N-glycosylation Modification Reveals Insights into the Oxidative Reactions of Liver in Wuzhishan Pigs
Abstract
1. Introduction
2. Results
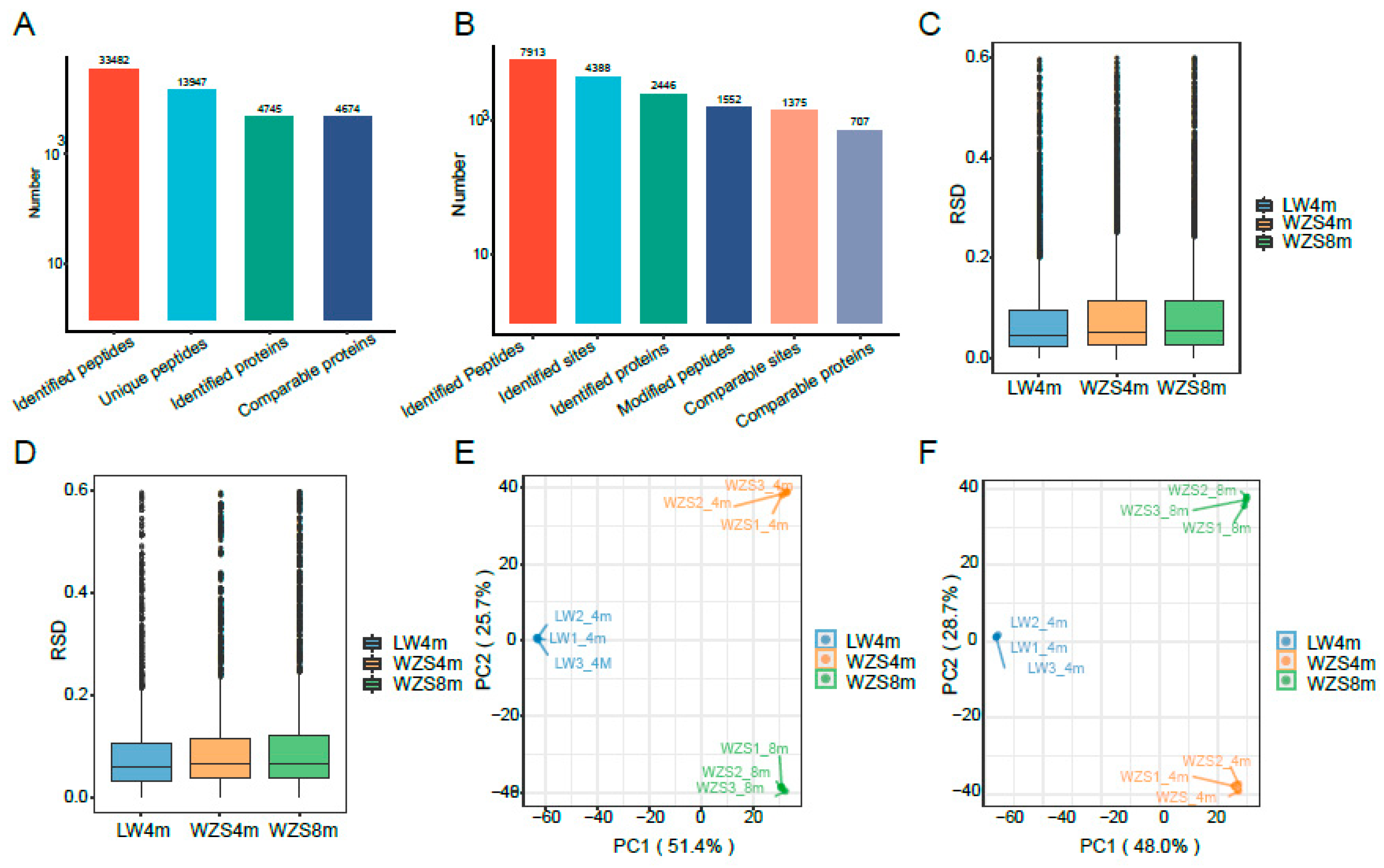
2.1. Proteome Analysis
2.2. Differentially Expressed Proteins (DEPs)
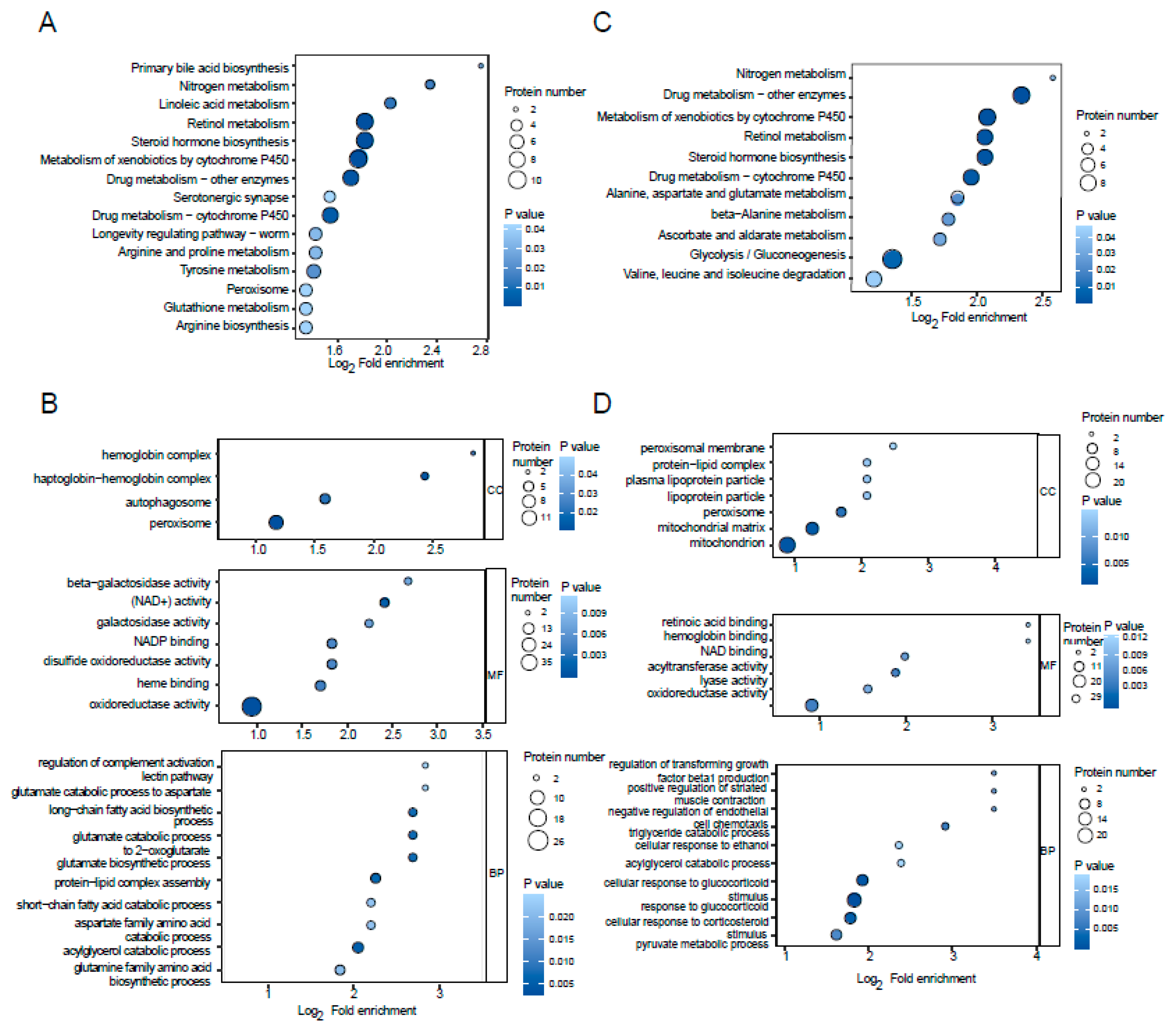
2.3. Functional Analysis of DEPs
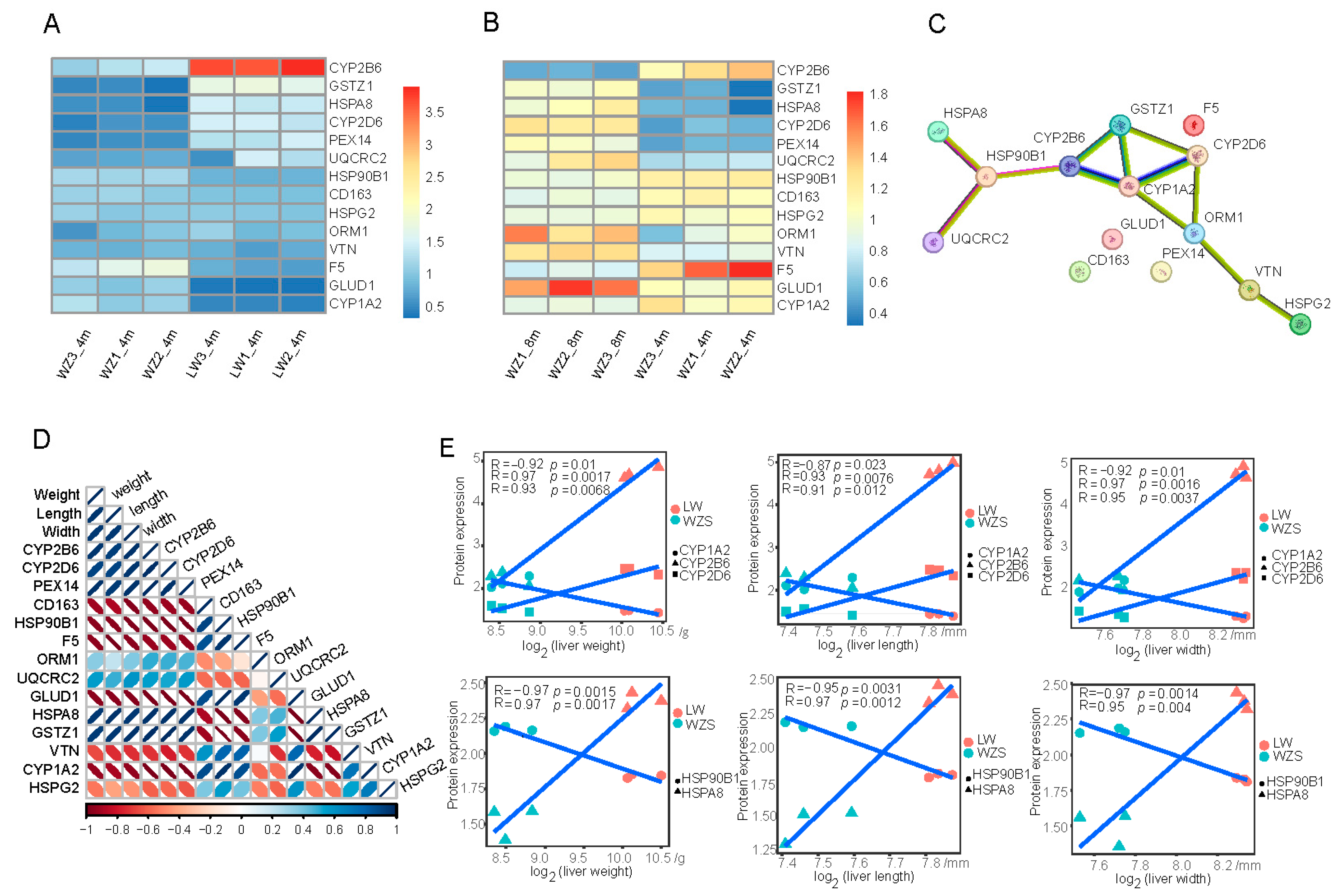
2.4. Oxidation DEP Selection
2.5. The Oxidation DEPs’ Domains and Motifs
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Peptide Preparation
4.3. LC-MS/MS Analysis
4.4. Database Search
4.5. Functional Analysis
4.6. Oxidation Proteins Selection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wada, Y.; Takeda, Y.; Kuwahata, M. Potential role of amino acid/protein nutrition and exercise in serum albumin redox state. Nutrients 2017, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Radovani, B.; Gudelj, I. N-glycosylation and inflammation; the not-so-sweet relation. Front. Immunol. 2022, 13, 893365. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in liver diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef]
- Sun, J.; Jia, L.; Chen, X. Efficient adsorption and extraction of glutathione s-transferases with glutathione-functionalized graphene oxide-polyhedral oligomeric silsesquioxane composite. Molecules 2023, 28, 340. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, L.; Zhang, N.Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.S.; Sun, L.H. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res. Rev. Mutat. Res. 2018, 778, 79–89. [Google Scholar] [CrossRef]
