Stability and Electronic Properties of Mixed Rare-Earth Tri-Metallofullerenes YxDy3-x@C80 (x = 1 or 2)
Abstract
1. Introduction
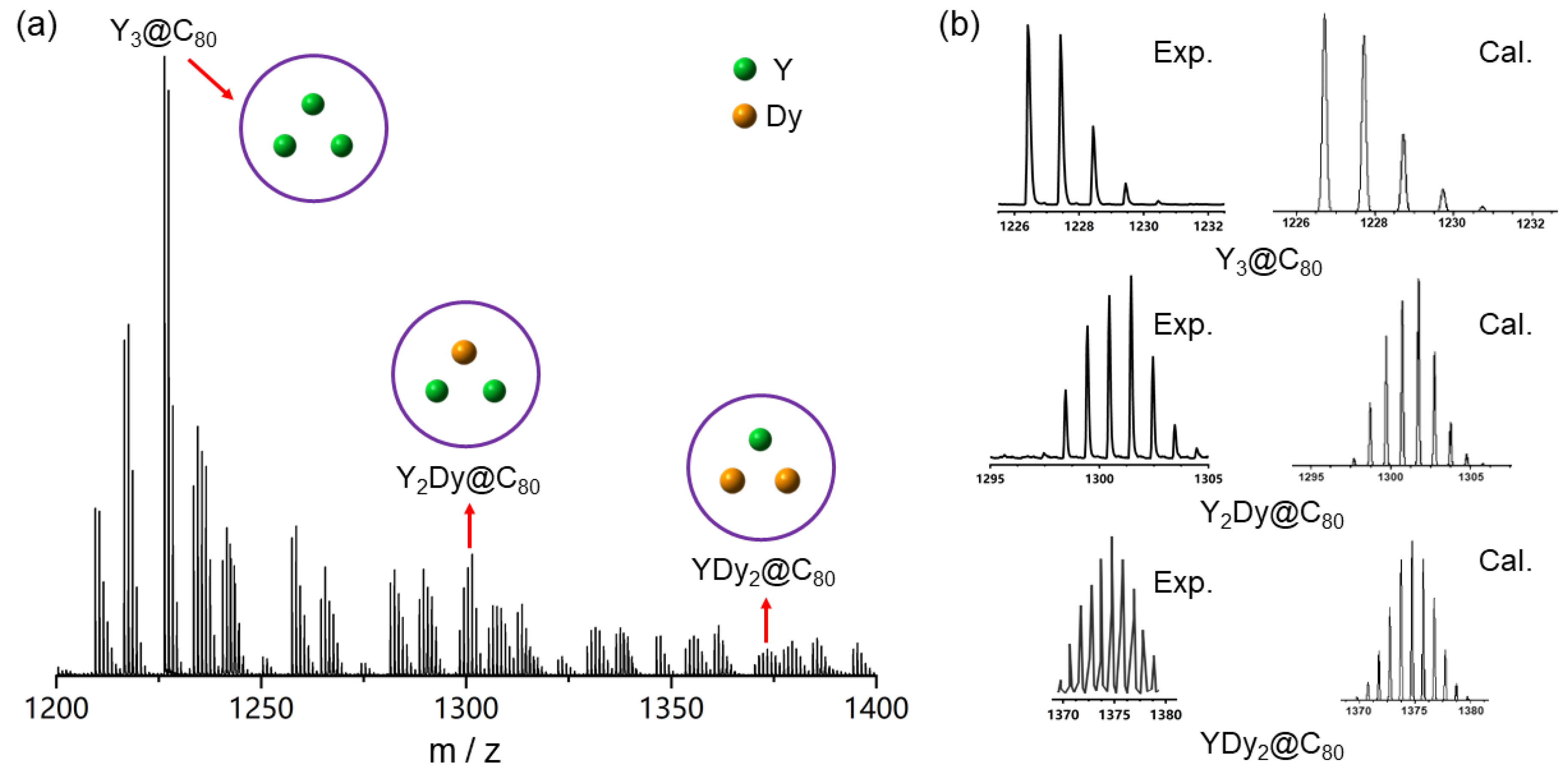
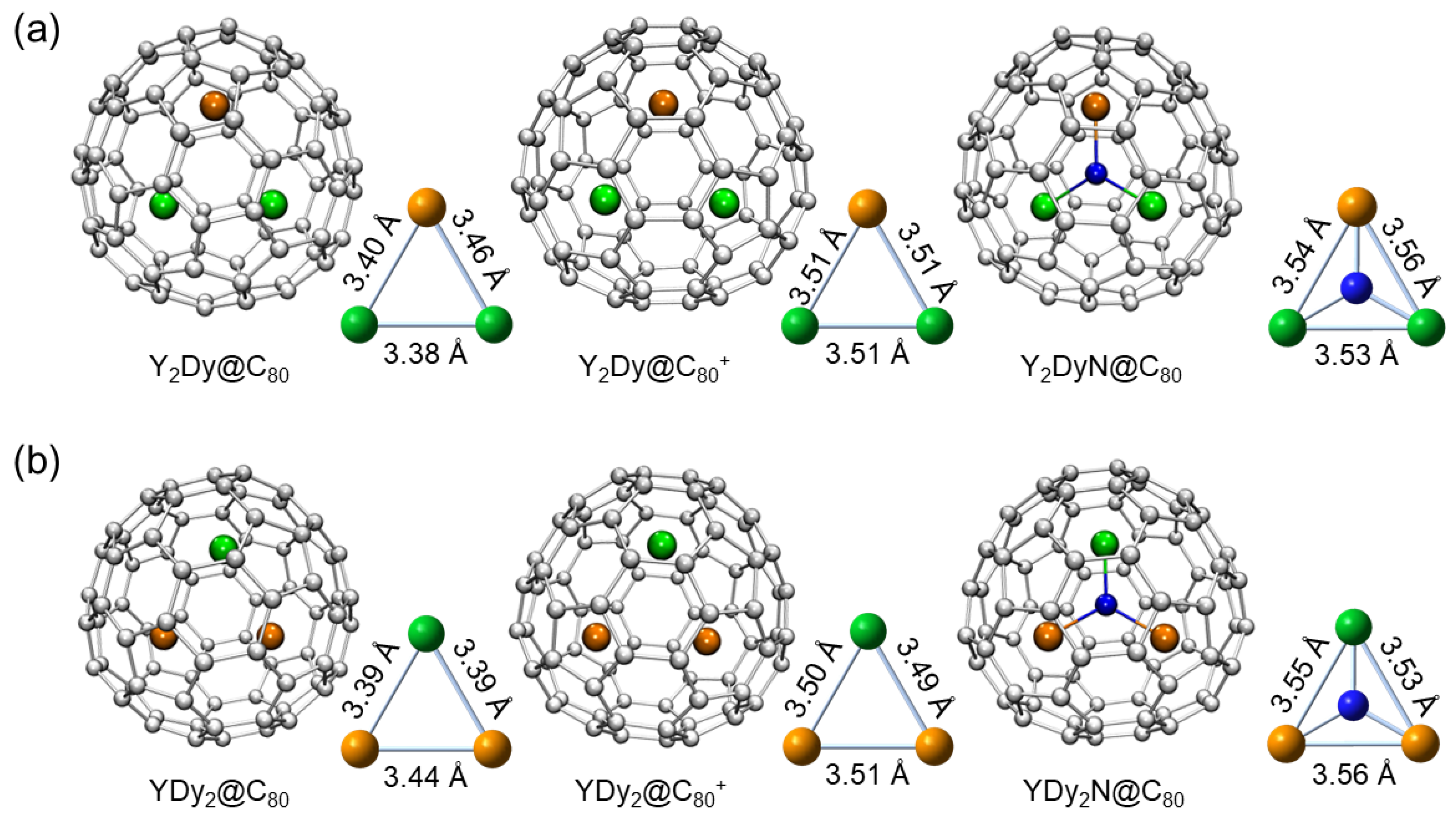
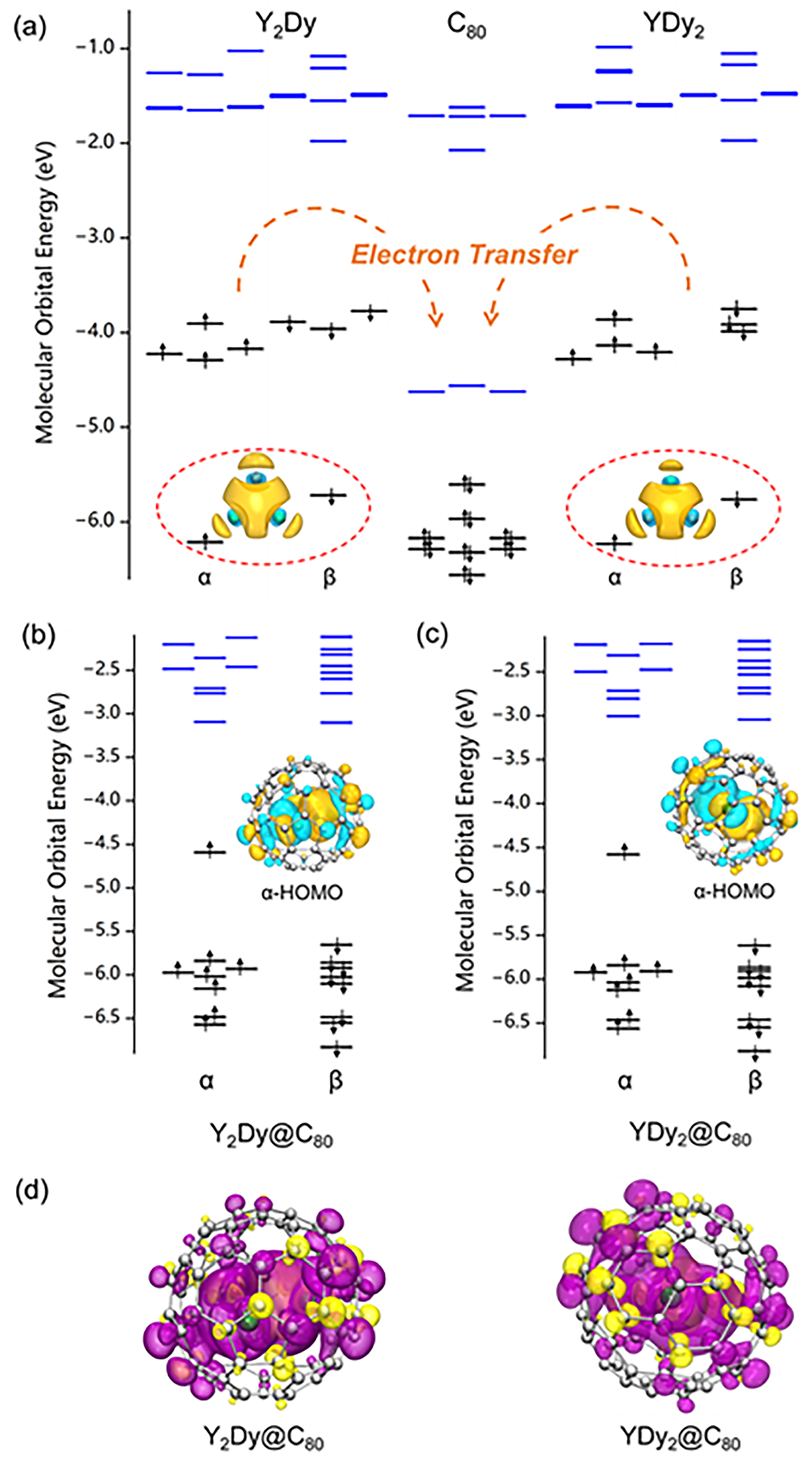
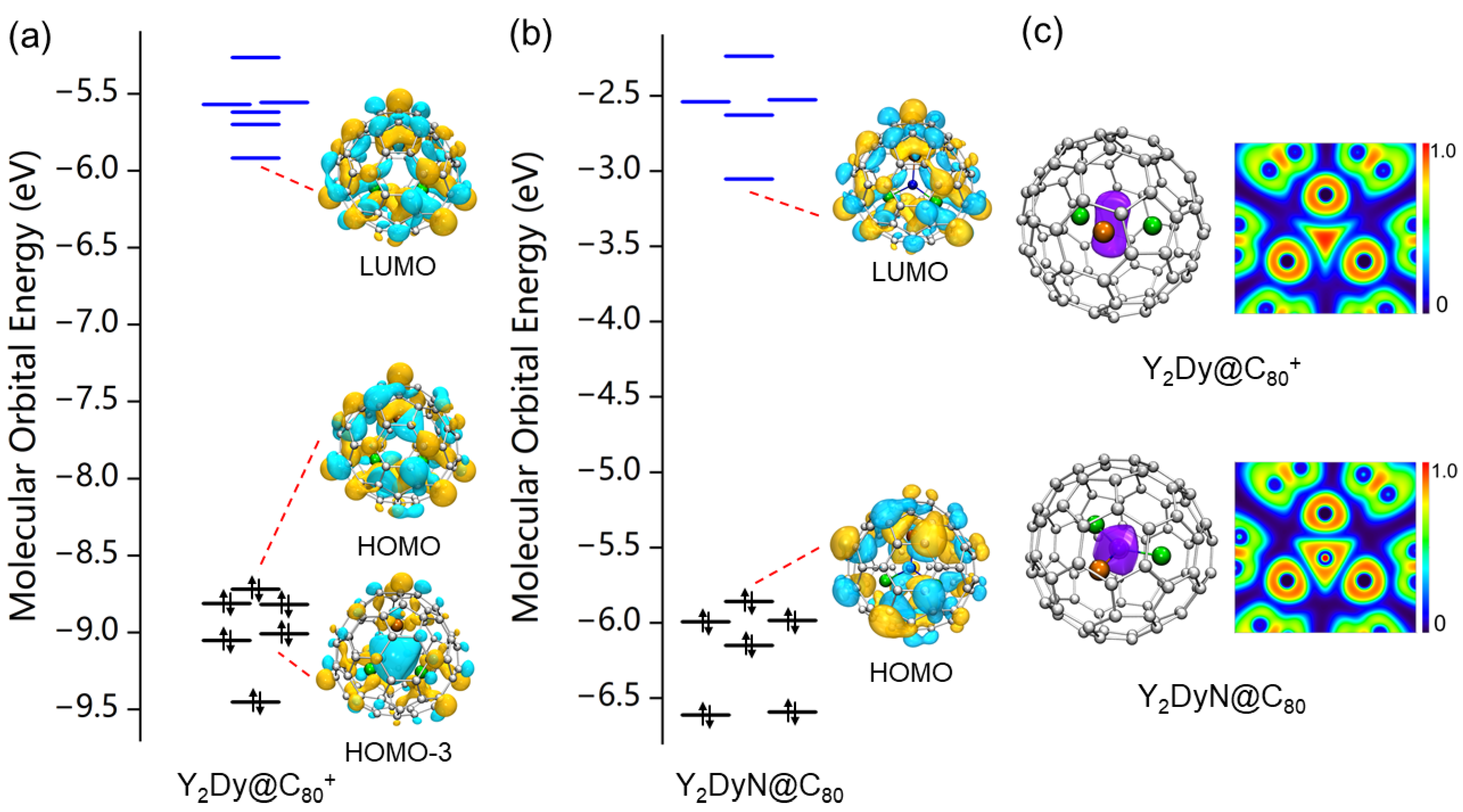
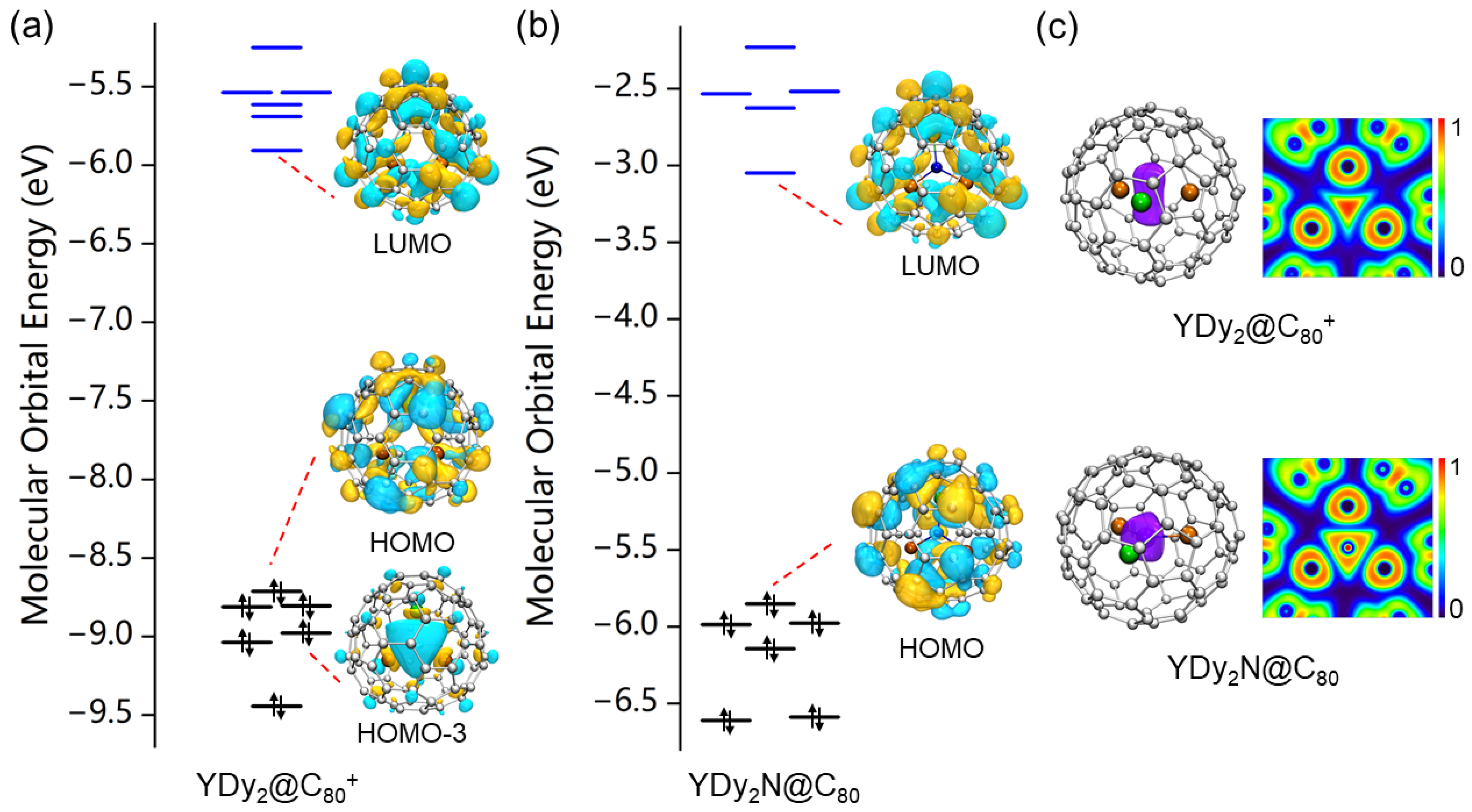
2. Results and Discussion
3. Experimental and Theoretical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shinohara, H. Endohedral metallofullerenes. Rep. Prog. Phys. 2000, 63, 843. [Google Scholar] [CrossRef]
- Chaur, M.N.; Melin, F.; Ortiz, A.L.; Echegoyen, L. Chemical, electrochemical, and structural properties of endohedral metallofullerenes. Angew. Chem. Int. Ed. 2009, 48, 7514–7538. [Google Scholar] [CrossRef]
- Cong, H.; Yu, B.; Akasaka, T.; Lu, X. Endohedral metallofullerenes: An unconventional core–shell coordination union. Coord. Chem. Rev. 2013, 257, 2880–2898. [Google Scholar]
- Popov, A.A.; Yang, S.; Dunsch, L. Endohedral fullerenes. Chem. Rev. 2013, 113, 5989–6113. [Google Scholar] [CrossRef]
- Wang, T.; Wang, C. Endohedral Metallofullerenes Based on Spherical Ih-C80 Cage: Molecular Structures and Paramagnetic Properties. Acc. Chem. Res. 2014, 47, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wei, T.; Jin, F. When metal clusters meet carbon cages: Endohedral clusterfullerenes. Chem. Soc. Rev. 2017, 46, 5005–5058. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.S.; Wang, W.W.; Zheng, J.J.; Zhao, X.; Nagase, S. Fused-Pentagon-Configuration-Dependent Electron Transfer of Monotitanium-Encapsulated Fullerenes. Inorg. Chem. 2017, 56, 6890–6896. [Google Scholar] [CrossRef]
- Jin, P.; Li, Y.; Magagula, S.; Chen, Z. Exohedral functionalization of endohedral metallofullerenes: Interplay between inside and outside. Coord. Chem. Rev. 2019, 388, 406–439. [Google Scholar] [CrossRef]
- Shen, W.; Hu, S.; Lu, X. Endohedral metallofullerenes: New structures and unseen phenomena. Chem. Eur. J. 2020, 26, 5748–5757. [Google Scholar] [CrossRef]
