Influence of pH on the Formation of Benzyl Ester Bonds Between Dehydrogenation Polymers and Konjac Glucomannan
Abstract
1. Introduction
2. Results and Discussion
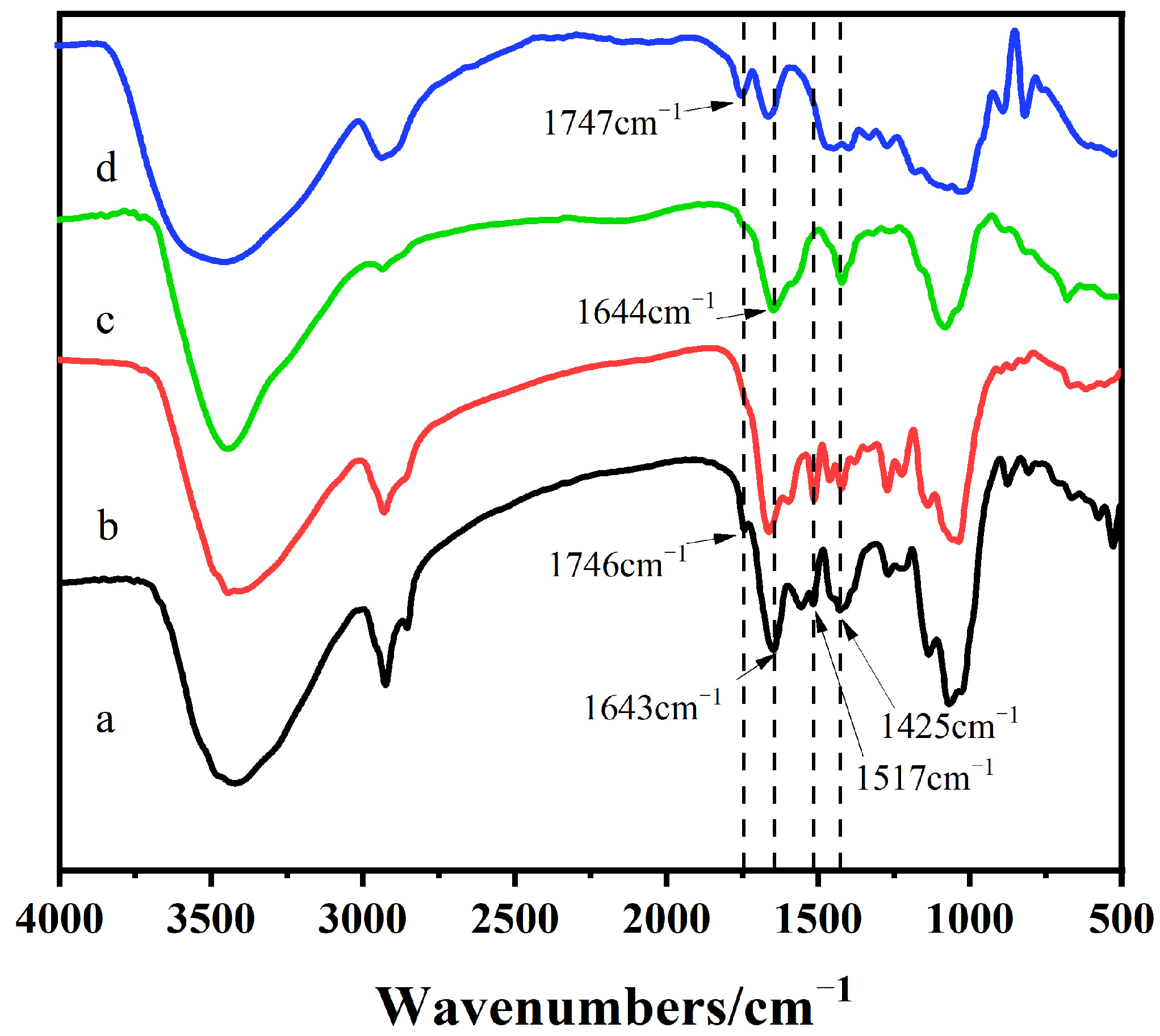
2.1. Infrared Spectrum Analysis of DHPKGC
2.2. NMR Analysis of DHP-KGC
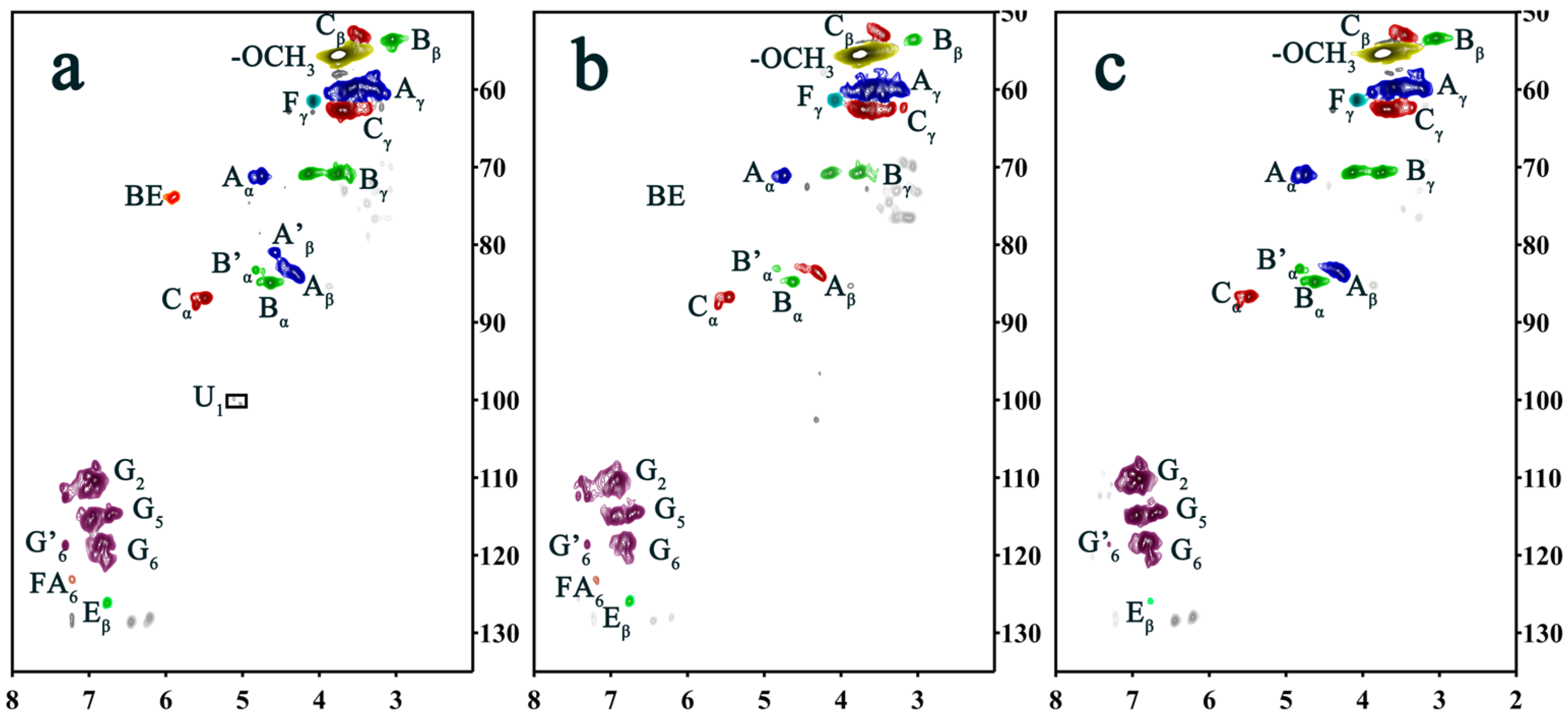
2.3. Two-Dimensional HSQC NMR Spectrum Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Oxidation of Konjac Glucomannan
3.2.2. Preparation of DHP–Konjac Glucomannan Complexes
3.2.3. Enzymatic Degradation of DHPKGC
3.2.4. Alkaline Treatment of EDDHPKGC
4. Analytical Procedure
4.1. Infrared Spectroscopic Analysis of DHPKGC
4.2. CP/MAS 13C-NMR Spectra Analysis of DHPCC
4.3. Two-Dimensional HSQC NMR Analysis of EDDHPCC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pang, Y.X.; Sharmin, N.; Wu, T.; Pang, C.H. An investigation on plant cell walls during biomass pyrolysis: A histochemical perspective on engineering applications. Appl. Energy 2023, 343, 121055. [Google Scholar] [CrossRef]
- Riseh, R.S.; Fathi, F.; Lagzian, A.; Vatankhah, M.; Kennedy, J.F. Modifying lignin: A promising strategy for plant disease control. Int. J. Biol. Macromol. 2024, 271, 132696. [Google Scholar]
- Sarkanen, K.V.; Ludwig, C.H. Lignins: Occurrence, Formation, Structure and Reactions; John Wiley & Sons: New York, NY, USA, 1971. [Google Scholar]
- Björkman, A. Lignin and lignin–carbohydrate complexes–extraction from wood meal with neutral solvents. Ind. Eng. Chem. 1957, 49, 1395–1398. [Google Scholar] [CrossRef]
- Zhao, Y.; Shakeel, U.; Rehman, M.S.U.; Li, H.; Xu, X.; Xu, J. Lignin-carbohydrate complexes (LCCs) and its role in biorefinery. J. Clean. Prod. 2020, 253, 120076. [Google Scholar] [CrossRef]
