Experimental and DFT Study of Monensinate and Salinomycinate Complexes Containing {Fe3(µ3–O)}7+ Core
Abstract
1. Introduction
2. Results and Discussion
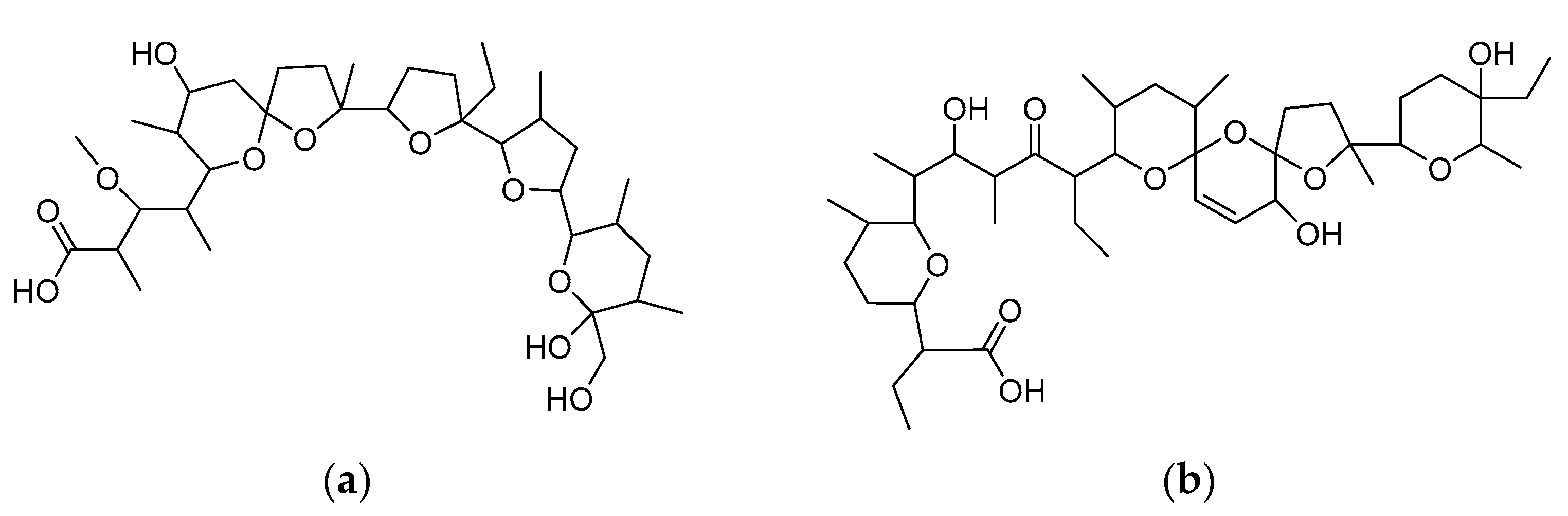
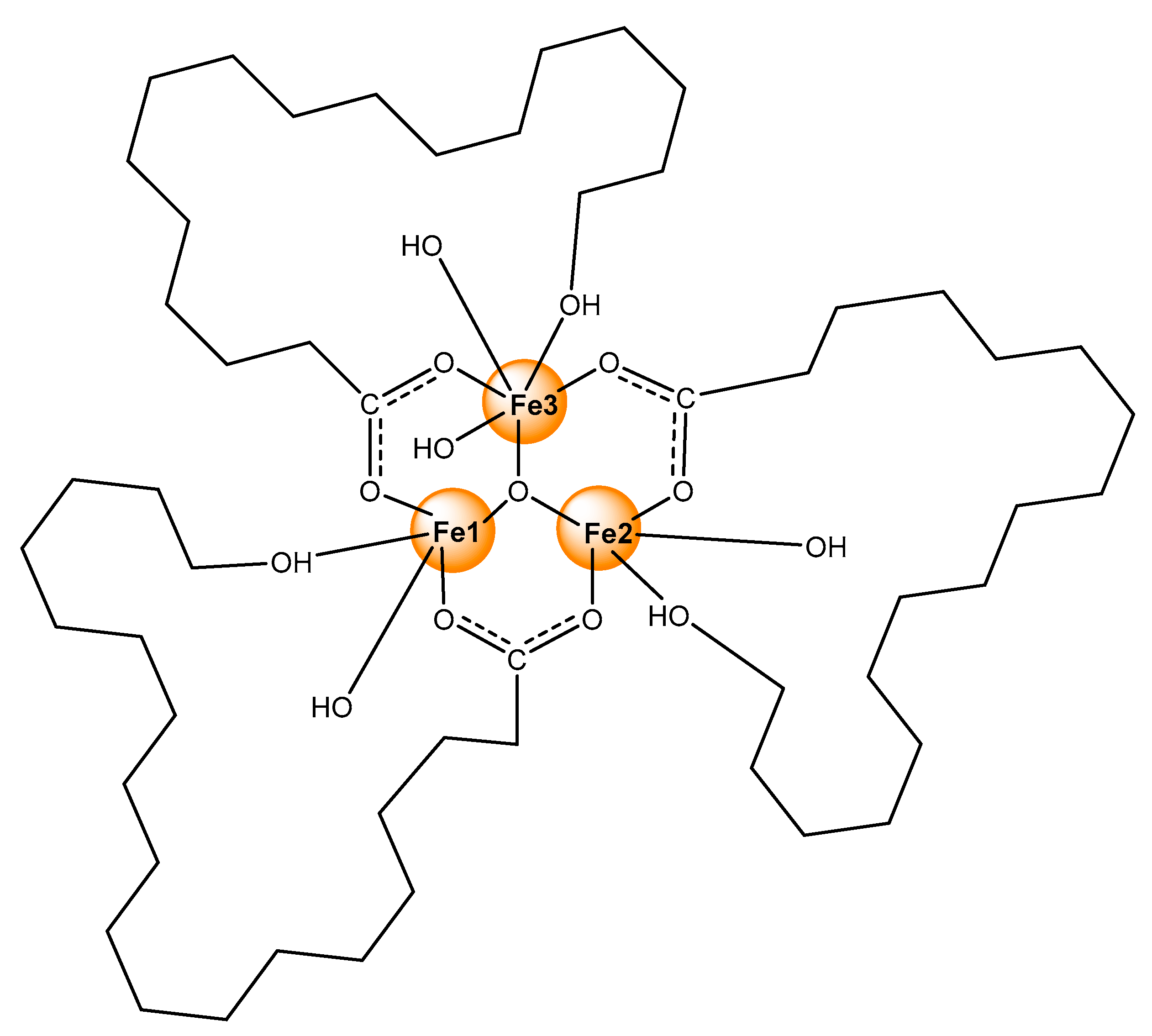
2.1. General
2.2. Elemental and Thermal Analysis
2.3. IR Spectroscopy
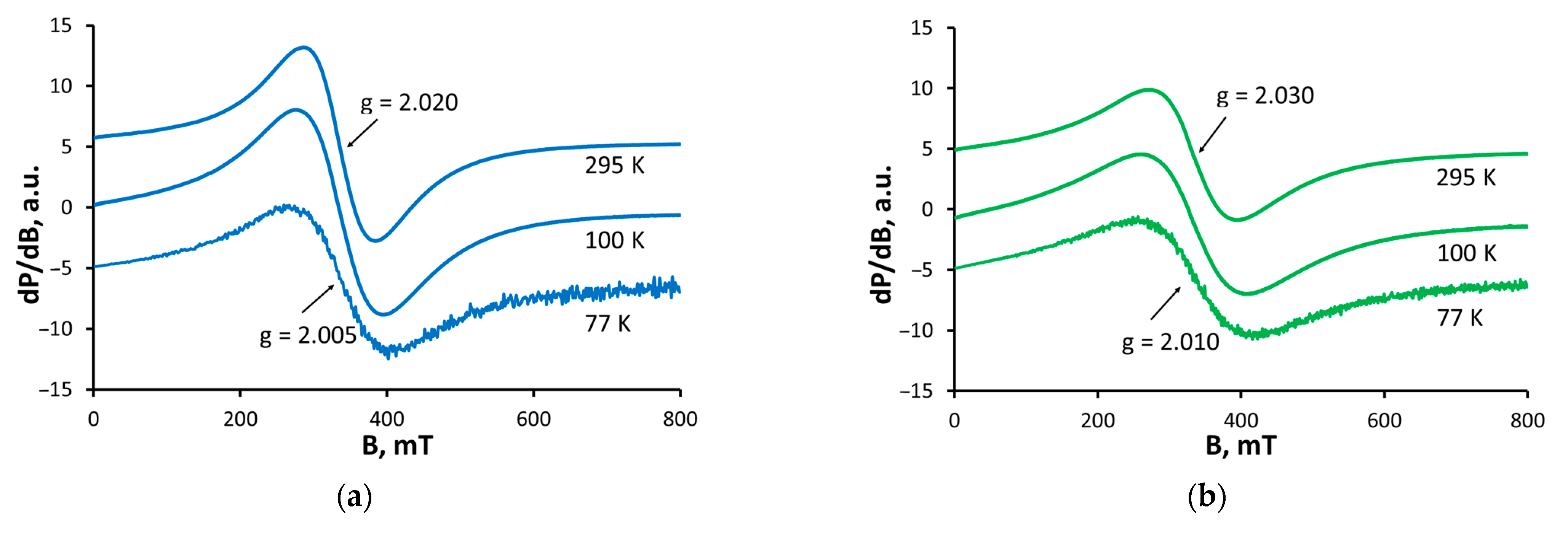
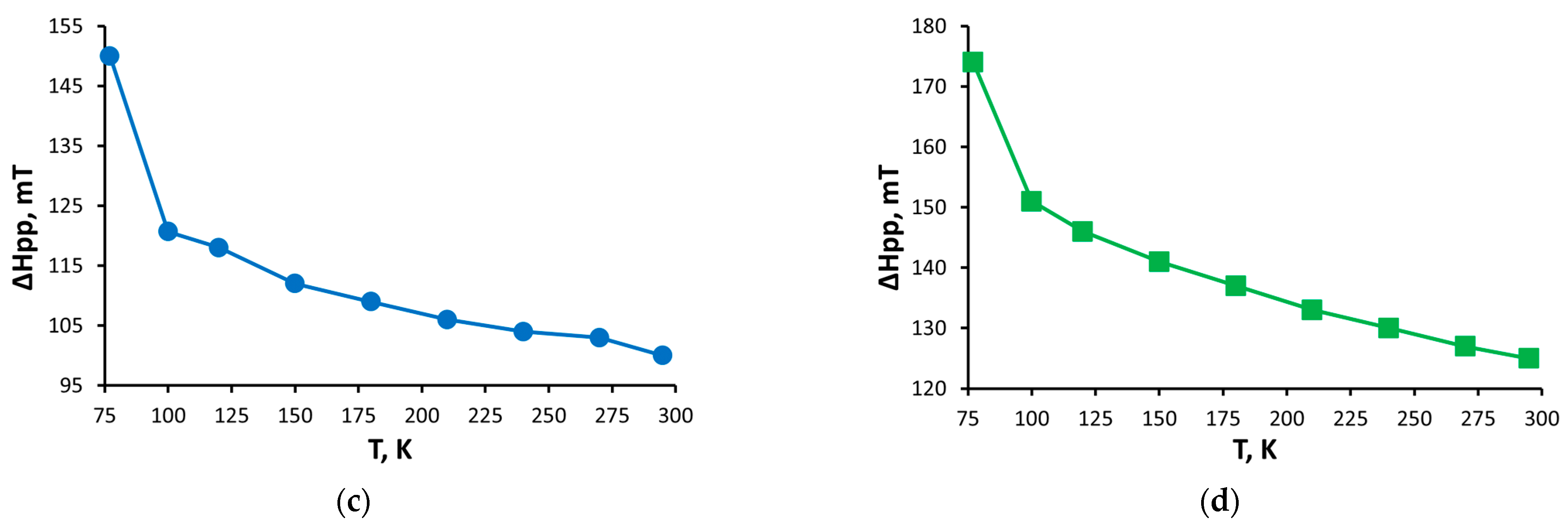
2.4. EPR Spectroscopy
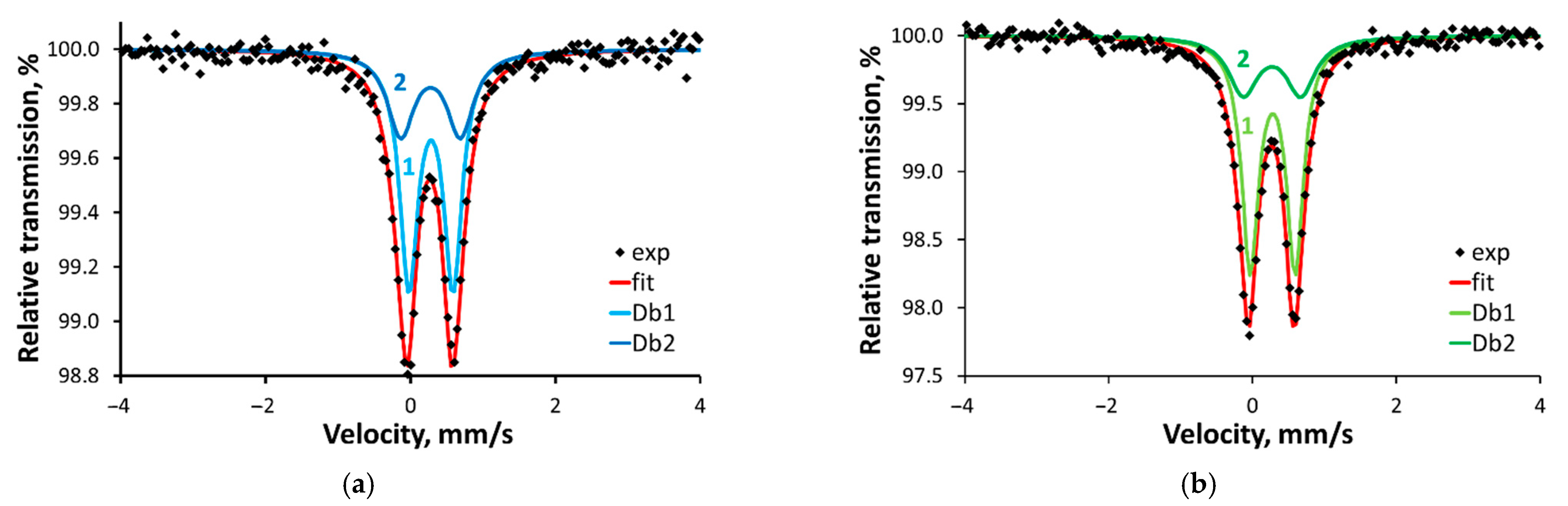
2.5. Mössbauer Spectroscopy
- (i)
- The antibiotics undergo deprotonation and act as bridging monoanions (IR);
- (ii)
- No water ligands are included in the primary coordination shell of the metal ions (TGV);
- (iii)
- An antiferromagnetic interaction between the metal centres occurs that can be realized within a triangular oxo-iron cluster (EPR);
- (iv)
- The three high-spin iron ions are inequivalent and possess non-symmetric charge distribution at 2:1 ratio (Mössbauer spectroscopy).
2.6. Theoretical Studies
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of Complexes 1–2
3.3. Spectroscopic Studies
3.4. Quantum Chemical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaaban, T.; Mohsen, Y.; Ezzeddine, Z.; Ghssein, G. Overview of Yersinia pestis metallophores: Yersiniabactin and Yersinopine. Biology 2023, 12, 598. [Google Scholar] [CrossRef]
- Chifman, J.; Laubenbacher, R.; Torti, S.V. A systems biology approach to iron metabolism. Adv. Exp. Med. Biol. 2014, 844, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Metalloproteomics, metalloproteomes, and the annotation of metalloproteins. Metallomics 2010, 2, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Crichton, R.R.; Pierre, J.L. Old iron, young copper: From Mars to Venus. BioMetals 2001, 14, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Archibald, F. Lactobacillus plantarum, an organism not requiring iron. FEMS Microbio. Lett. 1983, 19, 29–32. [Google Scholar] [CrossRef]
