Comparative Metabolomic Analysis of the Nutrient Composition of Different Varieties of Sweet Potato
Abstract
1. Introduction
2. Results
2.1. Comparison of the Major Nutrient Metabolites of Three Sweet Potato Species
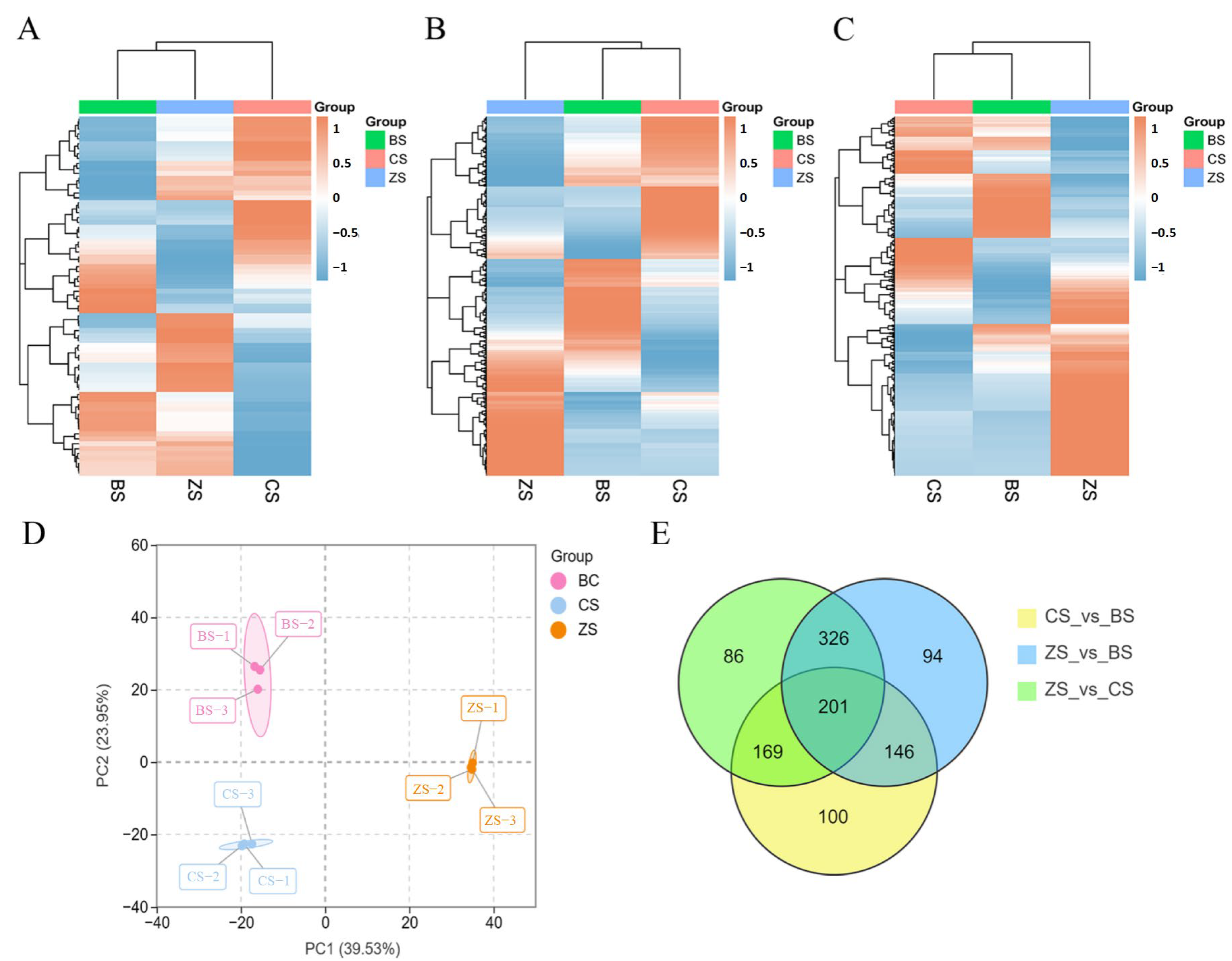
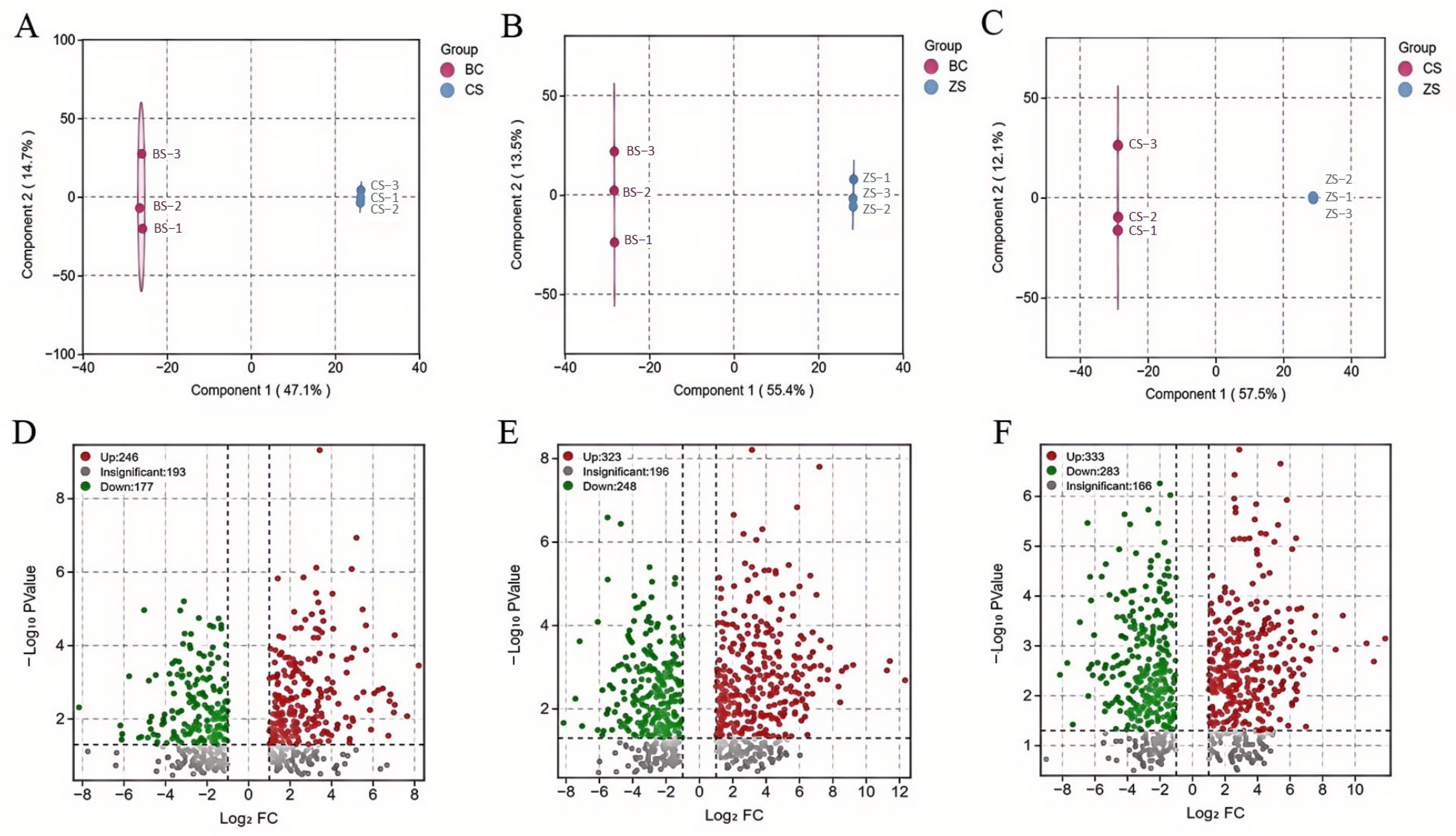
2.2. Multivariate Statistical Analysis
2.3. Identification of Differential Metabolites Among the Three Sweet Potato Varieties
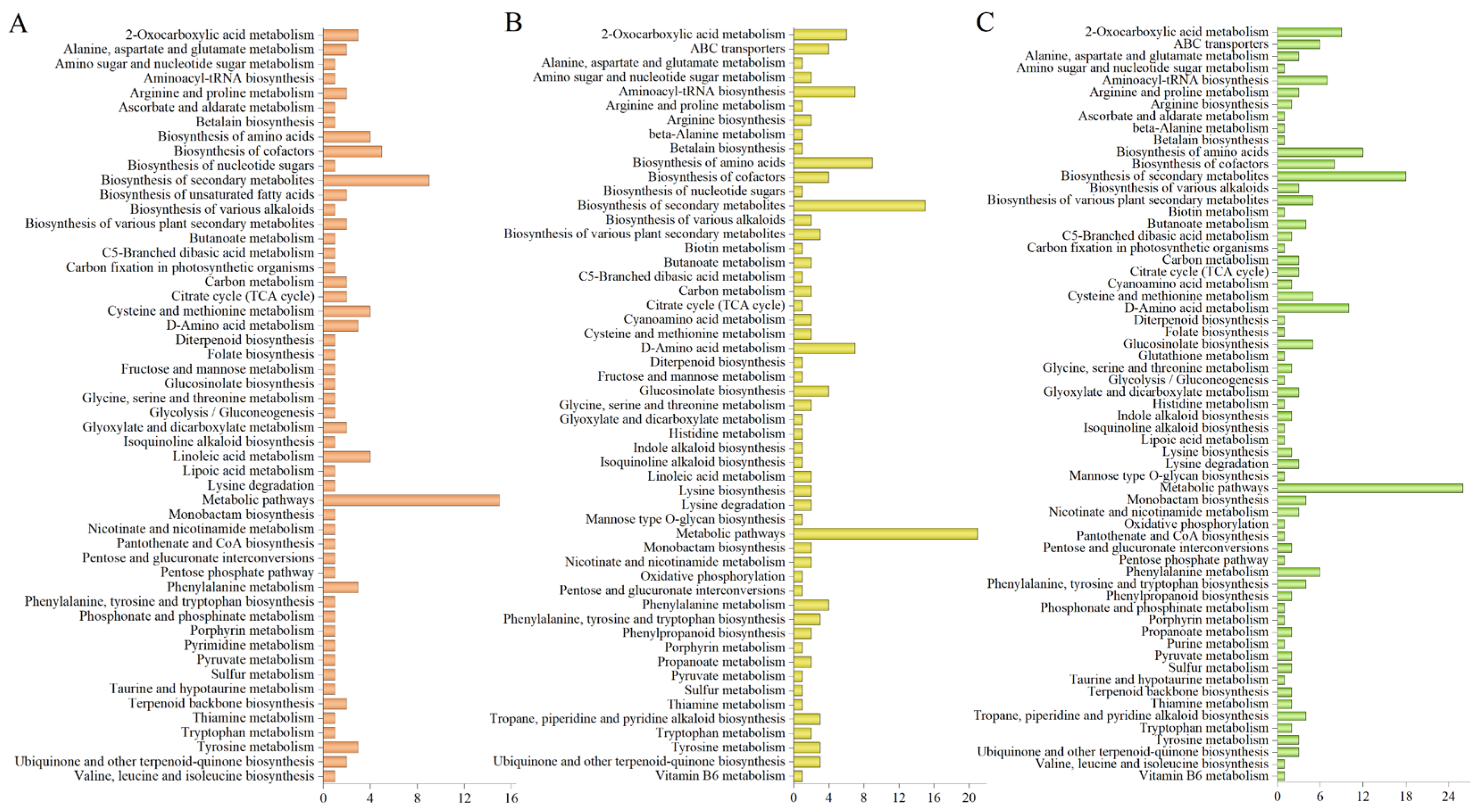
2.4. KEGG Annotation and Enrichment Analysis of Differential Metabolites
2.5. Significant Differences in Each Comparison Group for the Three Metabolites
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Dry Sample Extraction
4.3. Liquid Chromatography and Tandem Mass Spectrometry Conditions
4.4. Metabolite Identification and Quantification
4.5. Statistical Analysis
4.6. KEGG Annotation and Metabolic Pathway Analysis of Differential Metabolites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Zhao, H.; Jin, Y.; Zhu, J.; Ma, D. Analysis and perspectives of sweet potato industry contributing to national food security in China. Jiangsu J. Agric. Sci. 2022, 38, 1484–1491. [Google Scholar]
- Xiao, Y.; Zhu, M.; Gao, S. Genetic and Genomic Research on sweet potato for Sustainable Food and Nutritional Security. Genes 2022, 13, 1833. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Kourouma, V.; Mu, T.H.; Zhang, M.; Sun, H.N. Comparative study on chemical composition, polyphenols, flavonoids, carotenoids and antioxidant activities of various cultivars of sweet potato. Int. J. Food Sci. Technol. 2020, 55, 369–378. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, D.; Xiao, S.; Zhang, A.; Deng, Y.; Dai, X.; Zhou, Z.; Ji, Z.; Cao, Q. Comparative Metabolomic and Transcriptomic Analyses of Phytochemicals in Two Elite Sweet Potato Cultivars for Table Use. Molecules 2022, 27, 8939. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Reynolds, R.; Johanningsmeier, S.; Truong, V. Determination of free amino acids in five commercial sweet potato cultivars by hydrophilic interaction liquid chromatography-mass spectrometry. J. Food Compos. Anal. 2020, 92, 103522. [Google Scholar] [CrossRef]
- Song, J.; Yan, Y.; Wang, X.; Li, X.; Chen, Y.; Li, L.; Li, W. Characterization of fatty acids, amino acids and organic acids in three colored quinoas based on untargeted and targeted metabolomics. LWT-Food Sci. Technol. 2021, 140, 110690. [Google Scholar] [CrossRef]
- Lin, Z.; Li, G.; Zhang, H.; Ji, R.; Xu, Y.; Xu, G.; Li, H.; Liu, Z.; Luo, W.; Qiu, Y.; et al. Metabolic characteristics of taste differences under the soil and hydroponic cultures of sweet potato leaves by using non-targeted metabolomics. bioRxiv-Plant Biol. 2021, 2, 432602. [Google Scholar]
- Sabboh-Jourdan, H.; Valla, F.; Epriliati, I.; Gidley, M.J. Organic acid bioavailability from banana and sweet potato using an in vitro digestion and Caco-2 cell model. Eur. J. Nutr. 2011, 50, 31–40. [Google Scholar] [CrossRef]
- Wu, T.Y.; Tsai, C.C.; Hwang, Y.T.; Chiu, T.H. Effect of antioxidant activity and functional properties of Chingshey purple sweet potato fermented milk by Lactobacillus acidophilus, L. delbrueckii subsp. lactis, and L. gasseri strains. J. Food Sci. 2012, 77, M2–M8. [Google Scholar] [CrossRef]