- Giummarella, N.; Pu, Y.; Ragauskas, A.J.; Lawoko, M. A critical review on the analysis of lignin carbohydrate bonds. Green Chem. 2019, 21, 1573–1595. [Google Scholar] [CrossRef]
- Akpan, E.I.; Wetzel, B.; Friedrich, K. Eco-friendly and sustainable processing of wood-based materials. Green Chem. 2021, 23, 2198–2232. [Google Scholar] [CrossRef]
- Liu, C.; Luan, P.; Li, Q.; Cheng, Z.; Xiang, P.; Liu, D.; Hou, Y.; Yang, Y.; Zhu, H. Biopolymers derived from trees as sustainable multifunctional materials: A review. Adv. Mater. 2021, 33, 2001654. [Google Scholar] [CrossRef]
- Mäkelä, M.R.; Hildén, K.; Kowalczyk, J.E.; Hatakka, A. Progress and Research Needs of Plant Biomass Degradation by Basidiomycete Fungi. In Grand Challenges in Fungal Biotechnology; Springer: Cham, Switzerland, 2020; pp. 405–438. [Google Scholar]
- Liu, Y.; Wang, X.; Wu, Q.; Pei, W.; Teo, M.J.; Chen, Z.S.; Huang, C. Application of lignin and lignin-based composites in different tissue engineering fields. Int. J. Biol. Macromol. 2022, 222, 994–1006. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Wang, P.; Zeng, S.; Xie, Y. Lignin-Carbohydrate Complexes Based Spherical Biocarriers: Preparation, Characterization, and Biocompatibility. Int. J. Polym. Sci. 2017, 2017, 4915185. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, Q.; Xie, Y.; Li, J.; Chen, X. Preparation of biocompatible hydrogel from lignin-carbohydrate complex (LCC) as cell carriers. BioResources 2017, 12, 8490–8504. [Google Scholar] [CrossRef]
