Electrochemical Upgrading of Waste Polylactic Acid Plastic for the Coproduction of C2 Chemicals and Green Hydrogen
Abstract
1. Introduction
2. Results and Discussion
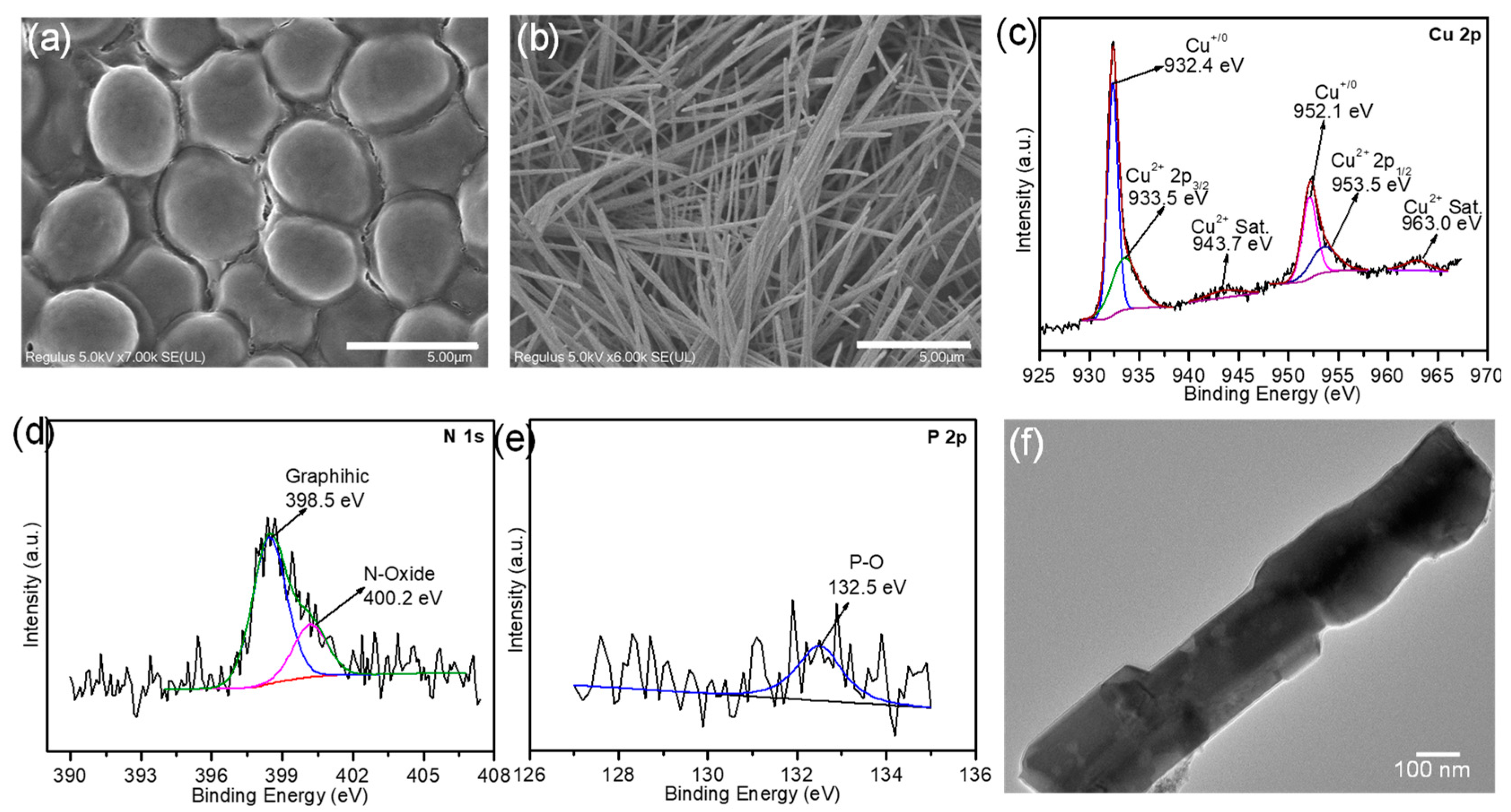
2.1. Material Fabrication and Characterization
2.2. Electrochemical Oxidation of PLA Monomer Coupled with H2 Production
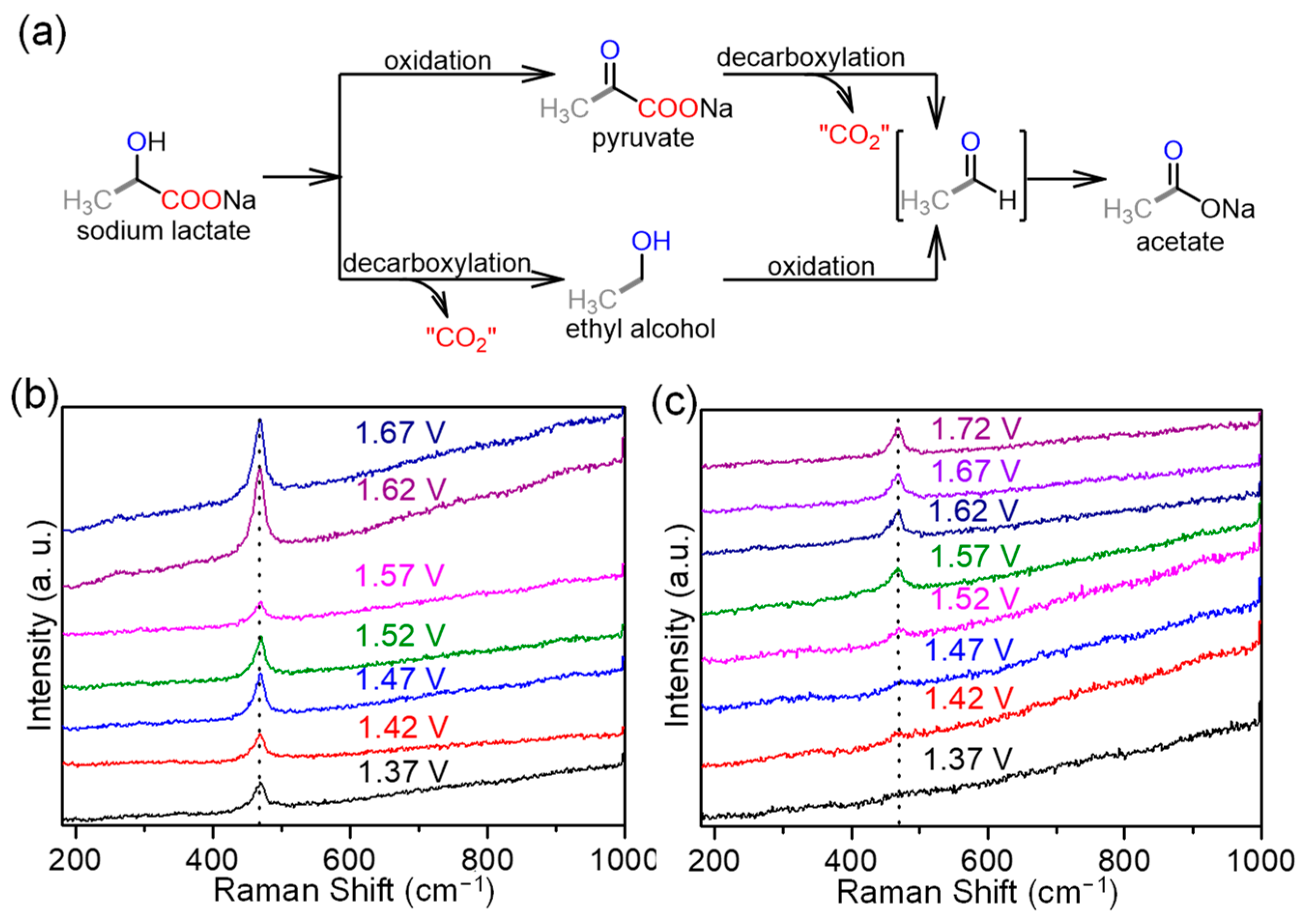
2.3. Electrochemical Mechanism of LOR
3. Experimental Section
3.1. Fabrication of Cu Cubes/NF
3.2. Preparation of Cu(OH)2 Nanowires/NF
3.3. Fabrication of CuO Nanowires/NF
3.4. Fabrication of P, N-Doped CuO Nanowires/NF
3.5. Material Characterization
3.6. Electrochemical Measurements
3.7. In Situ Raman Measurements
3.8. PLA Hydrolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Xiong, G.; Chen, Y.; Zhou, Z.; Liu, F.; Liu, X.; Yang, L.; Liu, Q.; Sang, Y.; Liu, H.; Zhang, X.; et al. Rapid synthesis of various electrocatalysts on Ni foam using a universal and facile induction heating method for efficient water splitting. Adv. Funct. Mater. 2021, 31, 2009580. [Google Scholar] [CrossRef]
- Gao, G.; Chen, X.; Han, L.; Zhu, G.; Jia, J.; Cabot, A.; Sun, Z. Advances in MOFs and their derivatives for non-noble metal electrocatalysts in water splitting. Coord. Chem. Rev. 2024, 503, 215639. [Google Scholar] [CrossRef]
- Xiao, C.; Hong, T.; Jia, J.; Jia, H.; Li, J.; Zhu, Y.; Ge, S.; Liu, C.; Zhu, G. Unlocking the potential of hydrogen evolution: Advancements in 3D nanostructured electrocatalysts supported on nickel foam. Appl. Catal. B Environ. 2024, 355, 124197. [Google Scholar] [CrossRef]
- Barnett, S.M.; Goldberg, K.I.; Mayer, J.M. A soluble copper-bipyridine water-oxidation electrocatalyst. Nat. Chem. 2012, 4, 498–502. [Google Scholar] [CrossRef]
- Du, J.; Xiang, D.; Zhou, K.; Wang, L.; Yu, J.; Xia, H.; Zhao, L.; Liu, H.; Zhou, W. Electrochemical hydrogen production coupled with oxygen evolution, organic synthesis, and waste reforming. Nano Energy 2022, 104, 107875. [Google Scholar] [CrossRef]
