Immunomodulatory Effects of Green Tea Catechins and Their Ring Fission Metabolites in a Tumor Microenvironment Perspective
Abstract
1. Introduction
2. Green Tea Consumption
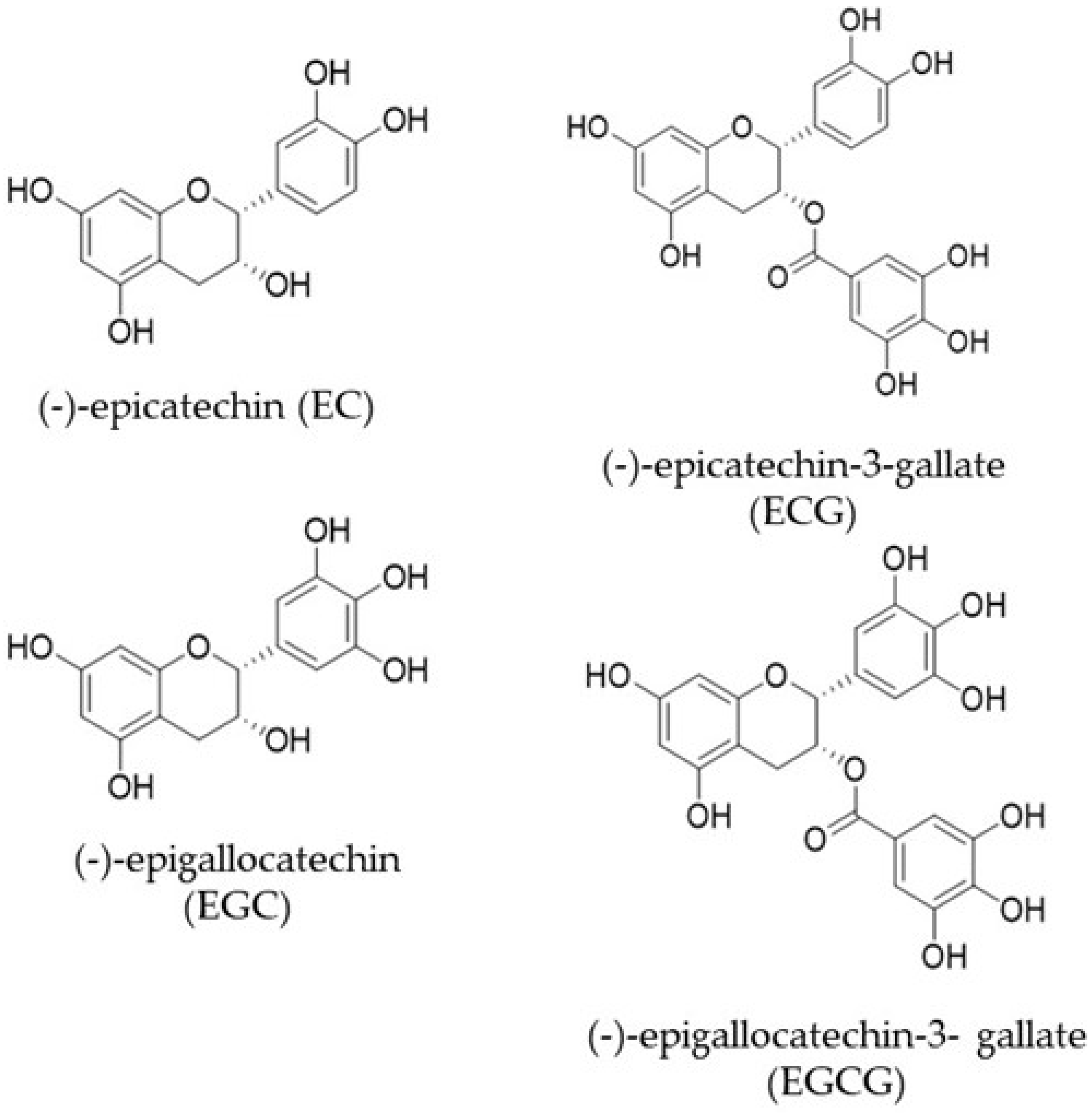
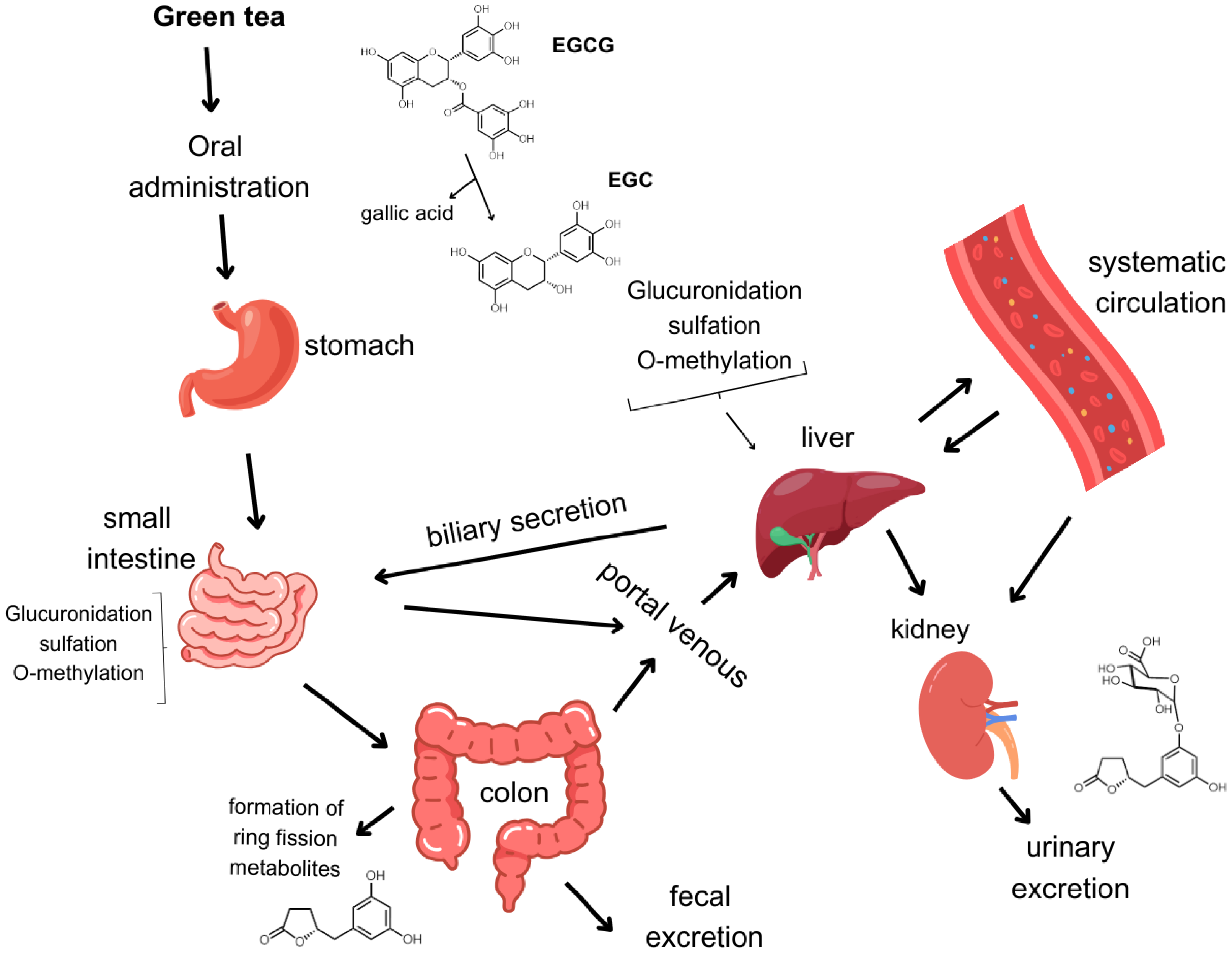
3. Bioavailability of Catechins
4. Catechin Ring Fission Metabolites and Their Biological Activity
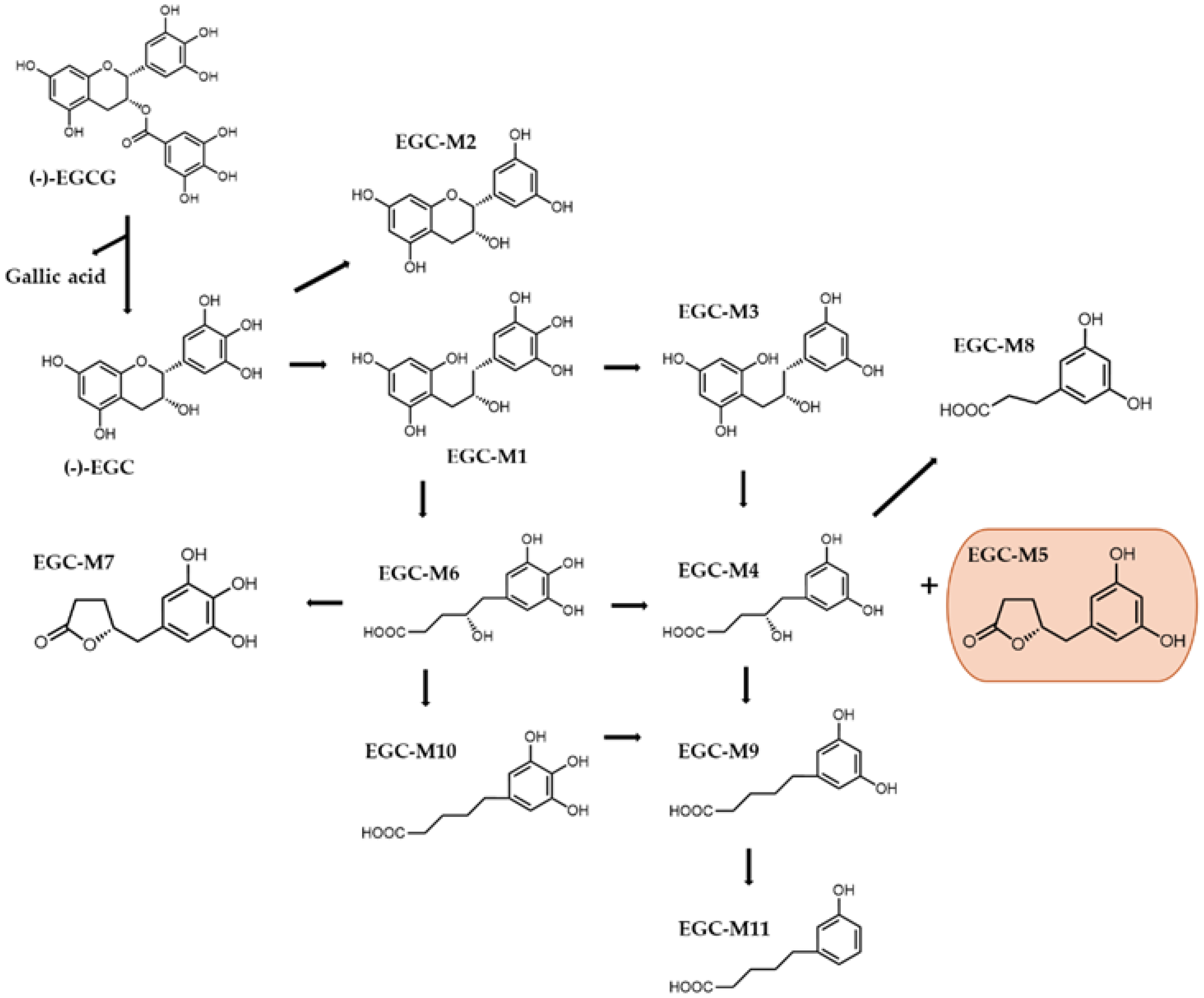
4.1. Antioxidant Properties of Catechins and Metabolites
4.2. AntiCancer Properties of Catechins and Metabolites
5. Modulation of Tumor Microenvironment by Green Tea Catechins and Major Metabolites
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gonçalves Bortolini, D.; Windson Isidoro Haminiuk, C.; Cristina Pedro, A.; De Andrade Arruda Fernandes, I.; Maria Maciel, G. Processing, Chemical Signature and Food Industry Applications of Camellia sinensis Teas: An Overview. Food Chem. X 2021, 12, 100160. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Shirakami, Y.; Shimizu, M. Possible Mechanisms of Green Tea and Its Constituents against Cancer. Molecules 2018, 23, 2284. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, A.; Nanjo, F. Metabolism of (−)-Epigallocatechin Gallate by Rat Intestinal Flora. J. Agric. Food Chem. 2010, 58, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Catterall, F.; King, L.J.; Clifford, M.N.; Ioannides, C. Bioavailability of Dietary Doses of 3 H-Labelled Tea Antioxidants (+)-Catechin and (−)-Epicatechin in Rat. Xenobiotica 2003, 33, 743–753. [Google Scholar] [CrossRef]
- Del Rio, D.; Calani, L.; Cordero, C.; Salvatore, S.; Pellegrini, N.; Brighenti, F. Bioavailability and Catabolism of Green Tea Flavan-3-Ols in Humans. Nutrition 2010, 26, 1110–1116. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and Its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef]
- Samynathan, R.; Thiruvengadam, M.; Nile, S.H.; Shariati, M.A.; Rebezov, M.; Mishra, R.K.; Venkidasamy, B.; Periyasamy, S.; Chung, I.-M.; Pateiro, M.; et al. Recent Insights on Tea Metabolites, Their Biosynthesis and Chemo-Preventing Effects: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 3130–3149. [Google Scholar] [CrossRef]
- Hajiboland, R. Environmental and Nutritional Requirements for Tea Cultivation. Folia Hortic. 2017, 29, 199–220. [Google Scholar] [CrossRef]
- Ran, W.; Li, Q.; Hu, X.; Zhang, D.; Yu, Z.; Chen, Y.; Wang, M.; Ni, D. Comprehensive Analysis of Environmental Factors on the Quality of Tea (Camellia sinensis var. sinensis) Fresh Leaves. Sci. Hortic. 2023, 319, 112177. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, P.; Zhong, N.; Huang, H.; Zheng, H. Impact of Storage Temperature on Green Tea Quality: Insights from Sensory Analysis and Chemical Composition. Beverages 2024, 10, 35. [Google Scholar] [CrossRef]
- Graham, H.N. Green Tea Composition, Consumption, and Polyphenol Chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.-Y.; Li, H.-B.; Sui, Z.-Q.; Corke, H. Absorption, Metabolism, Anti-Cancer Effect and Molecular Targets of Epigallocatechin Gallate (EGCG): An Updated Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.; Wang, M.-N.; Tseng, T.-Y.; Sung; Tsai, T.-H. Pharmacokinetics of (−)-Epigallocatechin-3-Gallate in Conscious and Freely Moving Rats and Its Brain Regional Distribution. J. Agric. Food Chem. 2007, 55, 1517–1524. [Google Scholar] [CrossRef]
