Oxidative Coupling of Methane: A Review Study on the Catalytic Performance
Abstract
1. Introduction: Framing the Analysis Context
2. Catalyst Functionality and Characteristics
2.1. Reaction Mechanism
2.1.1. Mechanism-Study of the Activating Roles of Different Oxygen Species
2.1.2. Catalyst Characterization to Track and Understand the Reaction Mechanism
2.2. Catalytic Materials
2.2.1. Chemical Functional Analysis of the Active Catalytic Materials
2.2.2. Material- and Structural-Based Analysis of the Catalysts
2.2.3. Synthesis-Recipes for OCM Catalysts
2.2.4. Spot-Reaction Intensity as a Measure for Tracking the Impacts of the Catalyst Characteristics on the Catalyst Selectivity
3. Catalyst Performance Analysis
3.1. Bulk-Reaction Intensity as a Measure for Tracking the Interactive Impacts of the Operating Parameters and Dimensional Factors of the Catalytic Bed on Its Catalytic Performance
3.1.1. Dimensional Factors: Gas Hourly Space Velocity (GHSV) Reflecting the Feed Flow and Dimensional Aspects of the Catalytic Bed
3.1.2. Operating Parameter: Methane-to-Oxygen Ratio
3.1.3. Operating Parameter: Reactor- and Reaction-Temperature
3.1.4. Typical OCM Reactor Performance Affected by the Interactive Impacts of the Operating Parameters and Dimensional Factors
3.2. Practical View on Testing and Analyzing the OCM Catalytic Performance
3.2.1. Reactor Engineering: Dimensional Factors Forging Selective Performance
3.2.2. Reactor Engineering: Thermal Management and Control
4. Conclusions and Visions
4.1. Summary and Conclusions
4.2. Vision: Priorities for Future Research and Development
Author Contributions
Funding
Conflicts of Interest
References
- Ortiz-Bravo, C.A.; Chagas, C.A.; Toniolo, F.S. Oxidative coupling of methane (OCM): An overview of the challenges and opportunities for developing new technologies. J. Nat. Gas Sci. Eng. 2021, 96, 104254. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Guan, N.; Li, L. Methane Activation and Utilization: Current Status and Future Challenges. Energy Technol. 2019, 8, 1900826. [Google Scholar] [CrossRef]
- Spallina, V.; Velarde, I.C.; Jimenez, J.A.M.; Godini, H.R.; Gallucci, F.; Van Sint Annaland, M. Techno-economic assessment of different routes for olefins production through the oxidative coupling of methane (OCM): Advances in benchmark technologies. Energy Convers. Manag. 2017, 154, 244–261. [Google Scholar] [CrossRef]
- Zavyalova, U.; Holena, M.; Schlög, R.; Baerns, M. Statistical analysis of past catalytic data on oxidative methane coupling for new insight into the composition of high-performance catalysts. ChemCatChem 2011, 3, 1935–1947. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Revisiting the oxidative coupling of methane to ethylene in the golden period of shale gas: A review. J. Ind. Eng. Chem. 2016, 37, 1–13. [Google Scholar] [CrossRef]
- Keller, G.E.; Bhasin, M.M. Synthesis of ethylene via oxidative coupling of methane: Determination of active catalysts. J. Catal. 1982, 73, 9–19. [Google Scholar] [CrossRef]
- Kim, M.; Repke, J.-U.; Schomäcker, R.; Khodadadi, A.; Wozny, G.; Görke, O.; Godini, H.R. Recognition of Oxidative Coupling of Methane Reactor Performance Patterns. Chem. Eng. Technol. 2022, 45, 694–708. [Google Scholar] [CrossRef]
- Stansch, Y.; Mleczko, L.; Baerns, M. Comprehensive Kinetics of Oxidative Coupling of Methane over the La2O3/CaO Catalyst. Ind. Eng. Chem. Res. 1997, 36, 2568–2579. [Google Scholar] [CrossRef]
- Kim, M.; Arndt, S.; Yildiz, M.; Schomäcker, R.; Görke, O.; Repke, J.-U.; Wozny, G.; Godini, H.R. Reaction Engineering of Oxidative Coupling of Methane, Experimental Observations and Analysis of the Impacts of Operating Parameters. Chem. Eng. Res. Des. 2021, 172, 84–98. [Google Scholar] [CrossRef]
- Cantrell, R.D.; Ghenciu, A.; Campbell, K.D.; Minahan, D.M.A.; Bhasin, M.M.; Westwood, A.D.; Nielsen, K.A. Catalyst for the Oxidative Dehydrogenation of Hydrocarbons. US Patent 6,576,803 B2, 10 June 2003. [Google Scholar]
- Si, J.; Zhao, G.; Sun, W.; Liu, J.; Guan, C.; Yang, Y.; Shi, X.-R.; Lu, Y. Oxidative Coupling of Methane: Examining the Inactivity of the MnOx-Na2WO4/SiO2 Catalyst at Low Temperature. Angew. Chem. Int. 2022, 61, e202117201. [Google Scholar] [CrossRef]