- Ribeiro, D.M.; Leclercqc, C.C.; Charton, S.A.B.; Costa, M.M.; Carvalho, D.F.P.; Sergeant, K.; Cocco, E.; Renaut, J.; Freire, J.P.B.; Prates, J.A.M.; et al. The impact of dietary Laminaria digitata and alginate lyase supplementation on the weaned piglet liver: A comprehensive proteomics and metabolomics approach. J. Proteom. 2024, 293, 105063. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; He, J.; Luo, Y.; Zheng, P.; Yu, J.; et al. Dietary apple polyphenols supplementation enhances antioxidant capacity and improves lipid metabolism in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1512–1520. [Google Scholar] [CrossRef]
- Fayyaz, A.; Makwinja, S.; Auriola, S.; Raunio, H.; Juvonen, R.O. Comparison of in vitro hepatic scoparone 7-o-demethylation between humans and experimental animals. Planta Med. 2018, 84, 320–328. [Google Scholar] [CrossRef]
- Esposito, S.; Krick, A.; Pasquier, O.; Bonche, F.; Ingenito, R.; Magotti, P.; Bianchi, E.; Monteagudo, E.; Gallo, M.; Cicero, D.O.; et al. Fatty acid acylated peptide therapeutics: Discovery of omega-n oxidation of the lipid chain as a novel metabolic pathway in preclinical species. J. Pharm. Biomed. Anal. 2023, 227, 115256. [Google Scholar] [CrossRef]
- Lyu, Q.; Feng, M.; Wang, L.; Yang, J.; Wu, G.; Liu, M.; Feng, Y.; Lin, S.; Yang, Q.; Hu, J. Taurine prevents liver injury by reducing oxidative stress and cytochrome c-mediated apoptosis in broilers under low temperature. Adv. Exp. Med. Biol. 2022, 1370, 145–152. [Google Scholar] [PubMed]
- Esmail, S.; Manolson, M.F. Advances in understanding N-glycosylation structure.; function.; and regulation in health and disease. Eur. J. Cell Biol. 2021, 100, 151186. [Google Scholar] [CrossRef] [PubMed]
