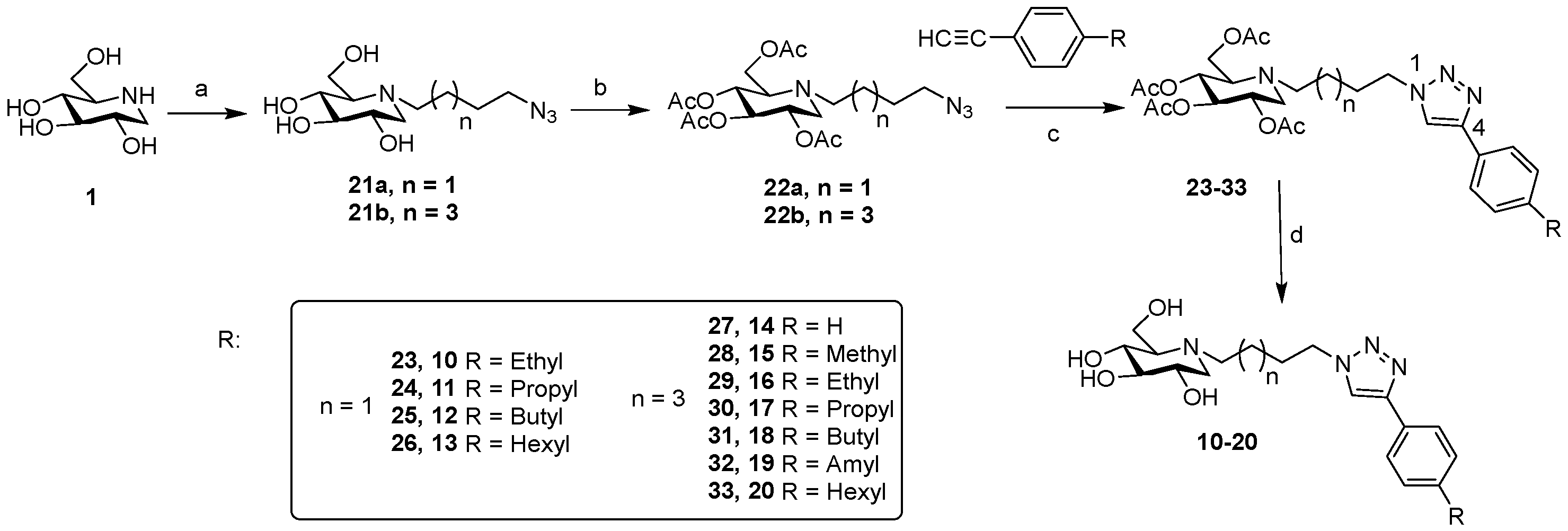
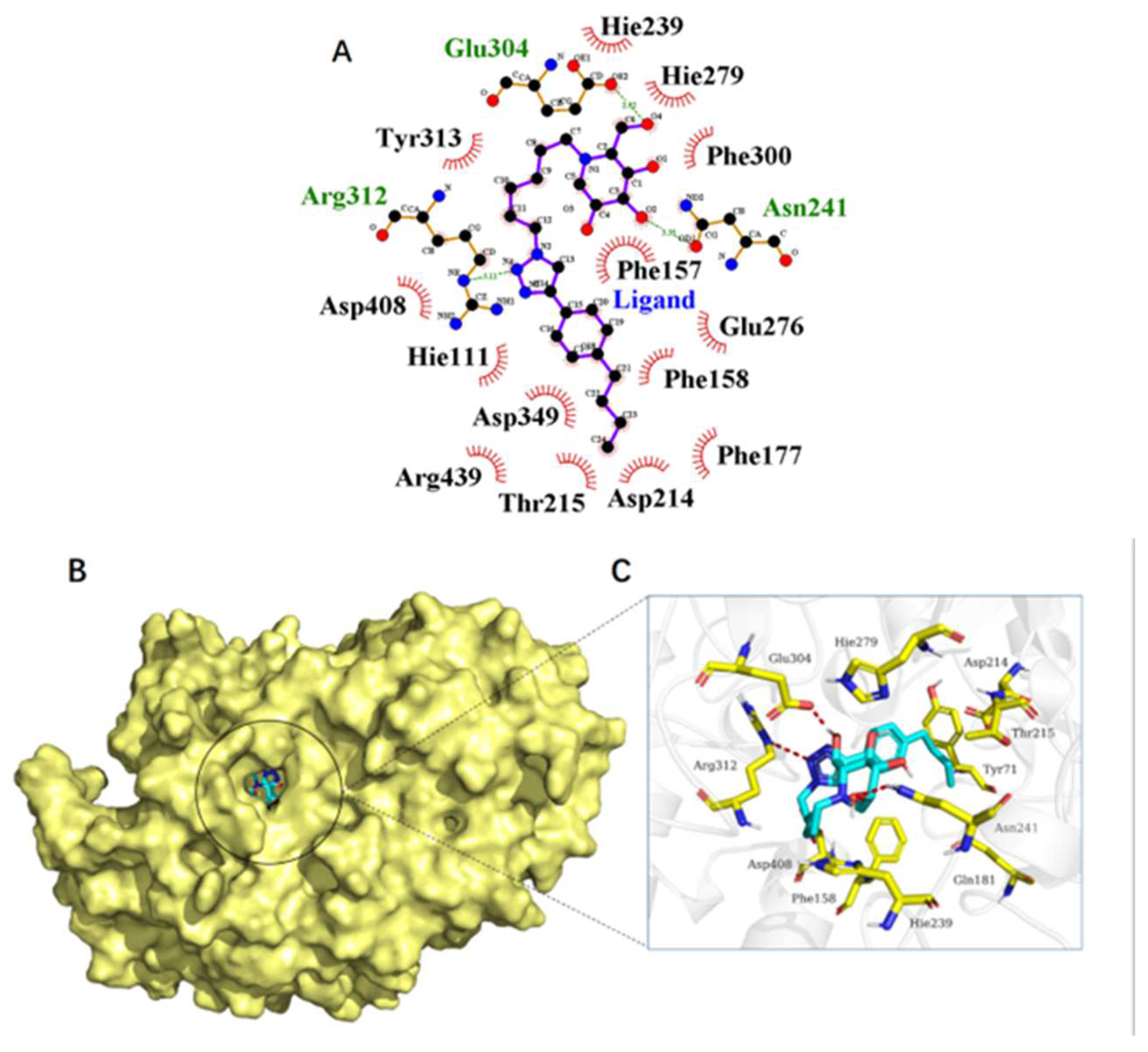
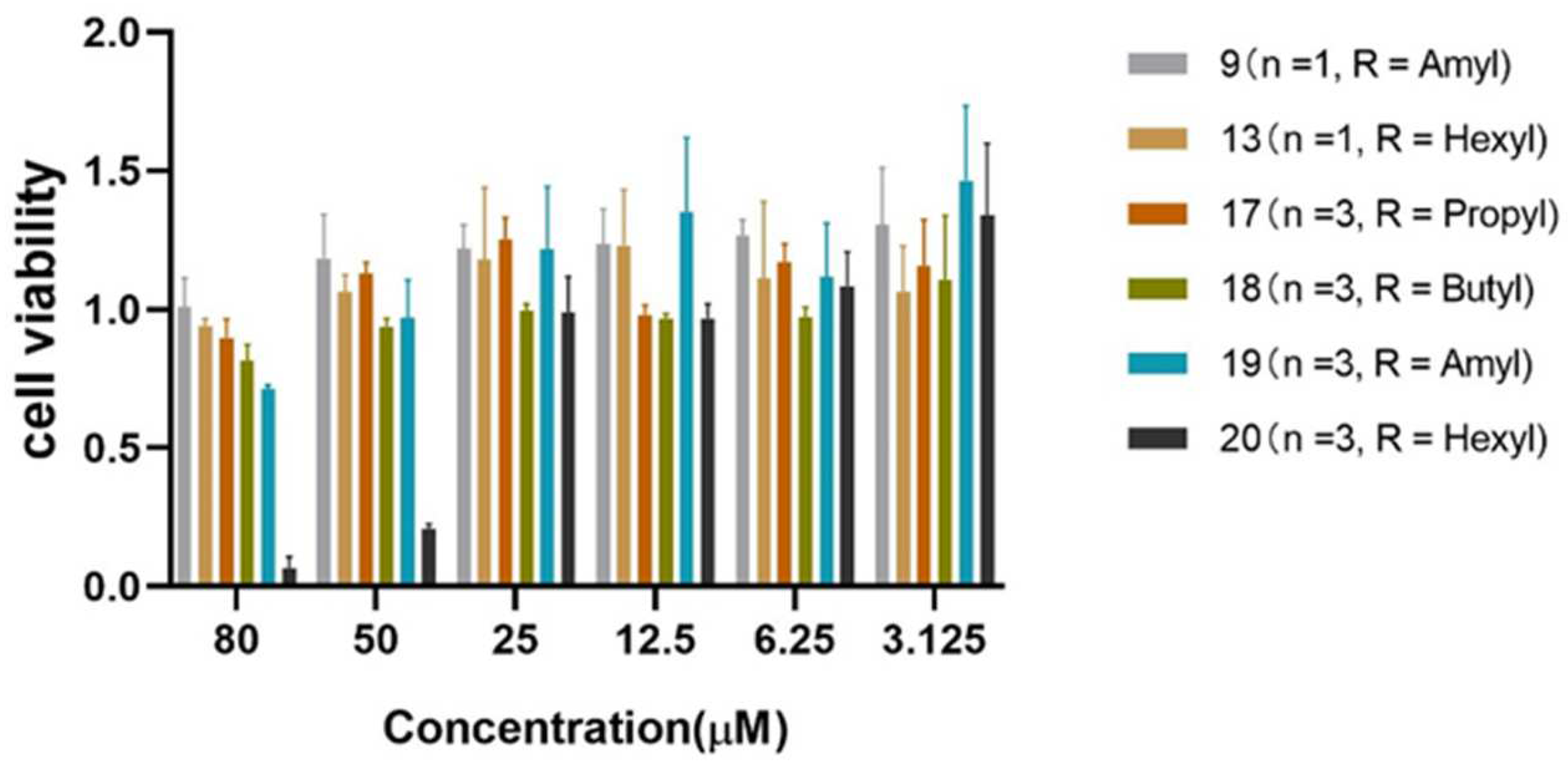
3.3. General Procedure B—CuAAC Reaction with 22a or 22b
To a solution of 22a or 22b (1.0 eq.) and substituted phenylacetylenes (2 eq.) in DMF/H2O (2:1) was added Copper (II) sulfate (0.3 eq.) and sodium ascorbate (0.6 eq.). The mixture was stirred at room temperature for 6 h. Saturated NaHCO3 solution and EtOAc were added. The organic layer was dried over Na2SO4 and concentrated. The residue was purified by flash column chromatography using EtOAc:PE (2:1 → 1:4).
(2R,3R,4R,5S)-2-(acetoxymethyl)-1-(4-(4-(4-ethylphenyl)-1H-1,2,3-triazol-1-yl)butyl)piperidine-3,4,5-triyl triacetate 23
Prepared according to procedure B. Compound 22a (200 mg, 0.47 mmol), 1-ethyl-4-ethynylbenzene (122 mg, 0.94 mmol), CuSO4·5H2O (34.96 mg, 0.14 mmol), sodium ascorbate (55.47 mg, 0.28 mmol), DMF/H2O (3 mL, 2:1). Yield: 210 mg, 0.38 mmol, 81%, white solid, Rf = 0.49 (1:2; PE:EtOAc). 1H NMR (500 MHz, CDCl3) δ 7.86–7.65 (m, 3H, ArH, CH- triazole), 7.39–7.10 (m, 2H, ArH), 5.14–5.00 (m, 2H, H-6), 4.94 (td, J = 9.9, 5.1 Hz, 1H, H-2), 4.42 (dq, J = 13.9, 6.8 Hz, 2H, H-10), 4.16 (qd, J = 12.9, 2.7 Hz, 2H, H-3, H-4), 3.17 (dd, J = 11.5, 5.0 Hz, 1H, H-1a), 2.84 (dt, J = 13.5, 7.9 Hz, 1H, H-7a), 2.68 (q, J = 7.6 Hz, 2H, H-13), 2.61 (dd, J = 6.5, 2.6 Hz, 1H, H-5), 2.53 (ddd, J = 13.2, 8.3, 4.8 Hz, 1H, H-7b), 2.23 (t, J = 10.9 Hz, 1H, H-1b), 2.09–1.97 (m, 12H, 4 × COCH3), 1.97–1.85 (m, 2H, H-9), 1.60–1.43 (m, 2H, H-8), 1.26 (t, 3H, H-14). 