- Xu, L.; Zanina, A.; Wu, K.; Li, J.; Chen, J.; Li, Y.; Jiang, G.; Kondratenko, E.V. Breaking the dilemma of low selectivity in the oxidative coupling of methane over Mn-Na2WO4/SiO2 at low temperatures. Chem. Eng. J. 2023, 473, 145372. [Google Scholar] [CrossRef]
- Cruellas, A.; Melchiori, T.; Gallucci, F.; van Sint Annaland, M. Advanced reactor concepts for oxidative coupling of methane. Catal. Rev. 2017, 59, 234–294. [Google Scholar] [CrossRef]
- Godini, H.R.; Kim, M.; Goerke, O.; Khadivi, M.; Schomaecker, R.; Repke, J.-U. Membrane Engineering for the Treatment of Gases Volume 2: Gas-Separation Issues Combined with Membrane Reactors, Chapter 3: Oxidative Coupling of Methane in Membrane Reactors; Royal Society of Chemistry: London, UK, 2018; ISBN 978-1-78262-875-0. [Google Scholar]
- Zhu, Z.; Guo, W.; Zhang, Y.; Pan, C.; Xu, J.; Zhu, Y.; Lou, Y. Research progress on methane conversion couplingphotocatalysis and thermocatalysis. Carbon Energy 2021, 3, 519–540. [Google Scholar] [CrossRef]
- Arinaga, A.M.; Ziegelski, M.C.; Marks, T.J. Alternative Oxidants for the Catalytic Oxidative Coupling of Methane. Angew. Chem. 2021, 60, 10502–10515. [Google Scholar] [CrossRef]
- Myrach, P.; Nilius, N.; Levchenko, S.V.; Gonchar, A.; Risse, T.; Dinse, K.-P.; Boatner, L.A.; Frandsen, W.; Horn, R.; Freund, H.J.; et al. Temperature-dependent morphology: Magnetic and optical properties of Li-doped MgO. ChemCatChem 2010, 2, 854–862. [Google Scholar] [CrossRef]
- Werny, M.J.; Wang, Y.; Girgsdies, F.; Schlögl, R.; Trunschke, A. Fluctuating Storage of the Active Phase in a Mn-Na2WO4/SiO2 Catalyst for the Oxidative Coupling of Methane. Angew. Chem. Int. Ed. 2020, 59, 14921–14926. [Google Scholar] [CrossRef] [PubMed]
- Zanina, A.; Kondratenko, V.A.; Lund, H.; Li, J.; Chen, J.; Li, Y.; Jiang, G.; Kondratenko, E.V. Elucidating the Role of Oxygen Species in Oxidative Coupling of Methane over Supported MnOx-Na2WO4-containing Catalysts. ChemCatChem 2023, 16, e202300885. [Google Scholar] [CrossRef]
- Mestl, G.; Knözinger, H.; Lunsford, J.H. High temperature in situ Raman spectroscopy of working oxidative coupling of catalysts. Berichte Bunsenges. Phys. Chem. 1993, 97, 319–321. [Google Scholar] [CrossRef]
- Lunsford, J.H.; Yang, X.; Haller, K.; Laane, M.J.G.; Knözinger, H. In situ Raman spectroscopy of peroxide ions on barium/magnesium oxide catalysts. J. Phys. Chem. 1993, 97, 13810–13813. [Google Scholar] [CrossRef]
- Pak, S.; Qiu, P.; Lunsford, J.H. Elementary Reactions in the Oxidative Coupling of Methane over Mn/Na2WO4/SiO2 and Mn/Na2WO4/MgO Catalysts. J. Catal. 1998, 179, 222–230. [Google Scholar] [CrossRef]
- Alexiadis, V.I.; Chaar, M.; van Veen, A.; Muhler, M.; Thybaut, J.W.; Marin, G.B. Quantitative screening of an extended oxidative coupling of methane catalyst library. Appl. Catal. B 2016, 199, 252–259. [Google Scholar] [CrossRef]
- Hesham, G.; Ibrahim, A.; Alagta, R. Optimal Design of Ethane Pyrolysis Reactor. Int. J. Sci. Adv. Technol. 2012, 2, 36–41. [Google Scholar]
- Li, J.; Chen, J.; Zanina, A.; Li, Y.; Yu, C.; Liu, M.; Cui, G.; Wang, Y.; Zhou, M.; Kondratenko, E.V.; et al. Fundamentals of enhanced oxygen releasability of Mn-Na2WO4/SiO2 through cofed water for efficient oxidative coupling of methane in a chemical looping mode. J. Catal. 2023, 428, 115176. [Google Scholar] [CrossRef]
- Driscoll, D.J.; Lunsford, J.H. Gas-phase radical formation during the reaction of methane, ethane, ethylene and propylene over selected oxide catalysts. J. Phys. Chem. 1985, 89, 4415–4418. [Google Scholar] [CrossRef]
- Driscoll, D.J.; Martir, W.; Wang, J.X.; Lunsford, J.H. Formation of gas-phase methyl radical over MgO. J. Am. Chem. Soc. 1985, 107, 58–63. [Google Scholar] [CrossRef]
- Louis, C.; Chang, T.L.; Kermarec, M.; Van, T.L.; Tatibouët, J.; Che, M. EPR study of the stability and the role of the O2– species on La2O3 in the oxidative coupling of methane. Catal. Today 1992, 13, 283–289. [Google Scholar] [CrossRef]
- Yang, T.; Feng, L.; Shen, S. Oxygen species on the surface of La2O3/CaO and its role in the oxidative coupling of methane. J. Catal. 1994, 145, 384–389. [Google Scholar] [CrossRef]
- Voskrenskaya, E.N.; Roguleva, V.G.; Anshits, A.G. Oxidant activation over structural defects of oxide catalyst in oxidation of methane coupling. Catal. Rev.-Sci. Eng. 1995, 37, 101–143. [Google Scholar] [CrossRef]