- López-Ruiz, R.; Romero-González, R.; Frenich, A.G. Metabolomics approaches for the determination of multiple contaminants in food. Curr. Opin. Food Sci. 2019, 28, 49–57. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Yun, E.J.; Kim, K.H. Food metabolomics: From farm to human. Curr. Opin. Biotechnol. 2016, 37, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Utpott, M.; Rodrigues, E.; Rios, A.O.; Mercali, G.D.; Flores, S.H. Metabolomics: An analytical technique for food processing evaluation. Food Chem. 2022, 366, 130685. [Google Scholar] [CrossRef]
- Wang, L.; Qin, Y.; Wang, Y.; Zhou, Y. Changes of anthocyanin and amino acid metabolites in saffron petals (Crocus sativus L.) during fermentation based on untargeted metabolomics. LWT-Food Sci. Technol. 2024, 19, 115724. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Chen, W.; Zhang, W.; Zhang, H.; Yu, H.; Yi, Y. Integrated metabolomics and transcriptomics reveal metabolites difference between wild and cultivated Ophiocordyceps sinensis. Food Res. Int. 2023, 163, 112275. [Google Scholar] [CrossRef]
- Li, B.; Fu, Y.; Xi, H.; Liu, S.; Zhao, W.; Li, P.; Fan, W.; Wang, D.; Sun, S. Untargeted Metabolomics Using UHPLC-HRMS Reveals Metabolic Changes of Fresh-Cut Potato during Browning Process. Molecules 2023, 28, 3375. [Google Scholar] [CrossRef]
- Chang, W.C.; Chen, Y.T.; Chen, H.J.; Hsieh, C.W.; Liao, P.C. Comparative UHPLC-Q-Orbitrap HRMS-Based Metabolomics Unveils Biochemical Changes of Black Garlic during Aging Process. J. Agric. Food Chem. 2020, 68, 14049–14058. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Qiao, S.; Kang, Z.; Luo, F.; Bian, Q.; Cao, G.; Zhao, G.; Wu, Z.; Yang, G.; Wang, Y.; et al. Transcriptome and Metabolome Analyses Reflect the Molecular Mechanism of Drought Tolerance in Sweet Potato. Plants 2024, 13, 315. [Google Scholar] [CrossRef]