- Yaku, F.; Yamada, Y.; Koshijima, T. Lignin carbohydrate complex Pt. II. Enzymic degradation of acidic polysaccharide in Björkman LCC. Holzforschung 1976, 30, 148–156. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Sipilä, J.; Brunow, G. On the mechanism of formation of non-cyclic benzyl ethers during lignin biosynthesis. Part 4. The reactions of a β-O-4-type quinone methide with carboxylic acids in the presence of phenols. The formation and stability of benzyl esters between lignin and carbohydrates. Holzforschung 1991, 45, 9–14. [Google Scholar]
- Tanaka, K.; Nakatsubo, F.; Higuchi, T. Reactions of guaiacylglycerol-beta-guaiacyl ether with several sugars. II. Reactions of quinonemethide with pyranohexoses. J. Wood Sci. 1979, 48, 32–37. [Google Scholar]
- Terashima, N.; Atalla, R.; Ralph, S.; Landucci, L.L.; Lapierre, C.; Monties, B. New preparations of lignin polymer models under conditions that approximate cell wall lignification. I. Synthesis of novel lignin polymer models and their structural characterization by 13C NMR. Holzforschung 1995, 49, 521–527. [Google Scholar] [CrossRef]
- Cathala, B.; Monties, B. Influence of pectins on the solubility and the molar mass distribution of dehydrogenative polymers (DHPs, lignin model compounds). Int. J. Biol. Macromol. 2001, 29, 45–51. [Google Scholar] [CrossRef]
- Freudenberg, K.; Grion, G. Beitrag zum Bildungsmechanismus des Lignins und der Lignin-Kohlenhydrat-Bindung. Chem. Ber. 1959, 92, 1355–1363. [Google Scholar] [CrossRef]
- Brunow, G.; Sipilä, J.; Mäkelä, T. On the Mechanism of Formation of Non-cyclic Benzyl Ethers During Lignin Biosynthesis. Part 1. The Reactivity of β-O-4 Quinone Methides with Phenols and Alcohols. Holzforschung 1989, 43, 55–59. [Google Scholar] [CrossRef]
