Ball Milling and Magnetic Modification Boosted Methylene Blue Removal by Biochar Obtained from Water Hyacinth: Efficiency, Mechanism, and Application
Abstract
1. Introduction
2. Results and Discussion
2.1. The Surface Structures of Different Biochars
2.2. Adsorption Efficiency and Kinetics of MB by Biochars
2.3. Effect of Temperature on MB Adsorption
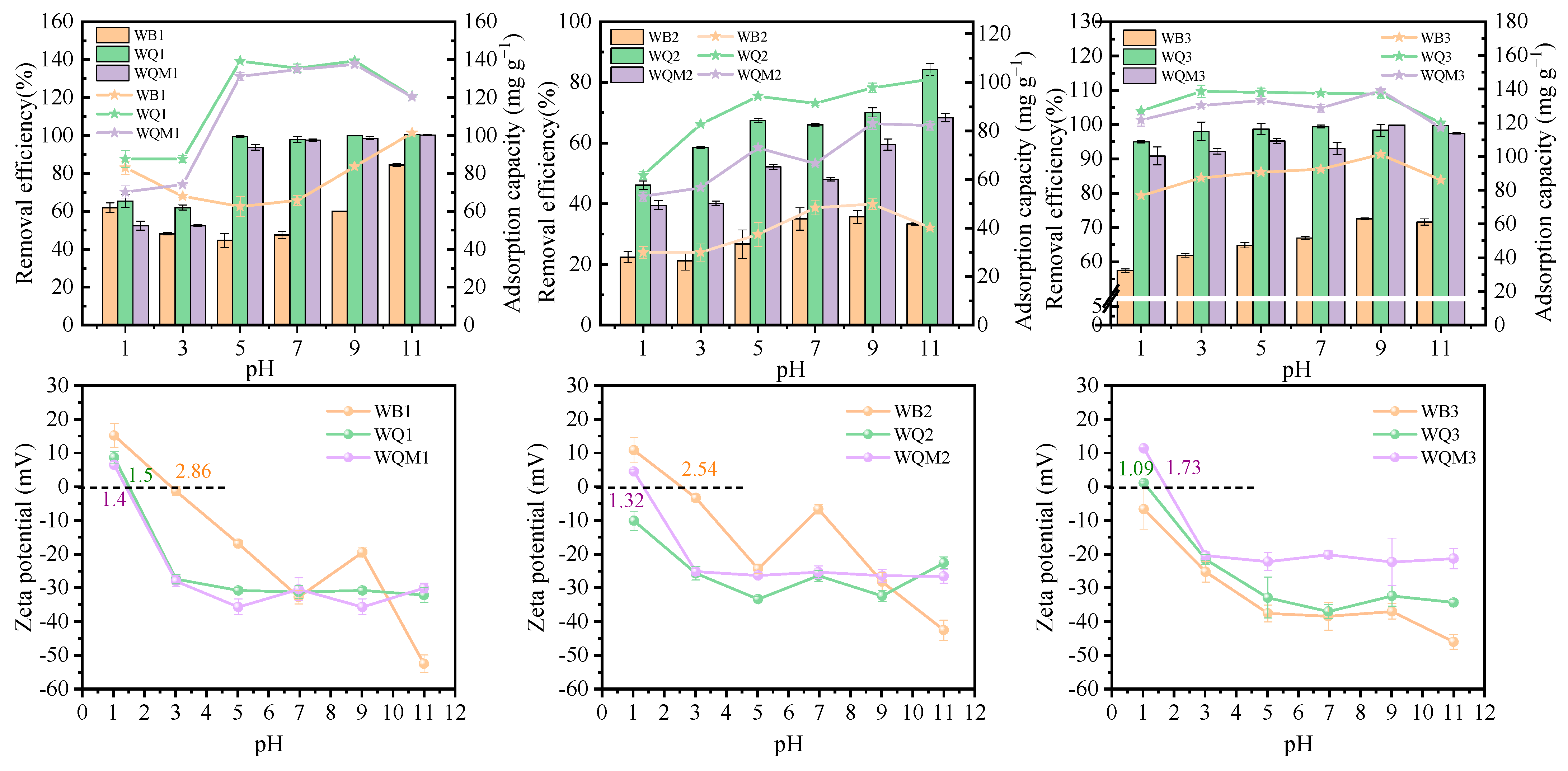
2.4. Effect of pH on MB Adsorption
2.5. Reusability of WQM1 and WQM3
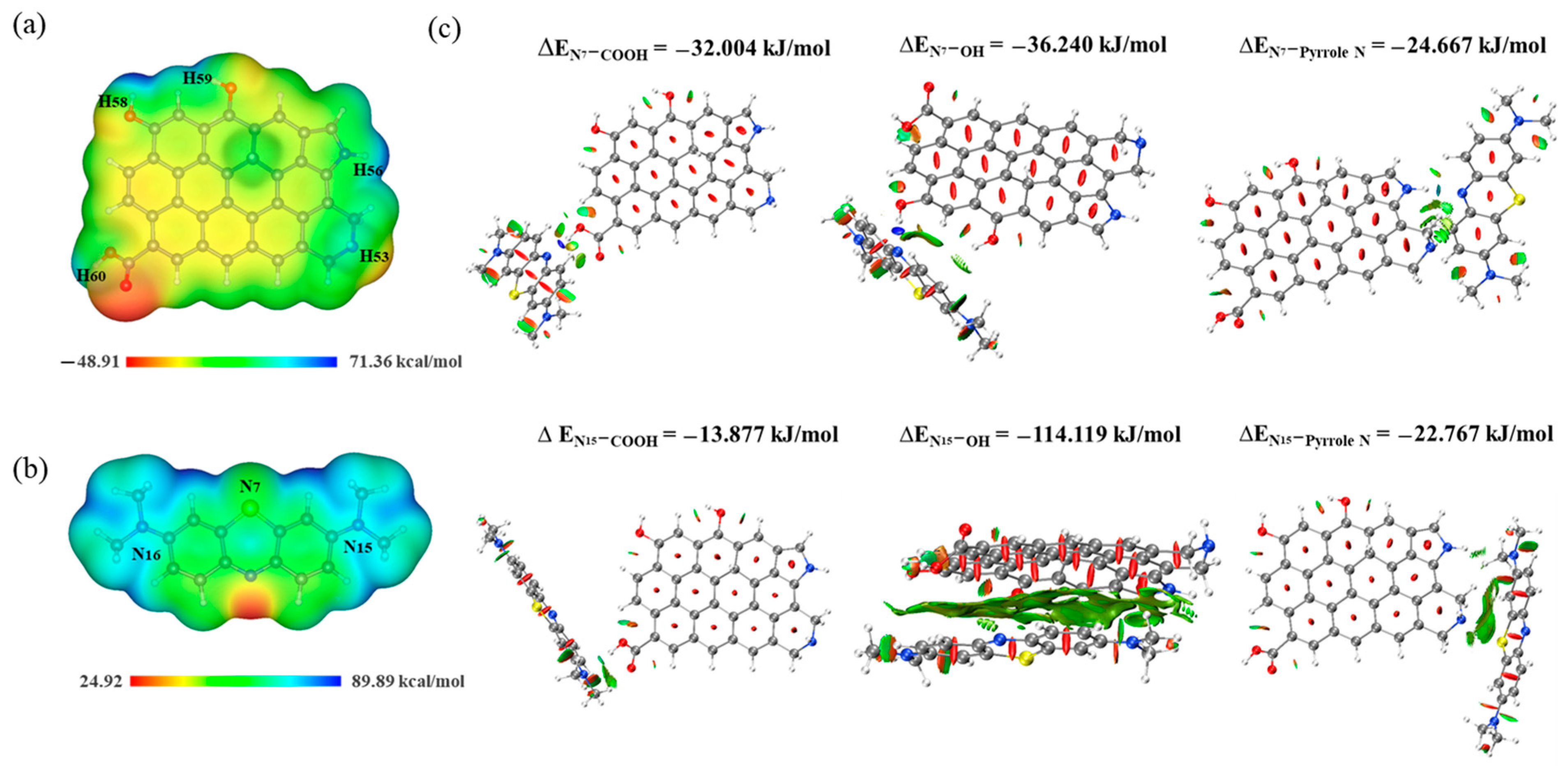
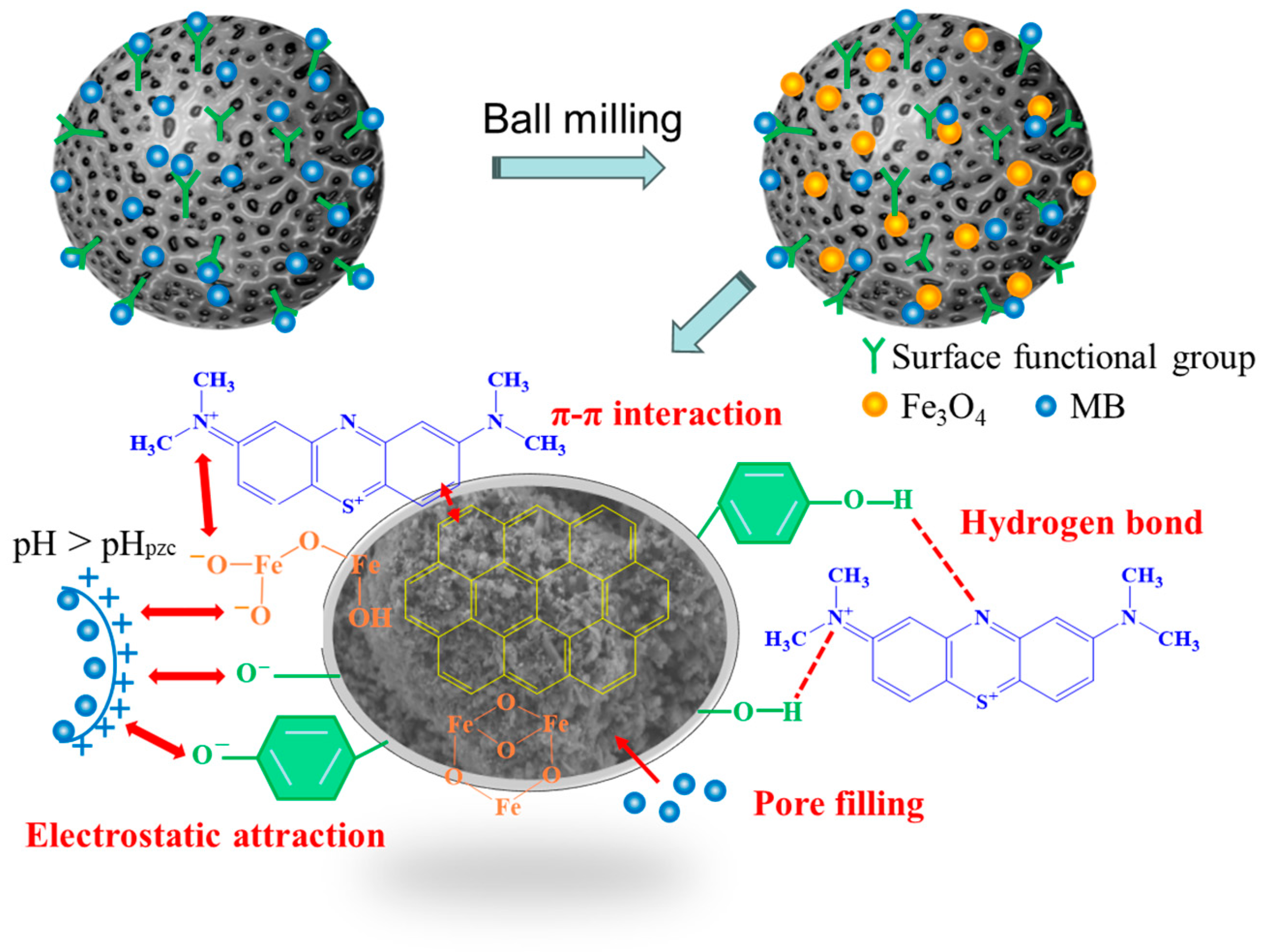
2.6. Removal Mechanism Analysis
3. Experimental Section
3.1. Preparation of Ball-Milled Magnetic Biochars
3.2. The Adsorption Efficiency and Kinetic Model
3.3. Adsorption Isotherm Model
3.4. Regeneration Studies
3.5. Characterization of Various Biochars
3.6. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benkhaya, S.; M’rabet, S.; El Harfi, A. A Review on Classifications, Recent Synthesis and Applications of Textile Dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Xiang, X.; Zhou, J.; Lin, S.; Zhang, N.; Abulipizi, G.; Chen, G.; Li, Z. Dual Drive Acute Lethal Toxicity of Methylene Blue to Daphnia Magna by Polystyrene Microplastics and Light. Sci. Total Environ. 2022, 840, 156681. [Google Scholar] [CrossRef]
- Oz, M.; Isaev, D.; Lorke, D.E.; Hasan, M.; Petroianu, G.; Shippenberg, T.S. Methylene Blue Inhibits Function of the 5-HT Transporter. Br. J. Pharmacol. 2012, 166, 168–176. [Google Scholar] [CrossRef]
- Fang, W.; Zhou, Y.; Cheng, M.; Yang, J.; Huang, Q.; Huang, Z.; Cui, Y.; Zhang, L.; Wang, Y.; Cen, Q.; et al. Effective Adsorption Performance and Mechanism of Methylene Blue from Dye Wastewater by Humic Acid Sucrose-Modified Red Mud. Process Saf. Environ. Prot. 2024, 191, 1168–1180. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of Methylene Blue on Low-Cost Adsorbents: A Review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated Lignin-Chitosan Extruded Blends for Efficient Adsorption of Methylene Blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef]
- Zhai, S.; Li, M.; Wang, D.; Zhang, L.; Yang, Y.; Fu, S. In Situ Loading Metal Oxide Particles on Bio-Chars: Reusable Materials for Efficient Removal of Methylene Blue from Wastewater. J. Clean. Prod. 2019, 220, 460–474. [Google Scholar] [CrossRef]
