Highly Efficient Reduction of Cr (VI) with C4H6O6
Abstract
1. Introduction
2. Results and Discussion
2.1. Reaction Mechanism
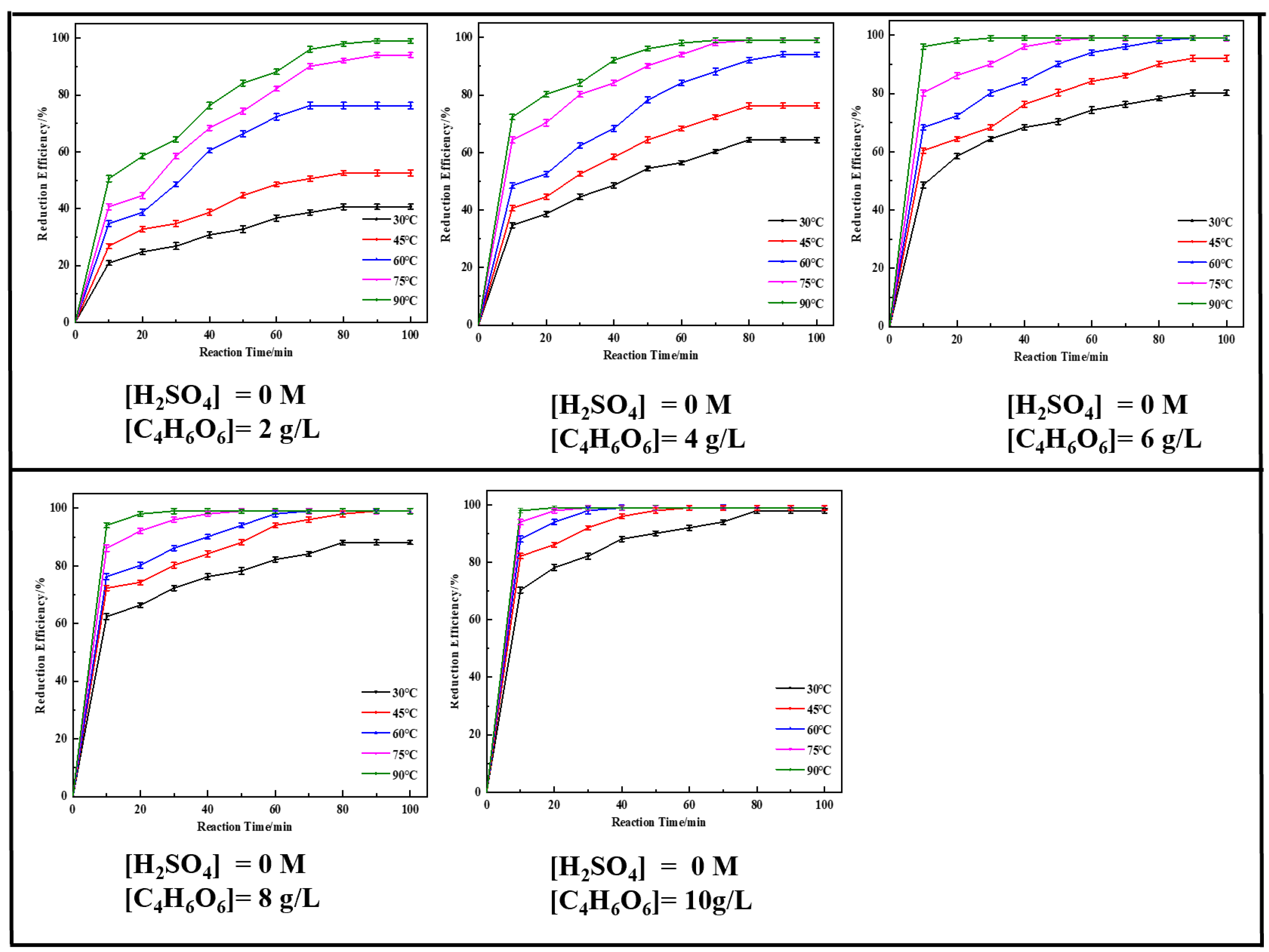
2.2. Single-Factor Experiments
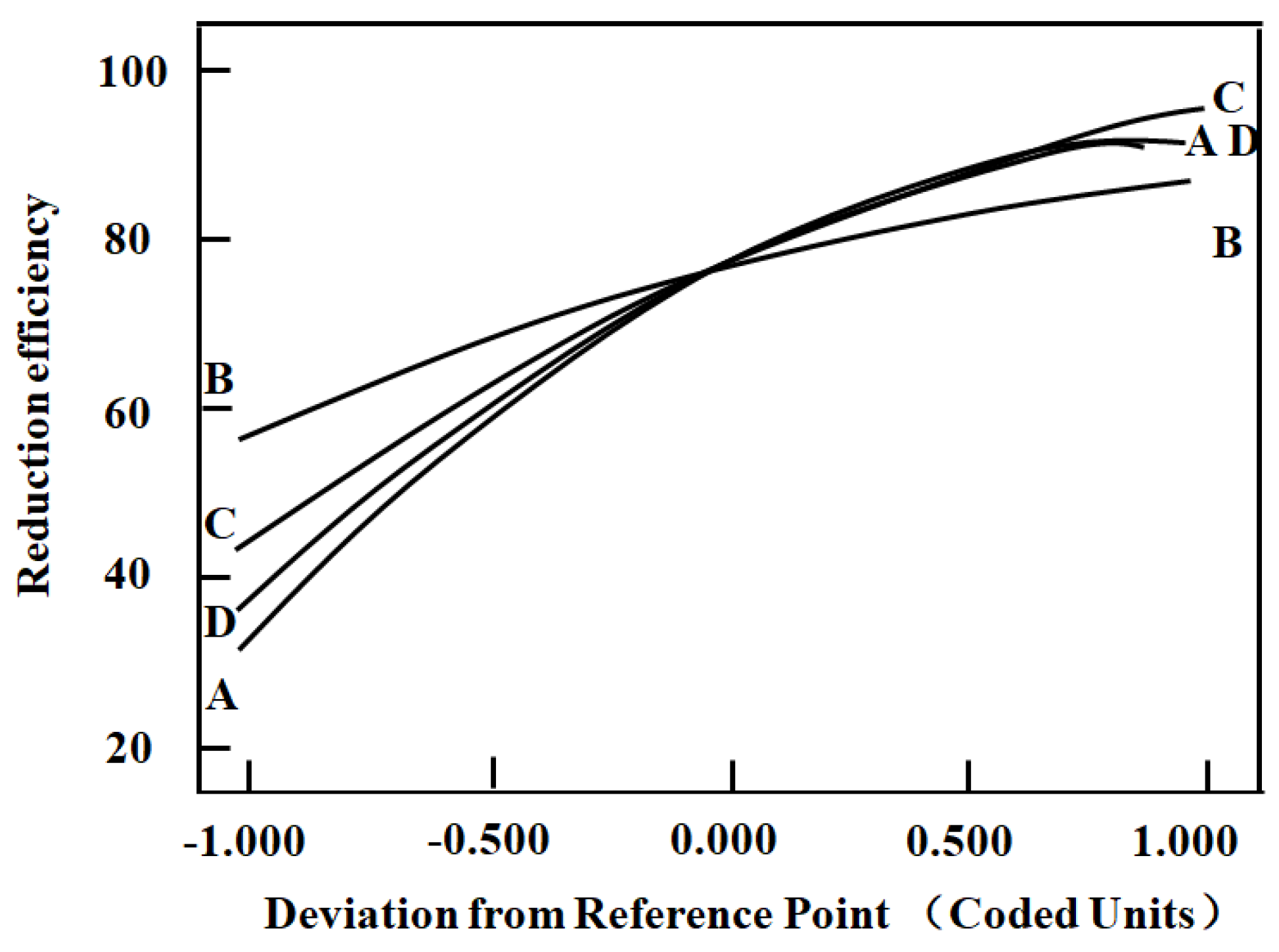