- Meng, X.; Sang, S.; Zhu, N.; Lu, H.; Sheng, S.; Lee, M.-J.; Ho, C.-T.; Yang, C.S. Identification and Characterization of Methylated and Ring-Fission Metabolites of Tea Catechins Formed in Humans, Mice, and Rats. Chem. Res. Toxicol. 2002, 15, 1042–1050. [Google Scholar] [CrossRef]
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of Tea Catechins after Ingestion of Green Tea and (−)-Epigallocatechin-3-Gallate by Humans: Formation of Different Metabolites and Individual Variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Lambert, J.D.; Sang, S.; Yang, C.S. Biotransformation of Green Tea Polyphenols and the Biological Activities of Those Metabolites. Mol. Pharm. 2007, 4, 819–825. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, Y.; Li, R.C. Oral Absorption and Bioavailability of Tea Catechins. Planta Medica 2000, 66, 444–447. [Google Scholar] [CrossRef]
- Yong Feng, W. Metabolism of Green Tea Catechins: An Overview. Curr. Drug Metab. 2006, 7, 755–809. [Google Scholar] [CrossRef]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Lu, H.; Meng, X.; Li, C.; Sang, S.; Patten, C.; Sheng, S.; Hong, J.; Bai, N.; Winnik, B.; Ho, C.-T.; et al. Glucuronides of Tea Catechins: Enzymology of Biosynthesis and Biological Activities. Drug Metab. Dispos. 2003, 31, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, J.B.; Walle, T. Glucuronidation and Sulfation of the Tea Flavonoid (−)-Epicatechin by the Human and Rat Enzymes. Drug Metab. Dispos. 2002, 30, 897–903. [Google Scholar] [CrossRef]
- Yang, C.S.; Lee, M.J.; Chen, L. Human Salivary Tea Catechin Levels and Catechin Esterase Activities: Implication in Human Cancer Prevention Studies. Cancer Epidemiol. Biomark. Prev. 1999, 8, 83–89. [Google Scholar]
- Zhu, B.T.; Patel, U.K.; Cai, M.X.; Conney, A.H. O-Methylation of Tea Polyphenols Catalyzed by Human Placental Cytosolic Catechol-O-Methyltransferase. Drug Metab. Dispos. 2000, 28, 1024–1030. [Google Scholar] [PubMed]
- Casanova, E.; Salvadó, J.; Crescenti, A.; Gibert-Ramos, A. Epigallocatechin Gallate Modulates Muscle Homeostasis in Type 2 Diabetes and Obesity by Targeting Energetic and Redox Pathways: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 532. [Google Scholar] [CrossRef] [PubMed]
- Holczer, M.; Besze, B.; Zámbó, V.; Csala, M.; Bánhegyi, G.; Kapuy, O. Epigallocatechin-3-Gallate (EGCG) Promotes Autophagy-Dependent Survival via Influencing the Balance of mTOR-AMPK Pathways upon Endoplasmic Reticulum Stress. Oxidative Med. Cell. Longev. 2018, 2018, 6721530. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Lee, M.-J.; Lu, H.; Meng, X.; Hong, J.J.J.; Seril, D.N.; Yang, C.S.; Sturgill, M.G. Epigallocatechin-3-Gallate Is Absorbed but Extensively Glucuronidated Following Oral Administration to Mice. J. Nutr. 2003, 133, 4172–4177. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Miyazawa, T. Chemiluminescence–High-Performance Liquid Chromatographic Determination of Tea Catechin, (−)-Epigallocatechin 3-Gallate, at Picomole Levels in Rat and Human Plasma. Anal. Biochem. 1997, 248, 41–49. [Google Scholar] [CrossRef]