- Xu, P.; Shang, Z.; Li, G.; Sun, Y.; He, K.; Li, X. A Novel Silica-Reinforced P(AM/AMPS/SA/TM-SiO2) Microspheres for Selective Adsorption of Methylene Blue from Aqueous Solution. Sep. Purif. Technol. 2023, 313, 123495. [Google Scholar] [CrossRef]
- Sivakumar, R.; Lee, N.Y. Adsorptive Removal of Organic Pollutant Methylene Blue Using Polysaccharide-Based Composite Hydrogels. Chemosphere 2022, 286, 131890. [Google Scholar] [CrossRef]
- Hawryluk-Sidoruk, M.; Raczkiewicz, M.; Krasucka, P.; Duan, W.; Mašek, O.; Zarzycki, R.; Kobyłecki, R.; Pan, B.; Oleszczuk, P. Effect of Biochar Chemical Modification (Acid, Base and Hydrogen Peroxide) on Contaminants Content Depending on Feedstock and Pyrolysis Conditions. Chem. Eng. J. 2024, 481, 148329. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Wang, X.; Zhao, Q.; Jiang, J.; Jiang, M. Effect of Different Production Methods on Physicochemical Properties and Adsorption Capacities of Biochar from Sewage Sludge and Kitchen Waste: Mechanism and Correlation Analysis. J. Hazard. Mater. 2024, 461, 132690. [Google Scholar] [CrossRef]
- Wang, B.; Zhai, Y.; Wang, T.; Li, S.; Peng, C.; Wang, Z.; Li, C.; Xu, B. Fabrication of Bean Dreg-Derived Carbon with High Adsorption for Methylene Blue: Effect of Hydrothermal Pretreatment and Pyrolysis Process. Bioresour. Technol. 2019, 274, 525–532. [Google Scholar] [CrossRef]
- Yan, N.; Hu, B.; Zheng, Z.; Lu, H.; Chen, J.; Zhang, X.; Jiang, X.; Wu, Y.; Dolfing, J.; Xu, L. Twice-Milled Magnetic Biochar: A Recyclable Material for Efficient Removal of Methylene Blue from Wastewater. Bioresour. Technol. 2023, 372, 128663. [Google Scholar] [CrossRef]
- Kamal, A.; Haroon, U.; Manghwar, H.; Alamer, K.H.; Alsudays, I.M.; Althobaiti, A.T.; Iqbal, A.; Akbar, M.; Farhana; Anar, M.; et al. Biological Applications of Ball-Milled Synthesized Biochar-Zinc Oxide Nanocomposite Using Zea mays L. Molecules 2022, 27, 5333. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.W.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball Milling as a Mechanochemical Technology for Fabrication of Novel Biochar Nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef]