13C NMR (125 MHz, CDCl3) δ 170.83, 170.32, 170.13, 169.79 (4 × C=O), 147.99 (C-12), 144.40 (Car), 128.36 (2 × CHar), 128.04 (Car), 125.73 (2 × CHar), 119.15 (C-11), 74.52 (C-3), 69.42 (C-4), 69.25 (C-2), 62.00 (C-6), 59.39 (C-5), 52.52 (C-1), 50.58 (C-7), 49.95 (C-10), 28.69 (C-13), 27.82 (C-9), 22.50 (C-8), 20.87, 20.84, 20.76, 20.70 (4 × COCH3), 15.56 (C-14).
(2R,3R,4R,5S)-2-(acetoxymethyl)-1-(4-(4-(4-propylphenyl)-1H-1,2,3-triazol-1-yl)butyl)piperidine-3,4,5-triyl triacetate 24
Prepared according to procedure B. Compound 22a (200 mg, 0.47 mmol), 1-eth-1-ynyl-4-propylbenzene (135 mg, 0.94 mmol), CuSO4·5H2O (34.96 mg, 0.14 mmol), sodium ascorbate (55.47 mg, 0.28 mmol), DMF/H2O (3 mL, 2:1). Yield: 220 mg, 0.39 mmol, 82%, white solid, Rf = 0.51 (1:2; PE:EtOAc). 1H NMR (500 MHz, CDCl3) δ 7.89–7.68 (m, 3H, ArH, CH- triazole), 7.25 (dd, J = 8.1, 4.0 Hz, 2H, ArH), 5.16–5.02 (m, 2H, H-6), 4.96 (dd, J = 9.2, 4.8 Hz, 1H, H-2), 4.43 (td, J = 11.7, 7.0 Hz, 2H, H-10), 4.26–4.10 (m, 2H, H-3, H-4), 3.18 (dt, J = 11.2, 4.5 Hz, 1H, H-1a), 2.90–2.80 (m, 1H, H-7a), 2.68–2.58 (m, 3H, H-5, H-13), 2.59–2.48 (m, 1H, H-7b), 2.25 (td, J = 11.4, 3.3 Hz, 1H, H-1b), 2.11–1.86 (m, 14H, 4 × COCH3, H-9), 1.68 (ddd, J = 15.1, 7.5, 4.1 Hz, 2H, H-14), 1.58–1.43 (m, 2H, H-8), 0.97 (td, J = 7.3, 4.0 Hz, 3H, H-15). 13C NMR (125 MHz, CDCl3) δ 170.83, 170.32, 170.13, 169.80 (4 × C=O), 147.97 (C-12), 142.83 (Car), 128.96 (2 × CHar), 128.01 (Car), 125.61 (2 × CHar), 119.19 (C-11), 74.51 (C-3), 69.41 (C-4), 69.25 (C-2), 61.95 (C-6), 59.37 (C-5), 52.49 (C-1), 50.57 (C-7), 49.93 (C-10), 37.81 (C-13), 27.80 (C-9), 24.50 (C-14), 22.44 (C-8), 20.86, 20.82, 20.74, 20.69 (4 × COCH3), 13.81 (C-15).
