Visible-Light-Induced Singlet Oxygen-Promoted Arylation and Alkylation of Quinoxalin-2(1H)-ones and Quinolines
Abstract
1. Introduction
2. Results
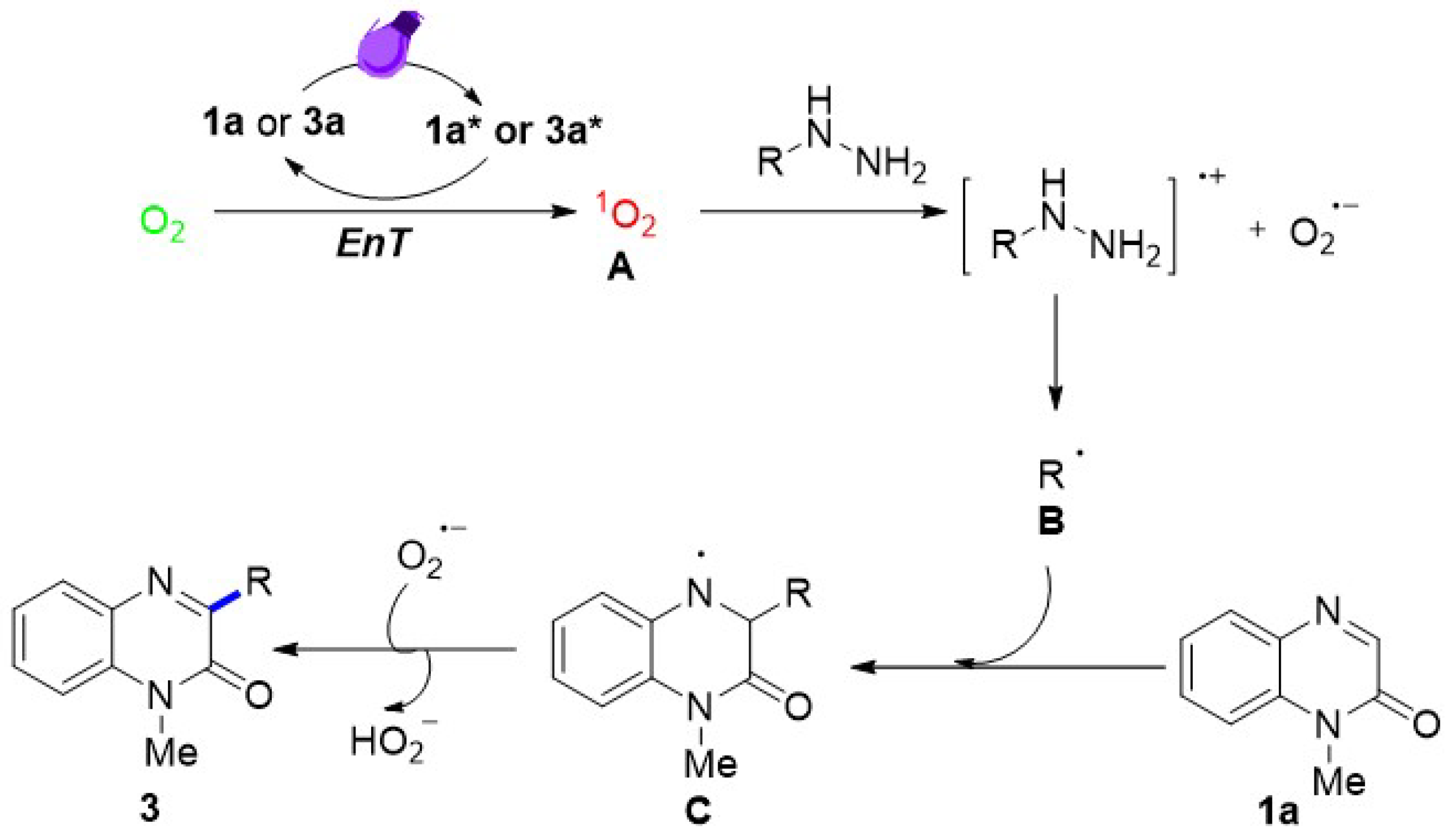
2.1. Electron Paramagnetic Resonance Studies of Reaction Intermediates
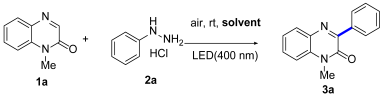
2.2. Reaction Condition Optimization C-3 Arylation of 1-Methylquinoxalin-2(1H)-One
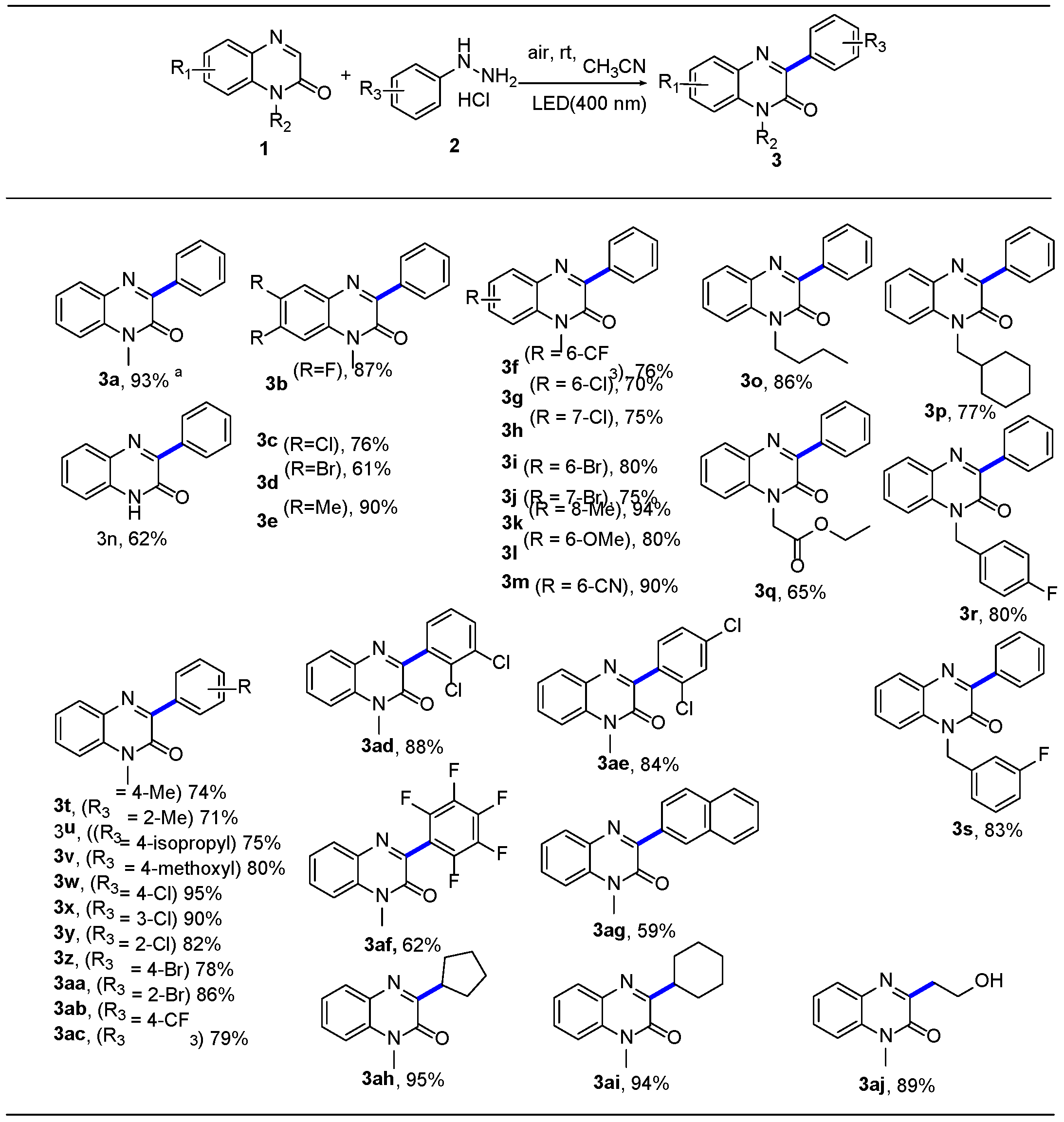
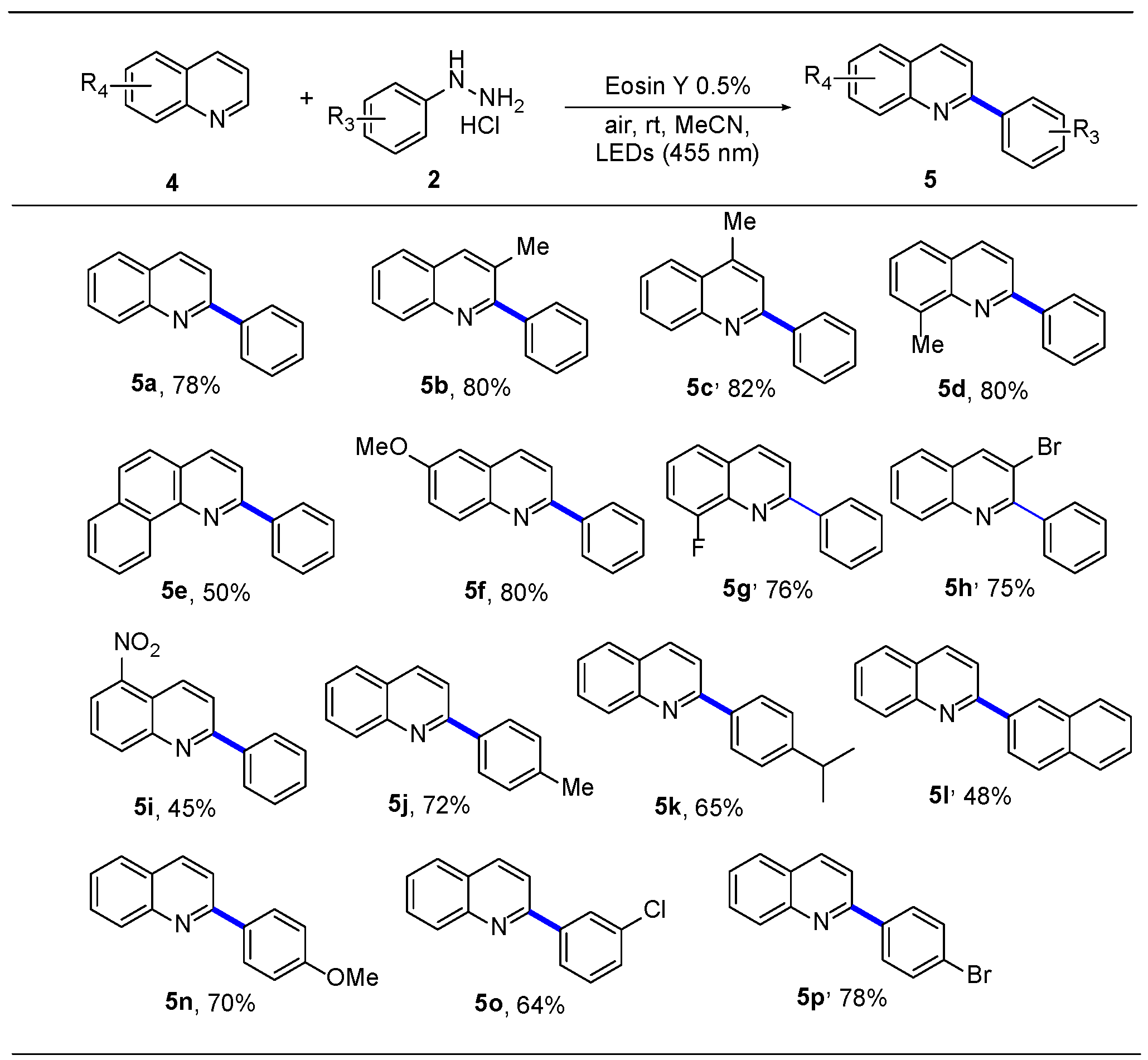
2.3. Substrate Expansion
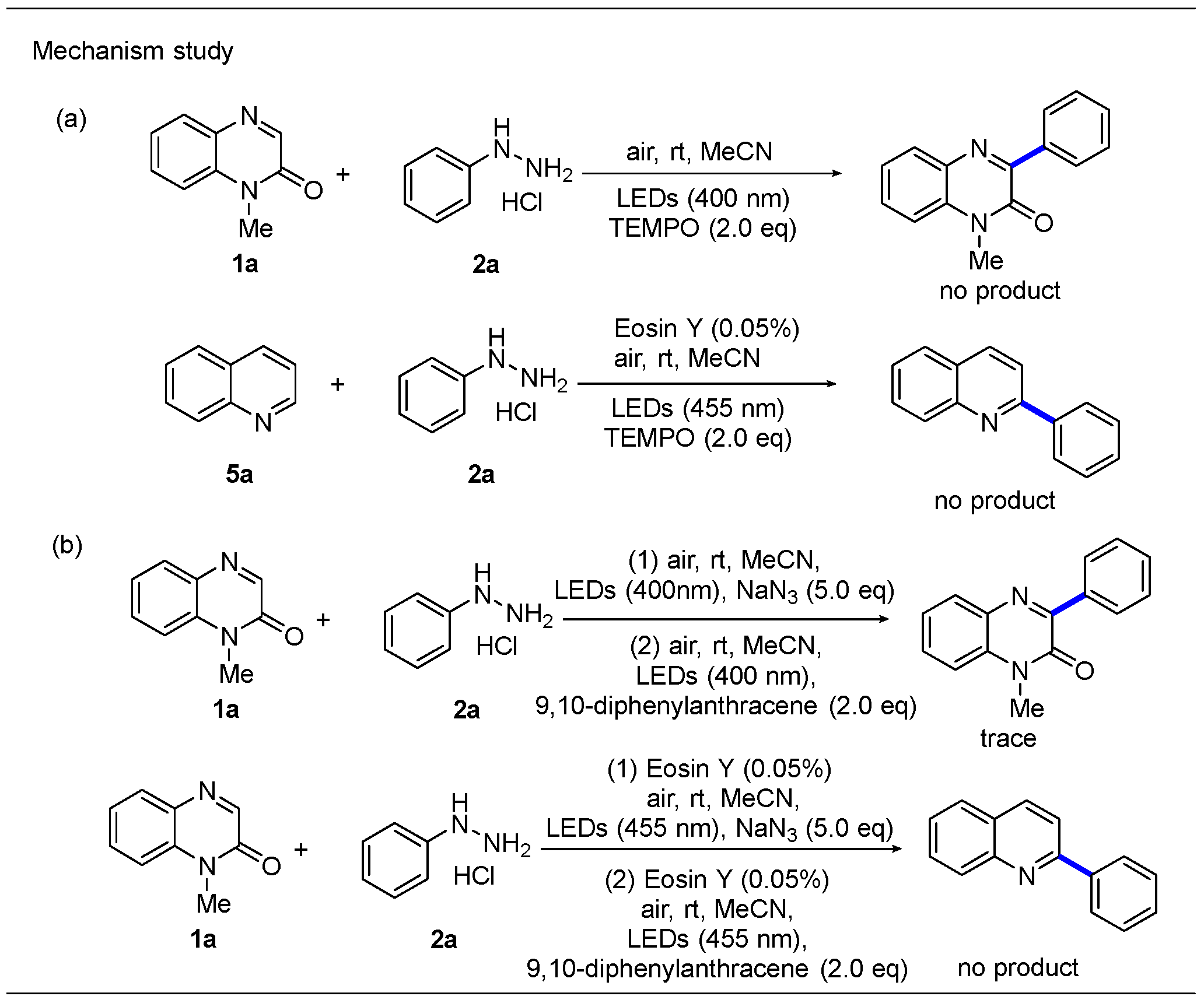
2.4. Mechanism Study
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badran, M.M.; Abouzid, K.A.M.; Hussein, M.H.M. Synthesis of Certain Substituted Quinoxalines as Antimicrobial Agents (Part II). Arch. Pharm. Res. 2003, 26, 107–113. [Google Scholar] [PubMed]
- Wu, B.; Yang, Y.; Qin, X.; Zhang, S.; Jing, C.; Zhu, C.; Ma, B. Synthesis and structure-activity relationship studies of quinoxaline derivatives as aldose reductase inhibitors. ChemMedChem 2013, 8, 1913–1917. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Hao, X.; Han, H.; Zhu, S.; Yang, Y.; Wu, B.; Hussain, S.; Parveen, S.; Jing, C.; Ma, B.; et al. Design and synthesis of potent and multifunctional aldose reductase inhibitors based on quinoxalinones. J. Med. Chem. 2015, 58, 1254–1267. [Google Scholar] [PubMed]
- Shen, C.; Wang, A.; Xu, J.; An, Z.; Loh, K.Y.; Zhang, P.; Liu, X. Recent Advances in the Catalytic Synthesis of 4-Quinolones. Chem 2019, 5, 1059–1107. [Google Scholar]
- Ke, Q.; Yan, G.; Yu, J.; Wu, X. Recent advances in the direct functionalization of quinoxalin-2(1H)-ones. Org. Biomol. Chem. 2019, 17, 5863–5881. [Google Scholar]
- Quinn, J.; Guo, C.; Ko, L.; Sun, B.; He, Y.; Li, Y. Pyrazino [2,3-g]quinoxaline-2,7-dione based π-conjugated polymers with affinity towards acids and semiconductor performance in organic thin film transistors. RSC Adv. 2016, 6, 22043–22051. [Google Scholar]
