Abstract
In cold methanol, energetic ionic liquid 1-n-propyl-3-vinyl-imidazol-1-ium perchlorate, 1, crystallizes in the presence of excess Ba(ClO4)2, 2, into tetrakis 1-propyl-3-vinyl-imidazol-1-ium·barium hexa-perchlorate, 3. Crystals of 3, with molecular formula (C8H13N2)4·BaCl6O24, are colorless and monoclinic, with space group P21/c. The crystal structure is characterized by a dodecahedral coordination around the barium atom, with each perchlorate chelating Ba2+ in a κ2O,O’ fashion, and the Ba(ClO4)64− anion is surrounded by four imidazolium cations.
1. Introduction
Numerous 1,3-dialkyl Imidazolium perchlorates and nitrates, as well as 1-alkyl-3-vinyl Imidazolium perchlorate and nitrate derivatives, have been shown to be room-temperature energetic ionic liquids (RTEILs) [1,2,3,4,5]. Recently, they have been suggested as monomers for effective fuel binders in propellant compositions that are also suitable for additive manufacturing of propellant grains [6,7,8,9,10]. The major advantage of this family of materials is that they contain both a cationic organic fuel and an anionic oxidizer in their molecular structure, thereby facilitating the initial steps of combustion and reducing the overall oxygen balance of the fuel part [11,12]. Despite their highly energetic nature, many of these materials fulfill the requirements for the safe handling of energetic materials in terms of shock and electrostatic initiation and are thermally stable up to temperatures exceeding 200 °C [13,14].