(2R,3R,4R,5S)-2-(acetoxymethyl)-1-(4-(4-(4-butylphenyl)-1H-1,2,3-triazol-1-yl)butyl)piperidine-3,4,5-triyl triacetate 25
Prepared according to procedure B. Compound 22a (200 mg, 0.47 mmol), 1-butyl-4-eth-1-ynylbenzene (148 mg, 0.94 mmol), CuSO4·5H2O (34.96 mg, 0.14 mmol), sodium ascorbate (55.47 mg, 0.28 mmol), DMF/H2O (3 mL, 2:1). Yield: 220 mg, 0.38 mmol, 80%, white solid, Rf = 0.53 (1:2; PE:EtOAc). 1H NMR (500 MHz, CDCl3) δ 7.85–7.67 (m, 3H, ArH, CH- triazole), 7.23 (d, J = 8.0 Hz, 2, ArH), 5.04 (dt, J = 15.5, 6.1 Hz, 2H, H-6), 4.94 (d, J = 4.4 Hz, 1H, H-2), 4.53–4.35 (m, 2H, H-10), 4.16 (qd, J = 12.9, 2.4 Hz, 2H, H-3, H-4), 3.16 (dd, J = 11.3, 4.7 Hz, 1H, H-1a), 2.92–2.77 (m, 1H, H-7a), 2.62 (m, 3H, H-5, H-13), 2.57–2.46 (m, 1H, H-7b), 2.23 (t, J = 10.9 Hz, 1H, H-1b), 2.09–1.82 (m, 14H, 4 × COCH3, H-9), 1.71–1.57 (m, 2H, H-14), 1.54–1.41 (m, 2H, H-8), 1.41–1.31 (m, 2H, H-15), 0.93 (t, J = 7.3 Hz, 3H, H-16). 13C NMR (125 MHz, CDCl3) δ 170.80, 170.29, 170.10, 169.77 (4 × C=O), 147.94 (C-12), 143.04 (Car), 128.89 (2 × CHar), 127.96 (Car), 125.61 (2 × CHar), 119.20 (C-11), 74.50 (C-3), 69.41 (C-4), 69.24 (C-2), 61.95 (C-6), 59.37 (C-5), 52.48 (C-1), 50.57 (C-7), 49.92 (C-10), 35.42 (C-13), 33.55 (C-14), 27.78 (C-9), 22.43 (C-15), 22.31 (C-8), 20.83, 20.80, 20.72, 20.66 (4 × COCH3), 13.96 (C-16).
(2R,3R,4R,5S)-2-(acetoxymethyl)-1-(4-(4-(4-hexylphenyl)-1H-1,2,3-triazol-1-yl)butyl)piperidine-3,4,5-triyl triacetate 26
Prepared according to procedure B. Compound 22a (200 mg, 0.47 mmol), 1-ethynyl-4-hexylbenzene (174 mg, 0.94 mmol), CuSO4·5H2O (34.96 mg, 0.14 mmol), sodium ascorbate (55.47 mg, 0.28 mmol), DMF/H2O (3 mL, 2:1). Yield: 260 mg, 0.42 mmol, 90%, white solid, Rf = 0.55 (1:2; PE:EtOAc). 1H NMR (500 MHz, CDCl3) δ 7.82–7.69 (m, 3H, ArH, CH- triazole), 7.24 (d, J = 8.1 Hz, 2H, ArH), 5.11–5.00 (m, 2H, H-6), 4.94 (td, J = 9.9, 5.1 Hz, 1H, H-2), 4.54–4.33 (m, 2H, H-10), 4.16 (qd, J = 12.9, 2.7 Hz, 2H, H-3, H-4), 3.16 (dd, J = 11.5, 5.0 Hz, 1H, H-1a), 2.83 (m, 1H, H-7a), 2.71–2.56 (m, 3H, H-5, H-13), 2.52 (m, 1H, H-7b), 2.34–2.16 (m, 1H, H-1b), 2.07–1.87 (m, 14H, 4 × COCH3, H-9), 1.62 (dt, J = 15.3, 7.6 Hz, 2H, H-14), 1.49 (m, 2H, H-8), 1.42–1.27 (m,6H, H-15, H-16, H-17), 0.88 (t, J = 6.9 Hz, 3H, H-18). 13C NMR (125 MHz, CDCl3) δ 170.83, 170.32, 170.12, 169.79 (4 × C=O), 147.99 (C-12), 143.11 (Car), 128.90 (2 × CHar), 127.97 (Car), 125.63 (2 × CHar), 119.16 (C-11), 74.51 (C-3), 69.42 (C-4), 69.25 (C-2), 61.97 (C-6), 59.37 (C-5), 52.50 (C-1), 50.57 (C-7), 49.93 (C-10), 35.76 (C-13), 31.73 (C-14), 31.39 (C-15), 28.96 (C-16), 27.80 (C-9), 22.62 (C-17), 22.45 (C-8), 20.86, 20.83, 20.75, 20.69 (4 × COCH3), 14.12 (C-18).
(2R,3R,4R,5S)-2-(acetoxymethyl)-1-(6-(4-phenyl-1H-1,2,3-triazol-1-yl)hexyl)piperidine-3,4,5-triyl triacetate 27
Prepared according to procedure B. Compound 22b (200 mg, 0.44 mmol), phenylacetylene (89 mg, 0.88 mmol), CuSO4·5H2O (33 mg, 0.13 mmol), sodium ascorbate (52 mg, 0.26 mmol), DMF/H2O (3 mL, 2:1). Yield: 190 mg, 0.34 mmol, 78%, white solid, Rf = 0.17 (1:1; PE:EtOAc). 1H NMR (500 MHz, CDCl3) δ 7.86–7.81 (m, 2H, ArH), 7.80 (s, 1H, CH- triazole), 7.42 (t, J = 7.6 Hz, 2H, ArH), 7.32 (dd, J = 10.3, 4.3 Hz, 1H, ArH), 5.16–5.00 (m, 2H, H-6), 4.95 (td, J = 9.8, 5.1 Hz, 1H, H-2), 4.38 (t, J = 7.1 Hz, 2H, H-12), 4.14 (qd, J = 12.9, 2.5 Hz, 2H, H-3, H-4), 3.18 (dd, J = 11.4, 5.0 Hz, 1H, H-1a), 2.77–2.66 (m, 1H, H-7a), 2.61 (d, J = 8.8 Hz, 1H, H-5), 2.57–2.46 (m, 1H, H-7b), 2.28 (t, J = 10.9 Hz, 1H, H-1b), 2.09–1.89 (m, 14H, 4×COCH3, H-11), 1.53–1.31 (m, 6H, H-8, H-9, H-10). 13C NMR (125 MHz, CDCl3) δ 170.86, 170.33, 170.07, 169.77 (4 × C=O), 147.70 (C-14), 130.70 (Car), 128.83 (2 × CHar), 128.09 (Car), 125.67 (2 × CHar), 119.59 (C-13), 74.63 (C-3), 69.51 (C-4), 69.38 (C-2), 61.62 (C-6), 59.49 (C-5), 52.79 (C-1), 51.49 (C-7), 50.23 (C-12), 30.25 (C-11), 26.56 (C-8), 26.32 (C-9), 24.78 (C-10), 20.86, 20.83, 20.74, 20.68 (4 × COCH3).