- Anshits, A.G.; Voskresenskaya, E.N.; Kondratenko, E.V.; Maksimov, N.G. The role of the defect structure of oxide catalysts for the oxidative coupling of methane. The activation of the oxidant. Catal. Today 1995, 24, 217–223. [Google Scholar] [CrossRef]
- Zanina, A.; Kondratenko, V.A.; Lund, H.; Li, J.; Chen, J.; Li, Y.; Jiang, G.; Kondratenko, E.V. The Role of Adsorbed and Lattice Oxygen Species in Product Formation in the Oxidative Coupling of Methane over M2WO4/SiO2 (M = Na, K, Rb, Cs). ACS Catal. 2022, 12, 15361–15372. [Google Scholar] [CrossRef]
- Dubois, J.L.; Cameron, C.J. Common features of oxidative coupling of methane co-feed catalysts. Appl. Catal. 1990, 67, 49–71. [Google Scholar] [CrossRef]
- Sourav, S.; Wang, Y.; Kiani, D.; Baltrusaitis, J.; Fushimi, R.R.; Wachs, I.E. New Mechanistic and Reaction Pathway Insights for Oxidative Coupling of Methane (OCM) over Supported Na2WO4/SiO2 Catalysts. Angew. Chem. 2021, 60, 21502–21511. [Google Scholar] [CrossRef] [PubMed]
- Gambo, Y.; Jalil, A.A.; Triwahyono, S.; Abdulrasheed, A.A. Recent advances and future prospect in catalysts for oxidative coupling of methane to ethylene: A review. J. Ind. Eng. Chem. 2018, 59, 218–229. [Google Scholar] [CrossRef]
- Liu, J.; Yue, J.; Lv, M.; Wang, F.; Cui, Y.; Zhang, Z.; Xu, G. From fundamentals to chemical engineering on oxidative coupling of methane for ethylene production: A review. Carbon Resour. Convers. 2022, 5, 1–14. [Google Scholar] [CrossRef]
- Vamvakeros, A.; Matras, D.; Jacques, S.D.M.; di Michiel, M.; Price, S.W.T.; Senecal, P.; Agote Aran, M.; Middelkoop, V.; Stenning, G.B.G.; Mosselmans, J.F.W.; et al. Real-time multi-length scale chemical tomography of fixed bed reactors during the oxidative coupling of methane reaction. J. Catal. 2020, 386, 39–52. [Google Scholar] [CrossRef]
- Matras, D.; Jacques, S.D.M.; Godini, H.R.; Khadivi, M.; Drnec, J.; Poulain, A.; Cernik, R.J.; Beale, A.M. Real-Time Operando Diffraction Imaging of La–Sr/CaO During the Oxidative Coupling of Methane. J. Phys. Chem. C 2018, 122, 2221–2230. [Google Scholar] [CrossRef]
- Zanina, A.; Kondratenko, V.A.; Makhmutov, D.; Lund, H.; Li, J.; Chen, J.; Li, Y.; Jiang, G.; Kondratenko, E.V. Front Cover: Elucidating the Role of Oxygen Species in Oxidative Coupling of Methane over Supported MnOx–Na2WO4-containing Catalysts. ChemCatChem 2024, 16, e202301610. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Baerns, M. Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Parishan, S.; Littlewood, P.; Arinchtein, A.; Fleischer, V.; Schomäcker, R. Chemical looping as a reactor concept for the oxidative coupling of methane over the MnxOy-Na2WO4/SiO2 catalyst, benefits and limitation. Catal. Today 2018, 311, 40–47. [Google Scholar] [CrossRef]
- Dardouri, R.; Gannouni, A.; Said Zina, M. Structural and Oxidative Properties of Manganese Incorporated Mesostructure Silica for Methane Oxidation. Adv. Mater. Sci. Eng. 2019, 2019, 6024876. [Google Scholar] [CrossRef]
- Matras, D.; Vamvakeros, A.; Jacques, S.; Grosjean, N.; Rollins, B.; Poulston, S.; Stenning, G.B.G.; Godini, H.R.; Drnec, J.; Cernik, R.J.; et al. Effect of thermal treatment on the stability of Na-MnW/SiO2 Catalyst for the Oxidative Coupling of Methane. Faraday Discuss. 2018, 229, 176–196. [Google Scholar] [CrossRef]
- Schmack, R.; Friedrich, A.; Kondratenko, E.V.; Polte, J.; Werwatz, A.; Kraehnert, R. A meta-analysis of catalytic literature data reveals property-performance correlations for the OCM reaction. Nat. Commun. 2019, 10, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Kondratenko, E.V.; Schlüter, M.; Baerns, M.; Linke, D.; Holena, M. Developing catalytic materials for the oxidative coupling of methane through statistical analysis of literature data. Catal. Sci. Technol. 2015, 5, 1668–1677. [Google Scholar] [CrossRef]
- Olivier, L.; Haag, S.; Pennemann, H.; Hofmann, C.; Mirodatos, C.; van Veen, A.C. High-temperature parallel screening of catalysts for the oxidative coupling of methane. Catal. Today 2008, 137, 80–89. [Google Scholar] [CrossRef]
- Wang, H. Property-Performance Correlations in the Oxidative Coupling of Methane: The Importance of Carbonate Formation and Stability. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 2018. Available online: http://d-nb.info/1161461906/34 (accessed on 20 July 2024).