- Li, M.; Zhao, R.; Dang, J.; Zhao, X. Theoretical study on the stabilities, electronic structures, and reaction and formation mechanisms of fullerenes and endohedral metallofullerenes. Coord. Chem. Rev. 2022, 471, 214762. [Google Scholar] [CrossRef]
- Shen, W.; Bao, L.; Lu, X. Endohedral Metallofullerenes: An Ideal Platform of Sub-Nano Chemistry. Chin. J. Chem. 2022, 40, 275–284. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Ullah, A.; Gutiérrez-Finol, G.M.; Bedoya-Pinto, A.; Gargiani, P.; Shi, D.; Yang, S.; Shi, Z.; Gaita-Ariño, A.; et al. High-temperature magnetic blocking in a monometallic dysprosium azafullerene single-molecule magnet. Chem 2023, 9, 3613–3622. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Morales-Martínez, R.; Zhong, J.; de Graaf, C.; Rodríguez-Fortea, A.; Poblet, J.M.; Echegoyen, L.; Feng, L.; Chen, N. U2@Ih(7)-C80: Crystallographic Characterization of a Long-Sought Dimetallic Actinide Endohedral Fullerene. J. Am. Chem. Soc. 2018, 140, 3907–3915. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Roselló, Y.; Yao, Y.R.; Zhuang, J.; Zhang, X.; Rodríguez-Fortea, A.; de Graaf, C.; Echegoyen, L.; Poblet, J.M.; Chen, N. U2N@Ih(7)-C80: Fullerene Cage Encapsulating an Unsymmetrical U (IV) = N = U (V) Cluster. Chem. Sci. 2021, 12, 282–292. [Google Scholar] [CrossRef]
- Junghans, K.; Rosenkranz, M.; Popov, A.A. Sc3CH@C80: Selective 13C enrichment of the central carbon atom. Chem. Commun. 2016, 52, 6561–6564. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Tan, K.; Nie, M.; Lu, Y.; Zhang, J.; Wang, C.; Lu, X.; Wang, T. Scandium Tetrahedron Supported by H Anion and CN Pentaanion inside Fullerene C80. Inorg. Chem. 2020, 59, 8284–8290. [Google Scholar] [CrossRef]