- Weiwer, M.; Spoonamore, J.; Wei, J.; Guichard, B.; Ross, N.T.; Masson, K.; Silkworth, W.; Dandapani, S.; Palmer, M.; Scherer, C.A.; et al. A Potent and Selective Quinoxalinone-Based STK33 Inhibitor Does Not Show Synthetic Lethality in KRAS-Dependent Cells. ACS Med. Chem. Lett. 2012, 3, 1034–1038. [Google Scholar] [CrossRef]
- Bao, H.; Lin, Z.; Jin, M.; Zhang, H.; Xu, J.; Chen, B.; Li, W. Visible-light-induced C H arylation of quinoxalin-2(1H)-ones in H2O. Tetrahedron Lett. 2021, 66, 1087–1092. [Google Scholar]
- Carrer, A.; Brion, J.D.; Messaoudi, S.; Alami, M. Palladium(II)-catalyzed oxidative arylation of quinoxalin-2(1H)-ones with arylboronic acids. Org. Lett. 2013, 15, 5605–5609. [Google Scholar]
- Tian, M.; Liu, S.; Bu, X.; Yu, J.; Yang, X. Covalent Organic Frameworks: A Sustainable Photocatalyst toward Visible-Light-Accelerated C3 Arylation and Alkylation of Quinoxalin-2(1H)-ones. Chem. Eur. J. 2020, 26, 369–373. [Google Scholar]
- Ramesh, B.; Reddy, C.R.; Kumar, G.R.; Reddy, B.V.S. Mn(OAc)3*2H2O promoted addition of arylboronic acids to quinoxalin-2-ones. Tetrahedron Lett. 2018, 59, 328–631. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Peng, S.; Yang, L.-H.; Peng, C.; Lin, Y.-W.; Yu, X.; Cao, Z.; Peng, Y.-Y.; He, W.-M. Aryl acyl peroxides for visible-light induced decarboxylative arylation of quinoxalin-2(1H)-ones under additive-, metal catalyst-, and external photosensitizer-free and ambient conditions. Green Chem. 2021, 23, 374–378. [Google Scholar] [CrossRef]
- Sun, K.; Xiao, F.; Yu, B.; He, W.-M. Photo-/electrocatalytic functionalization of quinoxalin-2(1H)-ones. Chin. J. Catal. 2021, 42, 1921–1943. [Google Scholar] [CrossRef]
- Yin, K.; Zhang, R. Transition-Metal-Free Direct C-H Arylation of Quinoxalin-2(1H)-ones with Diaryliodonium Salts at Room Temperature. Org. Lett. 2017, 19, 1530–1533. [Google Scholar] [CrossRef]
- Song, H.-Y.; Jiang, J.; Wu, C.; Hou, J.-C.; Lu, Y.-H.; Wang, K.-L.; Yang, T.-B.; He, W.-M. Semi-heterogeneous g-C3N4/NaI dual catalytic C-C bond formation under visible light. Green Chem. 2023, 25, 3292–3296. [Google Scholar] [CrossRef]