(2R,3R,4R,5S)-2-(acetoxymethyl)-1-(6-(4-(p-tolyl)-1H-1,2,3-triazol-1-yl)hexyl)piperidine-3,4,5-triyl triacetate 28
Prepared according to procedure B. Compound 22b (200 mg, 0.44 mmol), 4-ethynyltoluene (102 mg, 0.88 mmol), CuSO4·5H2O (33 mg, 0.13 mmol), sodium ascorbate (52 mg, 0.26 mmol), DMF/H2O (3 mL, 2:1). Yield: 180 mg, 0.32 mmol, 72%, white solid, Rf = 0.21 (1:1; PE:EtOAc). 1H NMR (500 MHz, CDCl3) δ 7.86–7.66 (m, 3H, ArH, CH- triazole), 7.24 (d, J = 7.9 Hz, 2H, ArH), 5.17–5.00 (m, 2H, H-6), 5.00–4.91 (m, 1H, H-2), 4.39 (t, J = 7.1 Hz, 2H, H-12), 4.16 (qd, J = 12.9, 2.4 Hz, 2H, H-3, H-4), 3.20 (dd, J = 11.4, 5.0 Hz, 1H, H-1a), 2.81–2.69 (m, 1H, H-7a), 2.63 (d, J = 8.8 Hz, 1H, H-5), 2.55–2.48 (m, 1H, H-7b), 2.39 (s, 3H, H-15), 2.30 (t, J = 10.9 Hz, 1H, H-1b), 2.12–1.88 (m, 14H, 4 × COCH3, H-11), 1.55–1.32 (m, 6H, H-8, H-9, H-10). 13C NMR (125 MHz, CDCl3) δ 170.89, 170.36, 170.09, 169.78 (4 × C=O), 147.81 (C-14), 137.92 (Car), 129.52 (2 × CHar), 127.87 (Car), 125.60 (2 × CHar), 119.21 (C-13), 74.65 (C-3), 69.52 (C-4), 69.39 (C-2), 61.63 (C-6), 59.51 (C-5), 52.81 (C-1), 51.51 (C-7), 50.22 (C-12), 30.27 (C-11), 26.58 (C-8), 26.34 (C-9), 24.78 (C-10), 21.29 (C-15), 20.87, 20.84, 20.75, 20.69 (4 × COCH3).
(2R,3R,4R,5S)-2-(acetoxymethyl)-1-(6-(4-(4-ethylphenyl)-1H-1,2,3-triazol-1-yl)hexyl)piperidine-3,4,5-triyl triacetate 29
Prepared according to procedure B. Compound 22b (200 mg, 0.44 mmol), 1-ethyl-4-ethynylbenzene (114 mg, 0.88 mmol), CuSO4·5H2O (33 mg, 0.13 mmol), sodium ascorbate (52 mg, 0.26 mmol), DMF/H2O (3 mL, 2:1). Yield: 220 mg, 0.38 mmol, 86%, white solid, Rf =0.25 (1:1; PE:EtOAc). 1H NMR (500 MHz, CDCl3) δ 7.89–7.67 (m, 3H, ArH, CH- triazole), 7.24 (d, J = 7.4 Hz, 2H, ArH), 5.13–4.99 (m, 2H, H-6), 4.95 (dd, J = 9.2, 4.9 Hz, 1H, H-2), 4.36 (t, J = 6.7 Hz, 2H, H-12), 4.21–4.06 (m, 2H, H-3, H-4), 3.17 (dd, J = 11.3, 4.4 Hz, 1H, H-1a), 2.72–2.50 (m, 5H, H-5, H-7, H-15), 2.28 (t, J = 10.8 Hz, 1H, H-1b), 2.16–1.87 (m, 14H, 4×COCH3, H-11), 1.55–1.23 (m, 8H, H-8, H-9, H-10, H-16). 13C NMR (125 MHz, CDCl3) δ 170.79, 170.26, 170.00, 169.72 (4 × C=O), 147.69 (C-14), 144.21 (Car), 128.28 (2 × CHar), 128.11 (Car), 125.63 (2 × CHar), 119.32 (C-13), 74.61 (C-3), 69.48 (C-4), 69.34 (C-2), 61.57 (C-6), 59.45 (C-5), 52.75 (C-1), 51.47 (C-7), 50.14 (C-12), 30.20 (C-11), 28.62 (C-15), 26.52 (C-8), 26.27 (C-9), 24.71 (C-10), 20.81, 20.77, 20.69, 20.63 (4 × COCH3), 15.52 (C-16).
(2R,3R,4R,5S)-2-(acetoxymethyl)-1-(6-(4-(4-propylphenyl)-1H-1,2,3-triazol-1-yl)hexyl)piperidine-3,4,5-triyl triacetate 30
Prepared according to procedure B. Compound 22b (200 mg, 0.44 mmol), 1-eth-1-ynyl-4-propylbenzene (126.9 mg, 0.88 mmol), CuSO4·5H2O (33 mg, 0.13 mmol), sodium ascorbate (52 mg, 0.26 mmol), DMF/H2O (3 mL, 2:1). Yield: 230 mg, 0.38 mmol, 87%, white solid, Rf = 0.31 (1:1; PE:EtOAc). 1H NMR (500 MHz, CDCl3) δ 7.82 (s, 1H, CH- triazole), 7.75 (d, J = 7.9 Hz, 2H, ArH), 7.22 (d, J = 7.9 Hz, 2H, ArH), 5.16–4.99 (m, 2H, H-6), 4.96 (dd, J = 9.4, 4.9 Hz, 1H, H-2), 4.36 (t, J = 6.9 Hz, 2H, H-12), 4.16 (d, J = 12.6 Hz, 2H, H-3, H-4), 3.18 (dd, J = 11.3, 4.9 Hz, 1H, H-1a), 2.72 (dt, J = 14.4, 7.6 Hz, 1H, H-7a), 2.60 (t, J = 7.5 Hz, 3H, H-5, H-15), 2.56–2.45 (m, 1H, H-7b), 2.29 (t, J = 10.8 Hz, 1H, H-1b), 2.13–1.81 (m, 14H, 4×COCH3, H-11), 1.66 (dd, J = 14.9, 7.4 Hz, 2H, H-16), 1.51–1.21 (m, 6H, H-8, H-9, H-10), 0.95 (t, J = 7.3 Hz, 3H, H-17). 13C NMR (125 MHz, CDCl3) δ 170.67, 170.16, 169.91, 169.65 (4 × C=O), 147.59 (C-14), 142.54 (Car), 128.84 (2 × CHar), 128.13 (Car), 125.48 (2 × CHar), 119.38 (C-13), 74.56 (C-3), 69.44 (C-4), 69.30 (C-2), 61.51 (C-6), 59.40 (C-5), 52.70 (C-1), 51.43 (C-7), 50.06 (C-12), 37.69 (C-15), 30.14 (C-11), 26.46 (C-8), 26.20 (C-9), 24.64 (C-10), 24.40 (C-16), 20.74, 20.71, 20.62, 20.57 (4 × COCH3), 13.72 (C-17).