2.3. Response Surface Methodology
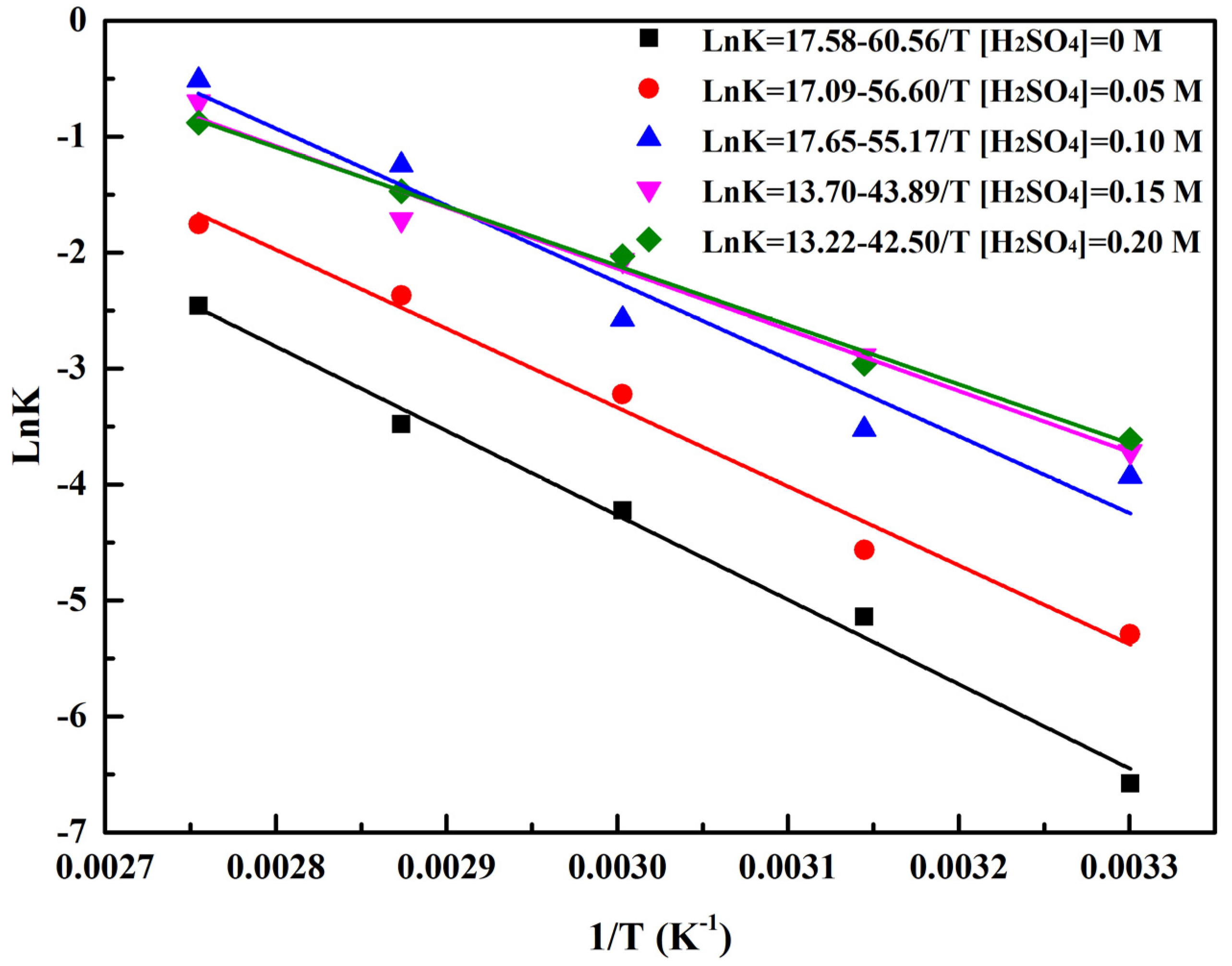
2.4. Reduction Kinetics Analysis
3. Experimental Procedure
3.1. Materials
3.2. Experimental Procedure
4. Conclusions
- Tartaric acid was an efficient reductant for Cr (VI) reduction in a strong acidic medium. Higher concentrations of tartaric acid and higher reaction temperatures could facilitate the reduction process and reduce reaction time.