- Liang, Y.-P.; He, Y.-N.; Jiang, Z.-Q.; Yang, L.-H.; Xie, L.-Y. Direct C-H Arylation ofQuinoxalin-2(H)-ones with Arylhrdrazine hydrochlorides. ChemistrySelect 2023, 8, e202204611. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Kuang, C. Direct arylation of pyridines without the use of a transition metal catalyst. Chem. Commun. 2014, 50, 7124–7127. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-Y.; Jiang, L.-L.; Tan, J.-X.; Wang, Y.; Xu, X.-Q.; Zhang, B.; Cao, Z.; He, W.-M. Visible-Light-Initiated Decarboxylative Alkylation of Quinoxalin-2(1H)-ones with Phenyliodine(III) Dicarboxylates in Recyclable Ruthenium(II) Catalytic System. ACS Sustain. Chem. Eng. 2019, 7, 14153–14160. [Google Scholar] [CrossRef]
- Yang, L.; Gao, P.; Duan, X.H.; Gu, Y.R.; Guo, L.N. Direct C-H Cyanoalkylation of Quinoxalin-2(1H)-ones via Radical C-C Bond Cleavage. Org. Lett. 2018, 20, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Pan, Y.L.; Yang, C.; Chen, L.; Li, X.; Cheng, J.P. Metal-Free Direct C-H Cyanoalkylation of Quinoxalin-2(1 H)-Ones by Organic Photoredox Catalysis. J. Org. Chem. 2019, 84, 7786–7795. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Zhou, M.; Zhao, J.; Zhang, P.; Li, W. The visible-light-triggered regioselective alkylation of quinoxalin-2(1H)-ones via decarboxylation coupling. Org. Biomol. Chem. 2019, 17, 10201–10208. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, L.; Pan, J.; Zeng, F.; Xie, Y.; Wei, W.; Yi, D. Visible-light-induced four-component difunctionalization of alenens to construct phosphorodithionate-containing quinoxalin-2(1H)-ones. Chin. Chem. Lett. 2024, 35, 110112–110117. [Google Scholar] [CrossRef]
- Niu, K.-K.; Cui, J.; Dong, R.-Z.; Yu, S.-S.; Liu, H.; Xing, L.-B. Visible-light-mediated direct C3 alkylation of quinoxalin-2(1H)-ones using alknes. Chem. Commun. 2024, 60, 2409–2412. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, Q.; Lin, Y.-W.; He, W.-M. The concept of dual roles design in clean organic preparation. Chin. Chem. Lett. 2019, 30, 2132–2138. [Google Scholar] [CrossRef]