(2R,3R,4R,5S)-2-(acetoxymethyl)-1-(6-(4-(4-butylphenyl)-1H-1,2,3-triazol-1-yl)hexyl)piperidine-3,4,5-triyl triacetate 31
Prepared according to procedure B. Compound 22b (200 mg, 0.44 mmol), 1-butyl-4-eth-1-ynylbenzene (138 mg, 0.88 mmol), CuSO4·5H2O (33 mg, 0.13 mmol), sodium ascorbate (52 mg, 0.26 mmol), DMF/H2O (3 mL, 2:1). Yield: 230 mg, 0.37 mmol, 85%, white solid, Rf =0.35 (1:1; PE:EtOAc). 1H NMR (500 MHz, CDCl3) δ 7.82–7.69 (m, 3H, ArH, CH- triazole), 7.22 (d, J = 6.3 Hz, 2H, ArH), 5.03 (dd, J = 15.8, 7.9 Hz, 2H, H-6), 4.95 (m, 1H, H-2), 4.37 (d, J = 5.0 Hz, 2H, H-12), 4.14 (d, J = 3.6 Hz, 2H, H-3, H-4), 3.17 (d, J = 6.7 Hz, 1H, H-1a), 2.72 (m, 1H, H-7a), 2.62 (m, 3H, H-5, H-15), 2.52 (m, 1H, H-7b), 2.28 (t, J = 10.7 Hz, 1H, H-1b), 2.14–1.87 (m, 14H, 4 × COCH3, H-11), 1.68–1.55 (m, 2H, H-16), 1.50–1.20 (m, 8H, H-8, H-9, H-10, H-17), 0.92 (t, J = 5.9 Hz, 3H, H-18). 13C NMR (125 MHz, CDCl3) δ 170.82, 170.30, 170.04, 169.74 (4 × C=O), 147.77 (C-14), 142.94 (Car), 128.86 (2 × CHar), 128.06 (Car), 125.57(2 × CHar), 119.27 (C-13), 74.63 (C-3), 69.50 (C-4), 69.36 (C-2), 61.60 (C-6), 59.47 (C-5), 52.78 (C-1), 51.49 (C-7), 50.17 (C-12), 35.40 (C-15), 33.54 (C-16), 30.23 (C-11), 26.55 (C-8), 26.30 (C-9), 24.74 (C-10), 22.30 (C-17), 20.84, 20.80, 20.72, 20.66 (4 × COCH3), 13.95 (C-18).
(2R,3R,4R,5S)-2-(acetoxymethyl)-1-(6-(4-(4-pentylphenyl)-1H-1,2,3-triazol-1-yl)hexyl)piperidine-3,4,5-triyl triacetate 32
Prepared according to procedure B. Compound 22b (200 mg, 0.44 mmol), 1-ethynyl-4-pentylbenzene (151 mg, 0.88 mmol), CuSO4·5H2O (33 mg, 0.13 mmol), sodium ascorbate (52 mg, 0.26 mmol), DMF/H2O (3 mL, 2:1). Yield: 250 mg, 0.4 mmol, 91%, white solid, Rf = 0.38 (1:1; PE:EtOAc). 1H NMR (500 MHz, CDCl3) δ 7.74 (m, 3H, ArH, CH-triazole), 7.23 (d, J = 8.0 Hz, 2H, ArH), 5.13–5.00 (m, 2H, H-6), 4.95 (dd, J = 14.0, 9.4 Hz, 1H, H-2), 4.38 (t, J = 6.9 Hz, 2H, H-12), 4.15 (qd, J = 13.0, 2.5 Hz, 2H, H-3, H-4), 3.18 (dd, J = 11.3, 4.9 Hz, 1H, H-1a), 2.72 (dd, J = 11.8, 7.8 Hz, 1H, H-7a), 2.62 (m, 3H, H-5, H-15), 2.51 (dd, J = 15.9, 6.9 Hz, 1H, H-7b), 2.28 (t, J = 10.8 Hz, 1H, H-1b), 2.12–1.85 (m, 14H, 4 × COCH3, H-11), 1.70–1.57 (m, 2H, H-16), 1.52–1.19 (m, 10H, H-8, H-9, H-10, H-17, H-18), 0.89 (d, J = 7.0 Hz, 3H, H-19). 13C NMR (125 MHz, CDCl3) δ 170.87, 170.34, 170.07, 169.77 (4 × C=O), 147.83 (C-14), 143.01 (Car), 128.87 (2 × CHar), 128.07 (Car), 125.60 (2 × CHar), 119.22 (C-13), 74.65 (C-3), 69.51 (C-4), 69.38 (C-2), 61.62 (C-6), 59.49 (C-5), 52.81 (C-1), 51.51 (C-7), 50.19 (C-12), 35.70 (C-15), 31.46 (C-16), 31.09 (C-17), 30.26 (C-11), 26.57 (C-8), 26.33 (C-9), 24.77 (C-10), 22.54 (C-18), 20.86, 20.83, 20.74, 20.68 (4 × COCH3), 14.05 (C-19).
(2R,3R,4R,5S)-2-(acetoxymethyl)-1-(6-(4-(4-hexylphenyl)-1H-1,2,3-triazol-1-yl)hexyl)piperidine-3,4,5-triyl triacetate 33
Prepared according to procedure B. Compound 22b (200 mg, 0.44 mmol), 1-ethynyl-4-hexylbenzene (163 mg, 0.88 mmol), CuSO4·5H2O (33 mg, 0.13 mmol), sodium ascorbate (52 mg, 0.26 mmol), DMF/H2O (3 mL, 2:1). Yield: 220 mg, 0.34 mmol, 78%, white solid, Rf =0.41 (1:1; PE:EtOAc). 1H NMR (500 MHz, CDCl3) δ 7.84–7.69 (m, 3H, ArH, CH-triazole), 7.31–7.17 (m, 2H, ArH), 5.15–5.01 (m, 2H, H-6), 4.97 (dd, J = 9.4, 4.6 Hz, 1H, H-2), 4.38 (q, J = 6.7 Hz, 2H, H-12), 4.24–4.08 (m, 2H, H-3, H-4), 3.26–3.10 (m, 1H, H-1a), 2.74 (m, 1H, H-7a), 2.63 (t, J = 10.0 Hz, 3H, H-5, H-15), 2.53 (td, J = 9.1, 4.6 Hz, 1H, H-7b), 2.30 (td, J = 11.2, 4.9 Hz, 1H, H-1b), 2.13–1.85 (m, 14H, 4×COCH3, H-11), 1.71–1.53 (m, 2H, H-16), 1.50–1.22 (m, 12H, H-8, H-9, H-10, H-17, H-18, H-19), 0.98–0.74 (m, 3H, H-20). 13C NMR (125 MHz, CDCl3) δ 170.80, 170.27, 170.01, 169.73 (4 × C=O), 147.76 (C-14), 142.94 (Car), 128.84 (2 × CHar), 128.08 (Car), 125.56 (2 × CHar), 119.25 (C-13), 74.63 (C-3), 69.50 (C-4), 69.36 (C-2), 61.60 (C-6), 59.47 (C-5), 52.78 (C-1), 51.49 (C-7), 50.15 (C-12), 35.71 (C-15), 31.69 (C-16), 31.35 (C-17), 30.23 (C-11), 28.91 (C-18), 26.54 (C-8), 26.29 (C-9), 24.75 (C-10), 22.58 (C-19), 20.82, 20.80, 20.70, 20.65 (4 × COCH3), 14.09 (C-20).