- Fuertes-Espinosa, C.; Gómez-Torres, A.; Morales-Martínez, R.; Rodríguez-Fortea, A.; García-Simón, C.; Gándara, F.; Imaz, I.; Juanhuix, J.; Maspoch, D.; Poblet, J.M.; et al. Purification of Uranium-based Endohedral Metallofullerenes (EMFs) by Selective Supramolecular Encapsulation and Release. Angew. Chem. 2018, 130, 11464–11469. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, L.; Ji, J.; Hou, Q.; Li, L.; Jin, P. Undiscovered Effect of C ↔ N Interchange Inside the Metal Carbonitride Clusterfullerenes: A Density Functional Theory Investigation. Inorg. Chem. 2020, 59, 6518–6527. [Google Scholar] [CrossRef]
- Wei, T.; Wang, S.; Lu, X.; Tan, Y.; Huang, J.; Liu, F.; Li, Q.; Xie, S.; Yang, S. Entrapping a group-VB transition metal, vanadium, within an endohedral metallofullerene: V x Sc3–x N@ I h-C80 (x= 1, 2). J. Am. Chem. Soc. 2016, 138, 207–214. [Google Scholar] [CrossRef]
- Takata, M.; Umeda, B.; Nishibori, E.; Sakata, M.; Saitot, Y.; Ohno, M.; Shinohara, H. Confirmation by X-ray diffraction of the endohedral nature of the metallofullerene Y@C82. Nature 1995, 377, 46–49. [Google Scholar] [CrossRef]
- Shinohara, H.; Sato, H.; Saito, Y.; Ohkohchi, M.; Ando, Y. Mass spectroscopic and ESR characterization of soluble yttrium-containing metallofullerenes YC82 and Y2C82. J. Phys. Chem. 2002, 96, 3571–3573. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, Y.; Wang, Y.; Zhou, T.; Shi, Z.; Omachi, H.; Shinohara, H.; Sun, B.; Wang, Z. Turning on the Near-Infrared Photoluminescence of Erbium Metallofullerenes by Covalent Modification. Inorg. Chem. 2019, 58, 14325–14330. [Google Scholar] [CrossRef]
- Lu, X.; Akasaka, T.; Nagase, S. Chemistry of endohedral metallofullerenes: The role of metals. Chem. Commun. 2011, 47, 5942–5957. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Liu, M.T.; Nagase, S.; Akasaka, T. New horizons in chemical functionalization of endohedral metallofullerenes. Molecules 2020, 25, 3626. [Google Scholar] [CrossRef]
- Akasaka, T.; Wakahara, T.; Nagase, S.; Kobayashi, K.; Waelchli, M.; Yamamoto, K.; Kondo, M.; Shirakura, S.; Okubo, S.; Maeda, Y.; et al. La@ C82 anion. An unusually stable metallofullerene. J. Am. Chem. Soc. 2000, 122, 9316–9317. [Google Scholar] [CrossRef]