- Lu, L.-H.; Wang, Z.; Xia, W.; Cheng, P.; Zhang, B.; Cao, Z.; He, W.-M. Sustainable routes for quantitative green selenocyanation of activated alkynes. Chin. Chem. Lett. 2019, 30, 1237–1240. [Google Scholar] [CrossRef]
- Shang, T.Y.; Lu, L.H.; Cao, Z.; Liu, Y.; He, W.M.; Yu, B. Recent advances of 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) in photocatalytic transformations. Chem. Commun. 2019, 55, 5408–5419. [Google Scholar] [CrossRef]
- Ye, S.; Xiang, T.; Li, X.; Wu, J. Metal-catalyzed radical-type transformation of unactivated alkyl halides with C–C bond formation under photoinduced conditions. Org. Chem. Front. 2019, 6, 2183–2199. [Google Scholar] [CrossRef]
- Dong, D.-Q.; Li, L.-X.; Li, G.-H.; Deng, Q.; Wang, Z.-L.; Long, S. Visible-light-induced deoxygenative C2-sulfonylation of quinoline N-oxides with sulfinic acids for the synthesis of 2-sulfonylquinoline via radical reactions. Chin. J. Catal. 2019, 40, 1494–1498. [Google Scholar] [CrossRef]
- Hao, S.-H.; Li, L.-X.; Dong, D.-Q.; Wang, Z.-L.; Yu, X.-Y. Synthesis of coumarins derivatives via decarboxylative cross-coupling of coumarin-3-carboxylic acid with benzylic C(sp3)-H bond. Tetrahedron Lett. 2018, 59, 4073–4075. [Google Scholar] [CrossRef]
- Li, G.-H.; Dong, D.-Q.; Yu, X.-Y.; Wang, Z.-L. Direct synthesis of 8-acylated quinoline N-oxidesviapalladium-catalyzed selective C–H activation and C(sp2)–C(sp2) cleavage. New J. Chem. 2019, 43, 1667–1670. [Google Scholar] [CrossRef]
- Dutta, N.B.; Bhuyan, M.; Baishya, G. K2S2O8 mediated C-3 arylation of quinoxalin-2(1H)-ones under metal-, photocatalyst-, light-free conditions. RSC Adv. 2020, 10, 3615–3624. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Ha, J.-H.; Park, G.-E.; Lee, Y.-R. Transition Metal-Free Iodosobenzene-Promoted Direct Oxidative 3-Arylation of Quinoxalin-2(H)-ones with Arylhydrazines. Adv. Synth. Catal. 2017, 9, 1515–1521. [Google Scholar] [CrossRef]