- He, L.; Liu, X.; Liu, S.; Zhang, J.; Zhang, Y.; Sun, Y.; Tang, R.; Wang, W.; Cui, H.; Li, R.; et al. Transcriptomic and targeted metabolomic analysis identifies genes and metabolites involved in anthocyanin accumulation in tuberous roots of sweet potato (Ipomoea batatas L.). Plant Physiol. Biochem. 2020, 156, 323–332. [Google Scholar] [CrossRef]
- Bennett, A.A.; Mahood, E.H.; Fan, K.; Moghe, G.D. Untargeted metabolomics of purple and orange-fleshed sweet potatoes reveals a large structural diversity of anthocyanins and flavonoids. Sci. Rep. 2021, 11, 16408. [Google Scholar] [CrossRef]
- Wan, X.; Wu, J.; Wang, X.; Cui, L.; Xiao, Q. Accumulation patterns of flavonoids and phenolic acids in different colored sweet potato flesh revealed based on untargeted metabolomics. Food Chem. X 2024, 23, 101551. [Google Scholar] [CrossRef]
- Ibrahim, R.; Sedeek, M.; Wareth, A.; Reda, M.; Gendy, A.; Farag, M. Impact of cultivar types and thermal processing methods on sweet potato metabolome, a comparative analysis via a multiplex approach of NIR and GC–MS based metabolomics coupled with chemometrics. Food Chem. 2024, 463, 141125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Z.; Li, Y.; Li, Z.; Zhou, W. Metabolite Profiling of Sorghum Seeds of Different Colors from Different Sweet Sorghum Cultivars Using a Widely Targeted Metabolomics Approach. Int. J. Genom. 2020, 2020, 6247429. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, T.; Wu, H.; Ge, Y.; Zhao, X.; Shen, X.; Zhou, W.; Wang, T.; Zhang, Y.; Ma, D.; et al. Exploring the metabolic changes in sweet potato during postharvest storage using a widely targeted metabolomics approach. J. Food Process. Preserv. 2021, 45, 15118. [Google Scholar] [CrossRef]