- Liu, Z.; Corva, M.; Amin, H.M.A.; Blanc, N.; Linnemann, J.; Tschulik, K. Single Co3O4 nanocubes electro- catalyzing the oxygen evolution reaction: Nano-impact insights into intrinsic activity and support effects. Int. J. Mol. Sci. 2021, 22, 13137. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Zan, L.; Baltruschat, H. Boosting the bifunctional catalytic activity of Co3O4 on silver and nickel substrates for the alkaline oxygen evolution and reduction reactions. Surf. Interfaces 2024, 54, 105218. [Google Scholar] [CrossRef]
- Soltani, M.; Amin, H.M.A.; Cebe, A.; Ayata, S.; Baltruschat, H. Metal-supported perovskite as an efficient bifunctional electrocatalyst for oxygen reduction and evolution: Substrate effect. J. Electrochem. Soc. 2021, 168, 034504. [Google Scholar] [CrossRef]
- Zhou, Z.; Zeng, L.; Xiong, G.; Yang, L.; Yuan, H.; Yu, J.; Xu, S.; Wang, D.; Zhang, X.; Liu, H.; et al. Multifunctional electrocatalyst of NiCo-NiCoP nanoparticles embedded into P-doped carbon nanotubes for energy-saving hydrogen production and upgraded conversion of formaldehyde. Chem. Eng. J. 2021, 426, 129214. [Google Scholar] [CrossRef]
- Li, G.; Han, G.; Wang, L.; Cui, X.; Moehring, N.K.; Kidambi, P.R.; Jiang, D.E.; Sun, Y. Dual hydrogen production from electrocatalytic water reduction coupled with formaldehyde oxidation via a copper-silver electrocatalyst. Nat. Commun. 2023, 14, 525. [Google Scholar] [CrossRef] [PubMed]
- Adam, D.B.; Tsai, M.-C.; Awoke, Y.A.; Huang, W.-H.; Lin, C.-H.; Alamirew, T.; Ayele, A.A.; Yang, Y.-W.; Pao, C.-W.; Su, W.-N.; et al. Engineering self-supported ruthenium-titanium alloy oxide on 3D web-like titania as iodide oxidation reaction electrocatalyst to boost hydrogen production. Appl. Catal. B Environ. 2022, 316, 121608. [Google Scholar] [CrossRef]