- Song, S.; Shi, X.; Zhu, Y.; Ren, Q.; Zhou, P.; Zhou, J.; Li, J. Electrochemical Oxidative C-H Arylation of Quinoxalin(on)es with Arylhydrazines Hydrochlorides under Mild Conditions. J. Org. Chem. 2022, 87, 4764–4776. [Google Scholar] [CrossRef]
- Shaw, M.H.; Shurtleff, V.W.; Terrett, J.A.; Cuthbertson, J.D.; MacMillan, D.W.C. Native functionality in triple catalytic cross-coupling: sp3 C–H bonds as latent nucleophiles. Science 2016, 47, 1304. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; MacMillan, D.W. Alcohols as alkylating agents in heteroarene C-H functionalization. Nature 2015, 525, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Ghosh, T.; Bardagi, J.I.; Koenig, B. Reduction of aryl halides by consecutive visible light-induced electron transfer processes. Science 2014, 346, 725–728. [Google Scholar] [CrossRef]
- Esser, P.; Pohlmann, B.; Scharf, H. The Photochemical Synthesis of Fine Chemicals with Sunlight Peter Esser, Bettina Pohlmann, and Hans-Dieter Scharf. Angew. Chem. Int. Ed. 1994, 33, 264–269. [Google Scholar] [CrossRef]
- Okada, M.; Fukuyama, T.; Yamada, K.; Ryu, I.; Ravelli, D.; Fagnoni, M. Sunlight photocatalyzed regioselective β-alkylation and acylation of cyclopentanones. Chem. Sci. 2014, 5, 2893–2898. [Google Scholar] [CrossRef]
- Narayanam, J.M.; Stephenson, C.R. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef]
- Zou, L.; Li, P.; Wang, B.; Wang, L. Visible-light-induced radical cyclization of N-allylbenzamides with CF3SO2Na to trifluoromethylated dihydroisoquinolinones in water at room temperature. Green Chem. 2019, 21, 3362–3369. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Bai, Y.-S.; Xu, X.-Q.; Peng, X.; Tang, H.-S.; Huang, Y.; Lin, Y.-W.; Cao, Z.; He, W.-M. Visible-light-induced decarboxylative acylation of quinoxalin-2(1H)-ones with α-oxo carboxylic acids under metal-, strong oxidant- and external photocatalyst-free conditions. Green Chem. 2020, 22, 1720–1725. [Google Scholar] [CrossRef]