- Sipilä, J.; Brunow, G. On the Mechanism of Formation of Non-Cyclic Benzyl Ethers During Lignin Biosynthesis. Part 2. The Effect of pH on the Reaction between a β-O-4-Type Quinone Methide and Vanillyl Alcohol in Water-Dioxane Solutions. The Stability of Non-Cyclic Benzyl Aryl Ethers During Lignin Biosynthesis. Holzforschung 1991, 45, 275–278. [Google Scholar]
- Wang, X.; Le, X.; Peng, W.; Ye, P.; An, J.; Zhang, G.; Wang, P.; Xie, Y. Effect of pH on the formation of benzyl ester bonds between glucuronic acid and dehydrogenation polymer. BioResources 2022, 17, 52. [Google Scholar] [CrossRef]
- Bu, X.; Pei, J.; Zhang, F.; Liu, H.; Zhou, Z.; Zhen, X.; Wang, J.; Zhang, X.; Chan, H. The hydration mechanism and hydrogen bonding structure of 6-carboxylate chitooligosaccharides superabsorbent material prepared by laccase/TEMPO oxidation system. Carbohydr. Polym. 2018, 188, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Terashima, N. Selective carbon 13-enrichment of side chain carbons of ginkgo lignin traced by carbon 13 nuclear magnetic resonance. J. Jpn. Wood Res. Soc. 1994, 37, 935–941. [Google Scholar]
- Xie, Y.; Yasuda, S.; Wu, H.; Liu, H. Analysis of the structure of lignin-carbohydrate complexes by the specific 13C tracer method. J. Wood Sci. 2000, 46, 130–136. [Google Scholar] [CrossRef]
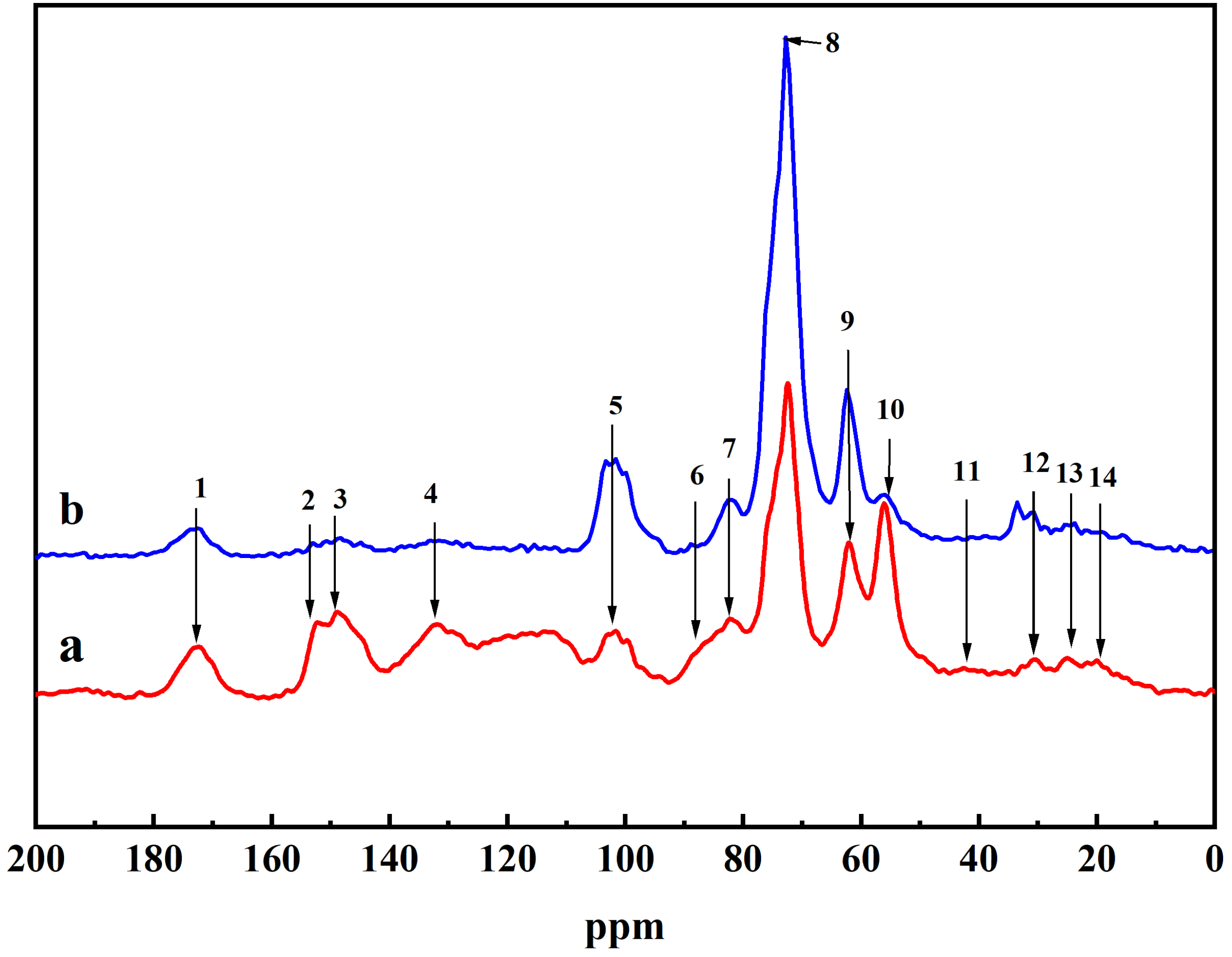

| Peak No. | pH 4 | pH 7 | Assignments |
|---|---|---|---|
| 1 | 172.7 | 173.8 | -COO- in aliphatic esters and acetyl group |
| 2 | 152.1 | 152.7 | C3/C4 in G-ring |
| 3 | 147.1 | 147.3 | C4 in G-ring |
| 4 | 131.2 | 131.6 | C1 in G-ring |
| 5 | 101–103 | 101–103 | C6 in G-ring |
| 6 | 88.3 | 88.6 | Cα(β-5, β-β) |
| 7 | 80.5 | 81.2 | Cβ in β-O-4 |
| 8 | 74 | 74.3 | Cα in β-O-4, Cα of ester |