- Martinez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Mas, D.; Valdivie, M.; Hu, C.A.; Ren, W.; Yin, Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 2017, 49, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Ohtani, Y.; Tatsukami, Y.; Aoki, W.; Amemiya, T.; Sukekiyo, Y.; Kubokawa, S.; Ueda, M. Folate Biofortification in Hydroponically Cultivated Spinach by the Addition of Phenylalanine. J. Agric. Food Chem. 2017, 65, 4605–4610. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kang, J.; Zhu, H.; Wang, K.; Han, Z.; Wang, L.; Liu, J.; Wu, Y.; He, P.; Tu, Y.; et al. L-Theanine and Immunity: A Review. Molecules 2023, 28, 3846. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Lin, L.; Li, H. Gambogic Acid as a Candidate for Cancer Therapy: A Review. Int. J. Nanomed. 2020, 15, 10385–10399. [Google Scholar] [CrossRef]
- Uto-Kondo, H.; Sakurai, A.; Ogawa, K.; Yamaguchi, Y.; Saito, T.; Kumagai, H. Suppressive Effect of Shiitake Extract on Plasma Ethanol Elevation. Nutrients 2020, 12, 2647. [Google Scholar] [CrossRef]
- Chim, N.; Habel, J.E.; Johnston, J.M.; Krieger, I.; Miallau, L.; Sankaranarayanan, R.; Morse, R.P.; Bruning, J.; Swanson, S.; Kim, H. The TB Structural Genomics Consortium: A decade of progress. Tuberculosis 2011, 91, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Grieb, B.; Thyagarajan, A.; Sliva, D. Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-kappaB signaling. Int. J. Mol. Med. 2008, 21, 577–584. [Google Scholar] [PubMed]