- A total of 100% of the Cr (VI) could be reduced by C4H6O6 in a strong acidic environment under high reaction temperatures. Response surface methodology analysis confirmed that the experimental parameters had a positive effect on the reduction process and followed the order [H2SO4] > [C4H6O6] > reaction temperature > reaction time.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, J.; Ma, X.; McDonald, T.J.; Huang, C.-H.; Sharma, V.K. Overlooked Role of Chromium(V) and Chromium(IV) in Chromium Redox Reactions of Environmental Importance. ACS EST Water 2022, 2, 932–942. [Google Scholar] [CrossRef]
- Hu, L.; Cai, Y.; Jiang, G. Occurrence and speciation of polymeric chromium(III), monomeric chromium(III) and chromium(VI) in environmental samples. Chemosphere 2016, 156, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Murthy, M.K.; Khandayataray, P.; Padhiary, S.; Samal, D. A review on chromium health hazards and molecular mechanism of chromium bioremediation. Rev. Environ. Health 2023, 38, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. The Biochemistry of Chromium. J. Nutr. 2000, 130, 715–718. [Google Scholar] [CrossRef]
- Vincent, J.B.; Lukaski, H.C. Chromium. Adv. Nutr. 2018, 9, 505–506. [Google Scholar] [CrossRef]
- Butera, S.; Trapp, S.; Astrup, T.F.; Christensen, T.H. Soil retention of hexavalent chromium released from construction and demolition waste in a road-base-application scenario. J. Hazard. Mater. 2015, 298, 361–367. [Google Scholar] [CrossRef]
- Mamais, D.; Noutsopoulos, C.; Kavallari, I.; Nyktari, E.; Kaldis, A.; Panousi, E.; Nikitopoulos, G.; Antoniou, K.; Nasioka, M. Biological groundwater treatment for chromium removal at low hexavalent chromium concentrations. Chemosphere 2016, 152, 238–244. [Google Scholar] [CrossRef]
- Uma, K.; Pawar, S. TiO2/g-C3N4 composites for the removal of chromium in wastewater. Results Chem. 2023, 5, 100743. [Google Scholar] [CrossRef]
- Panousi, E.; Mamais, D.; Noutsopoulos, C.; Mpertoli, K.; Kantzavelou, C.; Nyktari, E.; Kavallari, I.; Nasioka, M.; Kaldis, A. Biological groundwater treatment for hexavalent chromium removal at low chromium concentrations under anoxic conditions. Environ. Technol. 2019, 40, 365–373. [Google Scholar] [CrossRef]
- Zha, S.; Yu, A.; Wang, Z.; Shi, Q.; Cheng, X.; Liu, C.; Deng, C.; Zeng, G.; Luo, S.; Zhao, Z.; et al. Microbial strategies for effective hexavalent chromium removal: A comprehensive review. Chem. Eng. J. 2024, 489, 151457. [Google Scholar] [CrossRef]
- Peng, H.; Leng, Y.; Cheng, Q.; Shang, Q.; Shu, J.; Guo, J. Efficient Removal of Hexavalent Chromium from Wastewater with Electro-Reduction. Processes 2019, 7, 41. [Google Scholar] [CrossRef]
- da Silva, G.S.; dos Santos, F.A.; Roth, G.; Frankenberg, C.L.C. Electroplating for chromium removal from tannery wastewater. Int. J. Environ. Sci. Technol. 2020, 17, 607–614. [Google Scholar] [CrossRef]
- Del Pianta, D.; Frayret, J.; Gleyzes, C.; Cugnet, C.; Dupin, J.C.; Le Hecho, I. Determination of the chromium(III) reduction mechanism during chromium electroplating. Electrochim. Acta 2018, 284, 234–241. [Google Scholar] [CrossRef]
- Kumar, A.; Paul, P.; Nataraj, S.K. Bionanomaterial Scaffolds for Effective Removal of Fluoride, Chromium, and Dye. ACS Sustain. Chem. Eng. 2017, 5, 895–903. [Google Scholar] [CrossRef]