- Aldonza, M.B.D.; Cha, J.; Yong, I.; Ku, J.; Sinitcyn, P.; Le, D.; Cho, R.E.; Delos Reyes, R.D.; Kim, D.; Kim, S.; et al. Multi-targeted therapy resistance via drug-induced secretome fucosylation. eLife 2023, 12, e75191. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lan, L.; Li, Y.; Lu, J.; He, L.; Deng, Y.; Fei, M.; Lu, J.W.; Shangguan, F.; Lu, J.P.; et al. N-glycosylation stabilizes MerTK and promotes hepatocellular carcinoma tumor growth. Redox Biol. 2022, 54, 102366. [Google Scholar] [CrossRef]
- Jördens, M.S.; Keitel, V.; Karababa, A.; Zemtsova, I.; Bronger, H.; Häussinger, D.; Görg, B. Multidrug resistance-associated protein 4 expression in ammonia-treated cultured rat astrocytes and cerebral cortex of cirrhotic patients with hepatic encephalopathy. Glia 2015, 63, 2092–2105. [Google Scholar] [CrossRef]
- Coleman, T.; Podgorski, M.N.; Doyle, M.L.; Scaffidi-Muta, J.M.; Campbell, E.C.; Bruning, J.B.; De Voss, J.J.; Bell, S.G. Cytochrome P450-catalyzed oxidation of halogen-containing substrates. J. Inorg. Biochem. 2023, 244, 112234. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K.; Li, M. Effects of dietary epidermal growth factor supplementation on liver antioxidant capacity of piglets with intrauterine growth retardation. J. Anim. Sci. 2023, 101, skad323. [Google Scholar] [CrossRef]
- Uehara, S.; Yoneda, N.; Higuchi, Y.; Yamazaki, H.; Suemizu, H. Cytochrome P450-dependent drug oxidation activities and their expression levels in liver microsomes of chimeric TK-NOG mice with humanized livers. Drug Metab. Pharmacok. 2022, 44, 100454. [Google Scholar] [CrossRef]
- Bachour-El Azzi, P.; Chesné, C.; Uehara, S. Expression and functional activity of cytochrome P450 enzymes in human hepatocytes with sustainable reproducibility for in vitro phenotyping studies. Adv. Pharmacol. 2022, 95, 285–305. [Google Scholar]