- Atkins, P.W.; Overton, T.L.; Rourke, J.P.; Weller, M.T.; Armstrong, F.A. Shriver and Atkins’ Inorganic Chemistry, 5th ed.; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Koch, G.; Hävecker, M.; Kube, P.; Tarasov, A.; Schlögl, R.; Trunschke, A. The Influence of the Chemical Potential on Defects and Function of Perovskites in Catalysis. Front. Chem. 2021, 9, 746229. [Google Scholar] [CrossRef]
- Wang, Y.; Sourav, S.; Malizia, J.P.; Thompson, B.; Wang, B.; Kunz, M.R.; Nikolla, E.; Fushimi, R. Deciphering the mechanistic role of individual oxide phases and their combinations in supported Mn-Na2WO4 catalystsfor oxidative coupling of methane. ACS Catal. 2022, 12, 11886–11898. [Google Scholar] [CrossRef]
- Yildiz, M.; Aksub, Y.; Simon, U.; Otremba, T.; Kailasam, K.; Göbel, C.; Girgsdies, F.; Görke, O.; Rosowski, F.; Thomas, A.; et al. Silica material variation for the MnxOy-Na2WO4/SiO2. Appl. Catal. A Gen. General. 2016, 525, 168–179. [Google Scholar] [CrossRef]
- Godini, H.R.; Gili, A.; Görke, O.; Arndt, S.; Simon, U.; Thomas, A.; Schomäcker, R.; Wozny, G. Sol-gel method for synthesis of Mn-Na2WO4/SiO2 catalyst for methane oxidative coupling. Catal. Today 2014, 236, 12–22. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Yang, D.; Gao, R.; Wang, Y.; Yang, J. Scale up and stability test for oxidative coupling of methane over Na2WO4-Mn/SiO2 catalyst in a 200 mL fixed-bed reactor. J. Nat. Gas Chem. 2008, 17, 59–63. [Google Scholar] [CrossRef]
- Godini, H.R.; Gili, A.; Görke, O.; Simon, U.; Hou, K.; Wozny, G. Performance analysis of a porous packed-bed membrane reactor for Oxidative Coupling of Methane: Structural and operational characteristics. Energy Fuels 2014, 28, 877–890. [Google Scholar] [CrossRef]
- Kim, G.J.; Ausenbaugh, J.T.; Hwang, H.T. Effect of TiO2 on the Performance of Mn/Na2WO4 Catalysts in Oxidative Coupling of Methane. Ind. Eng. Chem. Res. 2021, 60, 3914–3921. [Google Scholar] [CrossRef]
- Zhao, K.; Gao, Y.; Wang, X.; Mosevitzky Lis, B.; Liu, J.; Jin, B.; Smith, J.; Huang, C.; Gao, W.; Wang, X.; et al. Lithium carbonate-promoted mixed rare earth oxides as a generalized strategy for oxidative coupling of methane with exceptional yields. Nat. Commun. 2023, 14, 7749. [Google Scholar] [CrossRef]
- Baser, D.S.; Cheng, Z.; Fan, J.A.; Fan, L.-S. Codoping Mg-Mn Based Oxygen Carrier with Lithium and Tungsten for Enhanced C2 Yield in a Chemical Looping Oxidative Coupling of Methane System. ACS Sustain. Chem. Eng. 2021, 9, 2651–2660. [Google Scholar] [CrossRef]
- Kolts, J.H.; Lunsford, J.H. Methane Conversion. Austr. Patent AU 1986054352 (1986), 30 November 1989. Available online: https://ipsearch.ipaustralia.gov.au/patents/1986054352 (accessed on 20 July 2024).