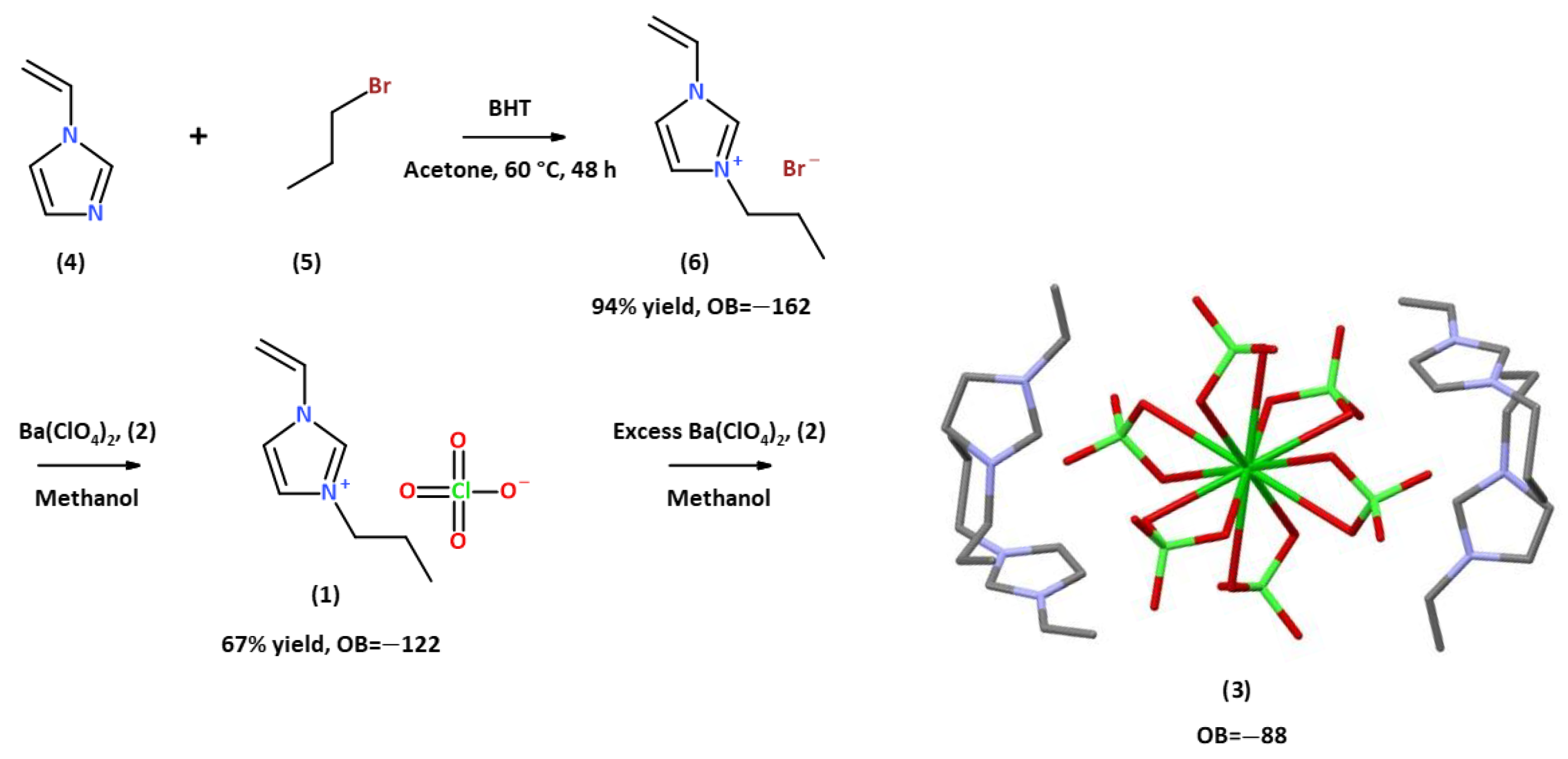
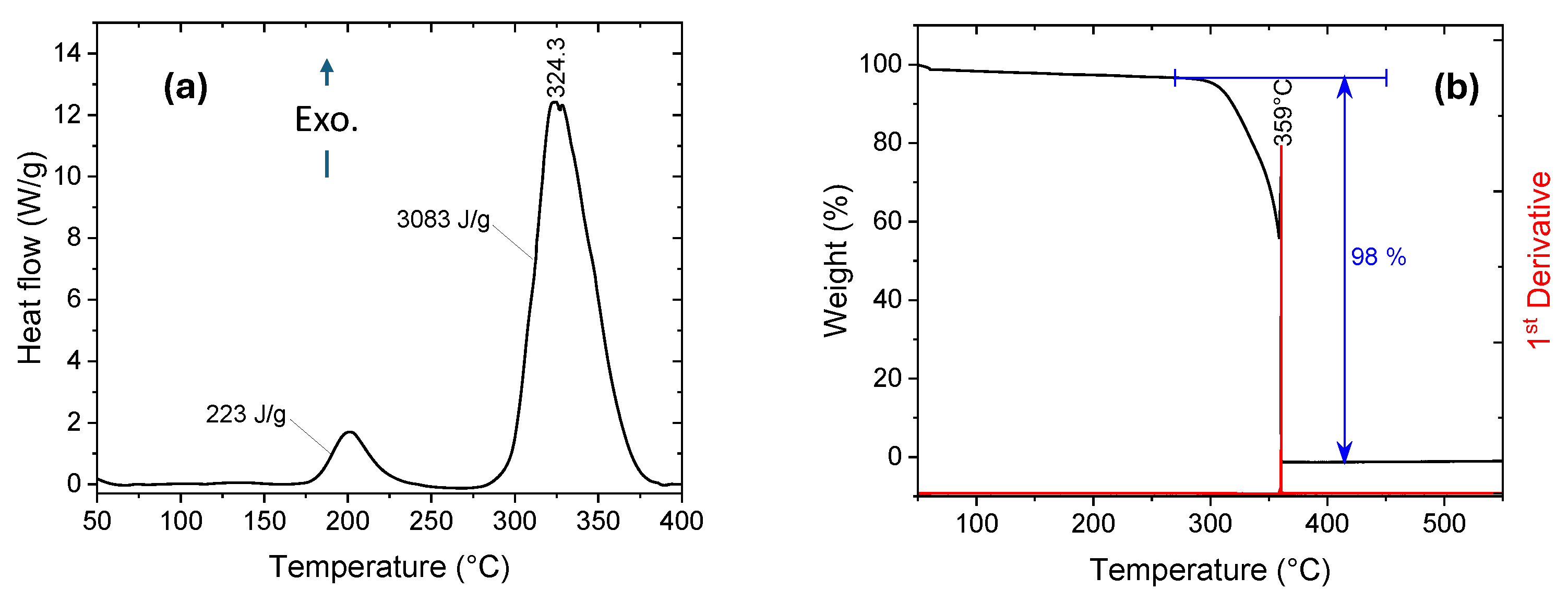
Yet another advantage of these materials is that the synthesis of 1-alkyl-3-vinyl Imidazolium perchlorates and nitrates is a simple, high-yield process, which is a significant advantage when preparing ingredients for propellants and explosives. For example, the synthesis of 1-n-propyl-3-vinyl Imidazolium perchlorate, 1, occurs through a two-step procedure from readily available and inexpensive materials; see Scheme 1. Ion metathesis using a solution of barium perchlorate, Ba(ClO4)2, 2, in methanol yields a room-temperature energetic ionic liquid (RTEIL), which is thermally stable up to ~175 °C. At around 200 °C, the material undergoes a thermally induced polymerization process, followed by a highly exothermic thermal decomposition of the resulting polymer with an onset at ~300 °C and a peak at 324 °C, ΔHd = 3083 J·g−1, which is >60% larger than the decomposition enthalpy of RDX measured on the same machine under the same conditions and calibration; see Figure 1a. TGA measurements reveal that the mass loss peaks sharply at 359 °C and reaches a constant level of 98% right after the weight loss peak; see Figure 1b.
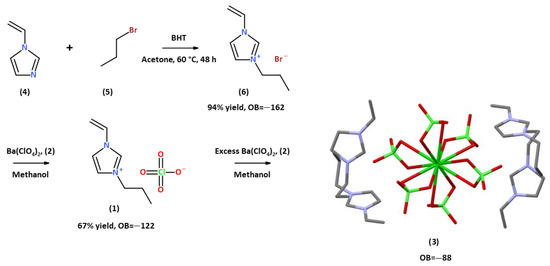
Scheme 1.