3.4. General Procedure C—Deacetylation Reaction of Compounds 23–33
Compounds 23–33 were dissolved in methanol, and NaOMe (1.0 M in methanol) was added until pH 9.0 was achieved. After stirring overnight at room temperature, the mixture was neutralized with ion exchange resin DOWEX® 50WX4-50 (H+), filtered and concentrated under reduced pressure. The resulting product (compounds 10–20) was obtained in quantitative yield.
(2R,3R,4R,5S)-1-(4-(4-(4-ethylphenyl)-1H-1,2,3-triazol-1-yl)butyl)-2-(hydroxymethyl)piperidine-3,4,5-triol 10
Prepared according to procedure C. Compound 23 (190 mg, 0.34 mmol). Yield: 130 mg, 0.33 mmol, 96%, white solid, Rf = 0.22 (3:1; CH2Cl2: MeOH + 1% NH4OH), [α]D25 22.6 (c 0.5, H2O). 1H NMR (500 MHz, D2O) δ 7.78 (s, 1H, CH- triazole), 7.42 (d, J = 7.4 Hz, 2H, ArH), 6.73 (d, J = 7.8 Hz, 2H, ArH), 4.09 (m, 2H, H-10), 3.69 (dd, J = 20.9, 12.1 Hz, 2H, H-6), 3.49 (d, J = 4.6 Hz, 1H, H-2), 3.33 (t, J = 9.4 Hz, 1H, H-3), 3.16 (t, J = 9.1 Hz, 1H, H-4), 2.86 (d, J = 6.2 Hz, 1H, H-1a), 2.58 (m, 1H, H-7a), 2.37 (m, 1H, H-7b), 2.17–1.88 (m, 4H, H-5, H-1b, H-13), 1.52 (m, 2H, H-9), 1.26 (m, 2H. H-8), 0.77 (t, J = 7.4 Hz, 3H, H-14). 13C NMR (125 MHz, D2O) δ 147.02 (C-12), 143.61 (Car), 128.09 (2 × CHar), 127.62 (Car), 125.40 (2 × CHar), 120.74 (C-13), 78.35 (C-3), 69.80 (C-4), 68.75 (C-2), 65.25 (C-6), 57.26 (C-5), 55.46 (C-1), 51.30 (C-7), 49.93 (C-10), 28.15 (C-13), 27.88 (C-9), 20.35 (C-8), 14.95 (C-14). HRMS (ESI) m/z calcd for C20H31N4O4+ [M+H]+ 391.23398, found 391.23346.
(2R,3R,4R,5S)-2-(hydroxymethyl)-1-(4-(4-(4-propylphenyl)-1H-1,2,3-triazol-1-yl)butyl)piperidine-3,4,5-triol 11
Prepared according to procedure C. Compound 24 (150 mg, 0.26 mmol). Yield:100 mg, 0.25 mmol, 95%, white solid, Rf = 0.25 (3:1; CH2Cl2: MeOH + 1% NH4OH), [α]D25 −10.7 (c 0.35, H2O). 1H NMR (500 MHz, D2O) δ 7.84 (s, 1H, CH-triazole), 7.45 (d, J = 6.6 Hz, 2H, ArH), 6.72 (d, J = 7.1 Hz, 2H, ArH), 4.11 (m, 2H, H-10), 3.70 (dd, J = 21.6, 12.4 Hz, 2H, H-6), 3.50 (dd, J = 13.8, 9.3 Hz, 1H, H-2), 3.34 (t, J = 9.3 Hz, 1H, H-3), 3.17 (t, J = 9.0 Hz, 1H, H-4), 2.87 (d, J = 5.5 Hz, 1H, H-1a), 2.59 (m, 1H, H-7a), 2.38 (m, 1H, H-7b), 2.16–1.93 (m, 4H, H-5, H-1b, H-13), 1.53 (m, 2H, H-9), 1.38–1.07 (m, 4H, H-8, H-14), 0.59 (t, J = 6.6 Hz, 3H, H-15). 13C NMR (125 MHz, D2O) δ 147.01 (C-12), 141.95 (Car), 128.63 (2 × CHar), 127.81(Car), 125.35(2 × CHar), 120.74 (C-13), 78.34 (C-3), 69.76 (C-4), 68.71 (C-2), 65.34 (C-6), 57.22 (C-5), 55.47 (C-1), 51.35 (C-7), 49.96 (C-10), 37.45 (C-13), 27.91 (C-9), 24.06 (C-14), 20.47 (C-8), 13.60 (C-15). HRMS (ESI) m/z calcd for C21H33N4O4+ [M+H]+ 405.24963, found 405.24997.
(2R,3R,4R,5S)-1-(4-(4-(4-butylphenyl)-1H-1,2,3-triazol-1-yl)butyl)-2-(hydroxymethyl)piperidine-3,4,5-triol 12
Prepared according to procedure C. Compound 25 (200 mg, 0.34 mmol). Yield: 130 mg, 0.31 mmol, 93%, white solid, Rf = 0.31 (3:1; CH2Cl2: MeOH + 1% NH4OH), [α]D25 −12.1 (c 0.39, H2O). 1H NMR (500 MHz, D2O) δ 7.83 (s, 1H, CH-triazole), 7.46 (s, 2H, ArH), 6.72 (s, 2H, ArH), 4.10 (m, 2H, H-10), 3.68 (d, J = 19.6 Hz, 2H, H-6), 3.49 (m, 1H, H-2), 3.33 (t, J = 8.8 Hz, 1H, H-3), 3.15 (t, J = 8.4 Hz, 1H, H-4), 2.86 (m, 1H, H-1a), 2.58 (m, 1H, H-7a), 2.36 (m, 1H, H-7b), 2.18–1.94 (m, 4H, H-5, H-1b, H-13), 1.51 (m, 2H, H-9), 1.39–0.91 (m, 6H, H-8, H-14, H-15), 0.65 (m, 3H, H-16). 13C NMR (125 MHz, D2O) δ 146.96 (C-12), 142.05 (Car), 128.52 (2 × CHar), 127.82(Car), 125.35(2 × CHar), 120.74 (C-13), 78.35 (C-3), 69.74 (C-4), 68.70 (C-2), 65.34 (C-6), 57.20 (C-5), 55.48 (C-1), 51.35 (C-7), 49.96 (C-10), 35.15 (C-13), 33.19 (C-14), 27.94 (C-9), 22.37 (C-15), 20.50 (C-8), 13.72 (C-16). HRMS (ESI) m/z calcd for C22H35N4O4+ [M+H]+ 419.26528, found 419.26492.