- Murata, K.; Hayakawa, T.; Fujita, K. Excellent effect of lithium-doped sulfated zirconia catalysts for oxidative coupling of methane to give ethene and ethane. Chem. Commun. 1997, 221–222. [Google Scholar] [CrossRef]
- Chu, P.; Landis, M.E. Selective Oxidative Coupling. US Patent 4 914 252, 3 April 1990. [Google Scholar]
- Schammel, W.P.; Wolfenbarger, J.; Ajinkya, M.; Ciczeron, J.M.; Mccarty, J.; Weinberger, S.; Edwards, J.D.; Sheridan, D.; Scher, E.C.; McCormick, J. Oxidative Coupling of Methane Systems and Methods. WO Patent 2013177433 A2, 28 November 2013. [Google Scholar]
- Vallet-Regi, M.; García, E.; González-Calbet, J.M. Synthesis and characterization of a new double perovskite: LaCaMnCoO6. J. Chem. Soc. Dalton Trans. 1998, 3, 775. [Google Scholar] [CrossRef]
- Bhasin, M.M.; Campbell, K.D. Oxidative coupling of methane—A progress report. In Methane and Alkane Conversion Chemistry; Bhasin, M.M., Slocum, D.W., Eds.; Springer Science Business Media: New York, NY, USA, 1995; pp. 3–17. [Google Scholar]
- Bagherzadeh, E.; Hassan, A.; Anthony, R.G.; Hassan, A.; Bozkurt, B.; Zhang, J. Catalyst and Method for Converting Natural Gas to Higher Carbon Compounds. US Patent 8129305, 6 March 2012. [Google Scholar]
- Campbell, K.D. Double Perovskite Catalysts for Oxidative Coupling. Patent No. 4,988,660, 29 January 1991. [Google Scholar]
- Jones, C.A.; Leonard, J.J.; Sofranko, J.A. The oxidative conversion of methane to higher hydrocarbons over alkali-promoted Mn/SiO2. J. Catal. 1987, 103, 311–319. [Google Scholar] [CrossRef]
- Langfeld, K.; Frank, B.; Strempel, V.E.; Berger-Karin, C.; Weinberg, G.; Kondratenko, E.V.; Schomacker, R. Comparison of oxidizing agents for the oxidative coupling of methane over state-of-the-art catalysts. Appl. Catal. A Gen. 2012, 417, 145–152. [Google Scholar] [CrossRef]
- Wolf, E.E. Methane Conversion by Oxidative Processes: Fundamental and Engineering Aspects; Springer: Dordrecht, The Netherlands, 1991; ISBN 978-94-015-7451-8. [Google Scholar] [CrossRef]
- Cai, X.; Hang, H.Y. Advances in catalytic conversion of methane and carbon dioxide to highly valuable products. Energy Sci. Eng. 2019, 7, 4–29. [Google Scholar] [CrossRef]
- Cong, L.; Zhao, Y.; Li, S.; Sun, Y. Sr-doping effects on La2O3 catalyst for oxidative coupling of methane. Chin. J. Catal. 2017, 38, 899–907. [Google Scholar] [CrossRef]
- Arndt, S.; Otremba, T.; Simon, U.; Yildiz, M.; Schubert, H.; Schomäcker, R. Mn–Na2WO4/SiO2 as catalyst for the oxidative coupling of methane. What is really known? Appl. Catal. A Gen. 2012, 425–426, 53–61. [Google Scholar] [CrossRef]