Synthesis of 1-n-propyl-3-vinyl Imidazolium perchlorate, 1, and preparation of tetrakis 1-propyl-3-vinyl-imidazol-1-ium·barium hexa-perchlorate, 3.
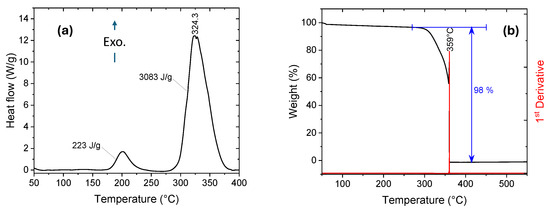
Figure 1.
(a) DSC and (b) TGA curves of 1-n-propyl-3-vinyl Imidazolium perchlorate, 1. Scan rate = 10 °C·min−1.
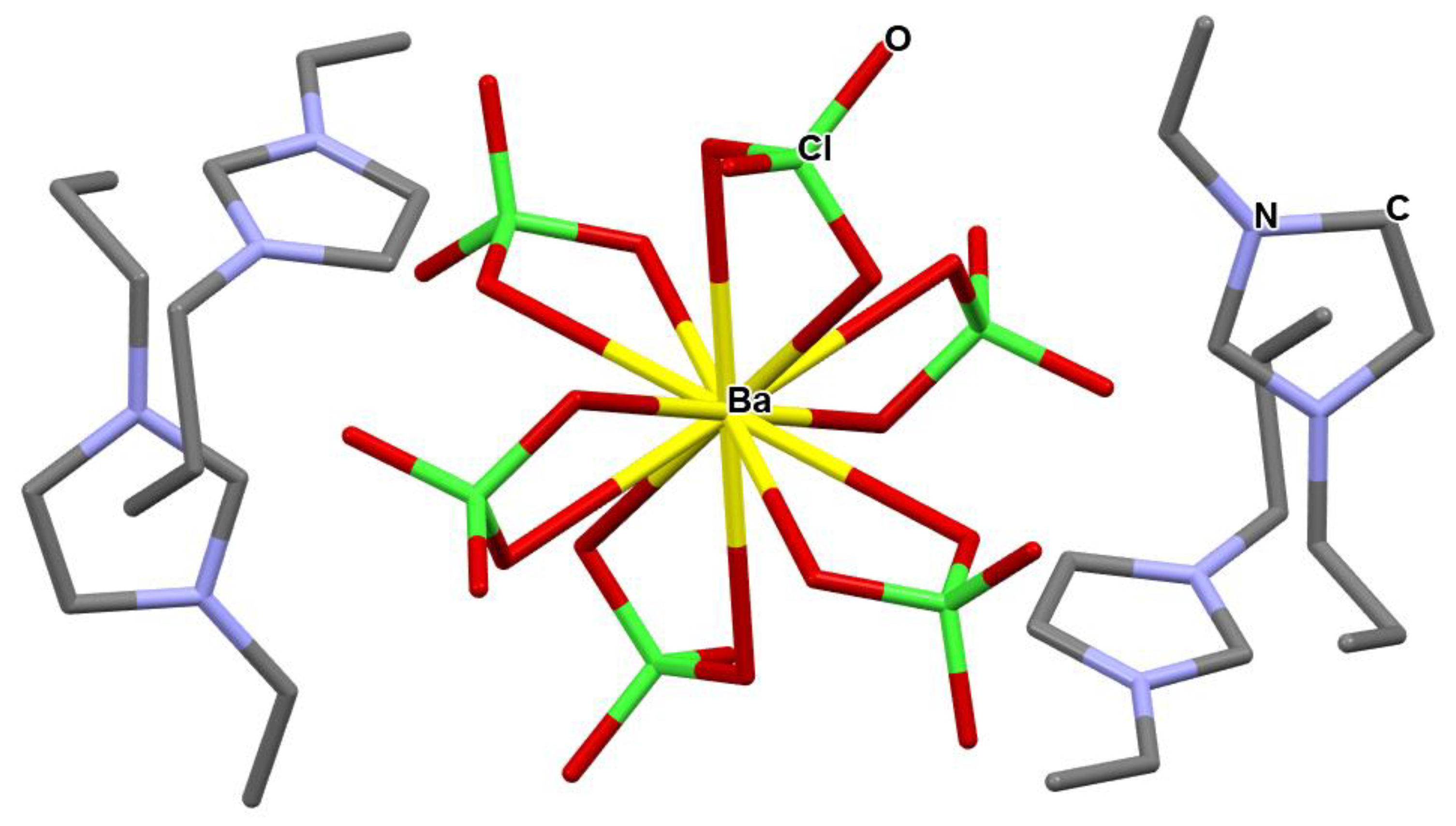
Interestingly, the addition of excess Ba(ClO4)2, 2, to solutions of 1 in cold methanol resulted in formation of a colorless crystalline material, which was identified as tetrakis 1-propyl-3-vinyl-imidazol-1-ium barium hexa-perchlorate, 3, (C8H13N2)4·BaCl6O24, characterized by an oxygen balance of OB = −88%, not far from the oxygen balance of TNT, OB = −74%. This involves the coordination of four perchlorate anions of four units of three to the barium atom of Ba(ClO4)2, forming a distorted dodecahedral coordination around it. At room temperature, the crystals melt and form a liquid phase.