- Pierre, J.L.; Fontecave, M.; Crichton, R.R. Chemistry for an essential biological process: The reduction of ferric iron. BioMetals 2002, 15, 341–346. [Google Scholar] [CrossRef]
- Paterson, S.; Armstrong, N.J.; Iacopetta, B.J.; McArdle, H.J.; Morgan, E.H. lntravesicular pH and iron uptake by immature erythroid cells. J. Cell. Physiol. 1984, 120, 225–232. [Google Scholar] [CrossRef]
- Page, M.G.P. The role of iron and siderophores in infection, and the development of siderophore antibiotics. Clin. Infect. Dis. 2019, 69, S529–S537. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Yang, M.; Dong, X. Recent progress in ferroptosis inducers for cancer therapy. Adv. Mater. 2019, 31, e1904197. [Google Scholar] [CrossRef]
- Qiu, Y.; Cao, Y.; Cao, W.; Jia, Y.; Lu, N. The application of ferroptosis in diseases. Pharmacol. Res. 2020, 159, 104919. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Pan, S.T. ROS-mediated therapeutic strategy in chemo-/radiotherapy of head and neck cancer. Oxid. Med. Cell. Longev. 2020, 2020, 5047987. [Google Scholar] [CrossRef]
- Guo, Q.; Li, L.; Hou, S.; Yuan, Z.; Li, C.; Zhang, W.; Zheng, L.; Li, X. The role of iron in cancer progression. Front. Oncol. 2021, 11, 778492. [Google Scholar] [CrossRef]
- Sacco, A.; Battaglia, A.M.; Botta, C.; Aversa, I.; Mancuso, S.; Costanzo, F.; Biamonte, F. Iron metabolism in the tumor microenvironment—Implications for anti-cancer immune response. Cells 2021, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Cosialls, E.; El Hage, R.; Dos Santos, L.; Gong, C.; Mehrpour, M.; Hamai, A. Ferroptosis: Cancer stem cells rely on iron until “to die for” it. Cells 2021, 10, 2981. [Google Scholar] [CrossRef]