- Zhu, Q.; Liang, Y.; Liu, H.; Guo, Y.; Zhang, Z.; Wang, C.; Liu, C.; Sun, H. Application of a Novel Ball-Milled Tourmaline-Biochar Composite Materials for Remediation of Groundwater and Bottom Mud Polluted with Heavy Metals. Sep. Purif. Technol. 2025, 352, 128278. [Google Scholar] [CrossRef]
- Mohd Faizal, A.N.; Putra, N.R.; Abdul Aziz, A.H.; Agi, A.; Ahmad Zaini, M.A. Giant Mud Crab Shell Biochar: A Promising Adsorbent for Methyl Violet Removal in Wastewater Treatment. J. Clean. Prod. 2024, 447, 141637. [Google Scholar] [CrossRef]
- Huang, J.; Zimmerman, A.R.; Chen, H.; Gao, B. Ball Milled Biochar Effectively Removes Sulfamethoxazole and Sulfapyridine Antibiotics from Water and Wastewater. Environ. Pollut. 2020, 258, 113809. [Google Scholar] [CrossRef]
- Ma, Y.; Yao, Y.; Qian, S.; Deng, Z.; Liu, Y.; Ma, J.; Zhang, Z. Ball Milling Boosted Hydrothermal N-Doped Sludge-Derived Biochar towards Efficiently Adsorptive Removal of Sulfamethoxazole from Waters: Behavior, Mechanism and DFT Study. Sep. Purif. Technol. 2024, 338, 126453. [Google Scholar] [CrossRef]
- Cao, G.; Sun, J.; Chen, M.; Sun, H.; Zhang, G. Co-Transport of Ball-Milled Biochar and Cd2+ in Saturated Porous Media. J. Hazard. Mater. 2021, 416, 125725. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Qi, W.; Zhai, L.; Wang, F.; Zhang, J.; Li, D. Preparation and Characterization of Sludge-Based Magnetic Biochar by Pyrolysis for Methylene Blue Removal. Nanomaterials 2021, 11, 2473. [Google Scholar] [CrossRef]
- Lohan, D.; Jain, R.; Srivastava, A.; Dutta, S.; Mohan, D.; Sharma, R.K. Surface Engineering Approaches for the Design of Magnetic Biochar-Composites for Removal of Heavy Metals: A Comprehensive Review. J. Environ. Chem. Eng. 2023, 11, 111448. [Google Scholar] [CrossRef]
- Qu, J.; Shi, J.; Wang, Y.; Tong, H.; Zhu, Y.; Xu, L.; Wang, Y.; Zhang, B.; Tao, Y.; Dai, X.; et al. Applications of Functionalized Magnetic Biochar in Environmental Remediation: A Review. J. Hazard. Mater. 2022, 434, 128841. [Google Scholar] [CrossRef]
- Zhong, C.; Guo, Z.; Hang, J.; Xu, S.; Song, H.; Liu, W.; Li, J. An Efficient and General Oxidative Magnetization for Preparation of Versatile Magnetic Porous Biochar at Low Temperature. J. Environ. Chem. Eng. 2024, 12, 112187. [Google Scholar] [CrossRef]