| 9 | 62.9 | 62.8 | Cα/Cβ in β-1, Cγ in β-5, and β-O-4 |
| 10 | 54.7 | 56.9 | -OCH3 |
| 11 | 42.1 | 42.9 | α-CH2- side chain of phenylpropane units |
| 12 | 30.9 | 30.4 | β-CH2- side chain of phenylpropane units |
| 13 | 24.1 | 24.1 | -CH2 in saturated alkyl |
| 14 | 20.7 | 20.1 | -CH3 of acetyl |
| Lable | pH = 4 | pH = 4 (Alkali) | pH = 7 | Assignments |
|---|---|---|---|---|
| δC/δH (ppm) | δC/δH (ppm) | δC/δH (ppm) | ||
| Cβ | 53.03/3.45 | 52.77/3.51 | 52.79/3.53 | Cβ–Hβ in phenylcoumaran (C) |
| Bβ | 53.48/3.03 | 53.46/3.05 | 53.54/3.06 | Cβ–Hβ in β-β (resinol) (B) |
| OCH3 | 55.46/3.75 | 55.44/3.74 | 55.49/3.74 | C–H in methoxyls |
| Aγ | 59.44/3.45 60.37/3.79 | 59.59/3.40 59.87/3.77 | 59.74/3.34 60.32/3.75 | Cγ–Hγ in β-O-4 substructures (A) |
| Fγ | 61.39/4.08 | 61.45/4.08 | 61.46/4.08 | Cγ–Hγ in cinnamyl alcohol end groups (F) |
| Cγ | 62.64/3.72 | 62.57/3.71 | 62.59/3.72 | Cγ–Hγ in phenylcoumaran (C) |
| Bγ | 70.86/3.75 70.81/4.13 | 70.77/3.74 70.81/4.13 | 70.74/3.74 70.83/4.13 | Cγ–Hγ in β-β resinol (B) |
| Aα | 71.05/4.74 | 71.06/4.74 | 70.13/4.73 | Cα–Hα in β-O-4 unit (A) |
| BE | 73.86/5.90 | ND | ND | Benzyl ester bond structure (BE) |
| Aβ | 83.99/4.33 | 83.66/4.28 | 83.58/4.28 | Cβ–Hβ in β-O-4 substructures (A) |
| A′β | 80.96/4.56 | ND | ND | Cβ–Hβ in β-O-4 linked to G (A) |
| Bα | 84.83/4.62 | 84.85/4.62 | 84.90/4.63 | Cα–Hα in β-β resinol (B) |
| B′α | 83.21/4.83 | 83.16/4.83 | 83.15/4.83 | Cα–Hα in β-β (B′, tetrahydrofuran) |
| Cα | 86.79/5.49 87.69/5.60 | 86.77/5.47 87.86/5.63 | 86.84/5.46 87.75/5.59 | Cα–Hα in phenylcoumaran (C) |
| G2 | 108.58/6.90 111.46/7.04 112.50/7.42 | 110.13/6.91 111.50/7.06 112.33/7.42 | 110.35/6.91 112.57/7.57 | C2–H2 in guaiacyl units (G) |