- Yan, H.F.; Zou, T.; Tuo, Q.Z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and links with diseases. Signal Transduct. Target Ther. 2021, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.L.; Chen, J.X.; Zhu, P.; Zhang, C.B.; Zhou, Y.; Duan, J.X. Focus on ferroptosis regulation: Exploring novel mechanisms and applications of ferroptosis regulator. Life Sci. 2022, 307, 120868. [Google Scholar] [CrossRef] [PubMed]
- Antoszczak, M.; Muller, S.; Caneque, T.; Colombeau, L.; Dusetti, N.; Santofimia-Castano, P.; Gaillet, C.; Puisieux, A.; Iovanna, J.L.; Rodriguez, R. Iron-sensitive prodrugs that trigger active ferroptosis in drug-tolerant pancreatic cancer cells. J. Am. Chem. Soc. 2022, 144, 11536–11545. [Google Scholar] [CrossRef]
- Wu, Z.; Zhong, M.; Liu, Y.; Xiong, Y.; Gao, Z.; Ma, J.; Zhuang, G.; Hong, X. Application of natural products for inducing ferroptosis in tumor cells. Biotechnol. Appl. Biochem. 2022, 69, 190–197. [Google Scholar] [CrossRef]
- Renshaw, J.C.; Robson, G.D.; Trinci, A.P.J.; Wiebe, M.G.; Livens, F.R.; Collison, D.; Taylor, R.J. Fungal siderophores: Structures, functions and applications. Mycol. Res. 2002, 106, 1123–1142. [Google Scholar] [CrossRef]
- De Lorenzo, V.; Martinez, J.L. Aerobactin production as a virulence factor: A reevaluation. Eur. J. Clin. Microbiol. Infect. Dis. 1988, 7, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Uptake of iron-siderophore complexes across the bacterial outer membrane. Trends Microbiol. 1993, 1, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Dreechsel, H.; Jung, G. Peptide siderophores. J. Peptide Sci. 1998, 4, 147–181. [Google Scholar] [CrossRef]
- De Luca, N.G.; Wood, P.M. Iron uptake by fungi: Contrasted mechanisms with internal or external reduction. Adv. Microb. Physiol. 2000, 43, 39–74. [Google Scholar] [CrossRef]
- Haas, H.; Eisendle, M.; Turgeon, B.G. Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 2008, 46, 149–187. [Google Scholar] [CrossRef] [PubMed]