- Kiani, D.; Sourav, S.; Baltrusaitis, J.; Wachs, I.E. Oxidative coupling of methane (OCM) by SiO2-supported tungsten oxide catalysts promoted with Mn and Na. ACS Catal. 2019, 9, 5912–5928. [Google Scholar] [CrossRef]
- Hiyoshi, N.; Sato, K. Oxidative coupling of methane over Mn–Na2WO4/SiO2 catalyst with continuous supply of alkali chloride vapor. Fuel Process. Technol. 2016, 151, 148–154. [Google Scholar] [CrossRef]
- Shurdumov, G.K.; Shurdumova, Z.V.; Cherkesov, Z.A.; Karmokov, A.M. Synthesis of alkaline-earth metal tungstates in melts of (NaNO3–M(NO3)2)eut–Na2WO4 (M = Ca, Sr, Ba) systems. Russ. J. Inorg. Chem. 2006, 51, 531–532. [Google Scholar] [CrossRef]
- Sokolovskii, V.D.; Mamedov, E.A. Oxidative dehydrodimerization of methane. Catal. Today 1992, 14, 415–419. [Google Scholar]
- Hiyoshi, N.; Ikeda, T. Oxidative coupling of methane over alkali chloride–Mn–Na2WO4/SiO2 catalysts: Promoting effect of molten alkali chloride. Fuel Process. Technol. 2015, 133, 29–34. [Google Scholar] [CrossRef]
- Maitra, A.M.; Campbell, I.; Tyler, R.J. Influence of basicity on the catalytic activity for oxidative coupling of methane. Appl. Catal. A Gen. 1992, 85, 27–46. [Google Scholar] [CrossRef]
- Palermo, A.; Holgado, J.; Lee, A.; Tikhov, M.; Lambert, R. Critical influence of the amorphous silica-to-cristobalite phase transition on the performance of Mn/Na2WO4/ SiO2 catalysts for the oxidative coupling of methane. J. Catal. 1998, 177, 259–266. [Google Scholar] [CrossRef]
- Tashjian, V.; Cassir, M.; Devynck, J.; Rummel, W. Oxidative dimerization of methane in molten Li2CO3-Na2CO3 eutectic supported by lithium aluminate at 700–850 °C. Appl. Catal. A 1994, 108, 157–169. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Grzybek, T.; Galvez, M.E.; Da Costa, P. A Short Review on the Catalytic Activity of Hydrotalcite-Derived Materials for Dry Reforming of Methane. Catalysts 2017, 7, 32. [Google Scholar] [CrossRef]
- Vamvakeros, A.; Jacques, S.D.M.; Middelkoop, V.; Di Michiel, M.; Egan, C.K.; Ismagilov, I.Z.; Vaughan, G.B.M.; Gallucci, F.; van Sint Annaland, M.; Shearing, P.R.; et al. Real Time chemical imaging of a working catalytic membrane reactor during oxidative coupling of methane. Chem. Commun. 2015, 51, 12752–12755. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Yokoyama, S.; Morikawa, A. Catalytic activity and selectivity-control for oxidative coupling of methane by oxygen-pumping through yttria-stabilized zirconia. Chem. Lett. 1985, 3, 319–322. [Google Scholar] [CrossRef]