- Sang, S.; Lee, M.; Yang, I.; Buckley, B.; Yang, C.S. Human Urinary Metabolite Profile of Tea Polyphenols Analyzed by Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry with Data-dependent Acquisition. Rapid Commun. Mass Spectrom. 2008, 22, 1567–1578. [Google Scholar] [CrossRef]
- Li, C.; Lee, M.-J.; Sheng, S.; Meng, X.; Prabhu, S.; Winnik, B.; Huang, B.; Chung, J.Y.; Yan, S.; Ho, C.-T.; et al. Structural Identification of Two Metabolites of Catechins and Their Kinetics in Human Urine and Blood after Tea Ingestion. Chem. Res. Toxicol. 2000, 13, 177–184. [Google Scholar] [CrossRef]
- Calani, L.; Del Rio, D.; Luisa Callegari, M.; Morelli, L.; Brighenti, F. Updated Bioavailability and 48 h Excretion Profile of Flavan-3-Ols from Green Tea in Humans. Int. J. Food Sci. Nutr. 2012, 63, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gan, R.-Y.; Chen, D.; Zheng, L.; Ng, S.B.; Rietjens, I.M.C.M. Gut Microbiota-Mediated Metabolism of Green Tea Catechins and the Biological Consequences: An Updated Review. Crit. Rev. Food Sci. Nutr. 2024, 64, 7067–7084. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Navajas-Porras, B.; López-Maldonado, A.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Green Tea and Its Relation to Human Gut Microbiome. Molecules 2021, 26, 3907. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Hu, K.; Li, D.; Guo, H.; Sun, L.; Xie, Z. Microbial-Transferred Metabolites and Improvement of Biological Activities of Green Tea Catechins by Human Gut Microbiota. Foods 2024, 13, 792. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Patán, F.; Tabasco, R.; Monagas, M.; Requena, T.; Peláez, C.; Moreno-Arribas, M.V.; Bartolomé, B. Capability of Lactobacillus Plantarum IFPL935 to Catabolize Flavan-3-Ol Compounds and Complex Phenolic Extracts. J. Agric. Food Chem. 2012, 60, 7142–7151. [Google Scholar] [CrossRef]
- Kohri, T.; Matsumoto, N.; Yamakawa, M.; Suzuki, M.; Nanjo, F.; Hara, Y.; Oku, N. Metabolic Fate of (−)-[4-3H]Epigallocatechin Gallate in Rats after Oral Administration. J. Agric. Food Chem. 2001, 49, 4102–4112. [Google Scholar] [CrossRef]
- Takagaki, A.; Yoshioka, Y.; Yamashita, Y.; Nagano, T.; Ikeda, M.; Hara-Terawaki, A.; Seto, R.; Ashida, H. Effects of Microbial Metabolites of (−)-Epigallocatechin Gallate on Glucose Uptake in L6 Skeletal Muscle Cell and Glucose Tolerance in ICR Mice. Biol. Pharm. Bull. 2019, 42, 212–221. [Google Scholar] [CrossRef]
- Takagaki, A.; Nanjo, F. Effects of Metabolites Produced from (−)-Epigallocatechin Gallate by Rat Intestinal Bacteria on Angiotensin I-Converting Enzyme Activity and Blood Pressure in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2015, 63, 8262–8266. [Google Scholar] [CrossRef]
- Sheng, Y.; Sun, Y.; Tang, Y.; Yu, Y.; Wang, J.; Zheng, F.; Li, Y.; Sun, Y. Catechins: Protective Mechanism of Antioxidant Stress in Atherosclerosis. Front. Pharmacol. 2023, 14, 1144878. [Google Scholar] [CrossRef]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and Mitochondria: An Update on Their Increasingly Emerging ROS-Scavenging Independent Actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef]