- Fan, Y.Y.; Chapkin, R.S. Importance of dietary gamma-linolenic acid in human health and nutrition. J. Nutr. 1998, 128, 1411–1414. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur. J. Pharmacol. 2016, 785, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Surucu, B.; Kuhnert, N. Characterization by LC-MSn of Four New Classes of Chlorogenic Acids in Green Coffee Beans: Dimethoxycinnamoylquinic Acids, Diferuloylquinic Acids, Caffeoyl-dimethoxycinnamoylquinic Acids, and Feruloyl-dimethoxycinnamoylquinic Acids. J. Agric. Food Chem. 2006, 54, 1957–1969. [Google Scholar] [CrossRef]
- Barea, P.; Melgosa, R.; Illera, A.E.; Alonso-Riano, P.; Diaz, D.C.E.; Benito-Roman, O.; Beltran, S.; Teresa, S.M. Production of small peptides and low molecular weight amino acids by subcritical water from fish meal: Effect of pressurization agent. Food Chem. 2023, 418, 135925. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Wang, X.; Xiao, Q. Comparative Metabolomic Analysis of the Nutrient Composition of Different Varieties of Sweet Potato. Molecules 2024, 29, 5395. https://doi.org/10.3390/molecules29225395
Wan X, Wang X, Xiao Q. Comparative Metabolomic Analysis of the Nutrient Composition of Different Varieties of Sweet Potato. Molecules. 2024; 29(22):5395. https://doi.org/10.3390/molecules29225395
Chicago/Turabian StyleWan, Xiaolin, Xiuzhi Wang, and Qiang Xiao. 2024. "Comparative Metabolomic Analysis of the Nutrient Composition of Different Varieties of Sweet Potato" Molecules 29, no. 22: 5395. https://doi.org/10.3390/molecules29225395
APA StyleWan, X., Wang, X., & Xiao, Q. (2024). Comparative Metabolomic Analysis of the Nutrient Composition of Different Varieties of Sweet Potato. Molecules, 29(22), 5395. https://doi.org/10.3390/molecules29225395