- Holinsworth, B.; Martin, J.D. Siderophore production by marine-derived fungi. Biometals 2009, 22, 625–632. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef]
- Codd, R.; Richardson-Sanchez, T.; Telfer, T.J.; Gotsbacher, M.P. Advances in the chemical biology of desferrioxamine B. ACS Chem. Biol. 2018, 13, 11–25. [Google Scholar] [CrossRef]
- Li, Z.; Chen, L.; Chen, C.; Zhou, Y.; Hu, D.; Yang, J.; Chen, Y.; Zhuo, W.; Mao, M.; Zhang, X.; et al. Targeting ferroptosis in breast cancer. Biomark. Res. 2020, 8, 58. [Google Scholar] [CrossRef]
- Hu, H.; Xu, Q.; Mo, Z.; Hu, X.; He, Q.; Zhang, Z.; Xu, Z. New anti-cancer explorations based on metal ions. J. Nanobiotechn. 2022, 20, 457. [Google Scholar] [CrossRef]
- Antoszczak, M.; Huczynski, A. Salinomycin and its derivatives—A new class of multiple-targeted “magic bullets”. Eur. J. Med. Chem. 2019, 176, 208–227. [Google Scholar] [CrossRef] [PubMed]
- Tefas, L.R.; Barbalata, C.; Tefas, C.; Tomuta, I. Salinomycin-based drug delivery systems: Overcoming the hurdles in cancer therapy. Pharmaceutics 2021, 13, 1120. [Google Scholar] [CrossRef] [PubMed]
- Petkov, N.; Pantcheva, I.; Ivanova, A.; Stoyanova, R.; Kukeva, R.; Alexandrova, R.; Abudalleh, A.; Dorkov, P. Novel cerium(IV) coordination compounds of monensin and salinomycin. Molecules 2023, 28, 4676. [Google Scholar] [CrossRef]
- Amani, V.; Safari, N.; Khavasi, H.R. Solution and solid state characterization of oxo-centered trinuclear iron(III) acetate complexes [Fe3(µ3-O)(µ-OAc)6(L)3]+. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 85, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kiana, S.; Yazdanbakhsh, M.; Jamialahmadi, M.; Tayyari, S.F. Vibrational assignment and structure of trinuclear oxo-centered of basic formate iron(III) and chromium(III) complexes: A density functional theory study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 287–294. [Google Scholar] [CrossRef]