- Xiang, L.-P.; Wang, A.; Ye, J.-H.; Zheng, X.-Q.; Polito, C.; Lu, J.-L.; Li, Q.-S.; Liang, Y.-R. Suppressive Effects of Tea Catechins on Breast Cancer. Nutrients 2016, 8, 458. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, D.; Wang, X.; Zhang, J.; Shan, Z.; Teng, W. (−)-Epigallocatechin Gallate Inhibits TNF-α-Induced PAI-1 Production in Vascular Endothelial Cells. J. Cardiovasc. Pharmacol. 2013, 62, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-M.; Gao, W.; Wang, H.; Zhao, D.; Nie, Z.-L.; Shi, J.-Q.; Zhao, S.; Lu, X.; Wang, L.-S.; Yang, Z.-J. Green Tea Polyphenol Epigallocatechin-3-Gallate Inhibits TNF-a-Induced Production of Monocyte Chemoattractant Protein-1 in Human Umbilical Vein Endothelial Cells. Cell Physiol. Biochem. 2014, 33, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, W.; Xiao, X.; Huang, Q.; Yu, J.; Yang, Y.; Han, T.; Zhang, D.; Niu, X. (−)-Epicatechin Gallate Blocks the Development of Atherosclerosis by Regulating Oxidative Stress In Vivo and In Vitro. Food Funct. 2021, 12, 8715–8727. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Morris, A.; Sunkara, M.; Layne, J.; Toborek, M.; Hennig, B. Epigallocatechin-Gallate Stimulates NF-E2-Related Factor and Heme Oxygenase-1 via Caveolin-1 Displacement. J. Nutr. Biochem. 2012, 23, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Perkins, J.T.; Hennig, B. EGCG Prevents PCB-126-Induced Endothelial Cell Inflammation via Epigenetic Modifications of NF-κB Target Genes in Human Endothelial Cells. J. Nutr. Biochem. 2016, 28, 164–170. [Google Scholar] [CrossRef]
- Varilek, G.W.; Yang, F.; Lee, E.Y.; deVilliers, W.J.S.; Zhong, J.; Oz, H.S.; Westberry, K.F.; McClain, C.J. Green Tea Polyphenol Extract Attenuates Inflammation in Interleukin-2–Deficient Mice, a Model of Autoimmunity. J. Nutr. 2001, 131, 2034–2039. [Google Scholar] [CrossRef]
- Takagaki, A.; Otani, S.; Nanjo, F. Antioxidative Activity of Microbial Metabolites of (−)-Epigallocatechin Gallate Produced in Rat Intestines. Biosci. Biotechnol. Biochem. 2011, 75, 582–585. [Google Scholar] [CrossRef]
- Cheng, W.-H. Green Tea: An Ancient Antioxidant Drink for Optimal Health? J. Nutr. 2019, 149, 1877–1879. [Google Scholar] [CrossRef]
- Talib, W.H.; Awajan, D.; Alqudah, A.; Alsawwaf, R.; Althunibat, R.; Abu AlRoos, M.; Al Safadi, A.; Abu Asab, S.; Hadi, R.W.; Al Kury, L.T. Targeting Cancer Hallmarks with Epigallocatechin Gallate (EGCG): Mechanistic Basis and Therapeutic Targets. Molecules 2024, 29, 1373. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Han, Z.; Li, X.; Xie, H.-H.; Zhu, S.-S. Mechanism of EGCG Promoting Apoptosis of MCF-7 Cell Line in Human Breast Cancer. Oncol. Lett. 2017, 14, 3623–3627. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.-D.; Yao, J.-J.; Wang, H.; Cui, W.-G.; Leng, J.; Ding, L.-Y.; Fan, K.-Y. Effects of EGCG on Proliferation and Apoptosis of Gastric Cancer SGC7901 Cells via Down-Regulation of HIF-1α and VEGF under a Hypoxic State. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, L.; Feng, J.; Li, J.; Liu, T.; Zhang, R.; Xu, S.; Cheng, K.; Zhou, Y.; Zhou, S.; et al. In Vitro and in Vivo Study of Epigallocatechin-3-Gallate-Induced Apoptosis in Aerobic Glycolytic Hepatocellular Carcinoma Cells Involving Inhibition of Phosphofructokinase Activity. Sci. Rep. 2016, 6, 28479. [Google Scholar] [CrossRef]