- Yu, W.; Yu, J.; Wang, Y.; Li, X.; Wang, Y.; Yuan, H.; Zhang, X.; Liu, H.; Zhou, W. Electrocatalytic upcycling of nitrate and hydrogen sulfide via a nitrogen-doped carbon nanotubes encapsulated iron carbide electrode. Appl. Catal. B Environ. 2022, 310, 121291. [Google Scholar] [CrossRef]
- Liang, X.; Li, X.; Dong, Q.; Gao, T.; Cao, M.; Zhao, K.; Lichtfouse, E.; Patrocinio, A.O.T.; Wang, C. Photo- and electrochemical processes to convert plastic waste into fuels and high-value chemicals. Chem. Eng. J. 2024, 482, 148827. [Google Scholar] [CrossRef]
- Shi, R.; Liu, K.S.; Liu, F.; Yang, X.; Hou, C.C.; Chen, Y. Electrocatalytic reforming of waste plastics into high value-added chemicals and hydrogen fuel. Chem. Commun. 2021, 57, 12595–12598. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, Y.; Li, Z.; Xu, M.; Wang, Y.; Ge, R.; Kong, X.; Zheng, L.; Duan, H. Electrocatalytic upcycling of polyethylene terephthalate to commodity chemicals and H2 fuel. Nat. Commun. 2021, 12, 4679. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Hu, M.-K.; Wei, W.; Zhou, S.-H.; Wu, X.-T.; Zhu, Q.-L. Ordered macroporous superstructure of bifunctional cobalt phosphide with heteroatomic modification for paired hydrogen production and polyethylene terephthalate plastic recycling. Appl. Catal. B Environ. 2022, 316, 121667. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Zhang, T.; Chen, Y.; Wang, T.; Zhao, Y. Electro-reforming polyethylene terephthalate plastic to co-produce valued chemicals and green hydrogen. J. Phys. Chem. Lett. 2022, 13, 622–627. [Google Scholar] [CrossRef]
- Liu, F.; Gao, X.; Shi, R.; Tse, E.C.M.; Chen, Y. A general electrochemical strategy for upcycling polyester plastics into added-value chemicals by a CuCo2O4 catalyst. Green Chem. 2022, 24, 6571–6577. [Google Scholar] [CrossRef]