- Abbas, H.; Azzis, H.; Bagherzadeh, E. Perovskite-based catalyst, its preparation and its use for conversion of methane to ethylene. Patent WO 2005 005042, 20 January 2005. [Google Scholar]
- Pak, S.; Lunsford, J.H. Thermal effects during the oxidative coupling of methane over Mn/Na2WO4/SiO2 and Mn/Na2WO4/MgO catalysts. Appl. Catal. A Gen. 1998, 168, 131–137. [Google Scholar] [CrossRef]
- Ji, S.; Xiao, T.; Li, S.; Chou, L.; Zhang, B.; Xu, C.; Liu, Y.; Hou, R.; York, A.P.E.; Green, M.L.H. Surface WO4 tetrahedron: The essence of the oxidative coupling of methane over M-W-Mn/SiO2 catalyst. J. Catal. 2003, 220, 47–56. [Google Scholar] [CrossRef]
- Machida, K.; Enyo, M. Oxidative dimerization of methane over cerium mixed oxides and its relation with their ion-conducting characteristics. J. Chem. Soc. Chem. Commun. 1987, 21, 1639–1640. [Google Scholar] [CrossRef]
- Au, C.T.; Zhou, X.P.; Liu, Y.W.; Ji, W.J.; Ng, C.F. The Characterization of BaF2/Y2O3 catalysts for the OCM reaction. J. Catal. 1998, 174, 153–163. [Google Scholar] [CrossRef]
- Gholipour, Z.; Malekzadeh, A.; Hatami, R.; Mortazavi, Y.; Khodadadi, A. Oxidative coupling of methane over Na2WO4 + Mn or Ce)/SiO2 catalysts: In situ measurement of electrical conductivity. J. Nat. Gas Chem. 2010, 19, 35–42. [Google Scholar] [CrossRef]
- Ferreira, V.J.; Tavares, P.; Figueiredo, J.L.; Faria, J.L. Ce-Doped La2O3 based catalyst for the oxidative coupling of methane. Catal. Commun. 2013, 42, 50–53. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Chou, L.; Zhang, B.; Song, H.; Zhao, J.; Jian, Y. Comparative study on oxidation of methane to ethane and ethylene over Na2WO4–Mn/SiO2 catalysts prepared by different methods. J. Mol. Catal. A Chem. 2006, 245, 272–277. [Google Scholar] [CrossRef]
- Thum, L.; Rudolph, M.; Schomäcker, R.; Wang, Y.; Tarasov, A.; Trunschke, A. Oxygen Activation in Oxidative Coupling of Methane on Calcium Oxide. J. Phys. Chem. C 2019, 123, 8018–8026. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Han, X.; Zheng, Y. Enhancing electrochemical methane coupling in solid oxide cells by tuning oxygen species in the catalyst. J. Mater. Chem. A 2024, 12, 5115–5123. [Google Scholar] [CrossRef]
- Mirodatos, C.; Xu, G.; Lacombe, S.; Ducarme, V.; Li, W.; Martin, G.A. Structure sensitivity of oxidative coupling of methane and dehydrogenation of ethane over lanthana catalysts. Stud. Surf. Sci. Catal. 1997, 107, 345–350. [Google Scholar]
- Sollier, B.M.; Bonne, M.; Khenoussi, N.; Michelin, L.; Miró, E.E.; Gómez, L.E.; Boix, A.V.; Lebeau, B. Synthesis and Characterization of Electrospun Nanofibers of Sr-La-Ce Oxides as Catalysts for the Oxidative Coupling of Methane. Ind. Eng. Chem. Res. 2020, 59, 11419–11430. [Google Scholar] [CrossRef]