- Dimiza, F.; Hatzidimitriou, A.G.; Sanakis, Y.; Papadopoulos, A.N.; Psomas, G. Trinuclear and tetranuclear iron(III) complexes with fenamates: Structure and biological profile. J. Inorg. Biochem. 2021, 218, 111410. [Google Scholar] [CrossRef]
- Feng, Q.; Li, B.; Du, R.; Jiang, F.; Liu, T. Syntheses, crystal structures, spectroscopic and magnetic properties of two trinuclear iron complexes with carboxylate and oximate mixed-bridge ligands. Trans. Met. Chem. 2019, 44, 49–55. [Google Scholar] [CrossRef]
- Chakradhar, R.P.; Sivaramaiah, G.; Rao, J.L.; Gopal, N.O. Fe3+ ions in alkali lead tetraborate glasses—An electron paramagnetic resonance and optical study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 62, 51–57. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Gibb, T.C. Mossbauer Spectroscopy; Springer: Dordrecht, The Netherlands, 1971. [Google Scholar] [CrossRef]
- Murray, K.S. Binuclear oxo-bridged iron(III) complexes. Coord. Chem. Rev. 1974, 12, 1–35. [Google Scholar] [CrossRef]
- Bill, E.; Krebs, C.; Winter, M.; Gerdan, M.; Trautwein, A.X.; Florke, U.; Haupt, H.-J.; Chaudhuri, P. A triangular iron(III) complex potentially relevant to iron(III)-binding sites in ferreascidin. Chem. Eur. J. 1997, 3, 193–201. [Google Scholar] [CrossRef]
- Dorkov, P.; Pantcheva, I.N.; Sheldrick, W.S.; Mayer-Figge, H.; Petrova, R.; Mitewa, M. Synthesis, structure and antimicrobial activity of manganese(II) and cobalt(II) complexes of the polyether ionophore antibiotic sodium monensin A. J. Inorg. Biochem. 2008, 102, 26–32. [Google Scholar] [CrossRef]
- Pantcheva, I.N.; Zhorova, R.; Mitewa, M.; Simova, S.; Mayer-Figge, H.; Sheldrick, W.S. First solid state alkaline-earth complexes of monensic acid A (MonH): Crystal structure of [M(Mon)2(H2O)2] (M = Mg, Ca), spectral properties and cytotoxicity against aerobic Gram-positive bacteria. Biometals 