In the past, a systematic study has been conducted on the crystal coordination of the barium ion in various compounds whose structures have been solved. It revealed that, although Ba2+ should normally exhibit a coordination number of nine or ten, one can find a variety of coordination modes with coordination numbers varying from six to twelve [15]. Some of the factors that might possibly explain this wide range of coordination numbers were discussed.
2. Results
2.1. Crystal Structure Determination of Tetrakis 1-N-Propyl-3-vinyl-imidazol-1-ium Barium Hexa-Perchlorate, 3
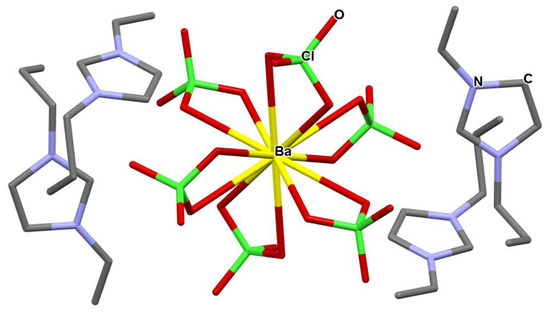
Figure 2.
Crystal structure of tetrakis 1-n-propyl-3-vinyl-imidazol-1-ium barium hexa-perchlorate, 3.

Table 1.
Structural information of the crystal of 3.
2.2. Supramolecular Features
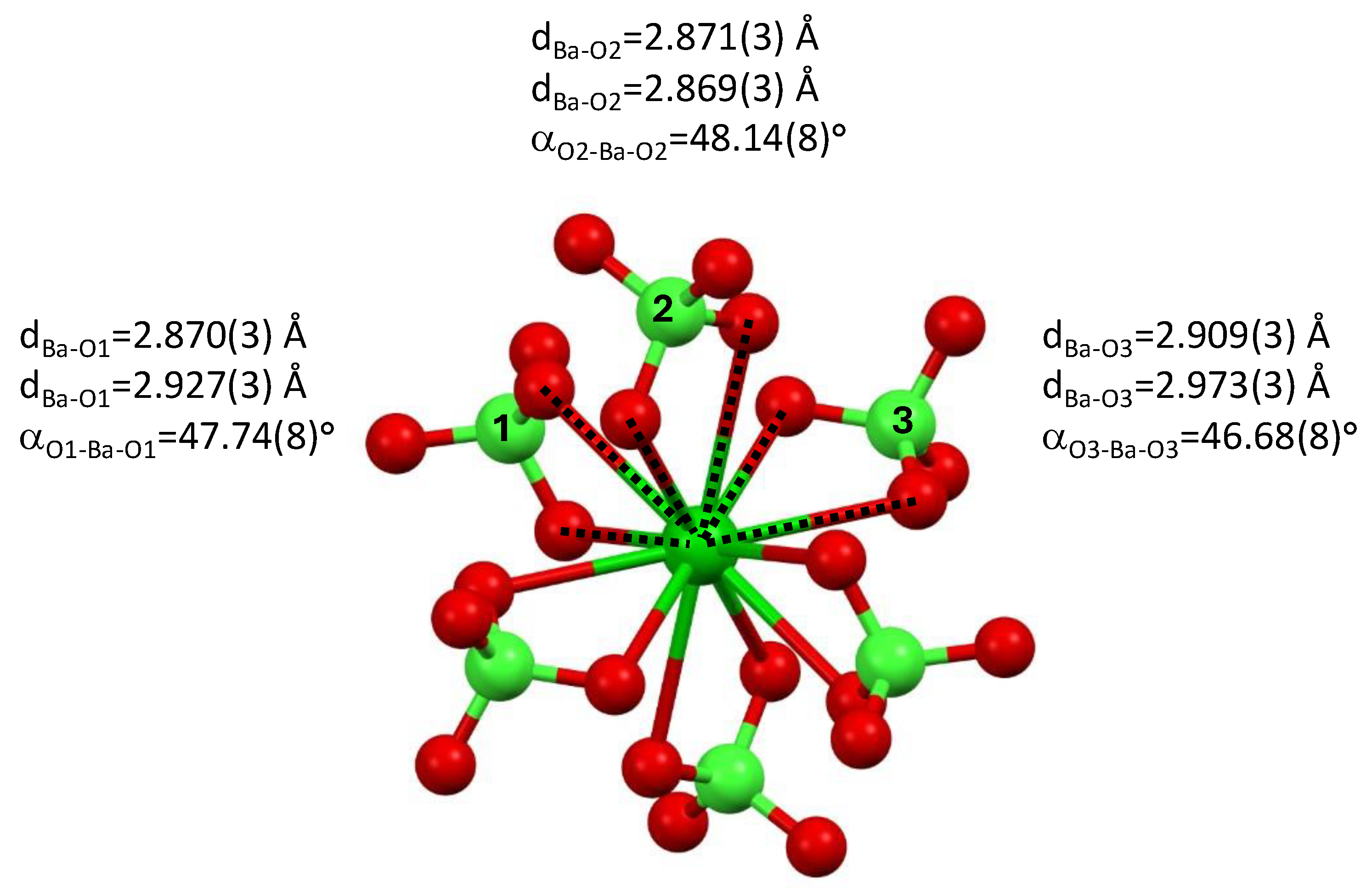
The perchlorate anions are bound to the barium cation through three almost equivalent, yet different, binding modes; see Figure 3. Two sets of perchlorate molecules, related to one another through an inversion center, are characterized by Ba-O bonds of dBa1-O = 2.870(3) Å and dBa-O1 = 2.927(3) Å and O-Ba-O angle of αO1-Ba-O1′ = 47.74(8)°. Two sets of perchlorate anions, related to one another through an inversion center, are characterized by Ba-O bonds of dBa-O2 = 2.871(3) Å and dBa-O2 = 2.869(3) Å and O-Ba-O angle of αO2-Ba-O2′ = 48.12(8)°. Two sets of perchlorate molecules, related to one another through an inversion center, are characterized by Ba-O bonds of dBa-O3 = 2.909(3) Å and dBa-O3 = 2.973(3) Å and O-Ba-O angle of αO3-Ba-O3′ = 46.68(8)°.