- Huang, L.; Xu, J.; He, L.; Liang, C.; Ouyang, Y.; Yu, Y.; Li, W.; Zhang, P. Rapid alkenylation of quinoxalin-2(1H)-ones enabled by the sequential Mannich-type reaction and solar photocatalysis. Chin. Chem. Lett. 2021, 32, 3627–3631. [Google Scholar] [CrossRef]
- Jiang, J.; Xiao, F.; He, W.-M.; Wang, L. The application of clean production in organic synthesis. Chin. Chem. Lett. 2021, 32, 1637–1644. [Google Scholar] [CrossRef]
- Gonzalez-de-Castro, A.; Xiao, J. Green and Efficient: Iron-Catalyzed Selective Oxidation of Olefins to Carbonyls with O2. J. Am. Chem. Soc. 2015, 137, 8206–8218. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-J.; Trost, B.M. Green chemistry for chemical synthesis. Proc. Natl. Acad. Sci. USA 2008, 105, 13197–13202. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liang, C.; Ouyang, Y.; Li, L.; Guo, Y.; Zhang, P.; Li, W. α-Functionalization of ketones promoted by sunlight and heterogeneous catalysis in the aqueous phase. Org. Biomol. Chem. 2022, 20, 790–795. [Google Scholar] [CrossRef]
- Xu, J.; Huang, L.; He, L.; Liang, C.; Ouyang, Y.; Shen, J.; Jiang, M.; Li, W. Direct para-C–H heteroarylation of anilines with quinoxalinones by metal-free cross-dehydrogenative coupling under an aerobic atmosphere. Green Chem. 2021, 23, 6632–6638. [Google Scholar] [CrossRef]
- Li, B.; Dixneuf, P.H. sp2 C-H bond activation in water and catalytic cross-coupling reactions. Chem. Soc. Rev. 2013, 42, 5744–5767. [Google Scholar] [CrossRef]
- Yang, Y.; Bao, Y.; Guan, Q.; Sun, Q.; Zha, Z.; Wang, Z. Copper-catalyzed S-methylation of sulfonyl hydrazides with TBHP for the synthesis of methyl sulfones in water. Green Chem. 2017, 19, 112–116. [Google Scholar] [CrossRef]
- Yang, F.; Klumphu, P.; Liang, Y.M.; Lipshutz, B.H. Copper-catalyzed trifluoromethylation of N-arylacrylamides “on water” at room temperature. Chem. Commun. 2014, 50, 936–938. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Ouyang, Y.; Yue, X.; Zhou, C.; Ni, Z.; Li, W. Molecular oxygen-mediated selective hydroxyalkylation and alkylation of quinoxalin-2(1H)-ones with alkylboronic acids. Chin. Chem. Lett. 2022, 33, 2036–2040. [Google Scholar] [CrossRef]