- Ma, Z.; Liang, H.; Wang, S.; Miao, W.; Yu, L.; Liu, S.; Luo, Z.; Su, S.; Wang, J.; Liu, S.; et al. Nardosinone relieves metabolic-associated fatty liver disease and promotes energy metabolism through targeting CYP2D6. Phytomedicine 2024, 130, 155748. [Google Scholar] [CrossRef]
- Prysyazhnyuk, V.; Sydorchuk, L.; Sydorchuk, R.; Prysiazhniuk, I.; Bobkovych, K.; Buzdugan, I.; Dzuryak, V.; Prysyazhnyuk, P. Glutathione-S-transferases genes-promising predictors of hepatic dysfunction. World J. Hepatol. 2021, 13, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Chang, X.; Yang, L.; Chamba, Y.; Geng, F. Quantitative proteomic analysis of Tibetan pig livers at different altitudes. Molecules 2023, 28, 1694. [Google Scholar] [CrossRef] [PubMed]
- Squirewell, E.J.; Mareus, R.; Horne, L.P.; Stacpoole, P.W.; James, M.O. Exposure of rats to multiple oral doses of dichloroacetate results in upregulation of hepatic glutathione transferases and NAD(P)H dehydrogenase [Quinone] 1. Drug Metab. Dispos. 2020, 48, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qi, Q.; Men, L.; Wang, S.; Li, M.; Xiao, M.; Chen, X.; Wang, S.; Wang, G.; Jia, H.; et al. Berberine protects Kawasaki disease-induced human coronary artery endothelial cells dysfunction by inhibiting of oxidative and endoplasmic reticulum stress. Vascul. Pharmacol. 2020, 127, 106660. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yu, M.; Li, J.; Guo, Y.; Wang, Z.; Hu, K.; Xu, F.; Liu, Y.; Li, L.; Wan, D.; et al. GLUD1 inhibits hepatocellular carcinoma progression via ROS-mediated p38/JNK MAPK pathway activation and mitochondrial apoptosis. Discov. Oncol. 2024, 15, 8. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.; Maimela, N.R.; Yang, L.; Zhang, Z.; Ping, Y.; Huang, L.; Zhang, Y. Molecular and clinical characterization of CD163 expression via large-scale analysis in glioma. Oncoimmunology 2019, 8, 1601478. [Google Scholar] [CrossRef]