2010, 23, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Bijelić, J.; Gašo-Sokač, D.; Djerdj, I. Single crystal growth, structural characterization and magnetic properties study of an antiferromagnetic trinuclear iron(III) acetate complex with uncoordinated hexamine. Inorg. Chim. Acta 2021, 520, 120292. [Google Scholar] [CrossRef]
- Raptopoulou, C.P.; Sanakis, Y.; Boudalis, A.K.; Psycharis, V. Salicylaldoxime (H2salox) in iron(III) carboxylate chemistry: Synthesis, X-ray crystal structure, spectroscopic characterization and magnetic behavior of trinuclear oxo-centered complexes. Polyhedron 2005, 24, 711–721. [Google Scholar] [CrossRef]
- Boudalis, A.K.; Sanakis, Y.; Raptopoulou, C.P.; Terzis, A.; Tuchagues, J.-P.; Perlepes, S.P. A trinuclear cluster containing the {Fe3(µ3-O)}7+ core: Structural, magnetic and spectroscopic (IR, Mössbauer, EPR) studies. Polyhedron 2005, 24, 1540–1548. [Google Scholar] [CrossRef]
- Lalia-Kantouri, M.; Papadopoulos, C.; Hatzidimitriou, A.; Bakas, T.; Pachin, S. A trinuclear iron(III) complex containing the semi-cubane [Fe3(μ3-O)]7+ core: Structural, spectroscopic, magnetic and electrochemical study. Z. Anorg. Allg. Chem. 2010, 636, 531–538. [Google Scholar] [CrossRef]
- Pap, J.S.; Kaizer, J.; Giorgi, M.; Speier, G. Molecular and electronic structure of a trinuclear oxo-centred iron(III) complex with trigonal bipyramidal metal sites. Inorg. Chem. Comm. 2010, 13, 1069–1073. [Google Scholar] [CrossRef]
- Gertenbach, P.G.; Popov, A.I. Solution chemistry of monensin and its alkali metal ion complexes. Potentiometric and spectroscopic studies. J. Am. Chem. Soc. 1975, 97, 4738–4744. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Zhao, H.; Fang, C.; Gao, J.; Liu, C. Spin-state energies of heme-related models from spin-flip TDDFT calculations. Phys. Chem. Chem. Phys. 2016, 18, 29486–29494. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ratner, M.A.; Schatz, G.C. Computational modeling of octahedral iron oxide clusters: Hexaaquairon(III) and its dimers. J. Phys. Chem. C 2013, 117, 21706–21717. [Google Scholar] [CrossRef]