- Xu, J.; Yang, H.; He, L.; Huang, L.; Shen, J.; Li, W.; Zhang, P. Synthesis of (E)-Quinoxalinone Oximes through a Multicomponent Reaction under Mild Conditions. Org. Lett. 2021, 23, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, H.; Cai, H.; Bao, H.; Li, W.; Zhang, P. Transition-Metal and Solvent-Free Oxidative C-H Fluoroalkoxylation of Quinoxalinones with Fluoroalkyl Alcohols. Org. Lett. 2019, 21, 4698–4702. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, H.; Zhao, J.; Ni, Z.; Zhang, P.; Shi, B.-F.; Li, W. Photocatalyst-, metal-, additive-free, direct C–H arylation of quinoxalin-2(1H)-ones with aryl acyl peroxides induced by visible light. Org. Chem. Front. 2020, 7, 4031–4042. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Liu, Y.-S.; Ding, H.-R.; Gong, S.-F.; Tan, J.-X.; He, J.-Y.; Cao, Z.; He, W.-M. C(sp2)–H/O–H cross-dehydrogenative coupling of quinoxalin-2(1H)-ones with alcohols under visible-light photoredox catalysis. Chin. J. Catal. 2020, 41, 1168–1173. [Google Scholar] [CrossRef]
- Peng, S.; Hu, D.; Hu, J.-L.; Lin, Y.-W.; Tang, S.-S.; Tang, H.-S.; He, J.-Y.; Cao, Z.; He, W.M. Metal-Free C3 Hydroxylation of Quinoxalin-2(1H)-ones in Water. Adv. Synth. Catal. 2019, 361, 5721–5726. [Google Scholar] [CrossRef]
- Lv, Y.; Bao, P.; Yue, H.; Wei, W. Direct decarboxylative C-H3-arylation of quinoxalin-2(H)-ones with aryl acyl peroxides leading to 3-arylquinoxalin-2(1H)-ones. Tetrahedron Lett. 2020, 61, 152559. [Google Scholar] [CrossRef]
- Sun, J.; Yang, H.; Zhang, B. Metal-free visible-light-initiated direct C3 alkylation of quinoxalin-2(1H)-ones and coumarins with unactivated alkyl iodides. Green Chem. 2022, 24, 858–863. [Google Scholar] [CrossRef]
- Galloway, J.D.; Mai, D.N.; Baxter, R.D. Silver-Catalyzed Minisci Reactions Using Selectfluor as a Mild Oxidant. Org. Lett. 2017, 19, 5772–5775. [Google Scholar] [CrossRef]