- Noah, N.F.M.; Sulaiman, R.N.R.; Othman, N.; Jusoh, N.; Rosly, M.B. Extractive continuous extractor for chromium recovery: Chromium (VI) reduction to chromium (III) in sustainable emulsion liquid membrane process. J. Clean. Prod. 2020, 247, 119167. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: A review. Environ. Chem. Lett. 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Pinos, V.; Dafinov, A.; Medina, F.; Sueiras, J. Chromium(VI) reduction in aqueous medium by means of catalytic membrane reactors. J. Environ. Chem. Eng. 2016, 4, 1880–1889. [Google Scholar] [CrossRef]
- Abinaya, M.; Govindan, K.; Kalpana, M.; Saravanakumar, K.; Prabavathi, S.L.; Muthuraj, V.; Jang, A. Reduction of hexavalent chromium and degradation of tetracycline using a novel indium-doped Mn2O3 nanorod photocatalyst. J. Hazard. Mater. 2020, 397, 122885. [Google Scholar] [CrossRef]
- Peng, H.; Du, Y.; Yong, J.; Huang, C.; Zheng, X.; Wen, J. ZnInGaS4 heterojunction with sulfide vacancies for efficient solar-light photocatalytic water splitting and Cr(VI) reduction. Chem. Eng. J. 2023, 452, 139386. [Google Scholar] [CrossRef]
- Peng, H.; Wang, L.; Li, H.; Zheng, X. Visible-light photoreduction of Cr(VI) over CdIn2S4/MOF-808 composites. Surf. Interfaces 2024, 51, 104567. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Karthik, R.; Chen, S.-M.; Vinoth Kumar, J.; Prakash, K.; Muthuraj, V. Construction of novel Pd/CeO2/g-C3N4 nanocomposites as efficient visible-light photocatalysts for hexavalent chromium detoxification. J. Colloid Interface Sci. 2017, 504, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, J.; Zhu, F.; Mu, F.; Zhang, L.; Dai, B.; Xu, J.; Zhu, A.; Sun, C.; Leung, D.Y.C. Study the photocatalytic mechanism of the novel Ag/p-Ag2O/n-BiVO4 plasmonic photocatalyst for the simultaneous removal of BPA and chromium(VI). Chem. Eng. J. 2019, 361, 1352–1362. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Akram, M.W.; Begum, R.; Wu, W.; Irfan, A. Inorganic nanoparticles for reduction of hexavalent chromium: Physicochemical aspects. J. Hazard. Mater. 2021, 402, 123535. [Google Scholar] [CrossRef]
- Treviño, P.; Ibanez, J.G.; Vasquez-Medrano, R. Chromium (VI) Reduction Kinetics by Zero-valent Aluminum. Int. J. Electrochem. Sci. 2014, 9, 2556–2564. [Google Scholar] [CrossRef]
- Wei, L.-L.; Gu, R.; Lee, J.-M. Highly efficient reduction of hexavalent chromium on amino-functionalized palladium nanowires. Appl. Catal. B Environ. 2015, 176–177, 325–330. [Google Scholar] [CrossRef]
- Boussouga, Y.-A.; Okkali, T.; Luxbacher, T.; Schäfer, A.I. Chromium (III) and chromium (VI) removal and organic matter interaction with nanofiltration. Sci. Total Environ. 2023, 885, 163695. [Google Scholar] [CrossRef]
- Gogoi, R.; Jena, S.K.; Singh, A.; Sharma, K.; Khanna, K.; Chowdhury, S.; Kumar, R.; Siril, P.F. Mechanically pulverized covalent organic framework as a metal-free photocatalyst for fenton-like degradation of organic pollutants and hexavalent chromium reduction. J. Environ. Chem. Eng. 2024, 12, 112006. [Google Scholar] [CrossRef]
- Li, W.-Q.; Wang, Y.-X.; Li, Y.-M.; He, C.-S.; Lai, B.; Chen, F.; Wang, H.-J.; Zhou, X.-G.; Mu, Y. Metal organic framework decorated with molybdenum disulfide for visible-light-driven reduction of hexavalent chromium: Performance and mechanism. J. Clean. Prod. 2021, 318, 128513. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Wu, Y.; Han, Y.; Lan, Y. Assessing the Photocatalytic Reduction of Cr(VI) by CuO in Combination with Different Organic Acids. Water Air Soil Pollut. 2017, 228, 363. [Google Scholar] [CrossRef]