- Neese, F. Prediction and interpretation of the 57Fe isomer shift in Mössbauer spectra by density functional theory. Inorg. Chim. Acta 2002, 337, 181–192. [Google Scholar] [CrossRef]



| Complex | Composition | MW, g/mol | C, % | H, % | Fe, % | |||
|---|---|---|---|---|---|---|---|---|
| Calc. | Found | Calc. | Found | Calc. | Found | |||
| [Fe3(µ3-O)Mon3(OH)4], 1 | C108H187Fe3O38 | 2261.18 | 57.37 | 56.33 | 8.34 | 7.35 | 7.41 | 7.39 |
| [Fe3(µ3-O)Sal3(OH)4], 2 | C126H211Fe3O38 | 2501.57 | 60.50 | 60.12 | 8.50 | 8.44 | 6.70 | 6.69 |
| Compound | Component | δ, mm/s | ∆, mm/s | Γ, mm/s | A, % | M, % |
|---|---|---|---|---|---|---|
| 1 | Db1 | 0.39 (0.59) | 0.61 (0.59) | 0.30 (0.28) | 65 (66) | 0.86 (5.21) |
| Db2 | 0.39 (0.58) | 0.82 (0.84) | 0.45 (0.33) | 35 (34) | 0.31 (2.25) | |
| 2 | Db1 | 0.39 (0.58) | 0.63 (0.61) | 0.28 (0.29) | 70 (65) | 1.69 (4.70) |
| Db2 | 0.38 (0.57) | 0.80 (0.93) | 0.49 (0.38) | 30 (35) | 0.42 (1.95) |
| Bond | Fe(1) | Fe(2) | Fe(3) |
|---|---|---|---|
| μ3-O−Fe | 1.9 | 1.9 | 2.0 |
| COO−−Fe | 2.0 | 2.0 | 2.1 |
| 2.1 | 2.1 | 2.1 | |
| ROH−Fe | 2.1 | 1.9 | 2.1 |
| OH−−Fe | 1.9 | 1.9 | 1.9 |
| − | − | 2.1 |
| Multiplicity | ΔG, kcal/mol 293 K | Population, % 293 K | Population, % 77 K | g-Factor 0 K |
|---|---|---|---|---|
| HS | 2.15 | 2.10 | 0.00 | 2.005 |
| AFM(Sx_1 + Sx_2) | 0.00 | 83.8 | 99.9 | 2.005 |
| AFMSx_3 | 1.04 | 14.1 | 0.10 | 2.006 |
| Centre/Spin State | HS | AFMSx_1 | AFMSx_2 | AFMSx_3 |
|---|---|---|---|---|
| Fe(1) | 4.43 | –4.42 | 4.42 | 4.42 |
| Fe(2) | 4.43 | 4.43 | –4.43 | 4.43 |
| Fe(3) | 4.49 | 4.48 | 4.49 | –4.48 |
| μ3–O | 0.40 | 0.02 | 0.11 | 0.27 |
| Fe(1) | Fe(2) | Fe(3) | |