- Li, Y.; Zimmerman, A.R.; He, F.; Chen, J.; Han, L.; Chen, H.; Hu, X.; Gao, B. Solvent-Free Synthesis of Magnetic Biochar and Activated Carbon through Ball-Mill Extrusion with Fe3O4 Nanoparticles for Enhancing Adsorption of Methylene Blue. Sci. Total Environ. 2020, 722, 137972. [Google Scholar] [CrossRef]
- Giraldo, S.; Robles, I.; Godínez, L.A.; Acelas, N.; Flórez, E. Experimental and Theoretical Insights on Methylene Blue Removal from Wastewater Using an Adsorbent Obtained from the Residues of the Orange Industry. Molecules 2021, 26, 4555. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional Heterogeneity of Different Biochar: Effect of Pyrolysis Temperature and Feedstocks. J. Environ. Manag. 2021, 278, 111501. [Google Scholar] [CrossRef]
- Fan, J.; Duan, T.; Zou, L.; Sun, J. Characteristics of Dissolved Organic Matter Composition in Biochar: Effects of Feedstocks and Pyrolysis Temperatures. Environ. Sci. Pollut. Res. 2023, 30, 85139–85153. [Google Scholar] [CrossRef]
- Zhou, J.; Fei, H.; He, Q.; Li, P.; Pan, Y.; Liang, X. Structural Characteristics and Thermal Performances of Lauric-Myristic-Palmitic Acid Introduced into Modified Water Hyacinth Porous Biochar for Thermal Energy Storage. Sci. Total Environ. 2023, 882, 163670. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, S.; Sun, B.; Han, Y.; Zhang, Z.; Shen, X.; Li, Z. Adsorption Efficiency and In-Situ Catalytic Thermal Degradation Behaviour of Microplastics from Water over Fe-Modified Lignin-Based Magnetic Biochar. Sep. Purif. Technol. 2025, 353, 128468. [Google Scholar] [CrossRef]
- Mu, R.; Qian, S.; Ma, Y.; Deng, Z.; Tang, J.; Zhang, Z. Functionally-Designed Metal Salt and Ball Milling Co-Modified Sludge Biochar for Adsorptive Removal of Trace Level Sulfamethoxazole: Behavior, Characterization, Mechanism and DFT Study. J. Environ. Chem. Eng. 2024, 12, 113479. [Google Scholar] [CrossRef]
- Li, H.; Kong, J.; Zhang, H.; Gao, J.; Fang, Y.; Shi, J.; Ge, T.; Fang, T.; Shi, Y.; Zhang, R.; et al. Mechanisms and Adsorption Capacities of Ball Milled Biomass Fly Ash/Biochar Composites for the Adsorption of Methylene Blue Dye from Aqueous Solution. J. Water Process Eng. 2023, 53, 103713. [Google Scholar] [CrossRef]
- Al-humaidi, J.Y.; Refat, M.S.; Abouzied, A.S. Facile Synthesis of ZIF-67 for the Adsorption of Methyl Green from Wastewater: Integrating Molecular Models and Removal Mechanism. Mol. Artic. 2022, 27, 8385. [Google Scholar]