- Feng, L.; Nakahodo, T.; Wakahara, T.; Tsuchiya, T.; Maeda, Y.; Akasaka, T.; Kato, T.; Horn, E.; Yoza, K.; Mizorogi, N.; et al. A singly bonded derivative of endohedral metallofullerene: La@ C82CBr (COOC2H5) 2. J. Am. Chem. Soc. 2005, 127, 17136–17137. [Google Scholar] [CrossRef]
- García-Hernández, D.A.; Manchado, A.; Cataldo, F. Hydrogenation of [Li@C60]PF6: A comparison with fulleranes derived from C60. Fuller. Nanotub. Carbon Nanostruct. 2022, 30, 1245–1251. [Google Scholar]
- Kobayashi, K.; Nagase, S.; Akasaka, T. A theoretical study of C80 and La2@C80. Chem. Phys. Lett. 1995, 245, 230–236. [Google Scholar] [CrossRef]
- Wang, Z.; Kitaura, R.; Shinohara, H. Metal-dependent stability of pristine and functionalized unconventional dimetallofullerene M2@Ih-C80. J. Phys. Chem. C 2014, 118, 13953–13958. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.; Zhang, J.; Wang, Z. Endohedral metallofullerene M2@C80: A new class of magnetic superhalogen. J. Phys. Chem. C 2019, 124, 2131–2136. [Google Scholar] [CrossRef]
- Liu, F.; Velkos, G.; Krylov, D.S.; Spree, L.; Zalibera, M.; Ray, R.; Samoylova, N.A.; Chen, C.H.; Rosenkranz, M.; Schiemenz, S.; et al. Air-stable redox-active nanomagnets with lanthanide spins radical-bridged by a metal–metal bond. Nat. Commun. 2019, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Dunsch, L. Isolation and characterisation of two Sc3N@C80 isomers. ChemPhysChem 2004, 5, 1445–1449. [Google Scholar] [CrossRef]
- Kurihara, H.; Iiduka, Y.; Rubin, Y.; Waelchli, M.; Mizorogi, N.; Slanina, Z.; Tsuchiya, T.; Nagase, S.; Akasaka, T. Unexpected formation of a Sc3C2@C80 bisfulleroid derivative. J. Am. Chem. Soc. 2012, 134, 4092–4095. [Google Scholar] [CrossRef]