| G5 | 114.80/6.73 114.92/6.95 | 114.49/6.71 114.89/6.95 | 114.55/6.75 115.13/6.96 | C5–H5 in guaiacyl units (G) |
| G6 | 118.31/6.85 120.30/6.75 | 118.50/6.84 | 118.63/6.76 | C6–H6 in guaiacyl units (G) |
| G′6 | 118.70/7.31 | 118.95/7.33 | 118.76/7.31 | α C6–H6 in G-type structural units with oxidized sites |
| Eβ | 126.04/6.76 | 126.06/6.76 | 126.06/6.75 | Cβ–Hβ in cinnamyl aldehyde end groups (E) |
| U1 | 99.82/5.11 100.42/5.02 | ND | ND | C1–H1 in 4-O-methyl-α-D-GlcUA (U) |
| FA6 | 123.03/7.22 | ND | 123.41/7.18 | C6–H6 in ferulate (p-FA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Zhang, X.; Le, X.; Chen, J.; Zhang, G.; An, J.; Feng, N.; Xie, J. Influence of pH on the Formation of Benzyl Ester Bonds Between Dehydrogenation Polymers and Konjac Glucomannan. Molecules 2024, 29, 5166. https://doi.org/10.3390/molecules29215166
Wang P, Zhang X, Le X, Chen J, Zhang G, An J, Feng N, Xie J. Influence of pH on the Formation of Benzyl Ester Bonds Between Dehydrogenation Polymers and Konjac Glucomannan. Molecules. 2024; 29(21):5166. https://doi.org/10.3390/molecules29215166
Chicago/Turabian StyleWang, Peng, Xu Zhang, Xi Le, Junjun Chen, Guangyan Zhang, Junjian An, Nianjie Feng, and Junxian Xie. 2024. "Influence of pH on the Formation of Benzyl Ester Bonds Between Dehydrogenation Polymers and Konjac Glucomannan" Molecules 29, no. 21: 5166. https://doi.org/10.3390/molecules29215166
APA StyleWang, P., Zhang, X., Le, X., Chen, J., Zhang, G., An, J., Feng, N., & Xie, J. (2024). Influence of pH on the Formation of Benzyl Ester Bonds Between Dehydrogenation Polymers and Konjac Glucomannan. Molecules, 29(21), 5166. https://doi.org/10.3390/molecules29215166






