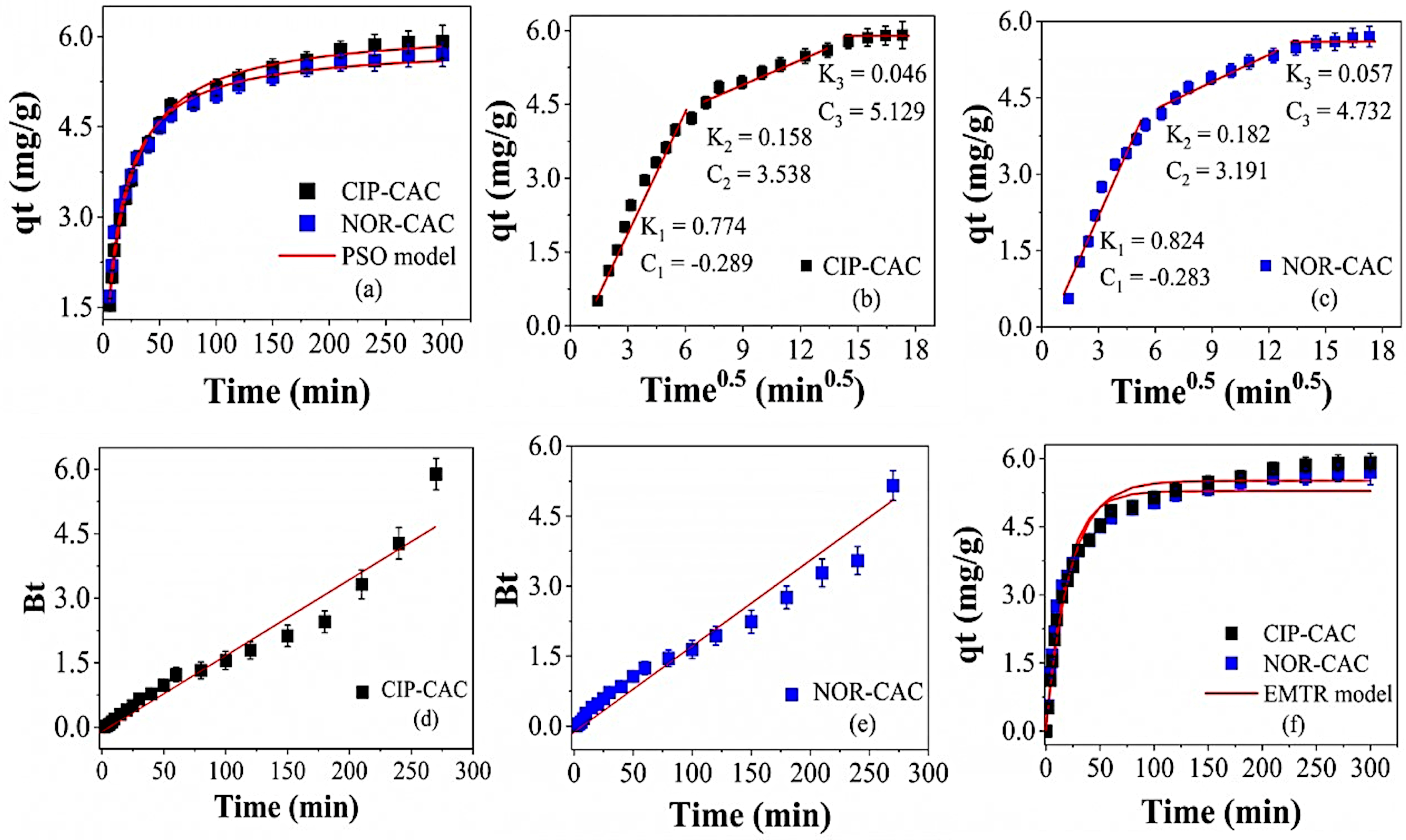

| Source | df | CIP-CAC | NOR-CAC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Removal | Adsorption Capacity | Removal | Adsorption Capacity | ||||||||||
| Coef | SS | p | Coef | SS | p | Coef | SS | p | Coef | SS | p | ||
| X1 | 1 | 6.07 | 294.52 | 0.0076 * | 0.36 | 1.02 | 0.0026 * | 6.54 | 342.30 | 0.0062 * | 0.39 | 1.19 | 0.0039 * |
| X2 | 1 | −9.66 | 745.75 | 0.0030 * | 0.33 | 0.85 | 0.0031 * | −9.69 | 750.98 | 0.0028 * | 0.29 | 0.68 | 0.0068 * |
| X3 | 1 | 5.23 | 218.82 | 0.0102 * | −0.28 | 0.64 | 0.0041 * | 5.16 | 212.59 | 0.0099 * | −0.31 | 0.76 | 0.0060 * |
| 1 | −11.45 | 483.72 | 0.0046 * | −0.27 | 0.27 | 0.0097 * | −11.47 | 485.59 | 0.0044 * | −0.21 | 0.16 | 0.0272 * | |
| 1 | −6.54 | 157.97 | 0.0140 * | −0.22 | 0.17 | 0.0149 * | −6.38 | 150.20 | 0.0140 * | −0.23 | 0.20 | 0.0223 * | |
| 1 | −11.27 | 468.62 | 0.0048 * | −0.19 | 0.14 | 0.0184 * | −11.75 | 509.81 | 0.0042 * | −0.20 | 0.15 | 0.0291 * | |
| X1X2 | 1 | −6.17 | 152.03 | 0.0145 * | 0.17 | 0.11 | 0.0227 * | −5.58 | 124.66 | 0.0168 * | 0.16 | 0.10 | 0.0424 * |
| X1X3 | 1 | 2.39 | 22.75 | 0.0865 | −0.16 | 0.10 | 0.0248 * | 2.08 | 17.31 | 0.1049 | −0.19 | 0.14 | 0.0306 * |
| X2X3 | 1 | −2.25 | 20.25 | 0.0957 | −0.04 | 0.01 | 0.2395 | −1.79 | 12.82 | 0.1345 | −0.10 | 0.04 | 0.0948 |
| Lack of fit | 3 | 35.26 | 0.1653 | 0.03 | 0.2029 | 37.98 | 0.01 | ||||||
| Pure error | 2 | 4.51 | 0.01 | 4.29 | 0.01 | ||||||||
| Total | 14 | 2468.55 | 3.27 | 2510.39 | 3.38 | ||||||||
| 95.29 | <0.0001 * | 5.81 | <0.0001 * | 92.99 | <0.0001 * | 5.54 | <0.0001 * | ||||||
| R2 | 0.9839 | 0.9885 | 0.9832 | 0.9956 | |||||||||
| Optimized Adsorption Conditions | CIP-CAC | NOR-CAC |
|---|---|---|
| Time (min.) | 266.40 | 273.60 |
| Initial adsorbate concentration (mg/L) | 192 | 186 |
| Adsorbent dosage (g/L) | 0.57 | 0.55 |
| Global desirability | 0.993 | 0.989 |
| Predicted values | ||
| Removal (%) | 93.44 | 90.36 |
| qe (mg/g) | 6.00 | 5.80 |
| Experimental values * | ||