(2R,3R,4R,5S)-1-(4-(4-(4-hexylphenyl)-1H-1,2,3-triazol-1-yl)butyl)-2-(hydroxymethyl)piperidine-3,4,5-triol 13
Prepared according to procedure C. Compound 26 (200 mg, 0.33 mmol). Yield: 140 mg, 0.31 mmol, 95%, white solid, Rf = 0.34 (3:1; CH2Cl2: MeOH + 1% NH4OH), [α]D25 7 (c 0.2, H2O). 1H NMR (500 MHz, D2O) δ 7.84 (s, 1H, CH-triazole), 7.49 (s, 2H, ArH), 6.78 (s, 2H, ArH), 4.09 (m, 2H, H-10), 3.71 (m, 2H, H-6), 3.50 (m, 1H, H-2), 3.34 (m, 1H, H-3), 3.16 (m, 1H, H-4), 2.87 (m, 1H, H-1a), 2.59 (m, 1H, H-7a), 2.36 (m, 1H, H-7b), 2.05 (m, 4H, H-5, H-1b, H-13), 1.25 (m, 12H, H-8, H-9, H-14, H-15, H-16, H-17), 0.80 (m, 3H, H-18). 13C NMR (125 MHz, D2O) δ 146.94(C-12), 142.05(Car), 128.49 (2 × CHar), 128.01(Car), 125.40(2 × CHar), 120.70 (C-13), 78.48 (C-3), 69.85 (C-4), 68.79 (C-2), 65.43 (C-6), 57.34 (C-5), 55.62 (C-1), 51.43 (C-7), 49.99 (C-10), 35.64 (C-13), 31.91 (C-14), 31.20 (C-15), 29.60 (C-16), 28.02 (C-9), 22.83 (C-17), 20.69 (C-8), 14.01 (C-18). HRMS (ESI) m/z calcd for C24H39N4O4+ [M+H]+ 447.29658, found 447.29654.
(2R,3R,4R,5S)-2-(hydroxymethyl)-1-(6-(4-phenyl-1H-1,2,3-triazol-1-yl)hexyl)piperidine-3,4,5-triol 14
Prepared according to procedure C. Compound 27 (122 mg, 0.22 mmol). Yield: 80 mg, 0.2 mmol, 93%, white solid, Rf = 0.21 (3:1; CH2Cl2: MeOH + 1% NH4OH), [α]D25 −8.2 (c 0.28, CH3OH). 1H NMR (500 MHz, CD3OD) δ 8.31 (s, 1H, CH- triazole), 7.81 (d, J = 7.4 Hz, 2H, ArH), 7.42 (t, J = 7.6 Hz, 2H, ArH), 7.33 (t, J = 7.4 Hz, 1H, ArH), 4.43 (t, J = 7.1 Hz, 2H, H-12), 3.85 (d, J = 2.1 Hz, 2H, H-6), 3.50 (td, J = 10.1, 4.8 Hz, 1H, H-2), 3.37 (t, J = 9.3 Hz, 1H, H-3), 3.16 (t, J = 9.1 Hz, 1H, H-4), 3.02 (dd, J = 11.2, 4.8 Hz, 1H, H-1a), 2.90–2.75 (m, 1H, H-7a), 2.65–2.56 (m, 1H, H-7b), 2.22 (m, 2H, H-5, H-1b), 1.94 (dd, J = 14.1, 7.0 Hz, 2H, H-11), 1.50 (m, 2H, H-8), 1.34 (m, 4H, H-9, H-10). 13C NMR (125 MHz, CD3OD) δ 147.42 (C-14), 130.35 (Car), 128.63 (2 × CHar), 127.97 (CHar), 125.27 (2 × CHar), 120.84 (C-13), 78.93 (C-3), 70.32 (C-4), 69.07 (C-2), 66.00 (C-6), 57.69 (C-5), 56.02 (C-1), 52.24 (C-7), 50.01 (C-12), 29.81 (C-11), 26.45 (C-8), 25.95 (C-9), 23.59 (C-10). HRMS (ESI) m/z calcd for C20H31N4O4+ [M+H]+ 391.23398, found 391.23373.
(2R,3R,4R,5S)-2-(hydroxymethyl)-1-(6-(4-(p-tolyl)-1H-1,2,3-triazol-1-yl)hexyl)piperidine-3,4,5-triol 15
Prepared according to procedure C. Compound 28 (152 mg, 0.27 mmol). Yield: 100 mg, 0.25 mmol, 94%, white solid, Rf = 0.25 (3:1; CH2Cl2: MeOH + 1% NH4OH), [α]D25 −7.1 (c 0.35, CH3OH). 1H NMR (500 MHz, CD3OD) δ 8.23 (s, 1H, CH- triazole), 7.68 (d, J = 7.9 Hz, 2H, ArH), 7.22 (d, J = 7.9 Hz, 2H, ArH), 4.39 (t, J = 7.0 Hz, 2H, H-12), 3.85 (m, 2H, H-6), 3.50 (td, J = 9.9, 4.8 Hz, 1H, H-2), 3.38 (t, J = 9.3 Hz, 1H, H-3), 3.17 (t, J = 9.1 Hz, 1H, H-4), 3.00 (dd, J = 11.1, 4.6 Hz, 1H, H-1a), 2.88–2.70 (m, 1H, H-7a), 2.58 (d, J = 6.6 Hz, 1H, H-7b), 2.33 (s, 3H, PhCH3, H-15), 2.25–2.09 (m, 2H, H-5, H-1b), 1.92 (dd, J = 14.2, 7.8 Hz, 2H, H-11), 1.47 (m, 2H, H-8), 1.28 (m, 4H, H-9, H-10). 13C NMR (125 MHz, CD3OD) δ 147.48 (C-14), 137.99 (Car), 129.26 (2 × CHar), 127.51 (Car), 125.26 (2 × CHar), 120.50 (C-13), 79.04 (C-3), 70.45 (C-4), 69.20 (C-2), 65.97 (C-6), 57.86 (C-5), 56.14 (C-1), 52.24 (C-7), 50.00 (C-12), 29.84 (C-11), 26.51 (C-8), 25.99 (C-9), 23.61 (C-10), 20.04 (C-15). HRMS (ESI) m/z calcd for C21H33N4O4+ [M+H]+ 405.24963, found 405.24973.
(2R,3R,4R,5S)-1-(6-(4-(4-ethylphenyl)-1H-1,2,3-triazol-1-yl)hexyl)-2-(hydroxymethyl)piperidine-3,4,5-triol 16
Prepared according to procedure C. Compound 29 (205 mg, 0.35 mmol). Yield: 140 mg, 0.33 mmol, 93%, white solid, Rf = 0.26 (3:1; CH2Cl2: MeOH + 1% NH4OH), [α]D25 −9.1 (c 0.5, CH3OH). 1H NMR (500 MHz, D2O) δ 7.88 (s, 1H, CH-triazole), 7.55 (s, 2H, ArH), 6.84 (s, 2H, ArH), 4.11 (m, 2H, H-12), 3.79 (m, 2H, H-6), 3.58 (m, 1H, H-2), 3.43 (m, 1H, H-3), 3.25 (d, J = 14.5 Hz, 1H, H-4), 2.95 (m, 1H, H-1a), 2.52 (m, 2H, H-7), 2.19 (m, 4H, H-5, H-1b, H-15), 1.58 (m, 2H, H-11), 1.23 (m, 2H, H-8), 0.92 (m, 7H, H-9, H-10, H-16). 13C NMR (125 MHz, D2O) δ 147.04 (C-14), 143.66 (Car), 128.13 (2 × CHar), 127.75 (Car), 125.42 (2 × CHar), 120.62 (C-13), 78.44 (C-3), 69.89 (C-4), 68.82 (C-2), 65.26 (C-6), 57.39 (C-5), 55.65 (C-1), 52.10 (C-7), 50.19 (C-12), 30.03 (C-11), 28.23 (C-15), 26.51 (C-8), 25.92 (C-9), 22.96 (C-10), 15.02 (C-16). HRMS (ESI) m/z calcd for C22H35N4O4+ [M+H]+ 419.26528, found 419.26465.