- Ma, F.; Wang, S.; Gong, X.; Liu, X.; Wang, Z.; Wang, P.; Liu, Y.; Cheng, H.; Dai, Y.; Zheng, Z.; et al. Highly efficient electrocatalytic hydrogen evolution coupled with upcycling of microplastics in seawater enabled via Ni3N/W5N4 janus nanostructures. Appl. Catal. B Environ. 2022, 307, 121198. [Google Scholar] [CrossRef]
- Liu, X.; Fang, Z.; Teng, X.; Niu, Y.; Gong, S.; Chen, W.; Meyer, T.J.; Chen, Z. Paired formate and H2 productions via efficient bifunctional Ni-Mo nitride nanowire electrocatalysts. J. Energy Chem. 2022, 72, 432–441. [Google Scholar] [CrossRef]
- Mao, Y.; Fan, S.; Li, X.; Shi, J.; Wang, M.; Niu, Z.; Chen, G. Trash to treasure: Electrocatalytic upcycling of polyethylene terephthalate (PET) microplastic to value-added products by Mn0.1Ni0.9Co2O4-δ RSFs spinel. J. Hazard. Mater. 2023, 457, 131743. [Google Scholar] [CrossRef]
- Liu, X.; Fang, Z.; Xiong, D.; Gong, S.; Niu, Y.; Chen, W.; Chen, Z. Upcycling PET in parallel with energy-saving H2 production via bifunctional nickel-cobalt nitride nanosheets. Nano Res. 2022, 16, 4625–4633. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K.; Liu, F.; Liu, C.; Shi, R.; Chen, Y. Selective electro-reforming of waste polyethylene terephthalate-derived ethylene glycol into C2 chemicals with long-term stability. Green Chem. 2023, 25, 5872–5877. [Google Scholar] [CrossRef]
- Liu, F.; Gao, X.; Shi, R.; Guo, Z.; Tse, E.C.M.; Chen, Y. Concerted and selective Electrooxidation of polyethylene-terephthalate-derived alcohol to glycolic acid at an industry-level current density over a Pd-Ni(OH)2 catalyst. Angew. Chem. Int. Ed. 2023, 62, e202300094. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, R.; Bao, T.; Ma, T.; Wei, W.; Shen, Y.; Ni, B.J. Dual-doped nickel sulfide for electro-upgrading polyethylene terephthalate into valuable chemicals and hydrogen fuel. Nano-Micro Lett. 2023, 15, 210. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Y.; Liu, F.; Liu, C.; Shi, R.; Chen, Y. Selective electrocatalytic reforming of PET-derived ethylene glycol to formate with a Faraday efficiency of 93.2% at industrial-level current densities. Chem. Eng. J. 2023, 473, 145292. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Li, X.; Deng, K.; Yu, H.; Xu, Y.; Wang, H.; Wang, Z.; Wang, L. Electrocatalytic upcycling of polyethylene terephthalate plastic to formic acid coupled with energy-saving hydrogen production over hierarchical Pd-doped NiTe nanoarrays. Appl. Catal. B Environ. 2024, 340, 123236. [Google Scholar] [CrossRef]
- Liu, K.; Gao, X.; Liu, C.X.; Shi, R.; Tse, E.C.M.; Liu, F.; Chen, Y. Energy-saving hydrogen production by seawater splitting coupled with PET plastic upcycling. Adv. Energy Mater. 2024, 14, 2304065. [Google Scholar] [CrossRef]
- Liu, X.; He, X.; Xiong, D.; Wang, G.; Tu, Z.; Wu, D.; Wang, J.; Gu, J.; Chen, Z. Electro-reforming of PET plastic to C2 chemicals with concurrent generation of hydrogen and electric energy. ACS Catal. 2024, 14, 5366–5376. [Google Scholar] [CrossRef]
- Kang, H.; He, D.; Yan, X.; Dao, B.; Williams, N.B.; Elliott, G.I.; Streater, D.; Nyakuchena, J.; Huang, J.; Pan, X.; et al. Cu promoted the dynamic evolution of Ni-based catalysts for polyethylene terephthalate plastic upcycling. ACS Catal. 2024, 14, 5314–5325. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, J.; Fang, Z.; Gong, S.; Xiong, D.; Chen, W.; Wu, D.; Chen, Z. Ultrafast activation of Ni foam by electro-corrosion and its use for upcycling PBT plastic waste. Appl. Catal. B Environ. 2023, 334, 122870. [Google Scholar] [CrossRef]
- Xiao, C.; Leow, W.R.; Chen, L.; Li, Y.; Li, C. Electrocatalytic conversion of waste polyamide-66 hydrolysates into high-added-value adiponitrile and hydrogen fuel. Electron 2023, 1, e14. [Google Scholar] [CrossRef]
- Global Production Capacities of Bioplastics. 2022. Available online: https://www.european-bioplastics.org/market/ (accessed on 10 February 2024).