| Removal (%) | 92.89 ± 2.56 | 89.21 ± 2.35 |
| qe (mg/g) | 5.90 ± 0.16 | 5.71 ± 0.19 |
| Models | Parameters | CIP-CAC | NOR-CAC |
|---|---|---|---|
| Experimental * | (mg/g) | 5.901 ± 0.275 | 5.682 ± 0.201 |
| PPO | (mg/g) | 5.511 | 5.287 |
| (min−1) | 0.045 | 0.054 | |
| R2 | 0.977 | 0.969 | |
| R2ajus | 0.976 | 0.968 | |
| RMSE | 0.291 | 0.316 | |
| AICC | −80.22 | −75.43 | |
| PSO | (mg/g) | 6.172 | 5.862 |
| (min−1) | 0.009 | 0.012 | |
| R2 | 0.998 | 0.996 | |
| R2ajus | 0.998 | 0.996 | |
| RMSE | 0.089 | 0.113 | |
| AICC | −90.47 | −86.69 | |
| Elovich | α (mg/g min) | 0.798 | 1.056 |
| β (mg/g) | 0.841 | 0.936 | |
| R2 | 0.979 | 0.975 | |
| R2ajus | 0.978 | 0.973 | |
| RMSE | 0.277 | 0.286 | |
| AICC | −50.89 | −43.21 |
| Models | Parameters | CIP-CAC | NOR-CAC | ||||
|---|---|---|---|---|---|---|---|
| 28 °C | 35 °C | 45 °C | 28 °C | 35 °C | 45 °C | ||
| Experimental * | (mg/g) | 6.020 ± 0.192 | 6.050 ± 0.199 | 6.130 ± 0.202 | 5.701 ± 0.181 | 5.790 ± 0.190 | 5.850 ± 0.195 |
| Langmuir | (mg/g) | 6.153 | 6.169 | 6.189 | 5.984 | 5.999 | 6.016 |
| (L/mg) | 0.073 | 0.080 | 0.090 | 0.046 | 0.050 | 0.054 | |
| R2 | 0.966 | 0.965 | 0.967 | 0.967 | 0.964 | 0.962 | |
| R2ajus | 0.962 | 0.961 | 0.963 | 0.963 | 0.963 | 0.958 | |
| RMSE | 0.313 | 0.310 | 0.297 | 0.318 | 0.328 | 0.334 | |
| AICc | −80.22 | −79.92 | −80.89 | −81.45 | −80.96 | −80.03 | |
| Freundlich | [(mg/g)(L/mg)1/nF] | 1.732 | 1.834 | 1.950 | 1.333 | 1.421 | 1.500 |
| n | 4.438 | 4.619 | 4.820 | 3.812 | 3.949 | 4.073 | |
| R2 | 0.947 | 0.944 | 0.945 | 0.962 | 0.960 | 0.959 | |
| R2ajus | 0.942 | 0.934 | 0.939 | 0.958 | 0.956 | 0.956 | |
| RMSE | 0.386 | 0.393 | 0.384 | 0.340 | 0.342 | 0.343 | |
| AICc | −71.57 | −65.87 | −66.99 | −80.86 | −79.44 | −79.32 | |
| Sips | (mg/g) | 7.155 | 7.096 | 7.081 | 7.123 | 7.223 | 7.241 |
| [(L/mg)1/ns] | 0.149 | 0.160 | 0.177 | 0.105 | 0.116 | 0.125 | |
| ns | 1.585 | 1.585 | 1.585 | 1.548 | 1.591 | 1.608 | |
| R2 | 0.988 | 0.989 | 0.992 | 0.994 | 0.993 | 0.993 | |