- Tabassam, N.; Mutahir, S.; Khan, M.A.; Khan, I.U.; Habiba, U.; Refat, M.S. Facile Synthesis of Cinnamic Acid Sensitized Rice Husk Biochar for Removal of Organic Dyes from Wastewaters: Batch Experimental and Theoretical Studies. Mater. Chem. Phys. 2022, 288, 126327. [Google Scholar] [CrossRef]
- Mu, Y.; Du, H.; He, W.; Ma, H. Functionalized Mesoporous Magnetic Biochar for Methylene Blue Removal: Performance Assessment and Mechanism Exploration. Diam. Relat. Mater. 2022, 121, 108795. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Z.; Huang, J.; Gao, B.; Zhao, L.; Qiu, H.; Cao, X. Sorption of Reactive Red by Biochars Ball Milled in Different Atmospheres: Co-Effect of Surface Morphology and Functional Groups. Chem. Eng. J. 2021, 413, 127468. [Google Scholar] [CrossRef]
- Cheng, L.; Liang, H.; Yang, W.; Yang, T.; Chen, T.; Gao, D. The Biochar/Fe-Modified Biocarrier Driven Simultaneous NDFO and Feammox to Remove Nitrogen from Eutrophic Water. Water Res. 2023, 243, 120280. [Google Scholar] [CrossRef]
- Rong, L.; Wu, L.; Zhang, T.; Hu, C.; Tang, H.; Pan, H.; Zou, X. Significant Differences in the Effects of Nitrogen Doping on Pristine Biochar and Graphene-like Biochar for the Adsorption of Tetracycline. Molecules 2024, 29, 173. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, M.; Feng, H.; Luo, X.; Yang, Y.; Hu, J.; Hu, Y. Effects and Mechanisms on Cr(VI) and Methylene Blue Adsorption by Acid (NH4)2S2O8 Modified Sludge Biochar. Sep. Purif. Technol. 2024, 345, 127100. [Google Scholar] [CrossRef]
- Viegas, R.M.C.; Campinas, M.; Costa, H.; Rosa, M.J. How Do the HSDM and Boyd’s Model Compare for Estimating Intraparticle Diffusion Coefficients in Adsorption Processes. Adsorption 2014, 20, 737–746. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Rethinking of the Intraparticle Diffusion Adsorption Kinetics Model: Interpretation, Solving Methods and Applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef]
- Srikaew, M.; Wongnongwa, Y.; Jungsuitiwong, S.; Kaiyasuan, C.; Promarak, V.; Saengsuwan, S. Improvement of Adsorption Performance and Selectivity of GO-COOH towards MB Dye through Effective Carboxylation Approach: Combined Experimental and DFT Studies. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 673, 131920. [Google Scholar] [CrossRef]