- Wang, J.; Man, G.C.W.; Chan, T.H.; Kwong, J.; Wang, C.C. A Prodrug of Green Tea Polyphenol (–)-Epigallocatechin-3-Gallate (Pro-EGCG) Serves as a Novel Angiogenesis Inhibitor in Endometrial Cancer. Cancer Lett. 2018, 412, 10–20. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, J.; Du, Y.; Ding, J.; Liu, J.-Y. The Green Tea Polyphenol EGCG Potentiates the Antiproliferative Activity of Sunitinib in Human Cancer Cells. Tumor Biol. 2016, 37, 8555–8566. [Google Scholar] [CrossRef]
- Zhu, B.-H. (−)-Epigallocatechin-3-Gallate Inhibits VEGF Expression Induced by IL-6 Бvia Stat3 in Gastric Cancer. World J. Gastroenterol. 2011, 17, 2315. [Google Scholar] [CrossRef]
- Luo, H.; Xu, M.; Zhong, W.; Cui, Z.; Liu, F.; Zhou, K.; Li, X. EGCG Decreases the Expression of HIF-1α and VEGF and Cell Growth in MCF-7 Breast Cancer Cells. J. BUON 2014, 19, 435–439. [Google Scholar]
- Pointner, A.; Mölzer, C.; Magnet, U.; Zappe, K.; Hippe, B.; Tosevska, A.; Tomeva, E.; Dum, E.; Gessner, D.; Lilja, S.; et al. The Green Tea Polyphenol EGCG Is Differentially Associated with Telomeric Regulation in Normal Human Fibroblasts versus Cancer Cells. Funct. Foods Health Dis. 2021, 11, 73. [Google Scholar] [CrossRef]
- Udroiu, I.; Marinaccio, J.; Sgura, A. Epigallocatechin-3-gallate Induces Telomere Shortening and Clastogenic Damage in Glioblastoma Cells. Environ. Mol. Mutagen. 2019, 60, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Oteiza, P.I. NADPH Oxidase 1: A Target in the Capacity of Dimeric ECG and EGCG Procyanidins to Inhibit Colorectal Cancer Cell Invasion. Redox Biol. 2023, 65, 102827. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Park, H.Y.; Hwang, H.S.; Kang, S.U.; Pyun, J.H.; Lee, M.H.; Choi, E.C.; Kim, C.-H. (−)-Epigallocatechin-3-Gallate (EGCG) Inhibits HGF-Induced Invasion and Metastasis in Hypopharyngeal Carcinoma Cells. Cancer Lett. 2008, 271, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Farabegoli, F.; Papi, A.; Orlandi, M. (–)-Epigallocatechin-3-Gallate down-Regulates EGFR, MMP-2, MMP-9 and EMMPRIN and Inhibits the Invasion of MCF-7 Tamoxifen-Resistant Cells. Biosci. Rep. 2011, 31, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Ravindran Menon, D.; Li, Y.; Yamauchi, T.; Osborne, D.G.; Vaddi, P.K.; Wempe, M.F.; Zhai, Z.; Fujita, M. EGCG Inhibits Tumor Growth in Melanoma by Targeting JAK-STAT Signaling and Its Downstream PD-L1/PD-L2-PD1 Axis in Tumors and Enhancing Cytotoxic T-Cell Responses. Pharmaceuticals 2021, 14, 1081. [Google Scholar] [CrossRef]
- Rawangkan, A.; Wongsirisin, P.; Namiki, K.; Iida, K.; Kobayashi, Y.; Shimizu, Y.; Fujiki, H.; Suganuma, M. Green Tea Catechin Is an Alternative Immune Checkpoint Inhibitor That Inhibits PD-L1 Expression and Lung Tumor Growth. Molecules 2018, 23, 2071. [Google Scholar] [CrossRef]