|---|---|---|---|
| Fe | 2.18 | 2.19 | 2.24 |
| μ3–O | –1.46 | –1.46 | –1.46 |
| COO− | –0.82 | –0.83 | –0.82 |
| COO− | –0.84 | –0.84 | –0.83 |
| ROH | –0.85/–0.31 * | –0.83/–0.30 * | –0.97/–0.29 * |
| OH− | –1.17/–0.70 * | –1.18/–0.71 * | –1.19/–0.71 * |
| OH− | – | – | –1.02/–0.65 * |
| Vibration | Monensinate Complex | Salinomycinate Complex | ||||
|---|---|---|---|---|---|---|
| Exp. | Calc. | SF | Exp. | Calc. | SF | |
| , COO¯ | 1530 | 1602 | 0.96 | 1522 | 1533 | 0.99 |
| , COO¯ | 1418 | 1501 | 0.94 | 1419 | 1436 | 0.99 |
| 112 | 101 | − | 103 | 97 | − | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkov, N.; Tadjer, A.; Encheva, E.; Cherkezova-Zheleva, Z.; Paneva, D.; Stoyanova, R.; Kukeva, R.; Dorkov, P.; Pantcheva, I. Experimental and DFT Study of Monensinate and Salinomycinate Complexes Containing {Fe3(µ3–O)}7+ Core. Molecules 2024, 29, 364. https://doi.org/10.3390/molecules29020364
Petkov N, Tadjer A, Encheva E, Cherkezova-Zheleva Z, Paneva D, Stoyanova R, Kukeva R, Dorkov P, Pantcheva I. Experimental and DFT Study of Monensinate and Salinomycinate Complexes Containing {Fe3(µ3–O)}7+ Core. Molecules. 2024; 29(2):364. https://doi.org/10.3390/molecules29020364
Chicago/Turabian StylePetkov, Nikolay, Alia Tadjer, Elzhana Encheva, Zara Cherkezova-Zheleva, Daniela Paneva, Radostina Stoyanova, Rositsa Kukeva, Petar Dorkov, and Ivayla Pantcheva. 2024. "Experimental and DFT Study of Monensinate and Salinomycinate Complexes Containing {Fe3(µ3–O)}7+ Core" Molecules 29, no. 2: 364. https://doi.org/10.3390/molecules29020364
APA StylePetkov, N., Tadjer, A., Encheva, E., Cherkezova-Zheleva, Z., Paneva, D., Stoyanova, R., Kukeva, R., Dorkov, P., & Pantcheva, I. (2024). Experimental and DFT Study of Monensinate and Salinomycinate Complexes Containing {Fe3(µ3–O)}7+ Core. Molecules, 29(2), 364. https://doi.org/10.3390/molecules29020364