- Fatima, R.; Kim, J.-O. Photocatalytic reduction of chromium by titanium metal organic frameworks in the presence of low-molecular-weight organic acids under UV and visible light. J. Environ. Chem. Eng. 2022, 10, 108796. [Google Scholar] [CrossRef]
- Wei, S.; Li, J.; Liu, L.; Shi, J.; Shao, Z. Photocatalytic effect of iron corrosion products on reduction of hexavalent chromium by organic acids. J. Taiwan Inst. Chem. Eng. 2014, 45, 2659–2663. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, F.; Peng, J.; Tang, B. The mobility of Cr(VI) on the ferrihydrite-Cr(VI) co-precipitates: The effect of co-existing tartaric acid and Cu(II). Appl. Geochem. 2023, 152, 105646. [Google Scholar] [CrossRef]
- Brose, D.A.; James, B.R. Hexavalent Chromium Reduction by Tartaric Acid and Isopropyl Alcohol in Mid-Atlantic Soils and the Role of Mn(III,IV)(hydr)oxides. Environ. Sci. Technol. 2013, 47, 12985–12991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Du, J.; Xing, B. Goethite catalyzed Cr(VI) reduction by tartaric acid via surface adsorption. Ecotoxicol. Environ. Saf. 2019, 171, 594–599. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J.; Qiu, H.; Wang, C.; Zhang, C.; Hao, Z.; Rao, Y.; Gong, Y. Efficient Removal of Cr (VI) with Biochar and Optimized Parameters by Response Surface Methodology. Processes 2021, 9, 889. [Google Scholar] [CrossRef]
- Peng, H.; Shang, Q.; Chen, R.; Zhang, L.; Chen, Y.; Guo, J. Highly efficient oxidative-alkaline-leaching process of vanadium-chromium reducing residue and parameters optimization by response surface methodology. Environ. Technol. 2020, 43, 2167–2176. [Google Scholar] [CrossRef]
- Peng, H.; Wang, L.; Guo, J.; Wu, Y.; Li, B.; Lin, Y. Efficient reduction of vanadium (V) with biochar and experimental parameters optimized by response surface methodology. Sci. Rep. 2024, 14, 8118. [Google Scholar] [CrossRef]
- Peng, H.; Tang, D.; Liao, M.; Wu, Y.; Fan, X.; Li, B.; Huang, H.; Shi, W. A Clean Method for Vanadium (V) Reduction with Oxalic Acid. Metals 2022, 12, 557. [Google Scholar] [CrossRef]
- Peng, H.; Wang, L.; Pan, W.; Yang, S.; Wang, J.; Qin, J.; Ao, L.; Lin, Y.; Tang, J. Highly Efficient Reduction of Vanadium (V) with Histidine. Water 2024, 16, 2227. [Google Scholar] [CrossRef]


| No. | Methods | Advantages | Disadvantages | |
|---|---|---|---|---|
| 1 | Physicochemical Processes | Chemical Precipitation | simple, effective | secondary pollution |
| 2 | Membrane | higher removal efficiency, no pollution loads and sometimes lower energy consumption | highly depend on materials, membrane pore size, and composition | |
| 3 | Ion Exchange | high efficiency, low cost, less sludge volume, and high selectivity | highly depends on resin structure and the solution environment | |
| 4 | Adsorption | high efficiency, simple operation and ease of regeneration | highly depends on the solution environment | |
| 5 | Electrochemical Technologies | Electrocoagulation | simple, productive, ease of operation | poor systematic reactor design and sacrifice of electrodes |
| 6 | Electrochemical Reduction | no further reagent | dependent on the electrode materials and electrochemical surface area of the electrode | |
| 7 | Electrodialysis | low energy consumption | high cost of electrodes | |