- Jašo, S.; Sadjadi, S.; Godini, H.R.; Simon, U.; Arndt, S.; Gorke, O.; Berthold, A.; Arellano-Garcia, H.; Schubert, H.; Schomacker, R.; et al. Experimental investigation of fluidized-bed reactor performance for oxidative coupling of methane. J. Nat. Gas Chem. 2012, 21, 534–543. [Google Scholar] [CrossRef]
- Tye, C.T.; Mohamed, A.R.; Bhatia, S. Modeling of catalytic reactor for oxidative coupling of methane using La2O3/CaO catalyst. Chem. Eng. J. 2002, 87, 49–59. [Google Scholar] [CrossRef]


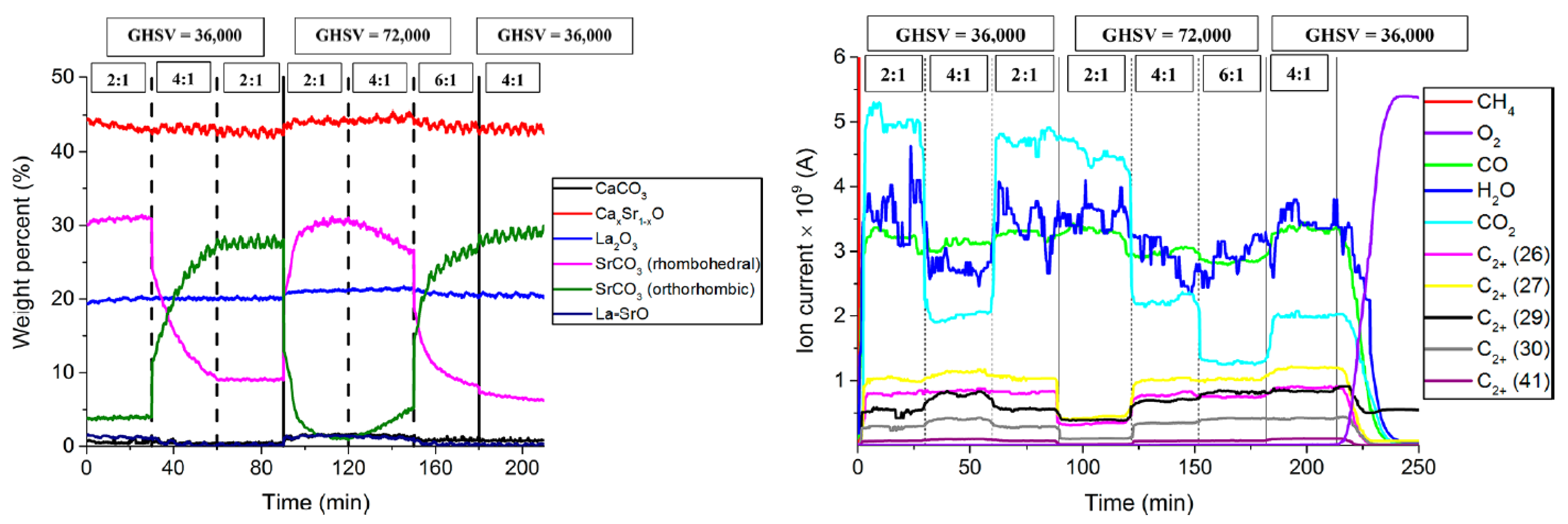
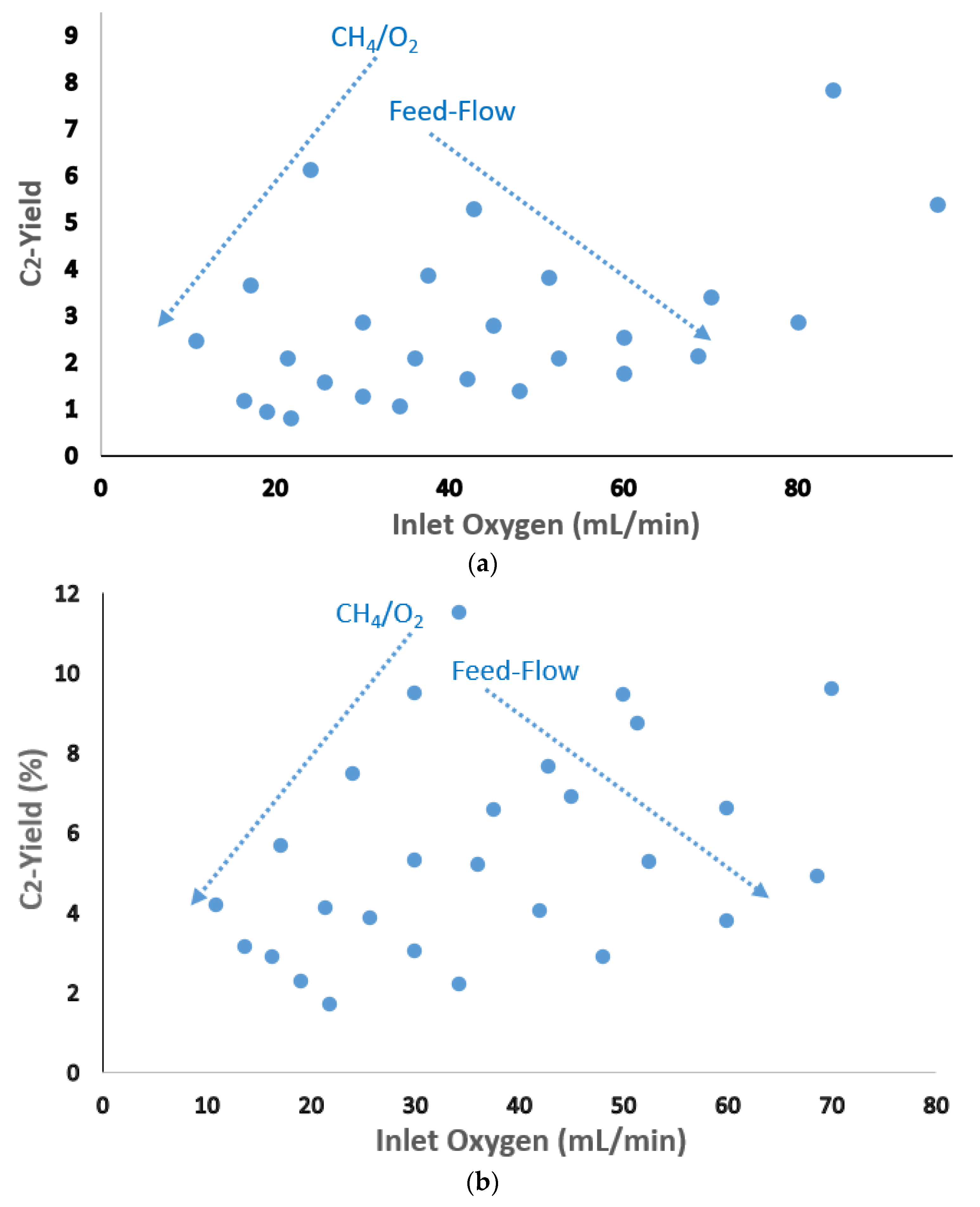
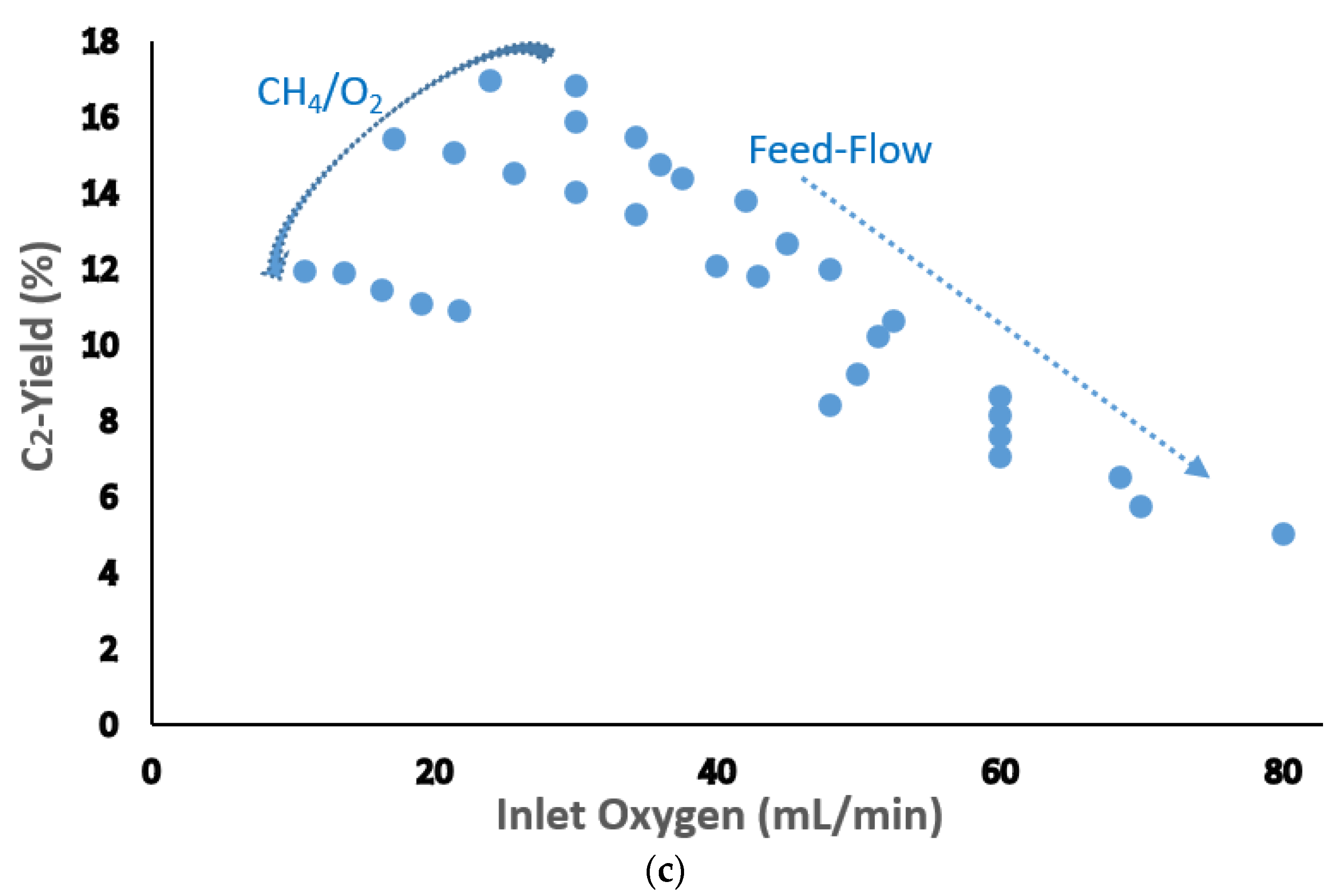
| Catalyst Benchmark-Type | Preparation Method | Reactor Type | T (°C), CH4/O2 | GHSV (h−1) | XCH4 (%) | SC2+ (%) | YC2H4 (%) | C2H4/C2H6 | Relative Stability | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Mn-Na-WOX/SiO2 | Impregnation | Fixed-bed Reactor | 800, 4 | 2700 | 37 | 66 | 20.2 | 5 | Medium | [53] |
| Mn-Na-WOX/SiO2 | Impregnation | Membrane Reactor | 820, 2.5 | 2000 | 39 | 66 | 20.3 | 3.9 | Medium | [54] |
| Mn-Na-WOX-SiO2 | Sol-gel | Membrane Reactor | 850, 3 | 2000 | 31 | 78 | 20 | 4.6 | Low | [52] |
| Mn-Na-Ce-WOX/TiO2 | Impregnation | Fixed-bed Reactor | 775, 2 | 4800 | 46 | 57 | 21.1 | 4.1 | - | [55] |
| La-PrOx-Li-COx | Impregnation | Chemical Looping | 700, - | 180 | 43 | 70 | 20 | 3.3 | High | [56] |
| Li-W-Mn/MgO | Impregnation | Chemical Looping | 850, - | 2400 | 50 | 58 | 23 | 5.5 | High | [57] |
| Li/MgO | - | Fixed-bed Reactor | 770, 3 | 655 | 59 | 53 | 21.2 | 2.1 | Low | [58] |
| Li-S/ZrO2 | Impregnation | Fixed-bed Reactor | 800, 2 | 6000 | 43 | 79 | 25.2 | 2.8 | - | [59] |
| Li-Mn | - | Fixed-bed Reactor | 760, 2.6 | 5540 | 41 | 68 | 27.9 | - | - | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godini, H.R.; Bhasin, M.M. Oxidative Coupling of Methane: A Review Study on the Catalytic Performance. Molecules 2024, 29, 4649. https://doi.org/10.3390/molecules29194649
Godini HR, Bhasin MM. Oxidative Coupling of Methane: A Review Study on the Catalytic Performance. Molecules. 2024; 29(19):4649. https://doi.org/10.3390/molecules29194649
Chicago/Turabian StyleGodini, Hamid Reza, and Madan Mohan Bhasin. 2024. "Oxidative Coupling of Methane: A Review Study on the Catalytic Performance" Molecules 29, no. 19: 4649. https://doi.org/10.3390/molecules29194649
APA StyleGodini, H. R., & Bhasin, M. M. (2024). Oxidative Coupling of Methane: A Review Study on the Catalytic Performance. Molecules, 29(19), 4649. https://doi.org/10.3390/molecules29194649