- McKeown, P.; Jones, M.D. The chemical recycling of PLA: A review. Sustain. Chem. 2020, 1, 1–22. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.F.; Roelands, M.C.P.; White, R.J.; van Harmelen, T.; de Wild, P.; van der Laan, G.P.; Meirer, F.; Keurentjes, J.T.F.; Weckhuysen, B.M. Beyond mechanical recycling: Giving new life to plastic waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, T.; Du, J.; Liu, H.; Yuan, H.; Wang, Y.; Jia, J.; Liu, H.; Zhou, W. Synthesis of CdS/MoS2 nanooctahedrons heterostructure with a tight interface for enhanced photocatalytic H2 evolution and biomass upgrading. Sol. RRL 2020, 5, 2000415. [Google Scholar] [CrossRef]
- Jiang, D.; Yuan, H.; Liu, Z.; Chen, Y.; Li, Y.; Zhang, X.; Xue, G.; Liu, H.; Liu, X.; Zhao, L.; et al. Defect-anchored single-atom-layer Pt clusters on TiO2−x/Ti for efficient hydrogen evolution via photothermal reforming plastics. Appl. Catal. B Environ. 2023, 339, 123081. [Google Scholar] [CrossRef]
- Liu, C.X.; Liu, K.; Xu, Y.; Wang, Z.; Weng, Y.; Liu, F.; Chen, Y. Photocatalytic upgrading of polylactic acid waste into alanine under mild conditions. Angew. Chem. Int. Ed. 2024, 63, e202401255. [Google Scholar] [CrossRef]
- Miao, Y.; Zhao, Y.; Gao, J.; Wang, J.; Zhang, T. Direct photoreforming of real-world polylactic acid plastics into highly selective value-added pyruvic acid under visible light. J. Am. Chem. Soc. 2024, 146, 4842–4850. [Google Scholar] [CrossRef]
- Chen, C.; Bloomfield, A.J.; Sheehan, S.W. Selective electrochemical oxidation of lactic acid using iridium-based catalysts. Ind. Eng. Chem. Res. 2017, 56, 3560–3567. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Yu, X.; Terekhina, I.; Henriksson, G.; Cornell, A. In situ catalyst reactivation for enhancing alcohol electro-oxidation and coupled hydrogen generation. Chem. Commun. 2020, 56, 4011–4014. [Google Scholar] [CrossRef]
- Yin, C.; Li, X.; Dai, Y.; Chen, Z.; Yang, D.; Liu, R.; Zou, W.; Tang, C.; Dong, L. The facet-regulated oxidative dehydrogenation of lactic acid to pyruvic acid on α-Fe2O3. Green Chem. 2021, 23, 328–332. [Google Scholar] [CrossRef]
- Li, G.; Yu, J.; Jia, J.; Yang, L.; Zhao, L.; Zhou, W.; Liu, H. Cobalt-cobalt phosphide nanoparticles@nitrogen-phosphorus doped carbon/graphene derived from cobalt ions adsorbed Saccharomycete yeasts as an efficient, stable, and large-current-density electrode for hydrogen evolution reactions. Adv. Funct. Mater. 2018, 28, 1801332. [Google Scholar] [CrossRef]
- Yu, J.; Li, G.; Liu, H.; Zhao, L.; Wang, A.; Liu, Z.; Li, H.; Liu, H.; Hu, Y.; Zhou, W. Ru-Ru2PΦNPC and NPC@RuO2 synthesized via environment-friendly and solid-phase phosphating process by Saccharomycetes as N/P sources and carbon template for overall water splitting in acid electrolyte. Adv. Funct. Mater. 2019, 29, 1901154. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Yu, J.; Liu, H.; Cao, Q.; Du, J.; Zhao, L.; Jia, J.; Liu, H.; Zhou, W. Ni-Ni3P nanoparticles embedded into N, P-doped carbon on 3D graphene frameworks via in situ phosphatization of saccharomycetes with multifunctional electrodes for electrocatalytic hydrogen production and anodic degradation. Appl. Catal. B Environ. 2020, 261, 118147. [Google Scholar] [CrossRef]