- Xu, P.; Yan, F.; Zhao, Y.; Chen, X.; Sun, S.; Wang, Y.; Ying, L. Green Tea Polyphenol EGCG Attenuates MDSCs-Mediated Immunosuppression through Canonical and Non-Canonical Pathways in a 4T1 Murine Breast Cancer Model. Nutrients 2020, 12, 1042. [Google Scholar] [CrossRef]
- Santos, R.A.; Andrade, E.D.S.; Monteiro, M.; Fialho, E.; Silva, J.L.; Daleprane, J.B.; Ferraz Da Costa, D.C. Green Tea (Camellia sinensis) Extract Induces P53-Mediated Cytotoxicity and Inhibits Migration of Breast Cancer Cells. Foods 2021, 10, 3154. [Google Scholar] [CrossRef]
- Santos, R.A.; Pessoa, H.R.; Daleprane, J.B.; De Faria Lopes, G.P.; Da Costa, D.C.F. Comparative Anticancer Potential of Green Tea Extract and Epigallocatechin-3-Gallate on Breast Cancer Spheroids. Foods 2023, 13, 64. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Ohishi, T.; Nakamura, Y.; Fukutomi, R.; Miyoshi, N. Anti-Cancer Effects of Dietary Polyphenols via ROS-Mediated Pathway with Their Modulation of MicroRNAs. Molecules 2022, 27, 3816. [Google Scholar] [CrossRef]
- Fix, L.N.; Shah, M.; Efferth, T.; Farwell, M.A.; Zhang, B. MicroRNA Expression Profile of MCF-7 Human Breast Cancer Cells and the Effect of Green Tea Polyphenon-60. Cancer Genom. Proteom. 2010, 7, 261–277. [Google Scholar]
- Siddiqui, I.A.; Asim, M.; Hafeez, B.B.; Adhami, V.M.; Tarapore, R.S.; Mukhtar, H. Green Tea Polyphenol EGCG Blunts Androgen Receptor Function in Prostate Cancer. FASEB J. 2011, 25, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Zan, L.; Chen, Q.; Zhang, L.; Li, X. Epigallocatechin Gallate (EGCG) Suppresses Growth and Tumorigenicity in Breast Cancer Cells by Downregulation of miR-25. Bioengineered 2019, 10, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.P.; Kwok, T.T. Epigallocatechin Gallate Up-Regulation of miR-16 and Induction of Apoptosis in Human Cancer Cells. J. Nutr. Biochem. 2010, 21, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Tran, H.-M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-Gallate Targets Cancer Stem-like Cells and Enhances 5-Fluorouracil Chemosensitivity in Colorectal Cancer. Oncotarget 2016, 7, 16158–16171. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Khandkar, M.; Banik, N.L.; Ray, S.K. Alterations in Expression of Specific microRNAs by Combination of 4-HPR and EGCG Inhibited Growth of Human Malignant Neuroblastoma Cells. Brain Res. 2012, 1454, 1–13. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Ai, W.; Banik, N.L.; Ray, S.K. Overexpression of miR-7-1 Increases Efficacy of Green Tea Polyphenols for Induction of Apoptosis in Human Malignant Neuroblastoma SH-SY5Y and SK-N-DZ Cells. Neurochem. Res. 2013, 38, 420–432. [Google Scholar] [CrossRef]
- Lambert, J.D.; Rice, J.E.; Hong, J.; Hou, Z.; Yang, C.S. Synthesis and Biological Activity of the Tea Catechin Metabolites, M4 and M6 and Their Methoxy-Derivatives. Bioorganic Med. Chem. Lett. 2005, 15, 873–876. [Google Scholar] [CrossRef]