| R2ajus | 0.985 | 0.986 | 0.991 | 0.992 | 0.991 | 0.992 | |
| RMSE | 0.193 | 0.185 | 0.146 | 0.147 | 0.156 | 0.148 | |
| AICc | −85.44 | −87.33 | −91.39 | −90.63 | −89.92 | −91.24 | |
| CIP–CAC | NOR–CAC | |||||
|---|---|---|---|---|---|---|
| 28 °C | 35 °C | 45 °C | 28 °C | 35 °C | 45 °C | |
| KL (L/mg) | 0.073 | 0.080 | 0.090 | 0.046 | 0.050 | 0.054 |
| (adim.) | 24,187.82 | 26,507.20 | 29,820.6 | 10,089.18 | 10,966.50 | 11,843.82 |
| ΔG° (kJ/mol) | −25.27 | −26.09 | −27.25 | −14.88 | −15.64 | −16.56 |
| ΔH° (kJ/mol) | 9.78 | 7.37 | ||||
| ΔS° (J/mol K) | 116.41 | 101.18 | ||||
| R2 | 0.999 | 0.988 | ||||
| R2ajus | 0.998 | 0.977 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Nascimento, R.A.; Novaes, N.d.R.d.O.; Morilla, D.P.; da Luz, P.T.S.; Costa, C.M.L.; Faria, L.J.G.d. Removal of Ciprofloxacin and Norfloxacin from Aqueous Solution with Activated Carbon from Cupuaçu (Theobroma grandiflorum) Bark. Molecules 2024, 29, 5853. https://doi.org/10.3390/molecules29245853
do Nascimento RA, Novaes NdRdO, Morilla DP, da Luz PTS, Costa CML, Faria LJGd. Removal of Ciprofloxacin and Norfloxacin from Aqueous Solution with Activated Carbon from Cupuaçu (Theobroma grandiflorum) Bark. Molecules. 2024; 29(24):5853. https://doi.org/10.3390/molecules29245853
Chicago/Turabian Styledo Nascimento, Rafael Alves, Nilson dos Reis de Oliveira Novaes, Demetrius Pereira Morilla, Patricia Teresa Souza da Luz, Cristiane Maria Leal Costa, and Lênio José Guerreiro de Faria. 2024. "Removal of Ciprofloxacin and Norfloxacin from Aqueous Solution with Activated Carbon from Cupuaçu (Theobroma grandiflorum) Bark" Molecules 29, no. 24: 5853. https://doi.org/10.3390/molecules29245853
APA Styledo Nascimento, R. A., Novaes, N. d. R. d. O., Morilla, D. P., da Luz, P. T. S., Costa, C. M. L., & Faria, L. J. G. d. (2024). Removal of Ciprofloxacin and Norfloxacin from Aqueous Solution with Activated Carbon from Cupuaçu (Theobroma grandiflorum) Bark. Molecules, 29(24), 5853. https://doi.org/10.3390/molecules29245853