- Li, G.; Yu, J.; Yu, W.; Yang, L.; Zhang, X.; Liu, X.; Liu, H.; Zhou, W. Phosphorus-doped iron nitride nanoparticles encapsulated by nitrogen-doped carbon nanosheets on iron foam in situ derived from Saccharomycetes cerevisiae for electrocatalytic overall water splitting. Small 2020, 16, e2001980. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, W.; Chang, B.; Li, X.; Jia, J.; Wang, D.; Xu, Z.; Zhang, X.; Liu, H.; Zhou, W. Waste-yeast biomass as nitrogen/phosphorus sources and carbon template: Environment-friendly synthesis of N,P-Mo2C nanoparticles on porous carbon matrix for efficient hydrogen evolution. Chin. Chem. Lett. 2022, 33, 3231–3235. [Google Scholar] [CrossRef]
- Yu, J.; Chang, B.; Yu, W.; Li, X.; Wang, D.; Xu, Z.; Zhang, X.; Liu, H.; Zhou, W. Chromium phosphide nanoparticles embedded in porous nitrogen-/phosphorus-doped carbon as efficient electrocatalysts for a nitrogen reduction reaction. Carbon Energy 2022, 4, 237–245. [Google Scholar] [CrossRef]
- Wang, S.; Dong, L.; Zhang, M.; Cheng, F.; Chen, S. N-doped carbon-coated Cu2O nanowire arrays on copper foam for rapid and stable water disinfection. J. Colloid Interface Sci. 2022, 625, 761–773. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, J.; Liu, Y.; Shen, J.; Wang, H.; Yang, X.; Zhu, Y.; Li, C. Multimetallic Ni-Mo/Cu nanowires as nonprecious and efficient full water splitting catalyst. J. Mater. Chem. A 2017, 5, 4207–4214. [Google Scholar] [CrossRef]
- Zhu, H.; Tang, Y.; Wang, J.J.; Sun, T.; Wang, M.; Wang, J.; Tan, Y.; Wang, J. Accelerating electrosynthesis of ammonia from nitrates using coupled NiO/Cu nanocomposites. Chem. Commun. 2024, 60, 2184–2187. [Google Scholar] [CrossRef] [PubMed]
- Rej, S.; Bisetto, M.; Naldoni, A.; Fornasiero, P. Well-defined Cu2O photocatalysts for solar fuels and chemicals. J. Mater. Chem. A 2021, 9, 5915–5951. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, B.; Wei, Z.; Zhou, W.; Wang, D.; Tian, J.; Wang, T.; Zhao, S.; Liu, J.; Tao, L.; et al. Coupling glucose-assisted Cu(I)/Cu(II) redox with electrochemical hydrogen production. Adv. Mater. 2021, 33, 2104791. [Google Scholar] [CrossRef]
- Zampardi, G.; Thoming, J.; Naatz, H.; Amin, H.M.A.; Pokhrel, S.; Madler, L.; Compton, R.G. Electrochemical behavior of single CuO nanoparticles: Implications for the assessment of their environmental fate. Small 2018, 14, e1801765. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, D.; Zhou, K.; Huang, J.; Kang, Q.; Li, H.; Duan, Y.; Du, J.; Liu, H. Electrochemical Upgrading of Waste Polylactic Acid Plastic for the Coproduction of C2 Chemicals and Green Hydrogen. Molecules 2024, 29, 5323. https://doi.org/10.3390/molecules29225323
Xiang D, Zhou K, Huang J, Kang Q, Li H, Duan Y, Du J, Liu H. Electrochemical Upgrading of Waste Polylactic Acid Plastic for the Coproduction of C2 Chemicals and Green Hydrogen. Molecules. 2024; 29(22):5323. https://doi.org/10.3390/molecules29225323
Chicago/Turabian StyleXiang, Daili, Kexin Zhou, Jiahui Huang, Qing Kang, Hao Li, Yuhui Duan, Jialei Du, and Hong Liu. 2024. "Electrochemical Upgrading of Waste Polylactic Acid Plastic for the Coproduction of C2 Chemicals and Green Hydrogen" Molecules 29, no. 22: 5323. https://doi.org/10.3390/molecules29225323
APA StyleXiang, D., Zhou, K., Huang, J., Kang, Q., Li, H., Duan, Y., Du, J., & Liu, H. (2024). Electrochemical Upgrading of Waste Polylactic Acid Plastic for the Coproduction of C2 Chemicals and Green Hydrogen. Molecules, 29(22), 5323. https://doi.org/10.3390/molecules29225323