- Hara-Terawaki, A.; Takagaki, A.; Kobayashi, H.; Nanjo, F. Inhibitory Activity of Catechin Metabolites Produced by Intestinal Microbiota on Proliferation of HeLa Cells. Biol. Pharm. Bull. 2017, 40, 1331–1335. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Alsahli, M.A.; Almatroudi, A.; Alrumaihi, F.; Al Abdulmonem, W.; Moawad, A.A.; Alwanian, W.; Almansour, N.M.; Rahmani, A.H.; Khan, A.A. Innovative Strategies of Reprogramming Immune System Cells by Targeting CRISPR/Cas9-Based Genome-Editing Tools: A New Era of Cancer Management. Int. J. Nanomed. 2023, 18, 5531–5559. [Google Scholar] [CrossRef]
- Zarrilli, G.; Businello, G.; Dieci, M.V.; Paccagnella, S.; Carraro, V.; Cappellesso, R.; Miglietta, F.; Griguolo, G.; Guarneri, V.; Lo Mele, M.; et al. The Tumor Microenvironment of Primitive and Metastatic Breast Cancer: Implications for Novel Therapeutic Strategies. Int. J. Mol. Sci. 2020, 21, 8102. [Google Scholar] [CrossRef] [PubMed]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Place, A.E.; Jin Huh, S.; Polyak, K. The Microenvironment in Breast Cancer Progression: Biology and Implications for Treatment. Breast Cancer Res. 2011, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Qian, B.-Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-Induced Chemokine Cascade Promotes Breast Cancer Metastasis by Enhancing Retention of Metastasis-Associated Macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Folgueira, M.A.A.K.; Maistro, S.; Katayama, M.L.H.; Roela, R.A.; Mundim, F.G.L.; Nanogaki, S.; De Bock, G.H.; Brentani, M.M. Markers of Breast Cancer Stromal Fibroblasts in the Primary Tumour Site Associated with Lymph Node Metastasis: A Systematic Review Including Our Case Series. Biosci. Rep. 2013, 33, e00085. [Google Scholar] [CrossRef]
- Deb, G.; Thakur, V.S.; Limaye, A.M.; Gupta, S. Epigenetic Induction of Tissue Inhibitor of Matrix Metalloproteinase-3 by Green Tea Polyphenols in Breast Cancer Cells: TIMP-3 induction by green tea polyphenols. Mol. Carcinog. 2015, 54, 485–499. [Google Scholar] [CrossRef]
- Bayraktar, S.; Batoo, S.; Okuno, S.; Glück, S. Immunotherapy in Breast Cancer. J. Carcinog 2019, 18, 2. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.-J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S.; et al. Safety and Tumor Responses with Lambrolizumab (Anti–PD-1) in Melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Powles, T.; Eder, J.P.; Fine, G.D.; Braiteh, F.S.; Loriot, Y.; Cruz, C.; Bellmunt, J.; Burris, H.A.; Petrylak, D.P.; Teng, S.; et al. MPDL3280A (Anti-PD-L1) Treatment Leads to Clinical Activity in Metastatic Bladder Cancer. Nature 2014, 515, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.-C.; Cheng, H.-Y.; Lin, T.-S.; Chen, W.-H.; Lin, J.-H.; Lin, J.-J.; Lu, C.-C.; Chiang, J.-H.; Hsu, S.-C.; Wu, P.-P.; et al. Epigallocatechin Gallate (EGCG), Influences a Murine WEHI-3 Leukemia Model In Vivo Through Enhancing Phagocytosis of Macrophages and Populations of T- and B-Cells. Vivo 2013, 27, 627–634. [Google Scholar]
- Lowe, G.M.; Gana, K.; Rahman, K. Dietary Supplementation with Green Tea Extract Promotes Enhanced Human Leukocyte Activity. J. Complement. Integr. Med. 2015, 12, 277–282. [Google Scholar] [CrossRef]
- Gana, K.; Rahman, K.; Lowe, G.M. Immunomodulation of Isolated Human Neutrophils by a Green Tea Extract. J. Nutraceuticals Funct. Med. Foods 2003, 4, 15–26. [Google Scholar] [CrossRef]