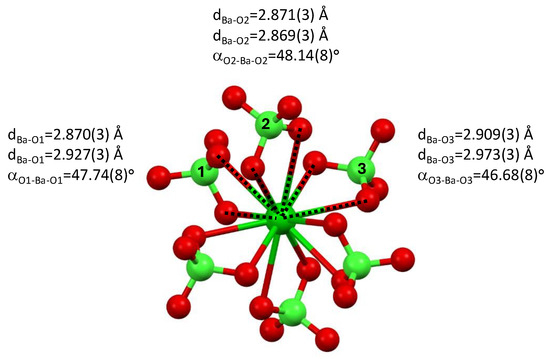
Figure 3.
Bonding of the perchlorate anions to the barium cation in tetrakis 1-n-propyl-3-vinyl-imidazol-1-ium barium hexa-perchlorate, 3.
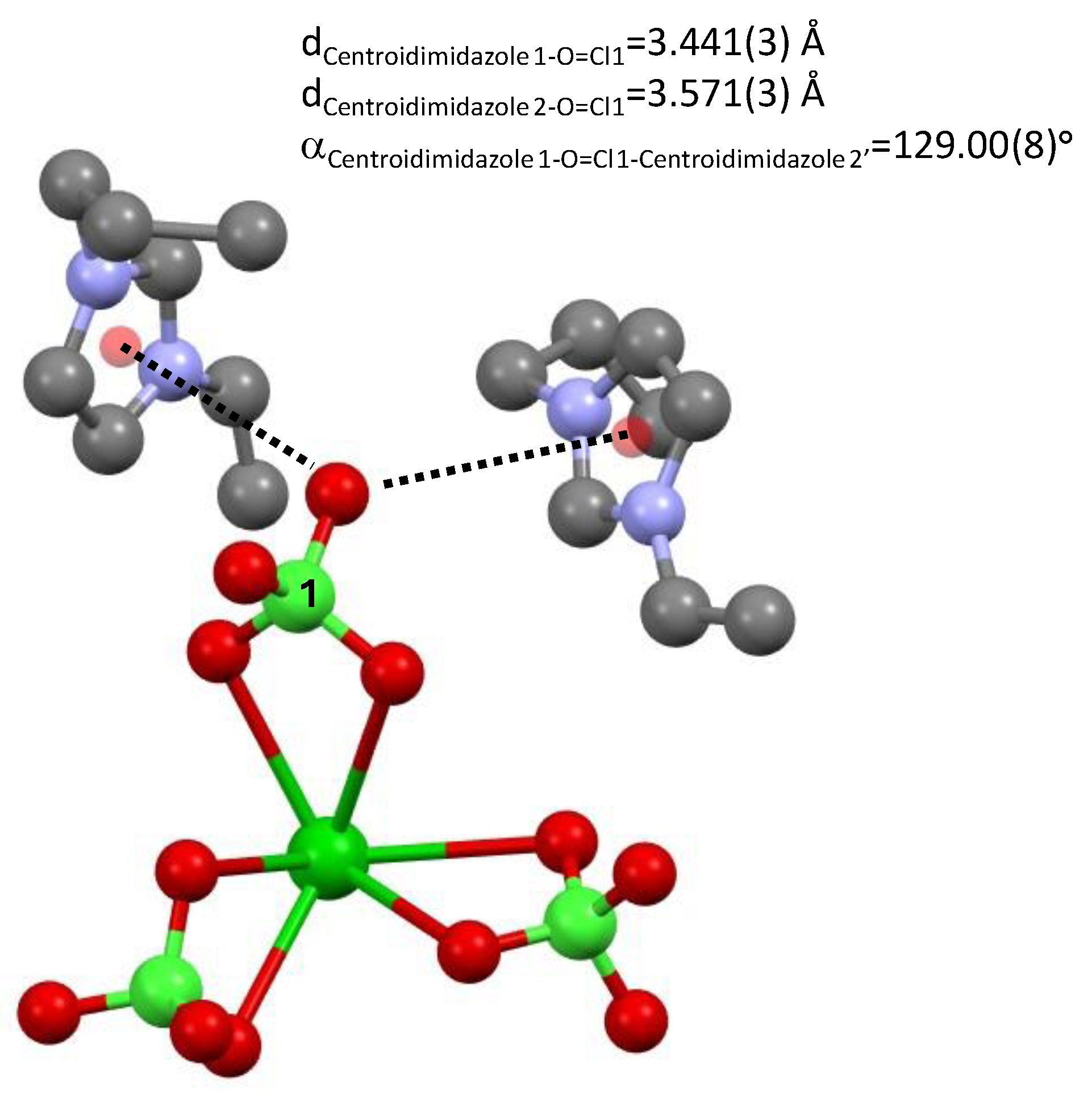
An oxygen atom of perchlorate 1 (and its symmetry equivalent) interacts with two π clouds of two imidazolium rings dCentroidimidazole1-O=Cl1 = 3.441(3) Å and dCentroidimidazole2-O=Cl1 = 3.571(3) Å, α Centroidimidazole1-O=Cl1-Centroidimidazole2′ = 129.00(8)°; see Figure 4.
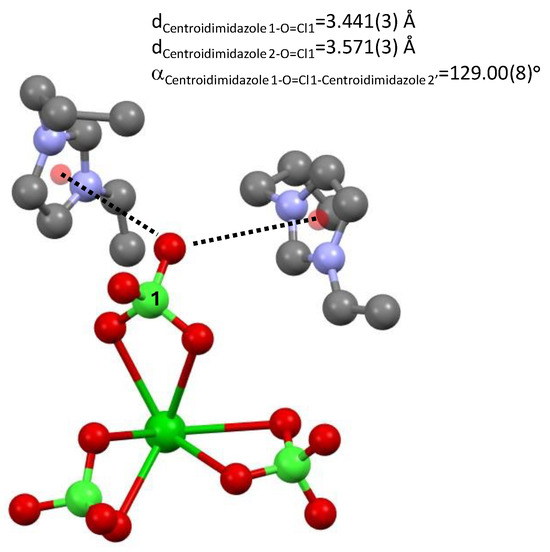
Figure 4.
Distances between an oxygen atom of perchlorate 1 and two imidazolium rings of tetrakis 1-n-propyl-3-vinyl-imidazol-1-ium barium hexa-perchlorate, 3.
3. Refinement
The single crystal of colorless plate material 3 was immersed in Paratone–N oil and mounted on a Rigaku Oxford Diffraction—XtaLAB Synergy-S at 100 K. Data collection was performed using monochromated Mo Kα radiation, λ = 0.71073 Å, using φ and ω scans to cover the Ewald sphere. Accurate cell parameters were obtained with the amount of indicated reflections. Using Olex2 [16], the structure was solved with the olex2.solve [17] structure solution program using charge flipping and refined with the ShelXL [18] refinement package using least squares minimization. All non-hydrogen atoms were refined with anisotropic displacement parameters. The hydrogen atoms were refined isotropically on calculated positions using a riding model with their Uiso values constrained to 1.5 times the Ueq of their pivot atoms for terminal sp3 carbon atoms and 1.2 times for all other carbon atoms. Software used for molecular graphics: Mercury 2022.3.0 [19].
4. Materials and Methods
All starting materials reported in the manuscript were purchased from Sigma-Aldrich (St. Louis, MO, USA). Solvents and starting materials were used as received unless otherwise noted.
NMR spectra were recorded on a Bruker-AVIII-400 spectrometer (Bremen, Germany). Liquid chromatography high-resolution mass spectrometry measurements were performed on Thermo Scientific UltiMate 3000 HPLC (Boston, MA, USA) using 70:30 Acetonitrile:H2O as solvent and a flow of 0.5 mL/min, with Brucker maxis impact HRMS ESI. Differential scanning calorimetry, DSC, measurements were conducted on a Q100 TA instrument, using aluminum crucibles with perforated covers. DSC curves were taken under an N2 atmosphere at 10 °C min−1 from ambient to 400 °C. Thermo-gravimetric analysis, TGA, measurements were conducted on a Q50 TA instrument (New Castle, DE, USA), using open aluminum crucibles. TGA curves were taken under an N2 atmosphere at 10 °C min−1 from ambient to 500 °C.