| 8 | Advanced Technologies | Photocatalysis | simple design, low-cost operation, high stability, and high removal efficiency | producing unwanted byproducts |
| 9 | Nanotechnology | higher removal efficiency, low waste generation, and specific uptake | increase the risk of nano-pollutants in the environment |
| Independent Variable | Unit | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| A: [H2SO4] | mol/L | 0 | 0.1 | 0.2 |
| B: Time | min | 10 | 25 | 40 |
| C: Temperature | °C | 30 | 60 | 90 |
| D: [C4H6O6] | g/L | 2 | 6 | 10 |
| Run | A: [H2SO4] | B: Time | C: Temperature | D: [C4H6O6] | Actual Reduction Efficiency (%) |
|---|---|---|---|---|---|
| 1 | 1 | 25 | 60 | 6 | 96.04 |
| 2 | 0 | 40 | 60 | 6 | 84.17 |
| 3 | 1 | 40 | 60 | 2 | 94.06 |
| 4 | 1 | 25 | 90 | 2 | 100.00 |
| 5 | 1 | 40 | 90 | 6 | 100.00 |
| 6 | 1 | 10 | 60 | 10 | 100.00 |
| 7 | 1 | 25 | 60 | 6 | 96.04 |
| 8 | 1 | 25 | 90 | 10 | 100.00 |
| 9 | 2 | 25 | 30 | 6 | 96.04 |
| 10 | 1 | 40 | 30 | 6 | 94.06 |
| 11 | 1 | 25 | 60 | 6 | 96.04 |
| 12 | 0 | 25 | 30 | 6 | 64.38 |
| 13 | 1 | 10 | 30 | 6 | 78.23 |
| 14 | 0 | 10 | 60 | 6 | 68.34 |
| 15 | 0 | 25 | 60 | 10 | 94.06 |
| 16 | 2 | 10 | 60 | 6 | 96.04 |
| 17 | 2 | 40 | 60 | 6 | 100.00 |
| 18 | 1 | 25 | 30 | 10 | 94.06 |
| 19 | 2 | 25 | 60 | 10 | 10.00 |
| 20 | 1 | 25 | 60 | 6 | 96.04 |
| 21 | 1 | 10 | 90 | 6 | 100.00 |
| 22 | 2 | 25 | 90 | 6 | 100.00 |
| 23 | 0 | 25 | 90 | 6 | 92.08 |
| 24 | 0 | 25 | 60 | 2 | 58.44 |
| 25 | 2 | 25 | 60 | 2 | 98.02 |
| 26 | 1 | 25 | 30 | 2 | 56.46 |
| 27 | 1 | 40 | 60 | 10 | 100.00 |
| 28 | 1 | 10 | 60 | 2 | 66.36 |
| 29 | 1 | 25 | 60 | 6 | 96.04 |
| Source | Sum of Squares | Df | Mean Square | F Value | p Value Prob > F |
|---|---|---|---|---|---|
| Model | 16.19 | 14 | 1.16 | 37.22 | <0.0001 |
| A | 4.20 | 1 | 4.20 | 135.06 | <0.0001 |
| B | 1.03 | 1 | 1.03 | 33.11 | <0.0001 |
| C | 3.02 | 1 | 3.02 | 97.07 | <0.0001 |
| D | 3.51 | 1 | 3.51 | 113.06 | <0.0001 |
| A × B | 0.13 | 1 | 0.13 | 4.03 | 0.0645 |
| A × C | 0.47 | 1 | 0.47 | 15.15 | 0.0016 |
| A × D | 0.95 | 1 | 0.95 | 30.73 | <0.0001 |
| B × C | 0.18 | 1 | 0.18 | 5.86 | 0.0296 |
| B × D | 0.60 | 1 | 0.60 | 19.39 | 0.0006 |
| A × A | 0.62 | 1 | 0.62 | 19.80 | 0.0005 |
| B × B | 0.055 | 1 | 0.055 | 1.78 | 0.2032 |
| C × C | 0.14 | 1 | 0.14 | 4.64 | 0.0491 |
| D × D | 0.43 | 1 | 0.43 | 13.94 | 0.0022 |
| Residual | 0.021 | 7 | 0.00294 | - | - |
| Lack-of-fit | 0.44 | 10 | 0.044 | - | - |
| Pure error | 0.000 | 4 | 0.000 | - | - |
| Cor Total | 16.63 | 28 | |||
| R-Squared | 0.9738 | ||||
| Adj-Squared | 0.9477 | ||||
| Pred R-Squared | 0.8493 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Qin, Z.; Jin, G.; Wang, J.; Qin, J.; Ao, L.; Li, B. Highly Efficient Reduction of Cr (VI) with C4H6O6. Molecules 2024, 29, 5459. https://doi.org/10.3390/molecules29225459
Peng H, Qin Z, Jin G, Wang J, Qin J, Ao L, Li B. Highly Efficient Reduction of Cr (VI) with C4H6O6. Molecules. 2024; 29(22):5459. https://doi.org/10.3390/molecules29225459
Chicago/Turabian StylePeng, Hao, Zonghui Qin, Guixuan Jin, Jingjing Wang, Jielin Qin, Lihua Ao, and Bing Li. 2024. "Highly Efficient Reduction of Cr (VI) with C4H6O6" Molecules 29, no. 22: 5459. https://doi.org/10.3390/molecules29225459
APA StylePeng, H., Qin, Z., Jin, G., Wang, J., Qin, J., Ao, L., & Li, B. (2024). Highly Efficient Reduction of Cr (VI) with C4H6O6. Molecules, 29(22), 5459. https://doi.org/10.3390/molecules29225459