- Yu, J.; Feng, H.; Tang, L.; Pang, Y.; Wang, J.; Zou, J.; Xie, Q.; Liu, Y.; Feng, C.; Wang, J. Insight into the Key Factors in Fast Adsorption of Organic Pollutants by Hierarchical Porous Biochar. J. Hazard. Mater. 2021, 403, 123610. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Khraisheh, M.A.M.; Allen, S.J.; Ahmad, M.N. The Removal of Dyes from Textile Wastewater: A Study of the Physical Characteristics and Adsorption Mechanisms of Diatomaceous Earth. J. Environ. Manag. 2003, 69, 229–238. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Wang, L.; Tuo, Y.; Yan, S.; Ma, J.; Zhang, X.; Shen, Y.; Guo, H.; Han, L. Experimental and DFT Insights into the Adsorption Mechanism of Methylene Blue by Alkali-Modified Corn Straw Biochar. RSC Adv. 2024, 14, 1854–1865. [Google Scholar] [CrossRef]
- Zhou, P.; Li, X.; Zhou, J.; Peng, Z.; Shen, L.; Li, W. Insights of the Adsorption Mechanism of Methylene Blue on Biochar from Phytoextraction Residues of Citrus aurantium L.: Adsorption Model and DFT Calculations. J. Environ. Chem. Eng. 2023, 11, 110496. [Google Scholar] [CrossRef]
- Makoś-Chełstowska, P.; Chromá, R.; Andruch, V. Closer Look into the Structures of Tetrabutylammonium Bromide–Glycerol-Based Deep Eutectic Solvents and Their Mixtures with Water. J. Mol. Liq. 2021, 338, 116676. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, C.; Wu, M.; Zhang, Z.; Chen, Z.; Liu, M.; Xie, T.; He, W.; Li, L.; Wang, F.; et al. Supramolecular Polymerization of C3-Symmetric, Triphenylene-Cored Aza-Polycyclic Aromatic Hydrocarbons with Excellent and Switchable Circularly Polarized Luminescence Performance. Macromolecules 2021, 54, 7291–7297. [Google Scholar] [CrossRef]
- Wang, B.; Dai, X.; Ma, Y.; Xin, J.; Pan, C.; Zhai, Y. Nitrogen-Rich Porous Biochar for Highly Efficient Adsorption of Perchlorate: Influencing Factors and Mechanism. J. Environ. Chem. Eng. 2023, 11, 110293. [Google Scholar] [CrossRef]