4.1. Synthesis of 1-N-Propyl-3-vinyl-imidazol-1-ium Bromide (6)
Vinyl imidazole, 4 (20.00 g, 212.52 mmol), and 2,6-di-tert-butyl-4-methylfenol (BHT) (0.0023 g, 0.0106 mmol, 100 ppm) were added to a 100 mL round bottom flask. The mixture was stirred for 5 min, and then, 1- bromopropane, 5 (33.18 g, 233.77 mmol), was added, and the mixture was heated and kept at 60 °C for 3 days, after which a phase separation occurred.
Excess 1- bromopropane was removed under reduced pressure, and the crude product was dissolved in methanol, placed in a separating funnel, and washed with hexane (3 × 50 mL). The methanol phase was collected, and 100 ppm of BHT was added before the solvent was removed under reduced pressure, affording the product (43.43 g, 200.04 mmol) in the form of a light yellow, thick oil, in 94% yield.
1H NMR (400 MHz, DMSO-d6, δ): 9.69 (d, J = 1.7 Hz, 1H, Ar H), 8.28 (d, J = 1.5 Hz, 1H, Ar H), 7.99 (d, J = 2.4 Hz, 1H, Ar H), 7.35 (dd, J = 15.6, 8.7 Hz, 1H, CH=CH2), 6.01 (dd, J = 15.7, 2.4 Hz, 1H, CH=CH2), 5.43 (dd, J = 8.8, 2.3 Hz, 1H, CH=CH2), 4.20 (t, J = 7.1 Hz, 2H, N-CH2-CH2), 1.85 (h, J = 7.3 Hz, 2H, -CH2-CH2-CH3), 0.88 (t, J = 7.3 Hz, 3H, -CH2-CH3); 13C NMR (101 MHz, DMSO-d6, δ): 135.81 (Ar C), 129.35 (Ar C), 123.74 (Ar C), 119.68 (CH=CH2), 109.13 (CH=CH2), 51.14 (N-CH2-CH2-), 23.06 (-CH2-CH2-CH3), 10.91 (-CH2-CH2-CH3); HRMS (ESI) m/z: [M]+ calcd. for C8H13N2: 137.1073; found: 137.1082. See Supplementary Materials.
4.2. Synthesis of 1-N-Propyl-3-vinyl-imidazol-1-ium Perchlorate (1)
6 (43.43 g, 200.04 mmol) was dissolved in 100 mL of methanol. Barium perchlorate, 2 (33.63 g, 100.02 mmol), was added to a separate beaker and dissolved in 120 mL of methanol. The solution of 6 was added to the beaker containing the barium perchlorate solution, and white precipitate formed. The mixture was diluted with 200 mL of methanol and stirred for 5 min before it was transferred to polypropylene tubes and centrifuged (4000 RPM, 15 min).
The liquid was filtered through a sintered glass Buchner (pore size: 10–16 μm), and the solvent was removed under reduced pressure, affording the product, 1 (31.66 g, 133.78 mmol), in the form of a light yellow, thick oil, in 67% yield.
1H NMR (400 MHz, DMSO) δ 9.42 (s, 1H), 8.16 (t, J = 1.8 Hz, 1H), 7.88 (t, J = 1.6 Hz, 1H), 7.26 (dd, J = 15.6, 8.8 Hz, 1H), 5.93 (dd, J = 15.6, 2.4 Hz, 1H), 5.42 (dd, J = 8.7, 2.4 Hz, 1H), 4.16 (t, J = 7.2 Hz, 2H), 1.92–1.75 (m, 2H), 0.88 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO) δ 135.24, 128.82, 123.26, 119.26, 108.87, 51.00, 22.71, 10.44; HRMS (ESI) m/z: [M]+ calcd. for C8H13N2: 137.1073; found: 137.1081. See Supplementary Materials.
4.3. Growth of Crystals of 3
A sample of 1 containing excess Ba(ClO4)2, 2, was kept at −18 °C in a freezer. After 24 h, solid was observed at the bottom of the vial. The liquid was decanted, and the crystalline solid was inspected under a light microscope, to select a crystal that was suitable for structure determination, using single-crystal x-ray diffraction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29215010/s1, Figure S1: 1H NMR spectrum of 6. Figure S2: 13C NMR spectrum of 6. Figure S3: 1H NMR spectrum of 1. Figure S4: 13C NMR spectrum of 1.
Author Contributions
Methodology, Y.Z.; formal analysis, N.F.; writing—original draft preparation, Y.Z. and N.F.; writing—review and editing, Y.E. and L.G.; conceptualization—L.G. and Y.E.; supervision, Y.E. and L.G.; project administration, Y.E. and Y.Z.; funding acquisition, Y.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by RAFAEL Advanced Defense Systems Ltd. (grant number: 348034809); The Center for Security Science & Technology, Technion—Israel Institute of Technology (CSST) (grant number: 20714270); and the Trudy Mandel Louis Charitable Trust.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
CCDC 2384212 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif (accessed on 1 October 2024).