- Kaminski, T.W.; Sivanantham, A.; Mozhenkova, A.; Smith, A.; Ungalara, R.; Dubey, R.K.; Shrestha, B.; Hanway, C.; Katoch, O.; Tejero, J.; et al. Hemoglobin scavenger receptor CD163 as a potential biomarker of hemolysis-induced hepatobiliary injury in sickle cell disease. Am. J. Physiol. Cell Physiol. 2024, 327, C423–C437. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, P.; Qiao, L.; Guan, H.; Gou, J.; Liu, X. Maternal protein deficiency impairs peroxisome biogenesis and leads to oxidative stress and ferroptosis in liver of fetal growth restriction offspring. J. Nutr. Biochem. 2023, 121, 109432. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, Y.; Wang, S.; Lin, Z.; Han, P.; Tian, D.; Wang, H.; Liu, M. L-lysine supplementation attenuates experimental autoimmune hepatitis in a chronic murine model. Exp. Anim. 2024, 73, 83–92. [Google Scholar] [CrossRef]
- Xu, S.; Hou, D.; Liu, J.; Ji, L. Age-associated changes in GSH S-transferase gene/proteins in livers of rats. Redox Rep. 2018, 23, 213–218. [Google Scholar] [CrossRef]
- Valliere-Douglass, J.F.; Eakin, C.M.; Wallace, A.; Ketchem, R.R.; Wang, W.; Treuheit, M.J.; Balland, A. Glutamine-linked and non-consensus asparagine-linked oligosaccharides present in human recombinant antibodies define novel protein glycosylation motifs. J. Biol. Chem. 2010, 285, 16012–16022. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, S.; Tang, J.; Tian, T.; Pan, Y.; Wu, L.; Zhang, J.; Liu, Y.; Huang, J.; Dai, H.; et al. A self-propagating c-met-sox2 axis drives cancer-derived igg signaling that promotes lung cancer cell stemness. Cancer Res. 2023, 83, 1866–1882. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Noga, S. Hydrophilic interaction liquid chromatography (HILIC)—A powerful separation technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Dolores-Hernández, M.; Morales-Hipólito, E.A.; Villaseñor, A.; López-Arellano, R. Determination of zilpaterol in a residue depletion study using LC-MS/MS in cattle plasma.; muscle.; liver and kidney. Food Chem. 2022, 382, 132287. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Bandeira, N.; Perez-Riverol, Y.; Sharma, V.; Carver, J.J.; Mendoza, L.; Kundu, D.J.; Wang, S.; Bandla, C.; Kamatchinathan, S.; et al. The ProteomeXchange consortium at 10 years: 2023 update. Nucleic Acids Res. 2023, 51, D1539–D1548. [Google Scholar] [CrossRef]
- Krug, K.; Mertins, P.; Zhang, B.; Hornbeck, P.; Raju, R.; Ahmad, R.; Szucs, M.; Mundt, F.; Forestier, D.; Jane-Valbuena, J.; et al. A curated resource for phosphosite-specific signature analysis. Mol. Cell. Proteom. 2019, 18, 576–593. [Google Scholar] [CrossRef]
- Castellani, F.; Antonucci, A.; Pindinello, I.; Protano, C.; Vitali, M. Determination of carbonyl compounds in different work environments: Comparison between LC-UV/DAD and LC-MS/MS detection methods. Int. J. Environ. Res. Public Health 2022, 19, 12052. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]

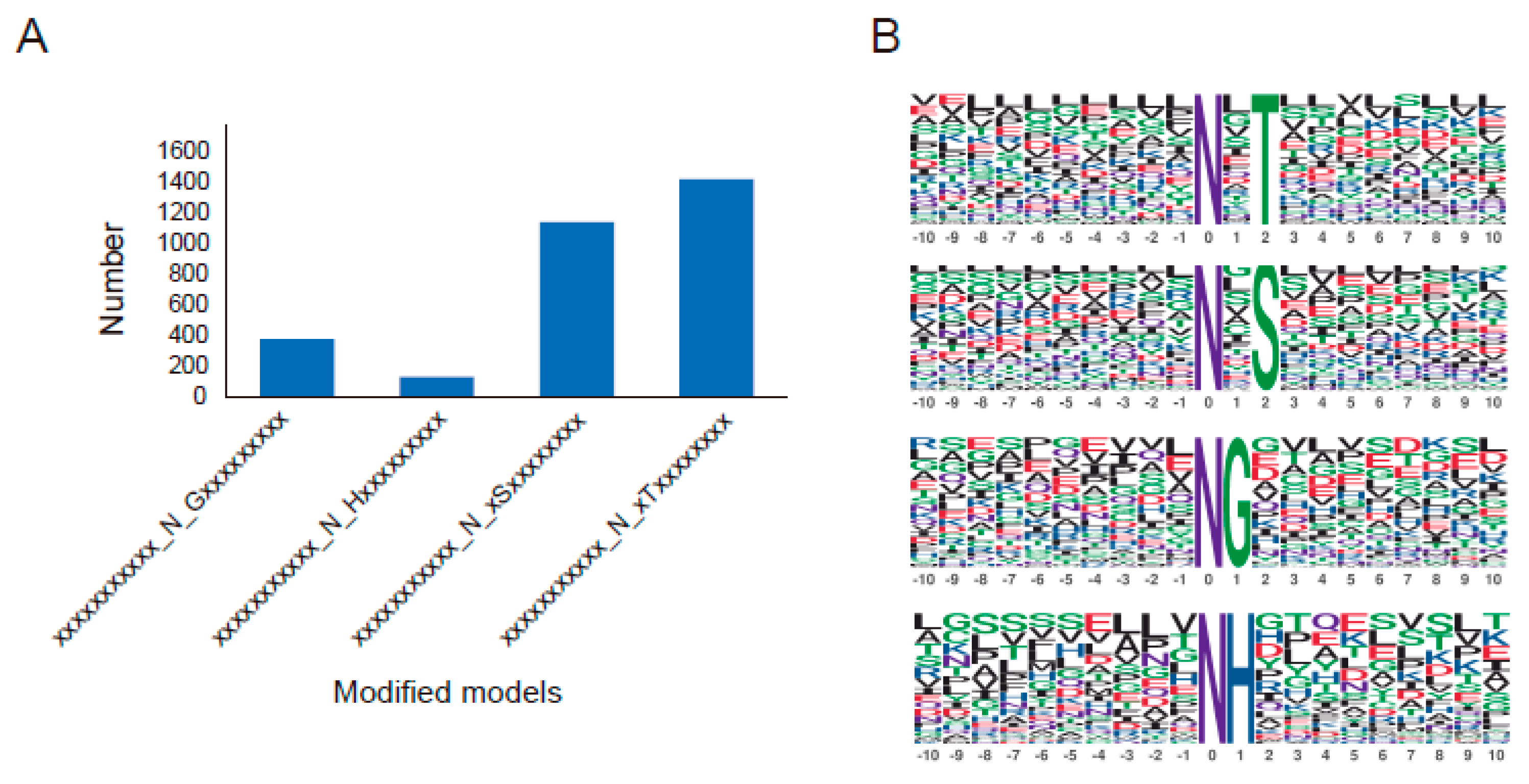
| Protein Name | Modified Sequence | Motif |
|---|---|---|
| CYP2B6 | _SQGALQDPTYYFHSSTAN[+O-H-N]CSIVFGK_ | xxxxxxxxxx_N_xSxxxxxxxx |
| CYP2D6 | _PVVVLN[+O-H-N]GLAAVR_ | xxxxxxxxxx_N_Gxxxxxxxxx |
| PEX14 | _ASSEQAEQPSQPN[+O-H-N]STPGSENVVPR_ | xxxxxxxxxx_N_xTxxxxxxxx |