- Alshabib, M.; Oluwadamilare, M.A.; Tanimu, A.; Abdulazeez, I.; Alhooshani, K.; Ganiyu, S.A. Experimental and DFT Investigation of Ceria-Nanocomposite Decorated AC Derived from Groundnut Shell for Efficient Removal of Methylene-Blue from Wastewater Effluent. Appl. Surf. Sci. 2021, 536, 147749. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, T.; Han, C.; Lv, X.; Bai, L.; Sun, X.; Zhang, P. Facile Synthesis of Fe-Modified Lignin-Based Biochar for Ultra-Fast Adsorption of Methylene Blue: Selective Adsorption and Mechanism Studies. Bioresour. Technol. 2022, 344, 126186. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, F.; Ji, B.; Zhu, L.; Song, H. Insights into the Mechanism of Methylene Blue Removed by Novel and Classic Biochars. Water Sci. Technol. 2019, 79, 1561–1570. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B. Application of Isotherms Models and Error Functions in Activated Carbon CO2 Sorption Processes. Microporous Mesoporous Mater. 2023, 354, 112513. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Jiang, R.; Li, X.; Li, C.; Fang, J.; Xing, Z.; Yu, G. An Insight into the Diazo Dye Direct Red 23 Adsorption on Ca-Modified Biochar in an Aqueous Solution: An Investigation Based on DFT and Molecular Dynamics. Sep. Purif. Technol. 2025, 354, 128655. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
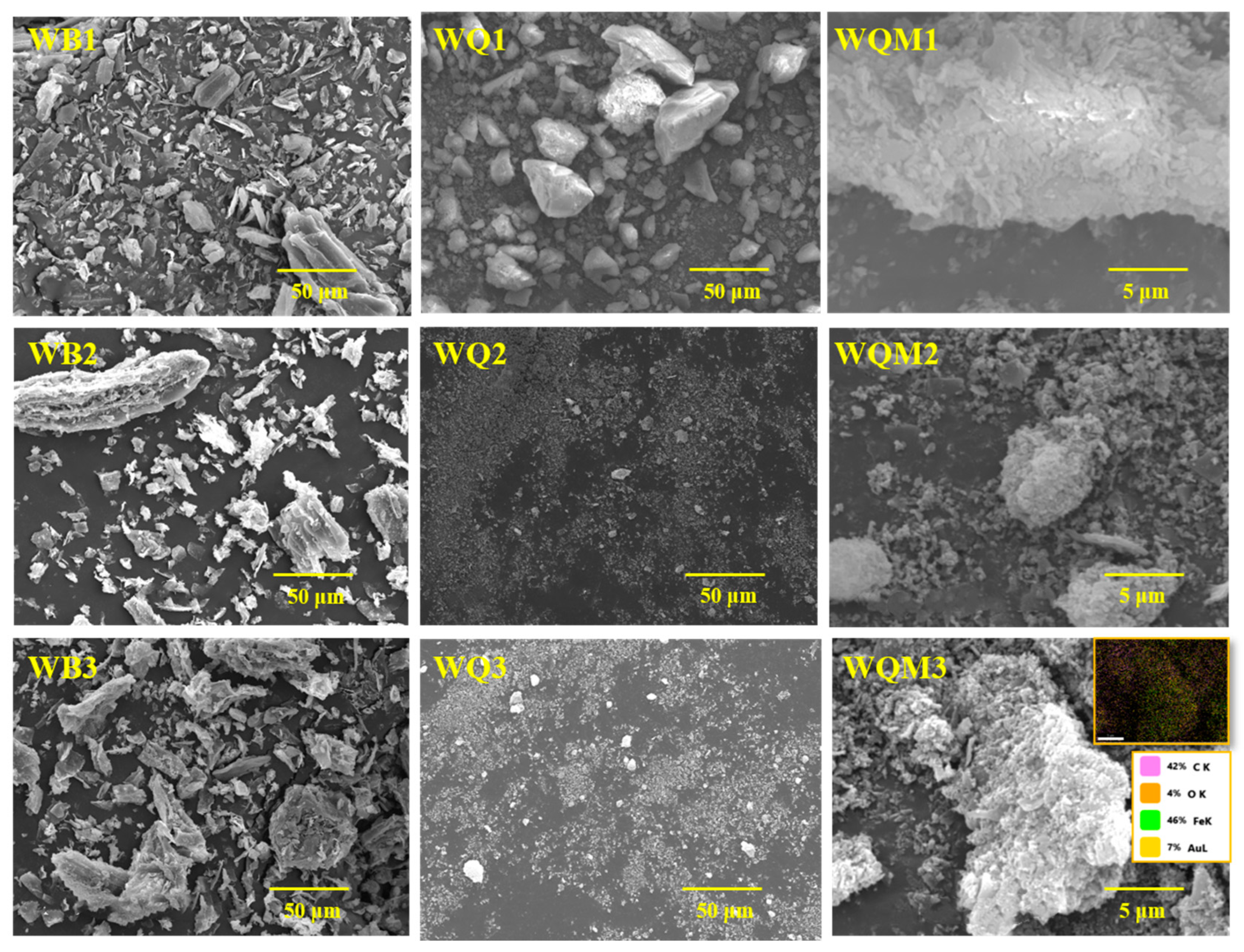
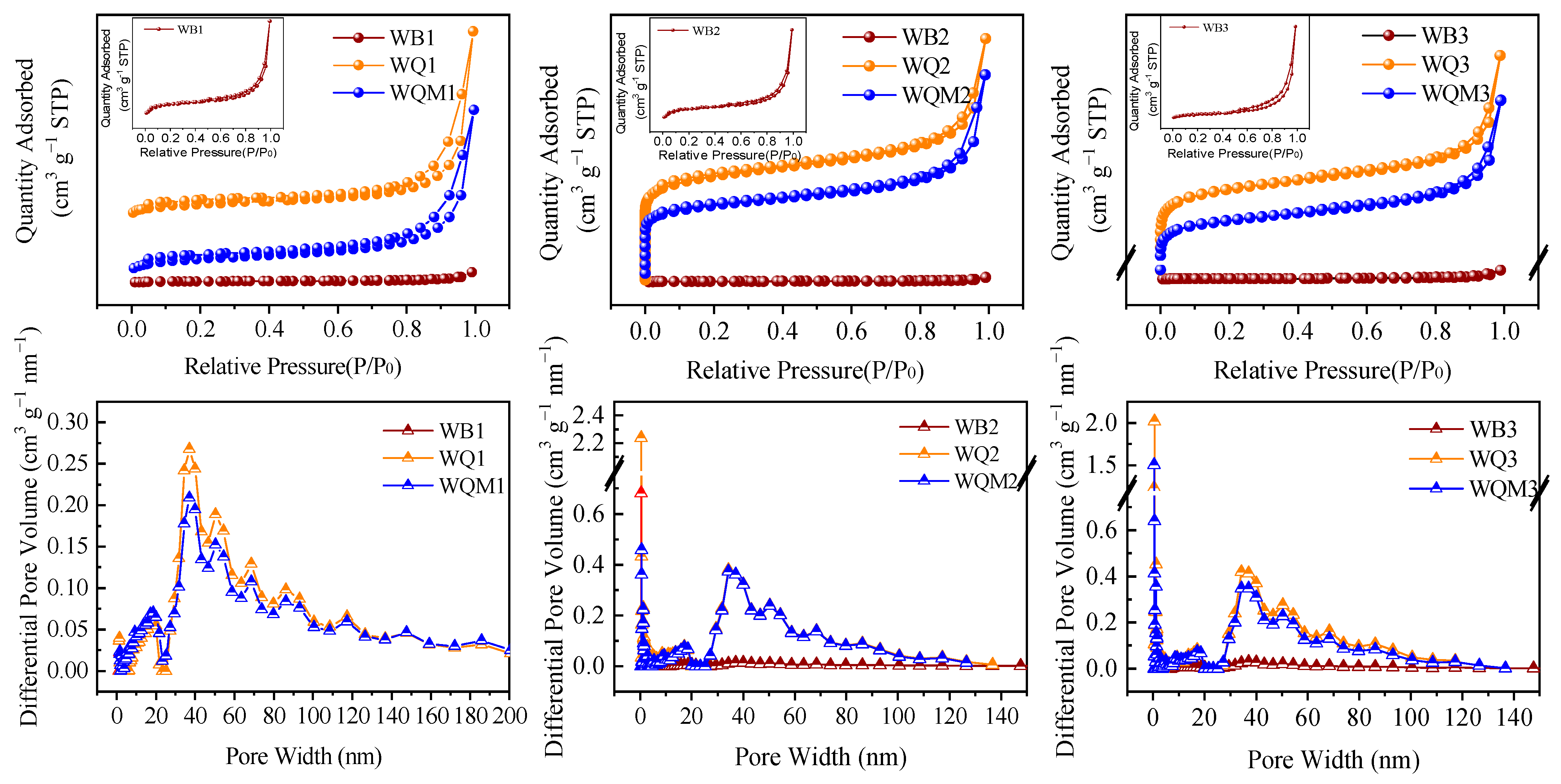
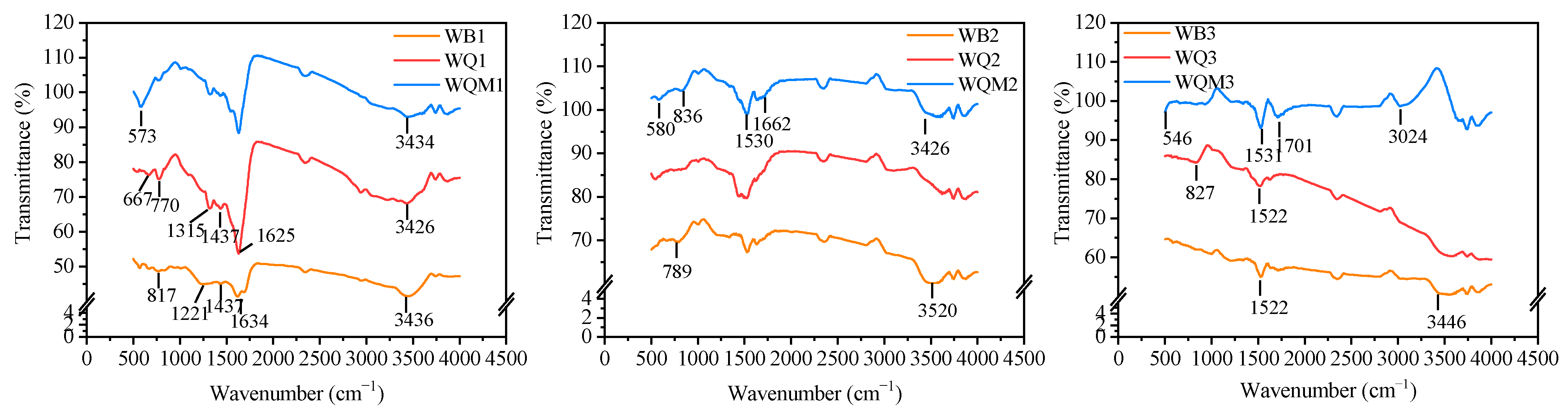
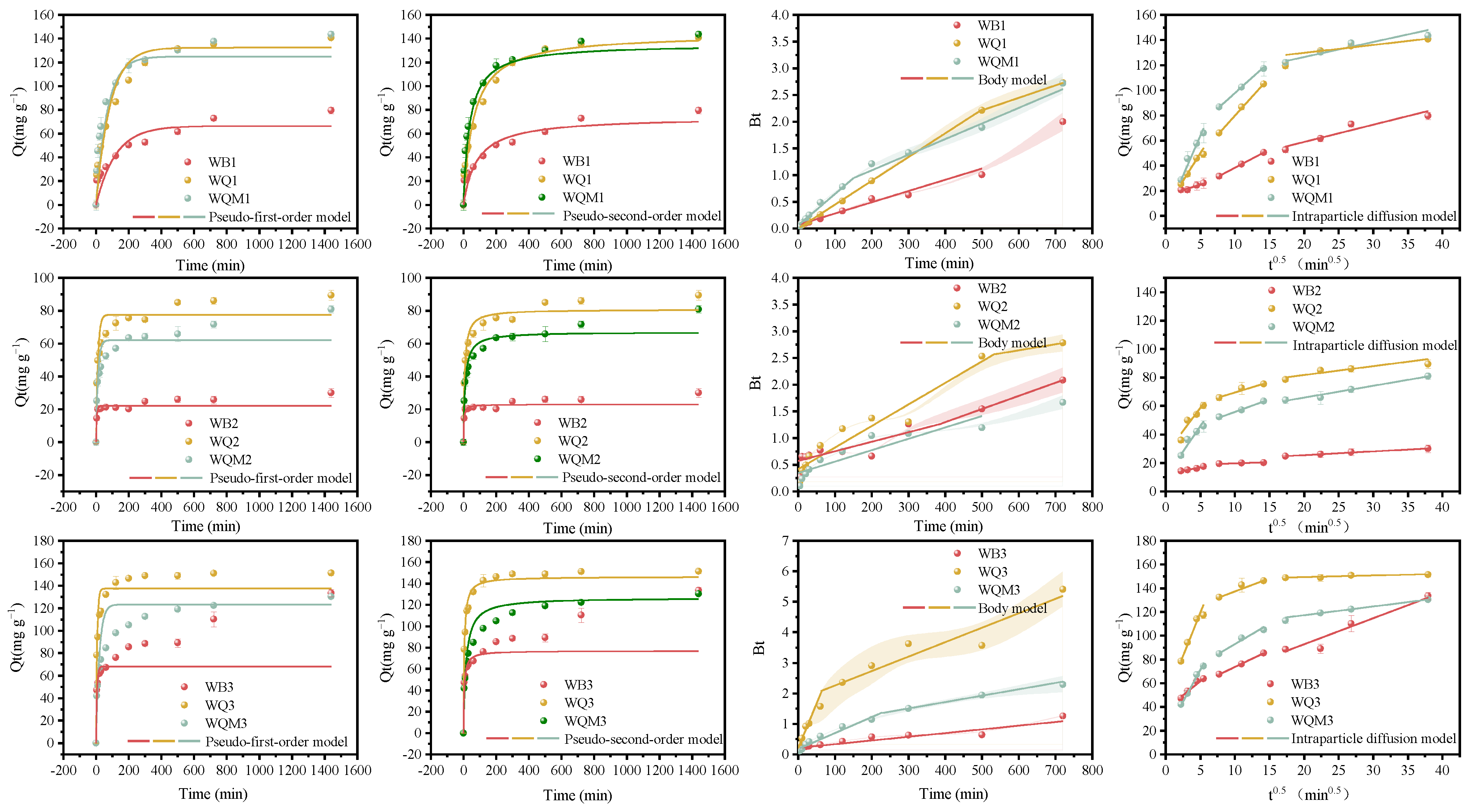

| Samples | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Size (nm) | XPS Atomic (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total Pore Volume | Micropore Volume | Mesopore Volume | C | N | O | Fe | |||
| WB1 | 0.30 | \ | \ | \ | \ | 82.45 | 3.71 | 13.84 | \ |
| WQ1 | 30.53 | 0.14 | 0.00 | 0.14 | 18.74 | 83.24 | 3.68 | 13.09 | \ |
| WQM1 | 32.59 | 0.13 | 0.00 | 0.13 | 15.46 | 69.85 | 2.92 | 25.41 | 1.82 |
| WB2 | 5.14 | 0.01 | 0.00 | 0.01 | 9.38 | \ | \ | \ | \ |
| WQ2 | 378.75 | 0.42 | 0.12 | 0.30 | 4.46 | \ | \ | \ | \ |
| WQM2 | 276.78 | 0.36 | 0.08 | 0.28 | 5.20 | \ | \ | \ | \ |
| WB3 | 8.87 | 0.02 | 0.00 | 0.02 | 9.97 | 83.14 | 3.7 | 13.16 | \ |
| WQ3 | 509.38 | 0.50 | 0.18 | 0.33 | 3.95 | 83.77 | 3.88 | 12.35 | \ |
| WQM3 | 389.48 | 0.42 | 0.13 | 0.29 | 4.30 | 71.63 | 2.74 | 23.72 | 1.91 |
| Pyrolysis Temperatures (°C) | Qe, exp (mg g−1) | Langmuir Model a | Freundlich Model b | ||||
|---|---|---|---|---|---|---|---|
| Qm (mg g−1) | KL (L mg−1) | R2 | KF | 1/n | R2 | ||