- Wang, T.S.; Chen, N.; Xiang, J.F.; Li, B.; Wu, J.Y.; Xu, W.; Jiang, L.; Tan, K.; Shu, C.Y.; Lu, X.; et al. Russian-doll-type metal carbide endofullerene: Synthesis, isolation, and characterization of Sc4C2@C80. J. Am. Chem. Soc. 2009, 131, 16646–16647. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Shen, W.; Bao, L.; Pan, C.; Slanina, Z.; Lu, X. Trapping an unprecedented Ti3C3 unit inside the icosahedral C80 fullerene: A crystallographic survey. Chem. Sci. 2019, 10, 10925–10930. [Google Scholar] [CrossRef]
- Jin, F.; Xin, J.; Guan, R.; Xie, X.M.; Chen, M.; Zhang, Q.; Popov, A.A.; Xie, S.Y.; Yang, S. Stabilizing a three-center single-electron metal–metal bond in a fullerene cage. Chem. Sci. 2021, 12, 6890–6895. [Google Scholar] [CrossRef] [PubMed]
- Tagmatarchis, N.; Aslanis, E.; Prassides, K.; Shinohara, H. Mono-, di- and trierbium endohedral metallofullerenes: Production, separation, isolation, and spectroscopic study. Chem. Mater. 2001, 13, 2374–2379. [Google Scholar] [CrossRef]
- Yang, S.; Dunsch, L. Di-and tridysprosium endohedral metallofullerenes with cages from C94 to C100. Angew. Chem. Int. Ed. 2006, 45, 1299–1302. [Google Scholar] [CrossRef]
- Guo, Y.J.; Zheng, H.; Yang, T.; Nagase, S.; Zhao, X. Theoretical Insight into the Ambiguous Endohedral Metallofullerene Er3C74: Covalent Interactions among Three Lanthanide Atoms. Inorg. Chem. 2015, 54, 8066–8076. [Google Scholar] [CrossRef]
- Lian, Y.; Shi, Z.; Zhou, X.; Gu, Z. Different extraction Behaviors between divalent and trivalent endohedral metallofullerenes. Chem. Mater. 2004, 16, 1704–1714. [Google Scholar] [CrossRef]
- Popov, A.A.; Zhang, L.; Dunsch, L. A pseudoatom in a cage: Trimetallofullerene Y3@C80 mimics Y3N@C80 with nitrogen substituted by a pseudoatom. ACS Nano 2010, 4, 795–802. [Google Scholar] [CrossRef]
- Xu, W.; Feng, L.; Calvaresi, M.; Liu, J.; Liu, Y.; Niu, B.; Shi, Z.; Lian, Y.; Zerbetto, F. An Experimentally Observed Trimetallofullerene Sm3@Ih-C80: Encapsulation of Three Metal Atoms in a Cage without a Nonmetallic Mediator. J. Am. Chem. Soc. 2013, 135, 4187–4190. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.; Wu, Y.; Wang, Z. Ln3@C80+(Ln= lanthanide): A new class of stable metallofullerene cations with multicenter metal–metal bonding in the sub-nanometer confined space. Inorg. Chem. Front. 2022, 9, 2173–2181. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Mu, L.; Ren, J.; Kong, X. A systematic study on the generation of multimetallic lanthanide fullerene ions by laser ablation mass spectrometry. Rapid Commun. Mass Spectrom. 2018, 32, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, J.; Mu, L.; Kong, X. Metallofullerene ions of Lun (1⩽n⩽9, 50⩽2m⩽198) generated by laser ablation of graphene/LuCl3. Int. J. Mass Spectrom. 2017, 422, 105–110. [Google Scholar] [CrossRef]
- Mu, L.; Yang, S.; Feng, R.; Kong, X. Encapsulation of Platinum in Fullerenes: Is That Possible? Inorg. Chem. 2017, 56, 6035–6038. [Google Scholar] [CrossRef]
- Kong, X.; Bao, X. Formation of endohedral metallofullerene (EMF) ions of (M = La, Y, n ⩽ 6, 50 ⩽ 2 m ⩽ 194) in the laser ablation process with graphene as precursor. Rapid Commun. Mass Spectrom. 2017, 31, 865–872. [Google Scholar] [CrossRef]
- Sabirov, D.S.; Tukhbatullina, A.A.; Shepelevich, I.S. Digitalizing Structure–Symmetry Relations at the Formation of Endofullerenes in Terms of Information Entropy Formalism. Symmetry 2022, 14, 1800. [Google Scholar] [CrossRef]
- Popov, A.A.; Dunsch, L. Structure, Stability, and Cluster-Cage Interactions in Nitride Clusterfullerenes M3N@C2n(M = Sc, Y; 2n = 68-98): A Density Functional Theory Study. J. Am. Chem. Soc. 2007, 129, 11835–11849. [Google Scholar] [CrossRef]
- Jin, P.; Tang, C.; Chen, Z. Carbon atoms trapped in cages: Metal carbide clusterfullerenes. Coord. Chem. Rev. 2014, 270, 89–111. [Google Scholar] [CrossRef]
- Osawa, E. Formation Mechanism of C60 under Nonequilibrium and Irreversible Conditions—An Annotation. Fuller. Nanotub. Carbon Nanostruct. 2012, 20, 299–309. [Google Scholar] [CrossRef]