| CD163 | _ATGWAN[+O-H-N]FSAGSGR_ | xxxxxxxxxx_N_xSxxxxxxxx |
| HSP90B1 | _EEEAIQLDGLN[+O-H-N]ASQIR_ | xxxxxxxxxx_N_xSxxxxxxxx |
| F5 | _HISQDN[+O-H-N]SSSSSIGPLEDLSSDLLLLER_ | xxxxxxxxxx_N_xSxxxxxxxx |
| GLUD1 | _IIAEGAN[+O-H-N]GPTTPEADK_ | xxxxxxxxxx_N_Gxxxxxxxxx |
| HSPA8 | _LLQDFFN[+O-H-N]GK_ | xxxxxxxxxx_N_Gxxxxxxxxx |
| GSTZ1 | _TISRIN[+O-H-N]KSLLALEAFQVSH_ | xxxxxxxxxx_N_xSxxxxxxxx |
| CYP1A2 | _HVLVNQWQVN[+O-H-N]HDPK_ | xxxxxxxxxx_N_Hxxxxxxxxx |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Wang, F.; Sun, R.; Zhang, Y.; Zheng, X.; Liu, H.; Chen, L.; Lin, Y.; Zhao, Y.; Liang, M.; et al. N-glycosylation Modification Reveals Insights into the Oxidative Reactions of Liver in Wuzhishan Pigs. Molecules 2024, 29, 5222. https://doi.org/10.3390/molecules29225222
Ren Y, Wang F, Sun R, Zhang Y, Zheng X, Liu H, Chen L, Lin Y, Zhao Y, Liang M, et al. N-glycosylation Modification Reveals Insights into the Oxidative Reactions of Liver in Wuzhishan Pigs. Molecules. 2024; 29(22):5222. https://doi.org/10.3390/molecules29225222
Chicago/Turabian StyleRen, Yuwei, Feng Wang, Ruiping Sun, Yan Zhang, Xinli Zheng, Hailong Liu, Linlin Chen, Yanning Lin, Yujie Zhao, Mingxia Liang, and et al. 2024. "N-glycosylation Modification Reveals Insights into the Oxidative Reactions of Liver in Wuzhishan Pigs" Molecules 29, no. 22: 5222. https://doi.org/10.3390/molecules29225222
APA StyleRen, Y., Wang, F., Sun, R., Zhang, Y., Zheng, X., Liu, H., Chen, L., Lin, Y., Zhao, Y., Liang, M., & Chao, Z. (2024). N-glycosylation Modification Reveals Insights into the Oxidative Reactions of Liver in Wuzhishan Pigs. Molecules, 29(22), 5222. https://doi.org/10.3390/molecules29225222