| WB1 | 29.81 | 31.54 | 5 | 0.92 | 13.28 | 0.19 | 0.64 |
| WQ1 | 204.44 | 143.97 | 3.45 | 0.95 | 51.74 | 0.27 | 0.61 |
| WQM1 | 244.58 | 197.59 | 0.62 | 0.94 | 107.23 | 0.13 | 0.67 |
| WB2 | 13.58 | 14.13 | 0.08 | 0.97 | 4.72 | 0.21 | 0.80 |
| WQ2 | 99.06 | 93.88 | 3.77 | 0.91 | 54.20 | 0.13 | 0.78 |
| WQM2 | 93.18 | 80.69 | 3.29 | 0.95 | 58.91 | 0.11 | 0.62 |
| WB3 | 76.98 | 29.91 | 18.28 | 0.91 | 18.30 | 0.22 | 0.96 |
| WQ3 | 192.17 | 185.04 | 17.25 | 0.92 | 109.41 | 0.11 | 0.76 |
| WQM3 | 177.6 | 137.49 | 27.94 | 0.79 | 94.08 | 0.10 | 0.64 |
| Materials | Pyrolysis Temperature | Surface Area (m2 g−1) | Adsorption Capacity (mg g−1) for MB | Reference |
|---|---|---|---|---|
| Sludge-based magnetic biochar | 600 °C | 70.6→20.2 | 47.4 | [21] |
| Ball-milled magnetic activated carbon | 600 °C | 544.9→75.4 | 304.2 | [25] |
| Ball-milled magnetic hickory wood-based biochar | 600 °C | 319.1→90.6 | 500.5 | [25] |
| Ball-milled magnetic straw-based biochar | 450 °C | 174.1→139.1 | 153.5 | [13] |
| WQM1 | 350 °C | 0.3→32.5 | 244.6 | This study |
| WQM3 | 750 °C | 3.9→389.5 | 177.6 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Ma, Y.; Cao, P.; Tang, X.; Xin, J. Ball Milling and Magnetic Modification Boosted Methylene Blue Removal by Biochar Obtained from Water Hyacinth: Efficiency, Mechanism, and Application. Molecules 2024, 29, 5141. https://doi.org/10.3390/molecules29215141
Wang B, Ma Y, Cao P, Tang X, Xin J. Ball Milling and Magnetic Modification Boosted Methylene Blue Removal by Biochar Obtained from Water Hyacinth: Efficiency, Mechanism, and Application. Molecules. 2024; 29(21):5141. https://doi.org/10.3390/molecules29215141
Chicago/Turabian StyleWang, Bei, Yayun Ma, Pan Cao, Xinde Tang, and Junliang Xin. 2024. "Ball Milling and Magnetic Modification Boosted Methylene Blue Removal by Biochar Obtained from Water Hyacinth: Efficiency, Mechanism, and Application" Molecules 29, no. 21: 5141. https://doi.org/10.3390/molecules29215141
APA StyleWang, B., Ma, Y., Cao, P., Tang, X., & Xin, J. (2024). Ball Milling and Magnetic Modification Boosted Methylene Blue Removal by Biochar Obtained from Water Hyacinth: Efficiency, Mechanism, and Application. Molecules, 29(21), 5141. https://doi.org/10.3390/molecules29215141