(2R,3R,4R,5S)-2-(hydroxymethyl)-1-(6-(4-(4-propylphenyl)-1H-1,2,3-triazol-1-yl)hexyl)piperidine-3,4,5-triol 17
Prepared according to procedure C. Compound 30 (228 mg, 0.38 mmol). Yield: 150 mg, 0.35 mmol, 92%, white solid, Rf = 0.28 (3:1; CH2Cl2: MeOH + 1% NH4OH), [α]D25 −8 (c 0.5, CH3OH). 1H NMR (500 MHz, D2O) δ 7.84 (s, 1H, CH- triazole), 7.49 (s, 2H, ArH), 6.73 (s, 2H, ArH), 4.06 (m, 2H, H-12), 3.74 (dd, J = 27.0, 10.8 Hz, 2H, H-6), 3.51 (m, 1H, H-2), 3.36 (t, J = 8.8 Hz, 1H, H-3), 3.19 (t, J = 8.5 Hz, 1H, H-4), 2.89 (m, 1H, H-1a), 2.46 (m, 2H, H-7), 2.11 (m, 4H, H-5, H-1b, H-15), 1.51 (m, 2H, H-11), 1.28–0.81 (m, 8H, H-8, H-9, H-10, H-16), 0.56 (m, 3H, H-17). 13C NMR (125 MHz, D2O) δ 147.00 (C-14), 141.88 (Car), 128.62 (2 × CHar), 127.94 (Car), 125.31 (2 × CHar), 120.61 (C-13), 78.46 (C-3), 69.89 (C-4), 68.82 (C-2), 65.30 (C-6), 57.42 (C-5), 55.71 (C-1), 52.09 (C-7), 50.20 (C-12), 37.44 (C-15), 30.09 (C-11), 26.54 (C-8), 25.97 (C-9), 24.07 (C-10), 22.99 (C-16), 13.56 (C-17). HRMS (ESI) m/z calcd for C23H37N4O4+ [M+H]+ 433.28093, found 433.28061.
(2R,3R,4R,5S)-1-(6-(4-(4-butylphenyl)-1H-1,2,3-triazol-1-yl)hexyl)-2-(hydroxymethyl)piperidine-3,4,5-triol 18
Prepared according to procedure C. Compound 31 (184 mg, 0.3 mmol). Yield: 120 mg, 0.27 mmol, 90%, white solid, Rf = 0.32 (3:1; CH2Cl2: MeOH + 1% NH4OH), [α]D25 −6.9 (c 0.35, CH3OH). 1H NMR (500 MHz, D2O) δ 7.86 (s, 1H, CH- triazole), 7.50 (s, 2H, ArH), 6.74 (s, 2H, ArH), 4.07 (m, 2H, H-12), 3.74 (dd, J = 15.4, 10.0 Hz, 2H, H-6), 3.52 (m, 1H, H-2), 3.36 (t, J = 8.6 Hz, 1H, H-3), 3.20 (t, J = 8.2 Hz, 1H, H-4), 2.90 (m, 1H, H-1a), 2.48 (m, 2H, H-7), 2.09 (m, 4H, H-5, H-1b, H-15), 1.53 (m, 2H, H-11), 1.09 (m, 10H, H-8, H-9, H-10, H-16, H-17), 0.63 (m, 3H, H-18). 13C NMR (125 MHz, D2O) δ 146.98 (C-14), 141.99 (Car), 128.54 (2 × CHar), 127.98 (Car), 125.35 (2 × CHar), 120.51 (C-13), 78.47 (C-3), 69.89 (C-4), 68.81 (C-2), 65.35 (C-6), 57.44 (C-5), 55.73 (C-1), 52.11(C-7), 50.23 (C-12), 35.16 (C-15), 33.18 (C-16), 30.15 (C-11), 26.59 (C-8), 26.02 (C-9), 23.08 (C-10), 22.34 (C-17), 13.71 (C-18). HRMS (ESI) m/z calcd for C24H39N4O4+ [M+H]+ 447.29658, found 447.29678.
(2R,3R,4R,5S)-2-(hydroxymethyl)-1-(6-(4-(4-pentylphenyl)-1H-1,2,3-triazol-1-yl)hexyl)piperidine-3,4,5-triol 19
Prepared according to procedure C. Compound 32 (214 mg, 0.34 mmol). Yield: 150 mg, 0.33 mmol, 96%, white solid, Rf = 0.36 (3:1; CH2Cl2: MeOH + 1% NH4OH), [α]D25 −7.5 (c 0.2, CH3OH). 1H NMR (500 MHz, D2O) δ 7.86 (s, 1H, CH- triazole), 7.51 (m, 2H, ArH), 6.75 (m, 2H, ArH), 4.04 (m, 2H, H-12), 3.73 (m, 2H, H-6), 3.51 (m, 1H, H-2), 3.34 (d, J = 7.6 Hz, 1H, H-3), 3.19 (m, 1H, H-4), 2.89 (m, 1H, H-1a), 2.47 (m, 2H, H-7), 2.10 (m, 4H, H-5, H-1b, H-15), 1.52 (m, 2H, H-11), 1.12 (m, 12H, H-8, H-9, H-10, H-16, H-17, H-18), 0.68 (m, 3H, H-19). 13C NMR (125 MHz, D2O) δ 149.40 (C-14), 144.41 (Car), 130.96 (2 × CHar), 130.52 (Car), 127.83 (2 × CHar), 123.01 (C-13), 80.97 (C-3), 72.37 (C-4), 71.29 (C-2), 67.85 (C-6), 59.93 (C-5), 58.23 (C-1), 54.59 (C-7), 52.69 (C-12), 37.95 (C-15), 34.29 (C-16), 33.32 (C-17), 32.66 (C-11), 29.08 (C-8), 28.55 (C-9), 25.60 (C-10), 24.97 (C-18), 16.41 (C-19). HRMS (ESI) m/z calcd for C25H41N4O4+ [M+H]+ 461.31223, found 461.31213.
(2R,3R,4R,5S)-1-(6-(4-(4-hexylphenyl)-1H-1,2,3-triazol-1-yl)hexyl)-2-(hydroxymethyl)piperidine-3,4,5-triol 20
Prepared according to procedure C. Compound 33 (160 mg, 0.25 mmol). Yield: 110 mg, 0.23 mmol, 92%, white solid, Rf = 0.4 (3:1; CH2Cl2: MeOH + 1% NH4OH), [α]D25 7 (c 0.2, CH3OH). 1H NMR (500 MHz, D2O) δ 7.86 (s, 1H, CH- triazole), 7.52 (s, 2H, ArH), 6.77 (s, 2H, ArH), 4.04 (m, 2H, H-12), 3.76 (m, 2H, H-6), 3.52 (m, 1H, H-2), 3.36 (m, 1H, H-3), 3.20 (m, 1H, H-4), 2.91 (m, 1H, H-1a), 2.48 (m, 2H, H-7), 2.11 (m, 4H, H-5, H-1b, H-15), 1.53 (m, 2H, H-11), 1.35–0.63 (m, 17H, H-8, H-9, H-10, H-16, H-17, H-18, H-19, H-20). 13C NMR (125 MHz, D2O) δ 146.95 (C-14), 141.78 (Car), 128.44 (2 × CHar), 127.99 (Car), 125.40 (2 × CHar), 120.50 (C-13), 78.56 (C-3), 69.95 (C-4), 68.87 (C-2), 65.42 (C-6), 57.52 (C-5), 55.85 (C-1), 52.13 (C-7), 50.21 (C-12), 35.66 (C-15), 31.88 (C-16), 31.18 (C-17), 30.23 (C-11), 29.59 (C-18), 26.67 (C-8), 26.13 (C-9), 23.20 (C-10), 22.81 (C-19), 13.98 (C-20). HRMS (ESI) m/z calcd for C26H43N4O4+ [M+H]+ 475.32788, found 475.32794.