- Sabirov, D.S.; Terentyev, A.O.; Sokolov, V.I. Activation energies and information entropies of helium penetration through fullerene walls. Insights into the formation of endofullerenes nX@C60/70 (n = 1 and 2) from the information entropy approach. RSC Adv. 2016, 6, 72230–72237. [Google Scholar]
- Aoyagi, S.; Nishibori, E.; Sawa, H.; Sugimoto, K.; Takata, M.; Miyata, Y.; Kitaura, R.; Shinohara, H.; Okada, H.; Sakai, T.; et al. A layered ionic crystal of polar Li@C60 superatoms. Nat. Chem. 2010, 2, 678–683. [Google Scholar] [CrossRef]
- Aoyagi, S.; Sado, Y.; Nishibori, E.; Sawa, H.; Okada, H.; Tobita, H.; Kasama, Y.; Kitaura, R.; Shinohara, H. Rock-Salt-Type Crystal of Thermally Contracted C60 with Encapsulated Lithium Cation. Angew. Chem. Int. Ed. 2012, 124, 3433–3437. [Google Scholar] [CrossRef]
- Okada, H.; Komuro, T.; Sakai, T.; Matsuo, Y.; Ono, Y.; Omote, K.; Yokoo, K.; Kawachi, K.; Kasama, Y.; Ono, S.; et al. Preparation of endohedral fullerene containing lithium (Li@C60) and isolation as pure hexafluorophosphate salt ([Li+@C60][]). RSC Adv. 2012, 2, 10624–10631. [Google Scholar]
- Kandrashkin, Y.E.; Zaripov, R.B. Low-temperature motion of the scandium bimetal in endofullerene Sc@C80(CH2Ph). Phys. Chem. Chem. Phys. 2023, 25, 31493–31499. [Google Scholar] [CrossRef]
- Bhusal, S.; Baruah, T.; Yamamoto, Y.; Zope, R.R. Electronic structure calculation of vanadium-and scandium-based endohedral fullerenes VSc2N@C2n(2n = 70, 76, 78, 80). Int. J. Quantum. Chem. 2018, 118, e25785. [Google Scholar] [CrossRef]
- Hao, Y.; Velkos, G.; Schiemenz, S.; Rosenkranz, M.; Wang, Y.; Büchner, B.; Avdoshenko, S.M.; Popov, A.A.; Liu, F. Using internal strain and mass to modulate Dy⋯Dy coupling and relaxation of magnetization in heterobimetallic metallofullerenes DyM2N@C80 and Dy2MN@C80 (M = Sc, Y, La, Lu). Inorg. Chem. Front. 2023, 10, 468–484. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Savin, A.; Nesper, R.; Wengert, S.; Fässler, T.F. ELF: The electron localization function. Angew. Chem. Int. Ed. Engl. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