- Yuan, J.-W.; Qu, L.-B. KMnO4-mediated direct selective radical cross-coupling: An effective strategy for C2 arylation of quinolone N-oxide with arylboronic acids. Chin. Chem. Lett. 2017, 28, 981–985. [Google Scholar] [CrossRef]
- Yuan, J.-W.; Yang, L.-R.; Mao, P.; Qu, L.-B. AgNO3-catalyzed direct C-H arylation of quinolones by oxidative decarboxylation of aromatic carboxylic acids. Org. Chem. Front. 2017, 4, 545–554. [Google Scholar] [CrossRef]

 | ||
|---|---|---|
| Entry | Solvent | Yield (%) b |
| 1 | Acetone | no |
| 2 | CH3CN | 93 |
| 3 | Dioxane | 43 |
| 4 | DCM | 56 |
| 5 | EtOH | 52 |
| 6 | DMSO | 49 |
| 7 | DMF | 55 |
| 8 | H2O | 50 |
| 9 | THF | 35 |
| 10 | TBME | 32 |
| 11 | Dioxane | 40 |
| 12 | CH3CN:EtOH = 1:1 | 62 |
| 13 | CH3CN:EtOH = 5:1 | 65 |
| 14 | HCl:H2O = 1:10 | 45 |
| 15 c | CH3CN | 72 |
| 16 d | CH3CN | 69 |
| 17 e | CH3CN | 72 |
| 18 f | CH3CN | 70 |
| 19 g | CH3CN | 69 |
| 20 h | CH3CN | 0 |
| 21 i | CH3CN | 95 |
| 22 j | CH3CN | trace |
| 23 k | CH3CN | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, R.; Yang, H.; Jiang, M.; Song, P. Visible-Light-Induced Singlet Oxygen-Promoted Arylation and Alkylation of Quinoxalin-2(1H)-ones and Quinolines. Molecules 2024, 29, 5113. https://doi.org/10.3390/molecules29215113
Tan R, Yang H, Jiang M, Song P. Visible-Light-Induced Singlet Oxygen-Promoted Arylation and Alkylation of Quinoxalin-2(1H)-ones and Quinolines. Molecules. 2024; 29(21):5113. https://doi.org/10.3390/molecules29215113
Chicago/Turabian StyleTan, Renjun, Hequn Yang, Min Jiang, and Peijun Song. 2024. "Visible-Light-Induced Singlet Oxygen-Promoted Arylation and Alkylation of Quinoxalin-2(1H)-ones and Quinolines" Molecules 29, no. 21: 5113. https://doi.org/10.3390/molecules29215113
APA StyleTan, R., Yang, H., Jiang, M., & Song, P. (2024). Visible-Light-Induced Singlet Oxygen-Promoted Arylation and Alkylation of Quinoxalin-2(1H)-ones and Quinolines. Molecules, 29(21), 5113. https://doi.org/10.3390/molecules29215113