- Kim, Y.H.; Won, Y.-S.; Yang, X.; Kumazoe, M.; Yamashita, S.; Hara, A.; Takagaki, A.; Goto, K.; Nanjo, F.; Tachibana, H. Green Tea Catechin Metabolites Exert Immunoregulatory Effects on CD4+ T Cell and Natural Killer Cell Activities. J. Agric. Food Chem. 2016, 64, 3591–3597. [Google Scholar] [CrossRef]
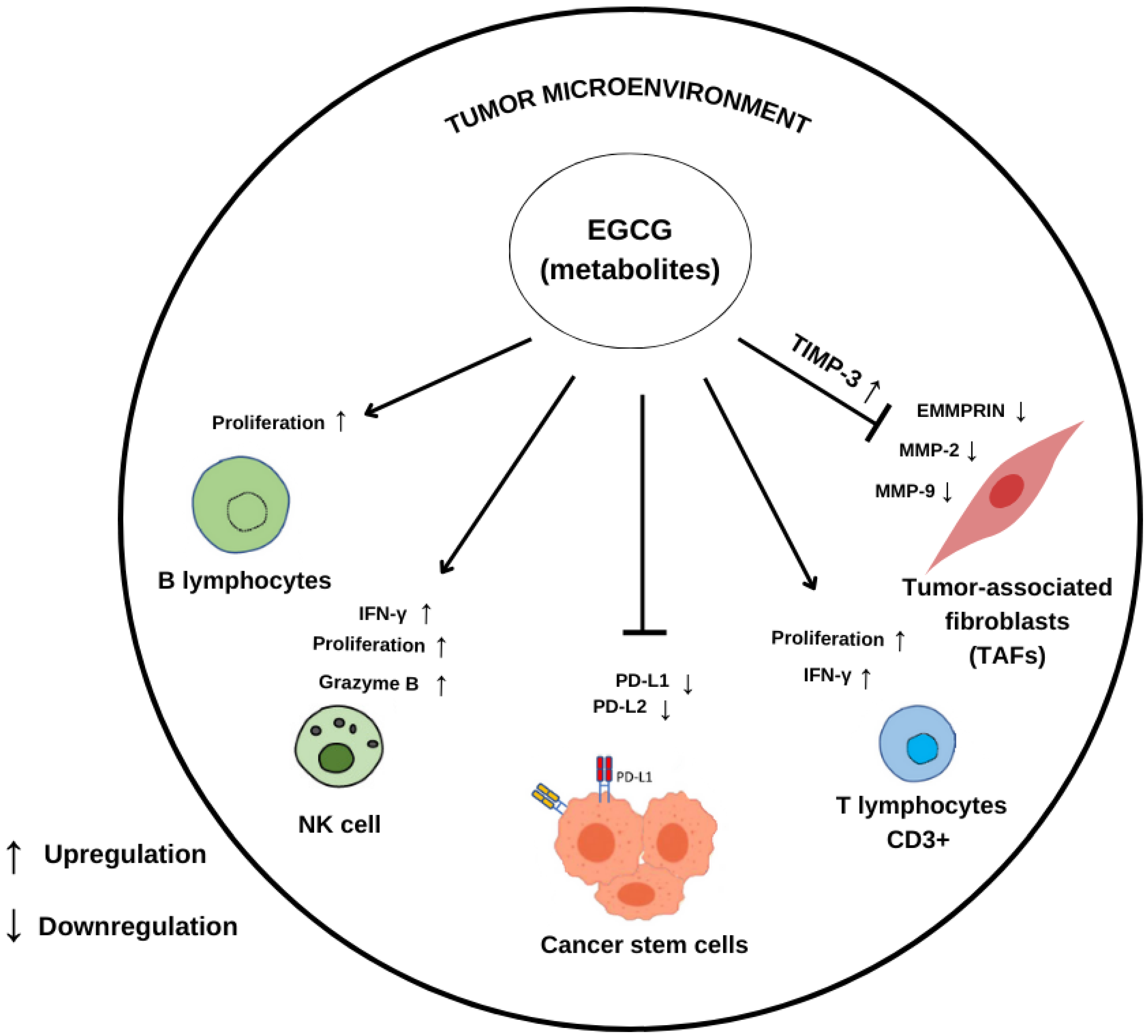
| Compound/ Concentration Used | Experimental Model | Modulatory Effects ↑ Upregulation ↓ Downregulation | Reference |
|---|---|---|---|
| EGCG 20 μM | Breast cancer cells MCF-7 MDA-MB-231 | ↑ TIMP-3 ↓ MMP-2 ↓ MMP-9 | [86] |
| GTP 10 μg/mL | Breast cancer cells MCF-7 MDA-MB-231 | ↑ TIMP-3 ↓ MMP-2 ↓ MMP-9 | [86] |
| EGCG 50 µg/mL | Tamoxifen-resistant breast cancer cell MCF-7 Tam | ↓ EGFR ↓ MMP-2 ↓ MMP-9 ↓ EMMPRIN | [64] |
| EGCG 10 µM | Melanoma cell lines 1205 Lu, A375, HS294T | Inhibits expression of PD-L1/L2 | [65] |
| EGCG 50 or 100 mg/kg | In vivo C57BL/6 mice | Inhibits JAK/STAT signaling ↓ PD-L1 T cell reactivation | [65] |
| EGCG 50 µM | NSCLC cell lines A549 and Lu99 | Inhibits the PD-1/PD-L1 checkpoint ↓ EGF-induced PD-L1 expression | [66] |
| GTE 0.3% | In vivo A/J mice with induced lung carcinogenesis | ↓ PD-L1 positive cells | [66] |
| EGCG 10 to 30 µM | Co-culture of Melanoma F10-OVA with CD3+ T cells | ↓ PD-L1 ↑ Interleukin-2 ↑ T lymphocytes activity | [66] |
| EGCG 5, 20, and 40 mg/kg | In vivo BALB/c mice with induced leukemia | ↑ B lymphocyte ↑ T lymphocyte ↑ NK cell activity ↑ Macrophages phagocytosis | [93] |
| GTE 300 mg/14 days | In vivo Human model | ↑ Leukocyte activation ↑ Myeloperoxidase ↑ Lactoferrin | [94] |
| GTE 10 µL | neutrophils | ↑ Superoxide ↑ Myeloperoxidase | [95] |
| EGC-M5 5, 10, 25, and 50 μM | CD4+ T cells | ↑ CD4+ T cell activity | [96] |
| EGC-M5 5, 10, 25, and 50 μM | Splenocytes | ↑ IFN-γ | [96] |
| EGC-M5 10 mg/kg | In vivo BALB/c mice | ↑ granzyme B+ NK cells | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, E.D.S.; Santos, R.A.; Guillermo, L.V.C.; Miyoshi, N.; Ferraz da Costa, D.C. Immunomodulatory Effects of Green Tea Catechins and Their Ring Fission Metabolites in a Tumor Microenvironment Perspective. Molecules 2024, 29, 4575. https://doi.org/10.3390/molecules29194575
Andrade EDS, Santos RA, Guillermo LVC, Miyoshi N, Ferraz da Costa DC. Immunomodulatory Effects of Green Tea Catechins and Their Ring Fission Metabolites in a Tumor Microenvironment Perspective. Molecules. 2024; 29(19):4575. https://doi.org/10.3390/molecules29194575
Chicago/Turabian StyleAndrade, Emmanuele D. S., Ronimara A. Santos, Landi V. C. Guillermo, Noriyuki Miyoshi, and Danielly C. Ferraz da Costa. 2024. "Immunomodulatory Effects of Green Tea Catechins and Their Ring Fission Metabolites in a Tumor Microenvironment Perspective" Molecules 29, no. 19: 4575. https://doi.org/10.3390/molecules29194575
APA StyleAndrade, E. D. S., Santos, R. A., Guillermo, L. V. C., Miyoshi, N., & Ferraz da Costa, D. C. (2024). Immunomodulatory Effects of Green Tea Catechins and Their Ring Fission Metabolites in a Tumor Microenvironment Perspective. Molecules, 29(19), 4575. https://doi.org/10.3390/molecules29194575