Acknowledgments
This paper is dedicated to the memory of Natalia’s parents, Fridman Boris (1944–2024) and Fridman Ludmila (1945–2023), who lived together for 52 years.
Conflicts of Interest
Author Levi Gottlieb was employed by the company RAFAEL, Advanced Defense Systems Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest The authors declare that this study received funding from RAFAEL Advanced Defense Systems Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
References
- Thomas, E.; Vijayalakshmi, K.P.; George, B.K. Imidazolium Based Energetic Ionic Liquids for Monopropellant Applications: A Theoretical Study. RSC Adv. 2015, 5, 71896–71902. [Google Scholar] [CrossRef]
- Papović, S.; Vraneš, M.; Armaković, S.; Armaković, S.J.; Szécsényi, K.M.; Bešter-Rogač, M.; Gadžurić, S. Investigation of 1,2,3-Trialkylimidazolium Ionic Liquids: Experiment and Density Functional Theory Calculations. New J. Chem. 2017, 41, 650–660. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Yang, H.; Zhang, D.; Kirichenko, K.; Smiglak, M.; Holbrey, J.D.; Reichert, W.M.; Rogers, R.D. Strategies toward the Design of Energetic Ionic Liquids: Nitro- and Nitrile-Substituted N,N′-Dialkylimidazolium Salts. New J. Chem. 2006, 30, 349. [Google Scholar] [CrossRef]
- Thomas, E.; Thomas, D.; Vijayalakshmi, K.P.; George, B.K. Mechanistic Outlook on Thermal Degradation of 1,3-Dialkyl Imidazolium Ionic Liquids and Organoclays. RSC Adv. 2016, 6, 9421–9428. [Google Scholar] [CrossRef]
- Chand, D.; Wilk-Kozubek, M.; Smetana, V.; Mudring, A.-V. Alternative to the Popular Imidazolium Ionic Liquids: 1,2,4-Triazolium Ionic Liquids with Enhanced Thermal and Chemical Stability. ACS Sustain. Chem. Eng. 2019, 7, 15995–16006. [Google Scholar] [CrossRef]
- Zertal, Y.; Yong, M.; Levi, A.; Sevilia, S.; Tsoglin, A.; Parvari, G.; Gottlieb, L.; Eichen, Y. Alkyl Vinyl Imidazolium Ionic Liquids as Fuel Binders for Photo-Curable Energetic Propellants. ACS Appl. Polym. Mater. 2022, 4, 4928–4939. [Google Scholar] [CrossRef]
- Sevilia, S.; Yong, M.; Grinstein, D.; Gottlieb, L.; Eichen, Y. Novel, Printable Energetic Polymers. Macromol. Mater. Eng. 2019, 304, 1900018. [Google Scholar] [CrossRef]
- Sevilia, S.; Parvari, G.; Bernstein, J.; Fridman, N.; Grinstein, D.; Gottlieb, L.; Eichen, Y.; Szpilman, A.M. Imidazolium-Based Energetic Materials. ChemistrySelect 2022, 7, e202200322. [Google Scholar] [CrossRef]
- Wang, B.; Feng, Y.; Qi, X.; Deng, M.; Tian, J.; Zhang, Q. Designing Explosive Poly(Ionic Liquid)s as Novel Energetic Polymers. Chem.—Eur. J. 2018, 24, 15897–15902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shreeve, J.M. Energetic Ionic Liquids as Explosives and Propellant Fuels: A New Journey of Ionic Liquid Chemistry. Chem. Rev. 2014, 114, 10527–10574. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Verma, R.D.; Meshri, D.T.; Shreeve, J.M. Energetic Nitrogen-Rich Salts and Ionic Liquids. Angew. Chem. Int. Ed. 2006, 45, 3584–3601. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Köhler, J.; Homburg, A. Explosives, 7th ed.; completely revised and updated; Wiley-VCH: Weinheim, Germany, 2016; ISBN 978-3-527-33776-7. [Google Scholar]
- Xue, H.; Gao, Y.; Twamley, B.; Shreeve, J.M. New Energetic Salts Based on Nitrogen-Containing Heterocycles. Chem. Mater. 2005, 17, 191–198. [Google Scholar] [CrossRef]
- Gao, H.; Shreeve, J.M. Azole-Based Energetic Salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef] [PubMed]
- Manohar, H.; Ramaseshan, S. Crystal Co-Ordination of the Barium Ion. Proc. Indian Acad. Sci.-Sect. A 1964, 60, 317–351. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The Anatomy of a Comprehensive Constrained, Restrained Refinement Program for the Modern Computing Environment–Olex2 Dissected. Acta Crystallogr. Sect. Found. Adv. 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).