| Molecular Formula | Relative Energy |
|---|---|
| Y2Dy@Ih-31924C80 | 0.00 |
| Y2Dy@D5h-31923C80 | 12.04 |
| Y2Dy@C2v-31922C80 | 17.13 |
| Y2Dy@C1-28325C80 | 19.50 |
| Y2Dy@C2-29591C80 | 24.54 |
| Y2Dy@C2v-31920C80 | 25.58 |
| Y2Dy@C1-31876C80 | 28.06 |
| Y2Dy@C2-28319C80 | 29.20 |
| Y2Dy@C1-28324C80 | 30.50 |
| Molecular Formula | Relative Energy |
|---|---|
| YDy2@Ih-31924C80 | 0.00 |
| YDy2@D5h-31923C80 | 12.08 |
| YDy2@C2v-31922C80 | 18.67 |
| YDy2@C1-28325C80 | 23.05 |
| YDy2@C2-29591C80 | 27.81 |
| YDy2@C2v-31920C80 | 28.11 |
| YDy2@C1-31876C80 | 29.76 |
| YDy2@C2-28319C80 | 30.49 |
| YDy2@C1-28314C80 | 32.32 |
| Molecular Formula | Relative Energy |
|---|---|
| Y2Dy@Ih-C80 | 0.00 |
| Y2DyC2@C2-22010C78 | 42.95 |
| Y2DyC2@C1-21975C78 | 59.17 |
| Y2DyC2@C2v-24107C78 | 59.25 |
| Y2DyC2@C2v-24088C78 | 71.57 |
| Y2DyC2@D3h-24109C78 | 90.81 |
| Molecular Formula | Relative Energy |
|---|---|
| YDy2@Ih-C80 | 0.00 |
| YDy2C2@C2-22010C78 | 49.10 |
| YDy2C2@C2v-24107C78 | 63.04 |
| YDy2C2@C1-21975C78 | 65.24 |
| YDy2C2@C2v-24088C78 | 78.04 |
| YDy2C2@D3h-24109C78 | 95.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhou, Z.; Wang, Z. Stability and Electronic Properties of Mixed Rare-Earth Tri-Metallofullerenes YxDy3-x@C80 (x = 1 or 2). Molecules 2024, 29, 447. https://doi.org/10.3390/molecules29020447
Wu Y, Zhou Z, Wang Z. Stability and Electronic Properties of Mixed Rare-Earth Tri-Metallofullerenes YxDy3-x@C80 (x = 1 or 2). Molecules. 2024; 29(2):447. https://doi.org/10.3390/molecules29020447
Chicago/Turabian StyleWu, Yabei, Zhonghao Zhou, and Zhiyong Wang. 2024. "Stability and Electronic Properties of Mixed Rare-Earth Tri-Metallofullerenes YxDy3-x@C80 (x = 1 or 2)" Molecules 29, no. 2: 447. https://doi.org/10.3390/molecules29020447
APA StyleWu, Y., Zhou, Z., & Wang, Z. (2024). Stability and Electronic Properties of Mixed Rare-Earth Tri-Metallofullerenes YxDy3-x@C80 (x = 1 or 2). Molecules, 29(2), 447. https://doi.org/10.3390/molecules29020447





